| Research Article | ||
Open Vet J. 2023; 13(11): 1416-1424 Open Veterinary Journal, (2023), Vol. 13(11): 1416– Original Research Variations in metabolic parameters of in vitro matured porcine oocytes after vitrification-warmingSergio Morado1,2*, Ailén Aparicio1, Daniela Pinchetti1, Claudia Cecilia Arraztoa1, Gabriel Alvarez1,2, Cynthia Gutnisky1,2, Deborah Neild1, Gabriel Dalvit1 and Pablo Cetica1,21Universidad de Buenos Aires, Facultad de Ciencias Veterinarias, Instituto de Investigación y Tecnología en Reproducción Animal (INITRA), Buenos Aires, Argentina 2CONICET-Universidad de Buenos Aires, Instituto de Investigaciones en Producción Animal (INPA), Buenos Aires, Argentina *Corresponding Author: Sergio Morado. Universidad de Buenos Aires, Facultad de Ciencias Veterinarias, Instituto de Investigación y Tecnología en Reproducción Animal (INITRA), Buenos Aires, Argentina. Email: smorado [at] fvet.uba.ar Submitted: 10/07/2023 Accepted: 20/10/2023 Published: 30/11/2023 © 2023 Open Veterinary Journal
AbstractBackground: As the porcine oocyte is the most sensitive to low-temperature damage, it has been difficult to cryopreserve compared to those from other domestic animals. However, at present, vitrification is used as a method for the cryopreservation of both oocytes and embryos in this species. Aim: Our aim was to analyze alterations in metabolic parameters in vitrified-warmed in vitro matured porcine oocytes at different post-warming recuperation times. In addition, metaphase II plate recovery time analysis, in vitro fertilization, and intracytoplasmic sperm injection were carried out to evaluate oocyte recovery capacity. Methods: Oocytes were vitrified-warmed and then incubated for 0, 3, or 21 hours post-warming to assess biochemical parameters. Results: Oocyte viability and morphology were not affected by vitrification-warming. Cytosolic oxidative status, active mitochondria, and reactive oxygen species levels presented changes at the different time points in control and vitrified-warmed oocytes (p < 0.05) as well as differences between both groups (p < 0.05). Nicotinamide adenine dinucleotide phosphate levels remained constant throughout different recuperation times but were significantly lower in vitrified-warmed oocytes (p < 0.05). Metaphase II plate recovery occurred mostly between 3 and 4 hours post-warming, but the percentage of metaphase II was reduced by vitrification-warming. Sperm head decondensation and pronuclear formation capacities were not modified. Conclusion: In conclusion, vitrification-warming generates biochemical alterations in porcine oocytes that would be, in part, responsible for affecting their performance. Therefore, although the technique is a valid alternative for porcine oocyte cryopreservation, the protocols should be adapted to minimize those alterations. Keywords: Cryopreservation, Mitochondria, Porcine oocytes, Reactive oxygen species, Vitrification. IntroductionThe successful cryopreservation of gametes and embryos is of great importance for the maintenance of valuable genetic resources and the implementation of assisted reproductive technologies (Wu et al., 2017). Cells remain metabolically quiescent during storage, allowing their subsequent use in programs of assisted reproduction and gene bank formation (Santos et al., 2017). As the porcine oocyte is the most sensitive to low temperatures, it has been difficult to cryopreserve compared to oocytes from other domestic animals (Zhou and Li, 2009). In vitro-produced porcine embryos are also notoriously difficult to freeze because of their low developmental potential compared with their in vivo-derived counterparts (Grupen, 2014) and their high cytoplasmic lipid content, which confers extreme chilling sensitivity (Somfai et al., 2012). However, at present, vitrification is used for the cryopreservation of porcine oocytes and embryos (Mito et al., 2015). Many structural abnormalities as well as metabolic alterations have been related to the loss of oocyte competence due to vitrification. Several reports have described cytoskeleton alterations and hardening of the zona pellucida (ZP), related to rearrangements of the protein secondary structure as well as the lipid and carbohydrate configuration of the ZP (Rusciano et al., 2017). It has also been demonstrated that vitrification may disturb the reduction-oxidation (redox) status, reduce glutathione content, and increase reactive oxygen species (ROS) levels leading to damage in biomolecules such as DNA, proteins, and membrane lipids, together with mitochondrial dysfunction, which may induce apoptotic responses and reduce embryo viability (Dehghani-Mohammadabadi et al., 2014; Zhao et al., 2016). Some cell abnormalities might be reversible after oocyte vitrification-warming with an adequate post-warming recuperation protocol. In the mouse, it has been demonstrated that incubation for 2 or 3 hours results in higher percentages of normal meiotic spindles (Chen et al., 2001). Many researchers are now focusing on how to improve the vitrification technique to optimize survival and pregnancy rates (Mito et al., 2015; Wu et al., 2017). In this regard, it is also important to fully understand how vitrification-warming may affect oocyte metabolism and to identify how post-warming recuperation protocols may compensate for changes in biochemical parameters induced by the process. Therefore, our aim was to analyze alterations in metabolic parameters in vitrified-warmed in vitro matured porcine oocytes at different post-warming recuperation times. Materials and MethodsMaterialsUnless specified, all chemicals and reagents were purchased from Sigma (Sigma Chemical Co., St. Louis, MO). Cumulus-oocyte complexes (COCs) recoveryOvaries from slaughtered gilts were transported in an isothermal solution to the laboratory within 2 hours. Immature COCs were obtained by aspiration of antral ovarian follicles and were selected under a stereomicroscope. Only oocytes completely surrounded by an integral and dense cumulus were collected (Alvarez et al., 2016). COCs in vitro maturation (IVM)COCs were matured in medium 199 (Earle’s salts, L-glutamine, 2.2 mgl−1 sodium bicarbonate; GIBCO, Grand Island, NY) supplemented with 50 μg/ml gentamicin sulfate, 10% (v/v) porcine follicular fluid, 0.5 μg/ml FSH, and 0.5 μg/ml LH under mineral oil at 39℃, 5% CO2 in a humidified atmosphere for 44 hours (Alvarez et al., 2016). To analyze nuclear maturation percentages, in each experiment, a group of oocytes was denuded and then stained with 10 mgl−1 Hoechst 33342 solution for 15 minutes. Oocyte nuclear status was observed at a ×400 magnification with 330–380 nm (excitation) and 410 nm (emission) filters using a Jenamed II epifluorescence microscope (Carl Zeiss Jena, Buenos Aires, Argentina). Oocyte vitrificationMatured oocytes were partially denuded by gentle pipetting, leaving only the corona radiata necessary for the fertilization process, and then were vitrified-warmed using the minimum volume vitrification system from Cryotech® (Gandhi et al., 2017; Gutnisky et al., 2020). After warming, oocytes were cultured in medium 199 supplemented with 50 μg/ml gentamicin sulfate, 10% (v/v) porcine follicular fluid, 0.5 μg/ml FSH, and 0.5 μg/ml LH under mineral oil at 39℃, 5% CO2 in a humidified atmosphere during different incubation times. Experimental designAfter vitrification-warming, oocytes were incubated in the same medium used for IVM, and a cohort was studied at three different time points to analyze their metabolic status during recuperation: 0 hours (to evaluate oocyte behavior immediately after warming), 3 hours (to determine oocyte metabolic recovery), and 21 hours (to confirm that vitrified-warmed oocytes may survive during the period required for pronuclear formation after fertilization). Fresh matured nonfertilized oocytes were analyzed at the same time points to serve as controls, starting at 44 hours of IVM as 0 hours. To evaluate oocyte metaphase II recovery, in vitro fertilization (IVF), and intracytoplasmic sperm injection, the control and vitrified-warmed oocytes used came from a different batch of ovaries to synchronize the warming time with the completion of maturation. For the morphological, viability, and biochemical evaluations, control and vitrified-warmed oocytes came from the same batch of ovaries but were processed at different moments because of the extra time needed for the vitrification-warming process. For both functional and metabolic determinations, three replicates were performed and a total number of 1,040 oocytes were used. Evaluation of oocyte morphology and viabilityVitrified-warmed and control oocytes were morphologically evaluated under a stereomicroscope with differential interferential contrast analyzing their cytoplasmic structure and volume recovery, as well as the integrity and definition of their plasma membrane, ZP, and perivitelline space. Oocytes with asymmetric or irregular forms, increased perivitelline space, or cytoplasm granularity were considered abnormal (Gutnisky et al., 2020). To determine if oocytes survived the vitrification-warming process, we used the fluorescein diacetate (FDA) fluorochrome assay. Vitrified-warmed oocytes were incubated in a solution containing 0.12 µM FDA for 15 minutes. After incubation, they were washed twice in a phosphate buffer saline (PBS) + polyvinyl alcohol (PVA) solution and loaded on a glass slide for their observation under an epifluorescent microscope. Live oocytes were distinguished based on their green fluorescence (Alvarez et al., 2009). Determination of active mitochondria and cytosolic oxidative statusMito Tracker Green FM, active mitochondria indicator, and RedoxSensor Red CC-1, cytosolic oxidative activity indicator, dual assay was used (Alvarez et al., 2016). Oocytes were incubated for 45 minutes in the presence of 0.5 nM Mitotracker Green and 1 nM Redox Sensor Red, then washed in PBS + 0.1% PVA, mounted on glass slides, and observed under an epifluorescence microscope. Digitalized images were analyzed using IMAGE J software to measure their fluorescence intensity (Alvarez et al., 2016). Determination of ROS levelsOocytes were incubated for 30 minutes in PBS + PVA with 5 μM 2′,7′-dichlorodihydro fluorescein diacetate (DCHFDA). To evaluate esterase activity, a group of oocytes was incubated for 15 minutes in PBS + PVA with 0.12 μM FDA. After exposing both groups to their respective fluorochrome, they were washed in PBS + PVA and mounted on glass slides. Fluorescence intensity was measured in digital microphotographs obtained using an epifluorescence microscope with a 450–490 nm excitation filter and a 520 nm barrier filter. Digitalized images were analyzed using IMAGE J software. As DCHFDA fluorescence levels are dependent on the activity of intracellular esterases, the rate between the values obtained by DCHFDA for each oocyte and the average FDA brightness of each analyzed group was considered as a relative measure for ROS levels in each oocyte (Morado et al., 2009). Determination of the mitochondrial redox stateOocyte mitochondrial redox state was determined by two endogenous autofluorescent compounds, reduced nicotinamide adenine dinucleotide phosphate (NAD(P)H) and flavin adenine dinucleotide (FAD). Fluorescence intensity was determined using blue (excitation 405 nm, emission 420–520 nm) and green (excitation 473 nm, emission 490–590 nm) filters, respectively. Digitalized images were analyzed using IMAGE J software to measure their fluorescence intensity (Sutton-McDowall et al., 2015). Analysis of metaphase II plate recoveryVitrified oocytes must first recover their metaphase II chromosome configuration before they may be activated by IVF or ICSI. Therefore, to study the time required for metaphase plate recovery, vitrified-warmed oocytes were incubated for 2, 3, or 4 hours in the same medium used for oocyte IVM and then stained with Hoechst 33342 fluorochrome to differentiate oocytes with a well-configured metaphase II plate from those which still presented unrecovered nuclear material. Denuded oocytes were fixed in 40 mgl−1 paraformaldehyde solution for 1 hour. Finally, the fixed oocytes were stained with 10 mgl−1 Hoechst 33342 solution for 15 minutes. Oocyte nuclear status was observed at a ×400 magnification using 330–380 nm (excitation) and 410 nm (emission) filters for a Jenamed II epifluorescence microscope (Carl Zeiss Jena, Buenos Aires, Argentina). Analysis of cytoplasmic functionality recoveryIVF was performed to evaluate the recovery of oocyte cytoplasmic functionality. Fresh semen was obtained from a Yorkshire boar of proven fertility as described in Alvarez et al. (2012). Oocytes were inseminated to a final concentration of 5 × 108L−1 spermatozoa, and coincubation was performed under mineral oil at 39℃ for 18 hours in a 5% CO2 atmosphere. Presumed fertilized oocytes were released from the attached spermatozoa by repetitive pipetting, fixed on a glass slide with Carnoy’s fixing solution (ethanol: acetic acid, 3:1) for at least 24 hours, incubated in a 10 mgl−1 Hoechst 33342 fluorochrome aqueous solution for 15 minutes at room temperature and observed under an epifluorescence microscope using 330–380 nm (excitation) and 420 nm (emission) filters at ×400 magnification. Oocytes were considered cytoplasmically matured when one or more decondensed sperm heads could be identified (Alvarez et al., 2012). Vitrified-warmed oocytes (treatment) were incubated for 3 additional hours before insemination to allow the metaphase II plate to recover, while the control group was inseminated at 44 hours of maturation.
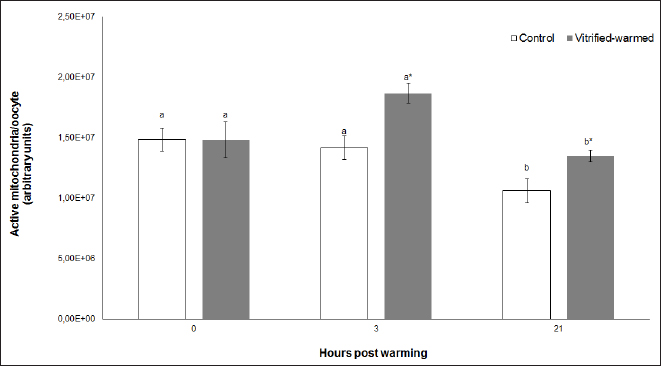
Fig. 1. Active mitochondria in control (white bars) and vitrified-warmed oocytes (grey bars) incubated for 0, 3, and 21 hours post-warming. Data are the mean ± SEM. Bars of the same color with different letters differ significantly (p < 0.05). Asterisks indicate significant differences between treatments at the same time point (p < 0.05).
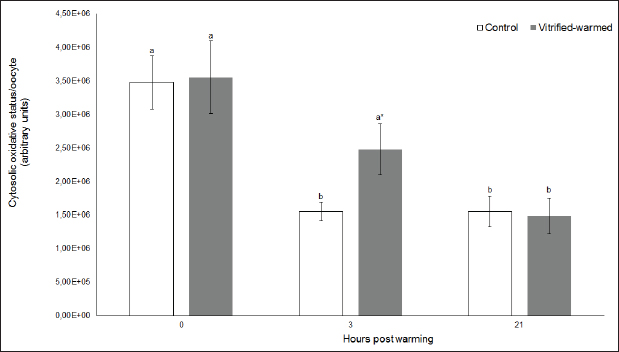
Fig. 2. Cytosolic oxidative status in control (white bars) and vitrified-warmed oocytes (grey bars) incubated for 0, 3, and 21 hours post-warming. Data are the mean ± SEM. Bars of the same color with different letters differ significantly (p < 0.05). Asterisks indicate significant differences between treatments at the same time point (p < 0.05).
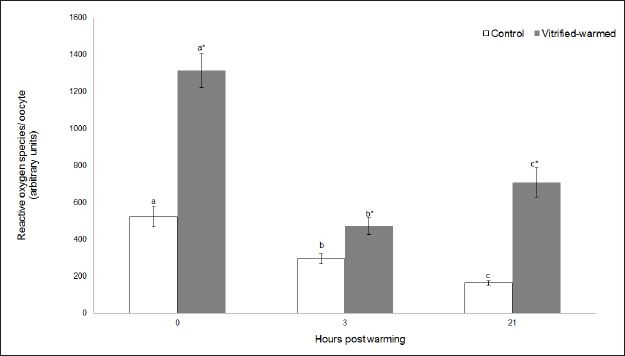
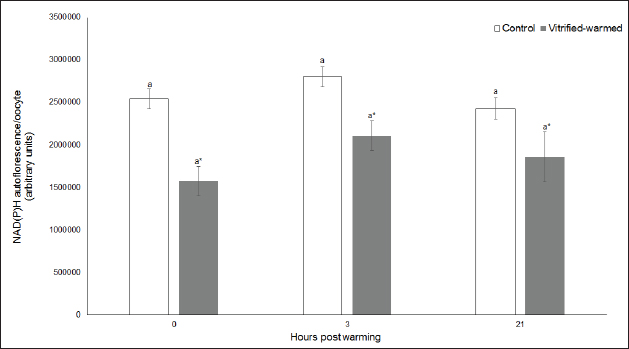
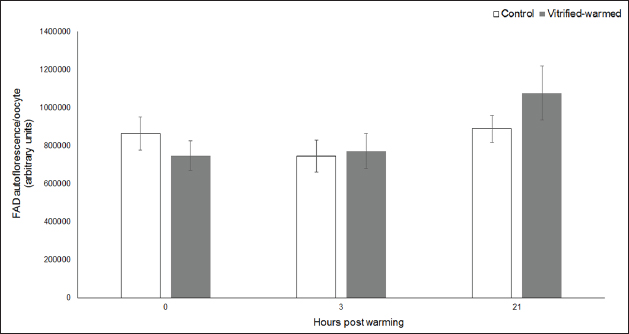
Fig. 3. ROS in control (white bars) and vitrified-warmed oocytes (grey bars) incubated for 0, 3, and 21 hours post-warming. Data are the mean ± SEM. Bars of the same color with different letters differ significantly (p < 0.05). Asterisks indicate significant differences between treatments at the same time point (p < 0.05). In addition, ICSI was performed to avoid the polyspermy usually obtained with IVF. ICSI was carried out using an inverted Leica® DMIL microscope equipped with Narishige® micromanipulators as described in Arraztoa et al. (2017). The presumed zygotes were fixed for 15 minutes (2% glutaraldehyde in PBS), incubated with 1% Hoechst 33342 in PBS for 15 minutes, washed in PBS containing 1 mgml−1 polyvinylpyrrolidone and mounted on glass slides. Oocytes were examined under an epifluorescence microscope using 330–380 nm (excitation) and 420 nm (emission) filters (Arraztoa et al., 2017). Oocytes were considered cytoplasmically matured when two pronuclei could be identified 18 hours after ICSI. Vitrified-warmed oocytes (treatment) were incubated for 3 additional hours before ICSI to let the metaphase plate II recover, while the control group was inseminated at 44 hours of maturation. Statistical analysisMetaphase II plate recovery, IVF, and pronuclei formation rates were compared using a chi-square analysis for nonparametric data. Cytosolic oxidative status, quantification of active mitochondria, ROS production, and redox state values were expressed as mean ± SEM, and interactions were analyzed by two-way ANOVA, using post-hoc general contrasts to compare different treatments (2 × 3 factorial analyses). All statistical tests were performed using the InfoStat software (Universidad de Córdoba, Córdoba, Argentina, see http://www.infostat.com.ar). A p-value < 0.05 was considered significant in all studies. Ethical approvalNo animals were used during this study. Ovaries were donated to our laboratory by a local slaughterhouse and semen was donated by a local farm. ResultsEvaluation of IVM and oocyte morphology and viabilityIn all the experiences after 44 hours of IVM, 20% of the oocytes were used to evaluate the maturation percentages, observing the presence of metaphase II in 70% of the in vitro matured oocytes. At 0, 3, and 21 hours of incubation 95%, 93%, and 90%, respectively, of the vitrified-warmed oocytes conserved a normal morphology, preserving their plasma membrane integrity, a reconstructed perivitelline space and a uniform cytoplasm. No significant differences were observed between the three-time points mentioned nor between vitrified-warmed and control oocytes. Regarding their survival capacity, vitrified-warmed oocytes showed similar behavior for the three time points studied and no differences were observed compared with control oocytes either. Active mitochondria and cytosolic oxidative statusControl and vitrified-warmed oocytes presented a similar behavior as regards their cytosolic oxidative status and their active mitochondria throughout the incubation time, observing that both levels decreased in both groups of oocytes from 0 to 21 hours (p < 0.05). However, active mitochondria were higher at 3 and 21 hours in vitrified-warmed oocytes compared with the control (p < 0.05; Fig. 1), while the cytosolic oxidative status only showed a significant increase at 3 hours (p < 0.05; Fig. 2). ROS levelsIn both groups under study, a higher ROS level was detected at 0 hours compared to 3 and 21 hours (p < 0.05). However, while in control oocytes, ROS levels decreased from 0 to 21 hours (p < 0.05), in the vitrified-warmed group ROS levels decreased from 0 to 3 hours and then increased to a higher level at 21 hours (p < 0.05). Comparing both groups under study, ROS production was higher in vitrified-warmed oocytes than in the control at three analyzed time points (p < 0.05) (Fig. 3). Mitochondrial redox stateNAD(P)H and FAD coenzyme levels were stable at the different evaluated time points in both groups of oocytes. However, vitrified-warmed oocytes showed a significantly lower NAD(P)H content in comparison with the control at every time point (p < 0.05; Fig. 4), while FAD levels presented no difference between groups (Fig. 5). Metaphase II plate recoveryTo study the time necessary for metaphase II plate recovery in vitrified-warmed oocytes, they were incubated for 2, 3, or 4 hours after warming. In most oocytes, normal metaphase II configuration recovered 3 hours after warming (52.6%). No further improvement was found after a 4-hour incubation (50%), while a 2-hour incubation post-warming proved to be insufficient to recover the metaphase II configuration (25%; p < 0.05). Taking into account that the oocyte maturation rate was 70%, only 75.1% of the vitrified-warmed oocytes were able to recover the metaphase II plate configuration after 3-hour incubation post-warming. Cytoplasmic functionality recoveryTo evaluate the recovery of oocyte cytoplasmic functionality, vitrified-warmed oocytes were co-incubated with fresh semen to determine their capacity to decondensed sperm heads. No significant differences in IVF rate were detected between control and vitrified-warmed oocytes (60% vs. 50%). Polyspermy rates were 30.6% and 36.7% for control and vitrified-warmed oocytes, respectively. To avoid polyspermy, the recovery of cytoplasmic functionality was also evaluated by ICSI, and no significant differences in pronuclei formation were detected between control and vitrified-warmed oocytes (44.1 vs. 38.9%). No significant differences were detected either by comparing IVF and ICSI results. DiscussionOur study represents a novel analysis regarding ROS production, oxidative cytosolic status, active mitochondria and redox state in in vitro matured porcine oocytes submitted to the vitrification-warming process using a minimum volume system. The changes observed in those metabolic parameters could partly explain the reduced developmental competence reported in the literature. We also found that the vitrification-warming process affects the recovery of the metaphase II chromosome configuration, but does not alter the cytoplasmic function to decondense sperm heads or to form pronuclei.
Fig. 4. NAD(P)H levels in control (white bars) and vitrified-warmed oocytes (grey bars) incubated for 0, 3, and 21 hours post-warming. Data are the mean ± SEM. Asterisks indicate significant differences between treatments at the same time point (p < 0.05).
Fig. 5. FAD levels in control (white bars) and vitrified-warmed oocytes (grey bars) incubated for 0, 3, and 21 hours post-warming. Data are the mean ± SEM. No significant differences between evaluated time points and between treatments at the same time point were observed. We first studied the porcine oocyte maturational capacity recovery after the vitrification-warming process. We determined that around 3 to 4 hours of incubation post-warming are necessary to achieve a higher metaphase II recovery rate in the porcine species. Likewise, it has been reported that the incubation of mouse oocytes for 2 or 3 hours post-warming results in higher percentages of normal meiotic spindles than the incubation for only 1 hour (Chen et al., 2001). Moreover, the rate of vitrified-warmed porcine oocytes that were able to recover the metaphase II plate configuration was lower than the meiotic maturation rate observed in fresh oocytes, suggesting that some type of metabolic or structural damage during this process may affect spindle formation. This coincides with what is reported in the literature (Chen et al., 2001). As regards oocyte cytoplasmic function, we observed no differences in decondensed sperm heads or pronuclei formation after the activation of vitrified-warmed oocytes compared with fresh oocytes neither by IVF nor ICSI. This suggests that the sperm head decondensation and pronuclear formation capacities of the oocyte would not be negatively affected by the vitrification-warming process in the porcine species. Then, we determined oocyte morphology and viability in matured vitrified-warmed and control porcine oocytes throughout 21 hours of extended incubation, as a survival test, and observed no differences between the studied groups. Thus, although other authors have reported alterations in oocyte survival (Park et al., 2005), nuclear morphology (Luna et al., 2001), microtubule formation (Chasombat et al., 2015), cytoplasmic organization (Palmerini et al., 2014), and membrane integrity (Succu et al., 2007) in oocytes submitted to different vitrification protocols, in our work Cryotech® method did not affect oocyte viability and macro-morphology. As regards biochemical parameters, vitrified-warmed oocytes presented an increase in active mitochondria 3 hours after warming compared with the control, which could be a consequence of a higher energy requirement for the reorganization of organelles, cytoplasm, and redox potential after the process. This coincides with previous studies in porcine oocyte IVM where we observed that the number of active mitochondria is modulated by the content of ATP or AMP (Alvarez et al., 2016). Similarly, using Mitotracker Red CMXRos high mitochondrial activity and low ATP content were detected in ovine oocytes after warming (Diez et al., 2005). The increase in active mitochondria at 3 hours was accompanied by an increase in cytosolic oxidative activity, coinciding with what we previously observed for bovine and porcine oocytes (Gutnisky et al., 2013; Alvarez et al., 2016). We observed higher ROS levels at the three studied time points compared with the control, which is consistent with other studies (Succu et al., 2007; Gupta et al., 2010). This increase would be related to changes in oocyte metabolism for their recovery after warming. Within the vitrified-warmed oocytes, the ROS peak at 0 hours could be due to the oxidative stress produced by the process, the decrease at 3 hours coincided with the time necessary for the recovery of the metaphase plate and the increase at 21 hours could be an initial indicator of oocyte aging. This ROS pattern associated with metabolic demands for oocyte recovery after warming is consistent with our previous studies in bovine oocytes in which we determined that ROS levels fluctuated during both maturation and activation after IVF, so that a higher mitochondrial respiratory chain activity is related to lower ROS production (Morado et al., 2009, 2013). Although no changes were observed in FAD autofluorescence, the reduction in NAD(P)H levels at the three studied time points could be attributed to an increased requirement to compensate for ROS production in vitrified oocytes (Veal et al., 2007) as well as to a higher consumption of reductive equivalents in the mitochondrial respiratory chain (Nelson and Cox, 2017). On the other hand, the higher NAD(P)H levels related to lower ROS production observed in control oocytes would be due to the fact that this group presented a quieter metabolic state (Madrid Gaviria et al., 2019). This statement is supported by a study that proposed that the tricarboxylic acid cycle activity would be related to NAD(P)H levels in the bovine oocyte (Sutton-McDowall et al., 2015). In conclusion, this study shows that porcine oocyte morphology and viability could be maintained through the vitrification-warming process and that the procedure did not affect the sperm head decondensation and pronuclear formation capacities of the oocyte. On the other hand, our results suggest that important metabolic changes occur during the recuperation process after warming in porcine oocytes, represented by interrelated variations in cytosolic oxidative status, active mitochondria, ROS production, and redox state throughout the different incubation time points, which could be partly responsible for the lower metaphase II plate recovery observed. Therefore, several improvements should be included in the vitrification-warming protocols to minimize variations in oocyte metabolism during this process. With this in consideration, our group will lead further studies to analyze the effect of the addition of antioxidants to the vitrification and warming media. AcknowledgmentsThe authors would like to thank Minguillon Slaughterhouse for the ovaries provided and Fabian Gentile for his technical assistance. Conflict of interestThe authors declare that there is no conflict of interest that could prejudice the impartiality of the research reported. FundingThis research was supported by funds from: University of Buenos Aires (UBACyT 20020130100693BA 2014-2017), Fondo para Investigación Científica y Tecnológica (FONCyT PICT-2017-3024), and Consejo Nacional de Investigaciones Científicas y Técnicas (PIP-CONICET 11220170100813CO 2017). Author contributionsAll authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Sergio Morado, Ailén Aparicio, Daniela Pinchetti, Claudia Arraztoa, Gabriel Álvarez, and Cynthia Gutnisky. The first draft of the manuscript was written by Sergio Morado and all authors commented on previous versions of the manuscript. Deborah Neild contributed also with resources and supervision; Gabriel Dalvit with resources, supervision, and project administration and Pablo Cetica with resources, supervision, project administration, and funding acquisition. All authors read and approved the final manuscript. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesAlvarez, G.M., Casiró, S., Gutnisky, C., Dalvit, G.C., Sutton Mc-Dowall, M.L., Thompson, J.G. and Cetica, P.D. 2016. Implications of glycolytic and pentose phosphate pathways on the oxidative status and active mitochondria of the porcine oocyte during IVM. Theriogenology 86, 2096–2106. Alvarez, G.M., Dalvit, G.C., Achi, M.V., Miguez, M.S. and Cetica, P.D. 2009. Immature oocyte quality and maturational competence of porcine cumulus-oocyte complexes subpopulations. Biocell 33, 167–177. Alvarez, G.M., Dalvit, G.C. and Cetica, P.D. 2012. Influence of the cumulus and gonadotropins on the metabolic profile of porcine cumulus–oocyte complexes during in vitro maturation. Reprod. Domest. Anim. 47, 856–864. Arraztoa, C.C., Baca Castex, C., Alvarez, G.M., Cetica, P.D. and Neild, D.M. 2017. In vitro production of porcine zygotes using intracytoplasmic injection of vitrified sperm. Reprod. Domest. Anim. 52, 775–780. Chasombat, J., Nagai, T., Parnpai, R. and Vongpralub, T. 2015. Pretreatment of in vitro matured bovine oocytes with docetaxel before vitrification: effects on cytoskeleton integrity and developmental ability after warming. Cryobiology 71, 216–223. Chen, S.U., Lien, Y.R., Cheng, Y.Y., Chen, H.F., Ho, H.N. and Yang, Y.S. 2001. Vitrification of mouse oocytes using closed pulled straws (CPS) achieves a high survival and preserves good patterns of meiotic spindles, compared with conventional straws, open pulled straws (OPS) and grids. Hum. Reprod. 16, 2350–2356. Dehghani-Mohammadabadi, M., Salehi, M., Farifteh, F., Nematollahi, S., Arefian, E., Hajjarizadeh, A., Parivar, K. and Nourmohammadi, Z. 2014. Melatonin modulates the expression of BCL-xl and improve the development of vitrified embryos obtained by IVF in mice. J. Assist. Reprod. Genet. 31, 453–461. Diez, C., Duque, P., Gomez, E., Hidalgo, C.O., Tamargo, C., Rodriguez, A., Fernández, L., de la Varga, S., Fernández, A., Facal, N. and Carbajo, M. 2005. Bovine oocyte vitrification before or after meiotic arrest: effects on ultrastructure and developmental ability. Theriogenology 64, 317–333. Gandhi, G., Kuwayama, M., Kagalwala, S. and Pangerkar, P. 2017. Appendix A: Cryotech® vitrification thawing. Methods. Mol. Biol. 1568, 281–295. Grupen, C.G. 2014. The evolution of porcine embryo in vitro production. Theriogenology 81, 24–37. Gupta, M.K., Uhm, S.J. and Lee, H.T. 2010. Effect of vitrification and beta-mercaptoethanol on reactive oxygen species activity and in vitro development of oocytes vitrified before or after in vitro fertilization. Fertil. Steril. 93, 2602–2607. Gutnisky, C., Morado, S., Dalvit, G.C., Thompson, J.G. and Cetica, P.D. 2013. Glycolytic pathway activity: effect on IVM and oxidative metabolism of bovine oocytes. Reprod. Fertil. Dev. 25, 1026–1035. Gutnisky, C., Morado, S., Gadze, T., Donato, A., Alvarez, G., Dalvit, G. and Cetica, P. 2020. Morphological, biochemical and functional studies to evaluate bovine oocyte vitrification. Theriogenology 143, 18–26. Luna, H.S., Ferrari, I. and Rumpf, R. 2001. Influence of stage of maturation of bovine oocytes at time of vitrification on the incidence of diploid metaphase II at completion of maturation. Anim. Reprod. Sci. 68, 23–28. Madrid Gaviria, S., Morado, S.A., López Herrera, A., Restrepo Betancur, G., Urrego Álvarez, R.A., Echeverri Zuluaga, J. and Cetica, P.D. 2019. Resveratrol supplementation promotes recovery of lower oxidative metabolism after vitrification and warming of in vitro produced bovine embryos. Reprod. Fertil. Dev. 31, 521–528. Mito, T., Yoshioka, K., Noguchi, M., Yamashita, S., Misumi, K., Hoshi, T. and Hoshi, H. 2015. Birth of piglets from in vitro-produced porcine blastocysts vitrified and warmed in a chemically defined medium. Theriogenology 84, 1314–1320. Morado, S.A., Cetica, P.D., Beconi, M.T. and Dalvit, G.C. 2009. Reactive oxygen species in bovine oocyte maturation in vitro. Reprod. Fertil. Dev. 21, 608–614. Morado, S., Cetica, P., Beconi, M., Thompson, J.G. and Dalvit, G. 2013. Reactive oxygen species production and redox state in parthenogenetic and sperm-mediated oocyte activation. Reproduction 145, 471–478. Nelson, D.L. and Cox, M.M. 2017. Lehninger principles of biochemistry, 7th ed. New York, NY: W.H. Freeman and Company. Palmerini, M.G., Antinori, M., Maione, M., Cerusico, F., Versaci, C., Nottola, S.A., Macchiarelli, G., Ali Khalili, M. and Antinori, S. 2014. Ultrastructure of immature and mature human oocytes after cryotop vitrification. J. Reprod. Dev. 60, 411–420. Park, K.E., Kwon, I.K., Han, M.S. and Niwa, K. 2005. Effects of partial removal of cytoplasmic lipid on survival of vitrified germinal vesicle stage pig oocytes. J. Reprod. Dev. 51, 151–160. Rusciano, G., De Canditiis, C., Zito, G., Rubessa, M., Roca, M.S., Carotenuto, R., Sasso, A. and Gasparrini, B. 2017. Raman-microscopy investigation of vitrification-induced structural damages in mature bovine oocytes. PloS One 12, e0177677. Santos, E.C.D.S., Somfai, T., Appeltant, R., Dang-Nguyen, T.Q., Noguchi, J., Kaneko, H. and Kikuchi, K. 2017. Effects of polyethylene glycol and a synthetic ice blocker during vitrification of immature porcine oocytes on survival and subsequent embryo development. Anim. Sci. J. 88, 1042–1048. Somfai, T., Kikuchi, K. and Nagai, T. 2012. Factors affecting cryopreservation of porcine oocytes. J. Reprod. Dev. 58, 17–24. Succu, S., Leoni, G.G., Bebbere, D., Berlinguer, F., Mossa, F., Bogliolo, L., Madeddu, M., Ledda, S. and Naitana, S. 2007. Vitrification devices affect structural and molecular status of in vitro matured ovine oocytes. Mol. Reprod. Dev. 74, 1337–1344. Sutton-McDowall, M.L., Purdey, M., Brown, H.M., Abell, A.D., Mottershead, D.G., Cetica, P.D., Dalvit, G.C., Goldys, E.M., Gilchrist, R.B., Gardner, D.K. and Thompson, J.G. 2015. Redox and anti-oxidant state within cattle oocytes following in vitro maturation with bone morphogenetic protein 15 and follicle stimulating hormone. Mol. Reprod. Dev. 82, 281–294. Veal, E.A., Day, A.M. and Morgan, B.A. 2007. Hydrogen peroxide sensing and signaling. Mol. Cell. 26, 1–14. Wu, G., Jia, B., Quan, G., Xiang, D., Zhang, B., Shao, Q. and Hong, Q. 2017. Vitrification of porcine immature oocytes: association of equilibration manners with warming procedures, and permeating cryoprotectants effects under two temperatures. Cryobiology 75, 21–27. Zhao, X.M., Hao, H.S., Du, W.H., Zhao, S.J., Wang, H.Y., Wang, N., Wang, D., Liu, Y., Qin, T. and Zhu, H.B. 2016. Melatonin inhibits apoptosis and improves the developmental potential of vitrified bovine oocytes. J. Pineal. Res. 60, 132–141. Zhou, G.B. and Li, N. 2009. Cryopreservation of porcine oocytes: recent advances. Mol. Hum. Reprod. 15, 279–285. | ||
| How to Cite this Article |
| Pubmed Style Morado S, Aparicio A, Pinchetti D, Arraztoa CC, Alvarez G, Gutnisky C, Neild D, Dalvit G, Cetica P. Variations in metabolic parameters of in vitro matured porcine oocytes after vitrification-warming. Open Vet J. 2023; 13(11): 1416-1424. doi:10.5455/OVJ.2023.v13.i11.4 Web Style Morado S, Aparicio A, Pinchetti D, Arraztoa CC, Alvarez G, Gutnisky C, Neild D, Dalvit G, Cetica P. Variations in metabolic parameters of in vitro matured porcine oocytes after vitrification-warming. https://www.openveterinaryjournal.com/?mno=160114 [Access: July 01, 2025]. doi:10.5455/OVJ.2023.v13.i11.4 AMA (American Medical Association) Style Morado S, Aparicio A, Pinchetti D, Arraztoa CC, Alvarez G, Gutnisky C, Neild D, Dalvit G, Cetica P. Variations in metabolic parameters of in vitro matured porcine oocytes after vitrification-warming. Open Vet J. 2023; 13(11): 1416-1424. doi:10.5455/OVJ.2023.v13.i11.4 Vancouver/ICMJE Style Morado S, Aparicio A, Pinchetti D, Arraztoa CC, Alvarez G, Gutnisky C, Neild D, Dalvit G, Cetica P. Variations in metabolic parameters of in vitro matured porcine oocytes after vitrification-warming. Open Vet J. (2023), [cited July 01, 2025]; 13(11): 1416-1424. doi:10.5455/OVJ.2023.v13.i11.4 Harvard Style Morado, S., Aparicio, . A., Pinchetti, . D., Arraztoa, . C. C., Alvarez, . G., Gutnisky, . C., Neild, . D., Dalvit, . G. & Cetica, . P. (2023) Variations in metabolic parameters of in vitro matured porcine oocytes after vitrification-warming. Open Vet J, 13 (11), 1416-1424. doi:10.5455/OVJ.2023.v13.i11.4 Turabian Style Morado, Sergio, Ailen Aparicio, Daniela Pinchetti, Claudia Cecilia Arraztoa, Gabriel Alvarez, Cynthia Gutnisky, Deborah Neild, Gabriel Dalvit, and Pablo Cetica. 2023. Variations in metabolic parameters of in vitro matured porcine oocytes after vitrification-warming. Open Veterinary Journal, 13 (11), 1416-1424. doi:10.5455/OVJ.2023.v13.i11.4 Chicago Style Morado, Sergio, Ailen Aparicio, Daniela Pinchetti, Claudia Cecilia Arraztoa, Gabriel Alvarez, Cynthia Gutnisky, Deborah Neild, Gabriel Dalvit, and Pablo Cetica. "Variations in metabolic parameters of in vitro matured porcine oocytes after vitrification-warming." Open Veterinary Journal 13 (2023), 1416-1424. doi:10.5455/OVJ.2023.v13.i11.4 MLA (The Modern Language Association) Style Morado, Sergio, Ailen Aparicio, Daniela Pinchetti, Claudia Cecilia Arraztoa, Gabriel Alvarez, Cynthia Gutnisky, Deborah Neild, Gabriel Dalvit, and Pablo Cetica. "Variations in metabolic parameters of in vitro matured porcine oocytes after vitrification-warming." Open Veterinary Journal 13.11 (2023), 1416-1424. Print. doi:10.5455/OVJ.2023.v13.i11.4 APA (American Psychological Association) Style Morado, S., Aparicio, . A., Pinchetti, . D., Arraztoa, . C. C., Alvarez, . G., Gutnisky, . C., Neild, . D., Dalvit, . G. & Cetica, . P. (2023) Variations in metabolic parameters of in vitro matured porcine oocytes after vitrification-warming. Open Veterinary Journal, 13 (11), 1416-1424. doi:10.5455/OVJ.2023.v13.i11.4 |