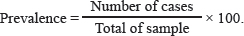| Research Article | ||
Open Vet J. 2023; 13(11): 1443-1450 Open Veterinary Journal, (2023), Vol. 13(11): 1443–1450 Original Research Tissue cysts and serological detection toxoplasmosis among wild rats from Surabaya, East Java, IndonesiaHeni Puspitasari1,2, Lucia Tri Suwanti2,3*, Mufasirin Mufasirin2,3, Kusnoto Kusnoto3, Ira Sari Yudaniayanti4, Boedi Setiawan4, Endang Suprihati3, Eduardus Bimo Aksono5, Dwi Priyo Widodo6 and Elly Nur Indasari21Doctoral Program of Veterinary Science, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 2Toxoplasma Study Group, Institute of Tropical Disease, Universitas Airlangga, Surabaya, Indonesia 3Division of Parasitology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 4Division of Clinic Veteriner, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 5Division of Veterinary Medicine, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 6Department of Parasitology, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia *Corresponding Author: Lucia Tri Suwanti. Toxoplasma Study Group, Institute of Tropical Disease, Universitas Airlangga, Surabaya, Indonesia. Email: lucia-t-s [at] fkh.unair.ac.id Submitted: 03/07/2023 Accepted: 27/10/2023 Published: 30/11/2023 © 2023 Open Veterinary Journal
AbstractBackground: The protozoan Toxoplasma gondii is the source of zoonosis toxoplasmosis and causes public health problems throughout the world. Environmental contamination by oocysts excreted by cats as definitive hosts affects the spread of this disease. Wild rats as rodents can be used as an indicator of environmental contamination by oocysts, considering that rats have a habit of living in dirty environments and can be infected by oocysts from the environment. Aim: This study aims to detect toxoplasmosis from tissue cysts and serological tests in wild rats as an indicator of environmental contamination in Surabaya. Methods: A total of 100 wild rats collected from Surabaya were collected in five areas (West, East, Central, North, and South of Surabaya) obtained from three trapping locations: housing, dense settlements, and markets. All samples were examined microscopically for parasitological tests through the brain tissue samples, and the serum was examined using the toxoplasma modified agglutination test to detect the presence of IgG and Immunoglobulin M (IgM). Results: This research used 100 wild rat samples, 77 Rattus tanezumi and 33 Rattus norvegicus, with evidence of 31% in serology and active infection with 19% tissue cyst. The results showed that the seroprevalence of T. gondii in wild rats was 31% (30% for IgG and 1% for IgM). Tissue cysts in the rat brain samples tested were 19% (19/100). The IgG prevalence rate in female rats was 25% (8/32), while for males, it was 32.3% (22/68). The highest seropositive IgG from densely populated settlements was 50%, markets were 25.8%, and housing was 12.1%. The highest seropositive IgM from densely populated settlements was 2.8%. Population density and the presence of cats are factors supporting the high seropositive rate at the trapping location. Conclusion: This study revealed that there has been toxoplasmosis contamination in Surabaya with evidence of 31% in serology and active infection with 19% tissue cyst. It is necessary for controlling with surveillance in cats to prevent transmission in humans. Keywords: Tissue cyst, Public health, ToMAT, Toxoplasmosis, Wild rats. IntroductionZoonotic toxoplasmosis is a global public health problem caused by infection with the protozoan Toxoplasma gondii (T. gondii) (Aguirre et al., 2019). This is because T. gondii is distributed globally, and besides human health, it also has an impact on animal health (Wormer et al., 2016). Toxoplasma gondii is an obligate intracellular parasite. Three methods are available for this parasite to infect a host during its life cycle: bradizoit by the consumption of tissue cysts, oocysts, and tachizoit through congenital infection (Al-Fertosi and Juma, 2006; Nurdianto et al., 2020). The definitive host for this parasite is the Felidae family (especially domestic cats), and the intermediate host is warm-blooded vertebrates, including humans, rodents, birds, livestock, and marine mammals (Saki and Khademvatan, 2014). Only the asexual stage has developed in the intermediate host, not the sexual stage. Cysts develop in the cat's intestine during the sexual period when it consumes rats (Hiswani, 2003; Praptiwi et al., 2016). Cats after infection can excrete oocysts in their feces, which contaminates the environment (Jiang et al., 2020). The cycle of toxoplasmosis involves the spread of the disease from rats to cats and people. Rats are the primary source of infection for the Felidae, which is why they are important in the epidemiology of toxoplasmosis. When oocysts sporulate in the environment, they become infectious for both humans and other animals, including rats. Wild forage on the ground can become infected with T. gondii. Rats, as an intermediate host and reservoir, play an important role in the life cycle and transmission of T. gondii infection. Toxoplasma gondii in the cystic stage can become a source of transmission for their offspring, predators (cats), and other animals (Galeh et al., 2021). Cats can be reinfected by Toxoplasma gondii and excrete oocysts back in large quantities into the environment (Zulpoa et al., 2018). Therefore, it is very important to conduct research on toxoplasmosis in rats as an indicator of environmental contamination by oocysts. Prevalence of toxoplasmosis in rats in several parts of the world is in Africa at 24.24%, Asia at 18.07%, Australia at 4.63%, Europa at 12.58%, North America at 8.21%, and South America at 19.40% (Galeh et al., 2020). Rodents are a key source of T. gondii infection for other hosts in addition to serving as intermediate hosts for the parasite (Bahadori et al., 2018). The incidence of toxoplasmosis in rats has been reported in several countries. However, research on toxoplasmosis in Indonesia is still very limited and the methods used are serological. Researchers only found two publications on the incidence of toxoplasmosis in rats, in Banjarnegara at 3.7% (Wijayanti and Marbawati, 2018) and in Yogyakarta at 4.8% (Agustin and Noor, 2009). Materials and MethodsSampling techniqueThis research was a cross-sectional study, conducted in the field (housing, dense settlements, and markets) to collect rat samples and in the Laboratory (Veterinary Parasitology and Balai Veteriner Lampung) to observe the presence of T. gondii parasitologically and serologically as well as molecularly. The experimental protocol was approved by the Animal Care and Use Committee of the Faculty of Veterinary Medicine, Airlangga University No. 2.KEH.080.07.2022. Study areaTrapping wild rats in the field is carried out in housing, dense settlements, and markets in Surabaya. Surabaya is divided into five regions; East, West, South, North, and Central Surabaya. Catching wild rats using mouse traps. Trapping ratsTrapping rats using a live trap. Traps were made at night in five areas in Surabaya (North, West, South, East, and Central Surabaya), and in each area, the sampling locations included housing, dense settlements, and markets. The number of samples depends on the trap at each location which was carried out for 2 months and it is estimated that there are more than 100 rats. Furthermore, the rats were sacrificed to take blood without giving anticoagulants for serological tests, parasitological tests, and bioassay. Blood sample collection and serological testRats were anesthetized using ketamine HCl at a dose of 50–100 mg/kg body weight by intramuscular injection in the thick muscle of the rat's thigh (Donald, 2002). A total of 100 wild rats trapped in the field (housing, dense settlements, and markets) were collected from June to September 2022. Next, approximately 3–5 ml of blood was taken from the heart using a 3 cc syringe then the blood was collected in a non-EDTA tube and stored overnight at room temperature to coagulate for serum separation. Separation of serum was obtained by centrifugation at 3,000 rpm for 10 minutes, which was carried out at the Institute of Tropical Disease and at the Parasitology Laboratory, Faculty of Veterinary Medicine, Airlangga University. The isolated serum was stored at −20ºC until it was used. After blood collection, followed by labeling and identification. Toxoplasmosis seroprevalence testing uses the toxoplasma modified agglutination test (ToMAT) to detect IgG and IgM (Syukran et al., 2017). The serum was directed with the ToMAT test reagent (red for IgG and blue for IgM) according to the instructions in Balai Veteriner Lampung. The results are read using a reading mirror, the negative results look like a small dot at the bottom of the well because the ToMAT reagent will settle to the bottom of the microplate well. Parasitological testThe parasitological test was carried out by a brain homogenization test (Maulana et al., 2017). Rat brains were homogenized in 1.5 ml of saline buffer (0.85% NaCl) with a mortar and passed through a 22-gauge needle and syringe. Two portions of the homogenate are separated. For the purpose of observing tissue cysts, one part was added to the same volume of 10% formalin and kept at room temperature. Brain pressure examination is done by taking a small part of the brain and placing it on a glass object. The brain is then covered with a cover glass and then another object glass is placed on top of the cover glass. Pressed with the thumb and viewed under a microscope with a magnification of 400× to 1,000× (Mufasirin and Suwanti, 2008). BioassayMicroscopically identified T. gondii bradyzoite cysts were isolated and then bioassayed on mice (Mus musculus) strain balb/c. Brain tissue was a specimen that was suspended and injected intraperitoneally into two healthy mice. One mouse was sacrificed 1 week after infection to see tachyzoites in the intraperitoneal fluid. Until day 60 after vaccination, the inoculated mice were monitored every day for the appearance of clinical symptoms. When mice died within 2 weeks of infection, a T. gondii isolate was deemed to be virulent. To determine whether there were any cysts in the mice's brains, the surviving mice were put to death 5–6 weeks after infection (Dubey et al., 2016). Ethical approvalThe experimental protocol was approved by the Animal Care and Use Committee of the Faculty of Veterinary Medicine, Airlangga University No. 2.KEH.080.07.2022. ResultsParasitological testThe results of the examination T. gondii cysts on 100 wild rats collected from Surabaya in June–September 2022. Microscopically showed several positive results with the discovery of cyst stages containing T. gondii bradyzoites in the rat brains. The results of the microscopic examination are shown in Figure 1. The average diameter of tissue cysts is 20.65 µm. The prevalence in the parasitological test is the percentage of rats infected with T. gondii compared to the total sample. The prevalence rate is calculated using the following equation:
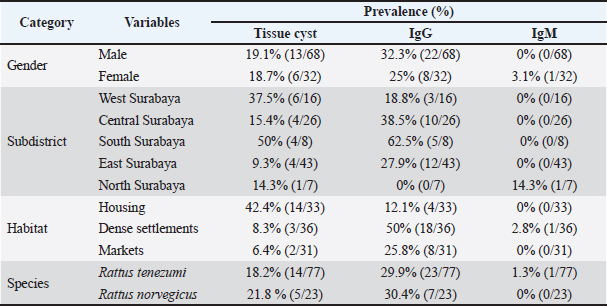
This research used 100 wild rat samples, 77 Rattus tanezumi and 33 Rattus norvegicus. The microscopic prevalence of T. gondii infection in R. tanezumi was 18.2% (14/77) and R. norvegicus was 21.8% (5/23). The general prevalence of T. gondii cysts microscopically was 19% (19/100) (Table 1). This value can be categorized in the prevalence of often (William and Bunkley, 1996. These results indicate that parasites often infect. Serological testMany methods can be used for serological testing of T. gondii antibodies. Serological test of T. gondii antibodies in this study used the To-MAT technique which has been standardized and validated by the Balai Besar Veteriner Lampung with a test accuracy of 94.89%, a sensitivity of 98.55%, and a specificity of 86.26%. The To-MAT method is one of the laboratory diagnostic methods for toxoplasmosis infection and is included in direct agglutination examination. IgM is the first antibody formed in an immune response (Nopitasari and Keman, 2014). This research used 100 wild rat samples, 77 R. tanezumi, and 33 R. norvegicus. The serological prevalence of T. gondii infection in R. tanezumi was 29.9% (23/77) for IgG and 1.3% (1/77) for IgM. Meanwhile, the serological prevalence of T. gondii infection in R. norvegicus was 30.4% (7/23) for IgG and 0% (0/23) for IgM (Table 1).
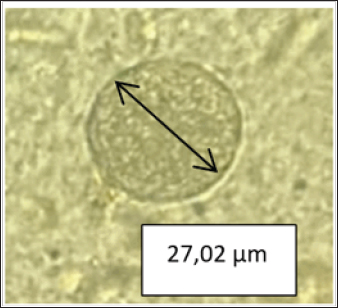
Fig. 1. tissue cysts in rat brain, 400× magnification with a diameter of 27.02 µm. Serological test for the presence of tissue cystThe results of IgG seroprevalence for the presence of bradyzoites in rat brains were 63.3% (19/30), while for IgM it was 0% or no bradyzoite cysts were found (Table 1). The presence of bradyzoites in samples with seropositive IgG and IgM although not significantly indicating a correlation, can indicate that indeed infection has occurred and is confirmed microscopically. Tachyzoites differentiate into bradyzoites via compartments and parasitophorous vacuoles that are thought to differentiate into the outer wall of the cyst. Initial infection and acute infection begin with the rapid division of tachyzoites (Lesmana, 2010). Approximately 10–14 days after infection, the tachyzoites differentiate into bradyzoites, which divide slowly to form tissue cysts. Tissue cysts can be in the body and do not cause clinical manifestations. Mice live in contact with the soil or are all scavengers such as pigs and can eat plants or drink water containing sporulated oocysts without being noticed. As soon as the sporulated oocyst is ingested by animals, it transforms into tachyzoites which are present in nerve and muscle tissue. Other research says that oocysts that are ingested by chickens will develop into cysts, settle in the chicken's body, and infect the organs of the chicken. Cysts are found in many organs, especially the brain, skeletal muscle, and heart. Wild rats can be infected with T. gondii from eating and drinking in dirty places contaminated with oocysts. Cat feces are not the only medium for the growth and transmission of T. gondii. Soil, plants, and even water are places where sporulated oocysts develop before being consumed by birds or rodents. The epidemiology and maintenance of T. gondii circulation in the country may be influenced by wild rats, which are potential T. gondii intermediate hosts and may be significant hosts (Galeh et al., 2020). The results of positive IgG seroprevalence 30 days later for the presence of bradyzoites on pressure examination of the rat brain showed 19 positive bradyzoites and 11 negatives. Of the 11 samples that were negative for bradyzoites, 13.6% were still false positives and 86.4% were false negatives. In 11 samples of negative bradyzoite results for the environment, a positive value of 21.2% was found in residential and housing trapping areas while in the market environment, it was not found. This is possible because the tissue cyst has not yet formed and microscopic examination of the brain pressure only takes a small part of the brain tissue even though it has been homogenized before. Table 1. Demographic data for prevalence toxoplasmosis of wild rats in Surabaya.
Table 2. Serological test.
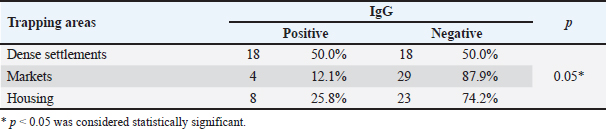
Serological test of the trapping areaWild rats in Surabaya were divided into five trapping areas. The seroprevalence among the five rats trapping that had the highest prevalence was South Surabaya at 62.5% (5/8), then Central Surabaya at 38.5% (10/26), East Surabaya at 27.9% (12/43), West Surabaya at 18.8% (3/16), and for North Surabaya 0 of 7 samples (Table 1). Immunoglobulin M (IgM) serological results for the trapping area showed positive only in the North Surabaya trapping area, it was not found in other trapping areas. This is due to the positive results of IgM only found in the trapping area of North Surabaya. IgM is formed when there is an acute infection and will disappear within 2–3 weeks, which is then replaced by immunoglobulin G which lasts a lifetime. IgM antibodies are formed early in the infection, appear around the third day, and can be stable in the blood for up to 3−4 months after infection (To et al., 2020). IgM seroprevalence from the five trappings was only in North Surabaya which had a positive result of 14.3% (1/7) and for the other rat trapping no IgM serology results were found. Toxoplasmosis cases in Northern Iran based on serological tests reached 56% of which 45% were males and 55% were females (Wijayanti and Marbawati, 2018). From Table 2, it can also be concluded that the p-value > 0.05 means that the IgM seroprevalence for the five trapping areas gives significantly different values. IgG seroprevalence from the five trappings had a positive result West Surabaya 18.8% (3/16), Central Surabaya 38.5% (10/26), South Surabaya 62.5% (5/8), East Surabaya 27.9% (12/43), and North Surabaya 0% (0/7). Serological test based on the environment of ratsIn this study, the trapping area was also used as a parameter to determine the serological prevalence of toxoplasmosis among wild rats of Surabaya. Dense settlements gave the highest IgG seroprevalence rate compared to the other 50% (18/36), markets had an IgG seroprevalence of 25.8% (8/31), while housing had an IgG seroprevalence of 12.1% (4/33) (Table 1). Based on the trapping area, IgG seroprevalence gives a significant value, this shows that there is an influence of the trapping environment of wild rats on the incidence of toxoplasmosis in Surabaya. A significant value is found in IgG seroprevalence in the market (Table 2). Serological tests based on the gender of ratsIgG seroprevalence rate in female rats was 25% (8/32), while for males, it was 32.3% (22/68) (Table 1). The seroprevalence of IgG in males was greater than in females although not significantly different, this indicated that gender did not have an effect on the value of IgG seroprevalence in rats in Surabaya. The percentage of male IgG is greater because most of the rat’s sample are male. The capture of rats is also influenced by the development and population of wild rats. Types of food, arrangement of goods, temperature, and humidity are factors that influence rat breeding. An intermediate host's behavior may be altered by Toxoplasma gondii infection of the host's brain. Toxoplasma gondii can survive in the host and change their behavior to ensure that transmission continues (Webster, 2001). DiscussionRat brains containing bradyzoite-filled, globular T. gondii cysts were discovered. Brain tissue analysis can be used to detect environmental contamination by determining the frequency of T. gondii infection in native and commensal rodents (Ivovic et al., 2019). Bradizoites are covered with thin cyst walls of varying sizes, with a cyst diameter of around 5–50 µm. Cyst sizes vary, there are small cysts containing only a few bradyzoites and 200 µm containing approximately 3,000 bradyzoites (Gandahusada et al., 1998). Tissue cysts form after 10–14 days of pasca infection. About 10–14 days after infection, the tachyzoites differentiate into bradyzoites which divide slowly to form tissue cysts. Tachyzoites will differentiate into tissue cysts in host cells (Dubey, 1998). A total of 100 samples were detected microscopically, and only 19 samples were positive for tissue cysts in the brain because the process of cyst formation in the body takes a long time and many factors. The process of changing tachyzoites into bradyzoites depends on the T. gondii strain. Non-virulent strains tend to change from the tachyzoite stage to become bradyzoites and then form cysts. The change in the tachyzoite stage to bradyzoite also depends on the speed of multiplication, pH, environmental temperature, and the presence of anti-mitochondrial drugs (NO) in the host's body (Susanto et al., 1998). A total of 19 samples with positive results indicated that the infection was chronic, characterized by the formation of brain tissue bradyzoite cysts. In line with research from chronic infections, parasites develop slowly and form bradyzoites in host tissue, and cysts can last for months in brain tissue, for example. Tachyzoite–bradyzoite stage differentiation or vice versa is the basis for chronic infection and the reactivation of chronic infection into acute infection (Hanafiah et al., 2013). Initial infection and acute infection are preceded by rapid division of tachyzoites. Approximately 10–14 days after infection, the tachyzoites differentiate into bradyzoites, which divide slowly to form tissue cysts. Tissue cysts can be in the body and do not cause clinical manifestations. In humans, tissue cysts form on average 10–14 days after infection. The number and location of tissue cysts depend on the host and strain of T. gondii. In mice, tissue cysts are found in the brain and internal organs. Meanwhile, in higher mammals such as goats, cows, and cats, there are more tissue cysts in the muscles than in the brain (Dubey, 1998). In this cross-sectional study, the results were 31% seropositive for T. gondii in 100 wild rat samples in Surabaya, 1% seropositive IgM, and 30% IgG. The results of serological testing of IgG against T. gondii in rat serum samples captured in Surabaya were 30% (30/100). These results indicate that there is still a potential for transmission of T. gondii. Due to the ease with which infected rats can spread T. gondii to other animals, the high prevalence of this unicellular parasite in wild rats is particularly significant in this case. Cats, dogs, and other animals may contract T. gondii tissue cysts from wild rats. On the other hand, because cats are considered to have contracted the illness by eating these infected mice, the prevalence of T. gondii infection in cats actually reflects infection rates in nearby rodents. A similar study conducted in Banjarnegara Regency produced 3.57% (3/84 mice) of toxoplasmosis positive sero (Wijayanti and Marbawati, 2018). In line with these results, another study stated that the seroprevalence of T. gondii in Northern Iran was 56% (Hosseini et al. 2021). Another study conducted in Poland on cases of Toxoplasmosis in Rodents stated that the seroprevalence was 5.5% (Grzybek et al., 2021). In line with research, regardless of the serological examination method used, the global seroprevalence of T. gondii infection in rodents; 0.3% in the USA (Smith et al., 1992), 0.8% in West India (Dubey et al., 2016), Southern China 3.2% (Webster, 1994), Panama 23.4% (Frenkel et al., 1995), England 35% (Webster, 1994), and Philippines 58% (Salibay and Claveria, 2005). Many studies have provided information regarding the seroprevalence of T. gondii in both humans and animals. The prevalence of T. gondii in animals in Indonesia, data obtained from dogs 75%, cats 35%–73%, goats 11%–61%, cattle 36.4%, pigs 11%–36%, and other livestock 10% (Setiati et al., 2014). Epidemiologically, this disease is widespread throughout the world. According to data from the center for disease control and prevention, it shows that as many as 60% of the population was infected with toxoplasmosis in 2018. The seroprevalence in Yogyakarta is 61.5% (Sujono, 2010). Meanwhile, the seroprevalence rate in Indonesia states that data in Surabaya is 58%, and in Jakarta, it is 70% (Terazawa et al., 2003). Wild rats that live in a human environment usually consume all human food ingredients so they are classified as omnivores. Rats can be infected with toxoplasmosis from contamination of T. gondii oocysts. Wild rats can become persistent intermediate hosts of T. gondii through vertical transmission, cannibalism, and horizontal transmission through insects as paratenic hosts. Rats infected with T. gondii can cause toxoplasmosis in cats through carnivorism. The high infection of toxoplasmosis rats indicates that rats may play a role in the transmission of toxoplasmosis in Surabaya epidemiologically and the positive number is highest in densely populated residential areas. This study indicates that toxoplasmosis in rats may be related to toxoplasmosis in cats found in densely populated environments. Rats can also be infected with toxoplasmosis from consuming cockroaches contaminated with T. gondii oocysts (Jittapalapong et al., 2009). Rats with chronic T. gondii infection lost their fear of the scent of cat urine, according to the findings of a behavioral study that contrasted rats with and without infection with rats who were infected with the parasite (Webster, 2001). Changes in behavior caused by this parasite can increase the risk of predation in infected rats, which leads to an increase in the transmission rate of the parasite to the definitive host (Berenreiterova et al., 2011). The mechanism underlying this change in behavior is still unclear, but one of the factors that is thought to cause the change in the behavior of the intermediate host is the increase in dopamine levels in chronic T. gondii infection (Prandovszky, 2011). According to Anasis (2019), a T. gondii infection can boost dopamine signals in the host brain by increasing the activity of the enzymes that produce them. The host's behavior is influenced by the elevated dopamine signal, which increases exploration of the surroundings, including cat urine. Behavior to explore the surrounding environment is the reason rats can be caught in the Surabaya environment. The prevalence of IgM also gave results that were not significantly different. In contrast to the research by Wijayanti and Marbawati (2018), it was stated that in his study the rats that were mostly caught were females. The prevalence of T. gondii did not statistically differ by species, location, or age (Wang et al., 2018). ConclusionOur findings indicate that T. gondii has been circulating in Surabaya, and wild rats have the potential to transmit the infection. It is necessary to control with surveillance environmental contamination of T. gondii to prevent transmission to humans. AcknowledgmentsThe authors express sincere gratitude to the Faculty of Veterinary Medicine, Universitas Airlangga, for supporting this study. Conflicts of interestThe authors declare that there is no conflict of interest. FundingThe authors are grateful to the Directorate General of Higher Education for the research grant for the doctoral program with contract number 759/UN3.15/PT/2022. Authors contributionsLTS concept and design the proposal, coordinating research, and wrote article manuscripts. HP collected data, biopsy, serology, and the literature review. ENI, MM, KK, ISY, and BS collected and analyzed the data. ES, EBA, and DPW revised and proofreading. All authors revised and proofreading manuscript. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesAguirre, A.A., Longcore, T., Barbieri, M., Dabritz, H., Hill, D., Klein, P.N., Lepczyk, C., Lilly, E.L., McLeod, R., Milcarsky, J., Murphy, C.E., Su, C., Wormer, E.V., Yolken, R. and Sizemore, G.C. 2019. The one health approach to toxoplasmosis: epidemiology, control, and prevention strategies. Eco. Health. 16, 378–390. Agustin, N.N. and Noor, Z. 2009. The difference of toxoplasmosis prevalence at mouse using serologic test with the ELISA method in kecamatan Wirobrajan and the surroundings. Mutiara Medika. 9(1), 1–5. Anasis, A.M. 2019. Changes in behavior in mice with Toxoplasma gondii infection. J. lIlmu Kedokteran dan Kesehatan. 6(1), 49–56. Al-Fertosi, R.B. and Juma, A.S.M. 2006. Possible cellular expression of IFNγ in a woman with abortion infected Toxoplasma gondii. Med. J. Islamic World Acad. Sci. 16(3), 121–134. Bahadori, E.S., Javid, S., Abdolhosein, D., Somayyeh, N. and Majid, P. 2018. Phylogenetic analysis of Toxoplasma gondii type II and type III by PCRRFLP plus sequencing on wild-rats of golestan forest, Iran. J. Vet. Sci. Technol. 9, 3. Berenreiterova, M., Flegr, J., Kubĕna, A.A. and Nĕmec, P. 2011. The distribution of Toxoplasma gondii cysts in the brain of a mouse with latent toxoplasmosis: implications for the behavioral manipulation hypothesis. PLoS One 6(12), e28925. Donald, C.P. 2002. Veterinary drug handbook, 4th ed. Lowa State Press. Dubey, J.P. 1998. Advances in the life cycle of Toxoplasma gondii. Int. J. Parasitol. 11, 267–299. Dubey, J.P., Ferreira, L.R., Alsaad, M., Verma, S.K., Alves, D.A., Holland, G.N. and McConkey, G.A. 2016. Experimental toxoplasmosis in rats induce orally with eleven stains of Toxoplasma gondii of seven genotype: tissue tropism, tissue cyst size, neural lesions, tissue cyst rupture without reaction, and ocular lesions. PLoS One 11(5), e0156255. Frenkel, K., Quintero-Nunez, R., Hassanein, R.S., Ulliez, P., Hassanein, K.M. and Quintero-Nunez, R. 1995. Transmission of Toxoplasma gondii in Panama city, Panama: a five-year prospective cohort study of children, cats, rodents, birds, and soil. Am. J. Trop. Med. Hyg. 53(5), 458–468. Galeh, T.M., Sarvi, S. and Montazeri, M. 2020. Global status of Toxoplasma gondii seroprevalence in rodent: a systematic review and meta-analysis. Front. Vet. Sci. 7, 461. Galeh, T.M., Shahabeddin, S., Seyed, A.H. and Ahmad, D. 2021. Genetic diversity of Toxoplasma gondii isolates from rodents in the world: a systematic review. Transbound. Emerg. Dis. 69(3), 943–957. Gandahusada, S., Ilahude, H. and Pribadi, W. 1998. Parasitologi Kedokteran. Edisi Ketiga. Jakarta, Indonesia: Fakultas Kedokteran Universitas Indonesia. Grzybek, M., Antolovo, D., Tolkacz, K., Alsarraf, M., Borowczyk, J.B., Nowicka, J., Paleolog, J., Biernat, B., Behmke, J.M. and Bajer, A. 2021. Seroprevalence of Toxoplasma gondii among sylvatic rodents in Poland. Animals 11(4), 1048. Hanafiah, M., Nurcahyo, W., Liza, D. and Karmil, T.F. 2013. Application of intradermal test diagnosis tool using protein membrane derived from bradizoite stage of Toxoplasma gondii to diagnose toxoplasmosis. J. Vet. 14(2), 213–220. Hiswani. 2003. Tesis: toxoplasmosis penyakit zoonosis Yang Perlu Diwaspadai Oleh Ibu Hamil. Kota Medan, Indonesia: Fakultas Kesehatan Masyarakat, Universitas Sumatera Utara. Hosseini, S.A., Abediankenari, S., Amauei, A., Sarvi, S., Sharif, M., Razaei, F. and Daryani, A. 2021. Seroprevalence of Toxoplasma gondii in wild rats (Rattus rattus) in Northern Iran. Vet. Med. Int. 27, 6655696. Ivovic, V., Potusek, S. and Buza, E. 2019. Prevalence and genotype identification of Toxoplasma gondii in suburban rodents collected at waste disposal sites. Parasite 26, 27. Jiang, N., Xin, S.L., Su, C., Zhang, L. and Yang, Y. 2020. Isolation and characterization of Toxoplasma gondii from captive caracals (caracal caracal). Int. J. Parasitol. Parasites. Widl. 13, 196–201. Jittapalapong, S., Herbreteau, V., Hugot, J.P., Areesrisom, P., Karnchanabanthoeng, A., Rerkamnuaychoke, W. and Morand, S. 2009. Rodent biodiversity human health and pest control in a changing environments relationship of parasites and pathogens diversity to rodents in Thailand. Kasetsart. J. Nat. Sci. 43(1), 106–117. Lesmana, S.D. 2010. Diferensiasi stadium Takizoit-Bradizoit pada Toxoplasma gondii. JIK. 4(2), 89–94. Maulana, D.M., Muchlisin, Z.A. and dan Sugito, S. 2017. Intensitas dan Prevalensi Parasit Pada Ikan Betok (Anabastestudineus) dari Perairan Umum Daratan Aceh Bagian Utara. J. Ilmiah Mahasiswa Kelautan dan Perikanan. 2(1), 1–11. Mufasirin, M. and Suwanti, S. 2008. Detection of Toxoplasma gondii on the local chicken eggs sold as the mixture of traditional medicine in Surabaya using biological test. Media. Kedokteran. Hewan. 24(1), 9–14. Nopitasari, R. and Keman, S. 2014. The IgM incidence and IgG prevalence of positive anti-toxoplasma in Kedurus Abattoir workers at Surabaya. J. Kesehatan. Lingkungan. 7(2), 98–106. Nurdianto, A.R., Aryati, A., Suryokusumo, M.G., Mufasirin, M., Suwanti, L.T., Sunarjo, Sardjono, T.W. and Dachlan, E.G. 2020. Effect of hyperbaric oxygen therapyon IL-17, fetal body weight and total fetus in pregnant Rattus norvegicus infected with tachizoit Toxoplasma gondii. Syst. Rev. Pharm. 11(3), 628–634. Prandovszky, E. 2011. The neurotropic parasite Toxoplasma gondii increases dopamine metabolism. PLoS One 6(9), e23866. Praptiwi, Y., Puspitasari, H., Suwanti, L.T. and Mufasirin, M. 2016. Production and characterization of immunologlobuline yolk as anti antigen membrane Toxoplasma gondii. Indones. J. Trop. Infect. Dis. 6(2), 29–33. Saki, J. and Khademvatan, S. 2014. Detection of Toxoplasma gondii by PCR and mouse bioassay in rodent of Ahvaz Distric, Southwestern Iran. Biomed. Res. Int. 2014, 1–5. Salibay, C.C. and Claveria, F.G. 2005. Serologic detection of Toxoplasma gondii infection in Rattus spp collected from free different sites in Dasmarinas, Cavite, Philippines, Southeast Asian. J. Trop. Med. Public. Health. 36(4), 46–49. Setiati, S., Alwi, I., Sudoyo, A.W., Simadibrata, M., Setiyohandi, B. and Syam, A.F. 2014. Buku ilmu penyakit dalam FKUI. Jakarta, Indonesia: Pusat Penerbitan Ilmu Penyakit Dalam. Smith, K.E., Zimmerman, J.J., Patton, S., Beran, G.W. and Hill, H.T. 1992. Epidemiology of toxoplasmosis in Iowa swine farms with an emphasis on the roles of free-living mammals. Vet. Parasitol. 42(3–4), 199–211. Sujono, S. 2010. Seroprevalensi toxoplasmosis dan Faktor-Faktor Risiko di Daerah Istimewa Yogyakarta dengan Metode ELISA Menggunakan Protein Rekombinan GRA-1 Takizoit Toxoplasma gondii Isolat Lokal. Thesis, Program Pasca Sarjana, Universitas Gadjah Mada, Yogyakarta, Indonesia. Susanto, L., Srisasi, G. and Rusli, M. 1998. Invasi Toxoplasma gondii kedalam sel hospes serta diferensiasinya dari takizoit ke bradizoit. Majalah. Kedokt. Indones. 49(6), 208–211. Syukran, M, Rahimi, S.A.E. and Wujaya, S. 2017. Intensity and prevalence of ectoparasites on betta fish (Betta splendens) in the district of Aceh Besar and Banda Aceh city waters. J. Ilmiah Mahasiswa. Kelautan dan Perikanan Unsyiah. 2(1), 221–228. Terazawa, A., Muljono, R., Susanto, L., Margono, S. and Konishi, E. 2003. High toxoplasma antibody prevalence among inhabitants in Jakarta, Indonesia. Japanese. J. Infect. Dis. 56(3), 107–109. To, K.K.W., Tsang, O.T.Y. and Leung, W.S. 2020. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS CoV-2: an observational cohort study. Lancet. Infect. Dis. 20(5), 565–574. Wang, X., Dong, L., Zhang, L., Yan, L.V., Qian, L. and Hailong, L. 2018. Genetic characterization of Toxoplasma gondii from wild rodents in Sichuan Province, Southwestern China. Iran. J. Parasitol. 14(1), 106–110. Webster, J.P. 1994. Prevalence and transmission of Toxoplasma gondii in wild brown rats, Rattus norvegicus. Parasitology 108(4), 407–411. Webster, J.P. 2001. Rats, cats, people and parasites: the impact of latent toxoplasmosis on behaviour. Microbes. Infect. 3, 1037–1045. Wijayanti, T. and Marbawati, D. 2018. Diversity, detection and the role of rats in the transmission of toxoplasmosis. BALABA 14(2), 169–180. Williams, E.H. and Bunkley, L.W. 1996. Parasites of offshore big game fishes of Puerto Rico and the western Atlantic. San Juan, Puerto Rico: University of Puerto Rico. Wormer, E.V., Carpenter, T.E., Singh, P., Shapiro, K., Wallender, W.W., Mazet, J.A.K., Conrad, P.A., Largier, J.L. and Maneta, M.P. 2016. Coastal development and precipitation drive pathogen flow from land to sea: evidence from a Toxoplasma gondii and felid host system. Sci. Rep. 6, 29252. Zulpoa, D.L., Sammia, A.S., Santosa, J.R., Sassea, J.P., Martinsa, T.A., Minuttia, A.F., Cardima, S.T., Barrosa, L.D., Navarroa, I.T. and Garcia, J.L. 2018. Toxoplasma gondii: a study of oocyte re-shedding in domestic cats. Vet. Parasitol. 249, 17–29. | ||
| How to Cite this Article |
| Pubmed Style Puspitasari H, Suwanti LT, Mufasirin M, Kusnoto K, Yudaniayanti IS, Setiawan B, Suprihati E, Aksono EB, Widodo DP, Indasari EN. Tissue cysts and serological detection toxoplasmosis among wild rats from Surabaya, East Java, Indonesia. Open Vet J. 2023; 13(11): 1443-1450. doi:10.5455/OVJ.2023.v13.i11.7 Web Style Puspitasari H, Suwanti LT, Mufasirin M, Kusnoto K, Yudaniayanti IS, Setiawan B, Suprihati E, Aksono EB, Widodo DP, Indasari EN. Tissue cysts and serological detection toxoplasmosis among wild rats from Surabaya, East Java, Indonesia. https://www.openveterinaryjournal.com/?mno=158991 [Access: July 03, 2025]. doi:10.5455/OVJ.2023.v13.i11.7 AMA (American Medical Association) Style Puspitasari H, Suwanti LT, Mufasirin M, Kusnoto K, Yudaniayanti IS, Setiawan B, Suprihati E, Aksono EB, Widodo DP, Indasari EN. Tissue cysts and serological detection toxoplasmosis among wild rats from Surabaya, East Java, Indonesia. Open Vet J. 2023; 13(11): 1443-1450. doi:10.5455/OVJ.2023.v13.i11.7 Vancouver/ICMJE Style Puspitasari H, Suwanti LT, Mufasirin M, Kusnoto K, Yudaniayanti IS, Setiawan B, Suprihati E, Aksono EB, Widodo DP, Indasari EN. Tissue cysts and serological detection toxoplasmosis among wild rats from Surabaya, East Java, Indonesia. Open Vet J. (2023), [cited July 03, 2025]; 13(11): 1443-1450. doi:10.5455/OVJ.2023.v13.i11.7 Harvard Style Puspitasari, H., Suwanti, . L. T., Mufasirin, . M., Kusnoto, . K., Yudaniayanti, . I. S., Setiawan, . B., Suprihati, . E., Aksono, . E. B., Widodo, . D. P. & Indasari, . E. N. (2023) Tissue cysts and serological detection toxoplasmosis among wild rats from Surabaya, East Java, Indonesia. Open Vet J, 13 (11), 1443-1450. doi:10.5455/OVJ.2023.v13.i11.7 Turabian Style Puspitasari, Heni, Lucia Tri Suwanti, Mufasirin Mufasirin, Kusnoto Kusnoto, Ira Sari Yudaniayanti, Boedi Setiawan, Endang Suprihati, Eduardus Bimo Aksono, Dwi Priyo Widodo, and Elly Nur Indasari. 2023. Tissue cysts and serological detection toxoplasmosis among wild rats from Surabaya, East Java, Indonesia. Open Veterinary Journal, 13 (11), 1443-1450. doi:10.5455/OVJ.2023.v13.i11.7 Chicago Style Puspitasari, Heni, Lucia Tri Suwanti, Mufasirin Mufasirin, Kusnoto Kusnoto, Ira Sari Yudaniayanti, Boedi Setiawan, Endang Suprihati, Eduardus Bimo Aksono, Dwi Priyo Widodo, and Elly Nur Indasari. "Tissue cysts and serological detection toxoplasmosis among wild rats from Surabaya, East Java, Indonesia." Open Veterinary Journal 13 (2023), 1443-1450. doi:10.5455/OVJ.2023.v13.i11.7 MLA (The Modern Language Association) Style Puspitasari, Heni, Lucia Tri Suwanti, Mufasirin Mufasirin, Kusnoto Kusnoto, Ira Sari Yudaniayanti, Boedi Setiawan, Endang Suprihati, Eduardus Bimo Aksono, Dwi Priyo Widodo, and Elly Nur Indasari. "Tissue cysts and serological detection toxoplasmosis among wild rats from Surabaya, East Java, Indonesia." Open Veterinary Journal 13.11 (2023), 1443-1450. Print. doi:10.5455/OVJ.2023.v13.i11.7 APA (American Psychological Association) Style Puspitasari, H., Suwanti, . L. T., Mufasirin, . M., Kusnoto, . K., Yudaniayanti, . I. S., Setiawan, . B., Suprihati, . E., Aksono, . E. B., Widodo, . D. P. & Indasari, . E. N. (2023) Tissue cysts and serological detection toxoplasmosis among wild rats from Surabaya, East Java, Indonesia. Open Veterinary Journal, 13 (11), 1443-1450. doi:10.5455/OVJ.2023.v13.i11.7 |