| Research Article | ||
Open Vet J. 2023; 13(10): 1259-1267 Open Veterinary Journal, (2023), Vol. 13(10): 1259–1267 Original Research Syringe immersion test as in vitro bioassay against Rhipicephalus microplus: Macrocyclic lactones dose-response relationshipDiego Robaina, Jessica Caballero, and Gonzalo Suárez*Unidad de Farmacología y Terapéutica, Departamento Hospital y Clínicas Veterinarias, Facultad de Veterinaria, Universidad de la República, Montevideo, Uruguay *Corresponding Author: Gonzalo Suárez. Unidad de Farmacología y Terapéutica, Departamento Hospital y Clínicas Veterinarias, Facultad de Veterinaria, Universidad de la República, Montevideo, Uruguay. Email: suarezveirano [at] gmail.com Submitted: 27/07/2023 Accepted: 07/09/2023 Published: 31/10/2023 © 2023 Open Veterinary Journal
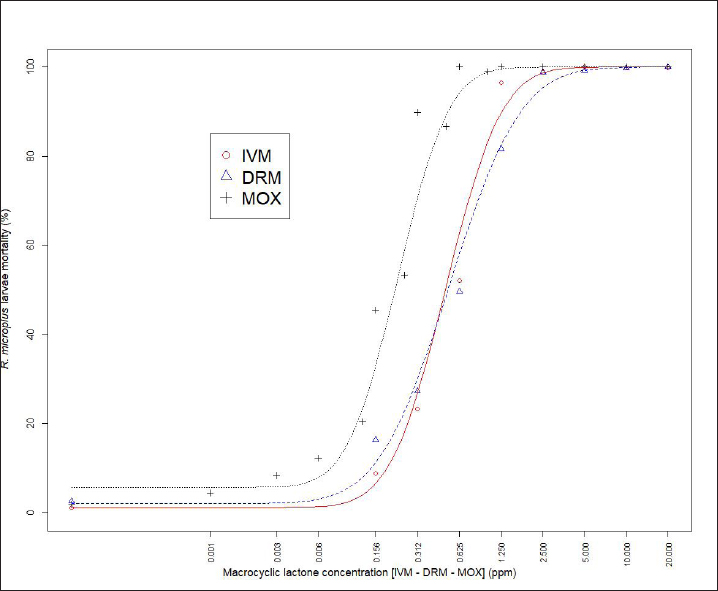
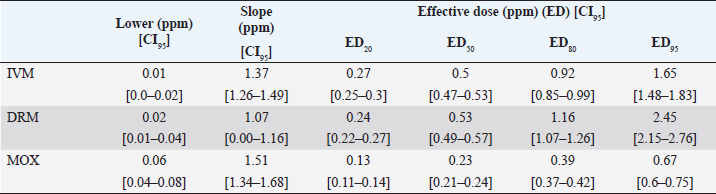
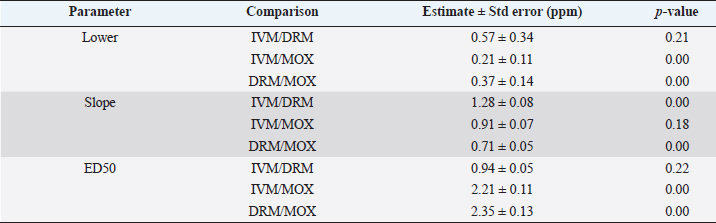
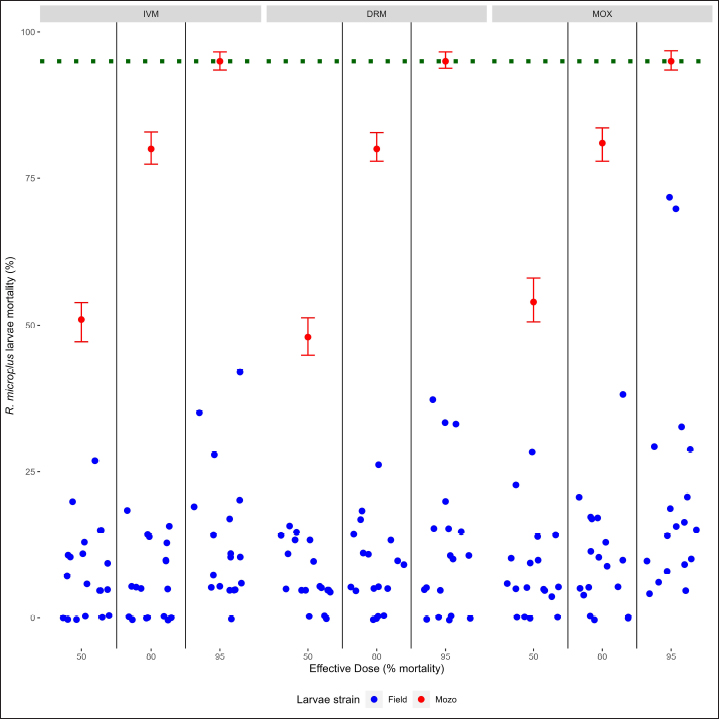
AbstractBackground: For the diagnosis of tick sensitivity against different acaricides, there are in vitro and in vivo methods. The main in vivo method, the stable test, is considered a defining methodology. In Uruguay, the Rhipicephalus microplus (R. microplus) strain Mozo is used as the standard susceptible strain by the regulatory authorities. In vitro techniques applied both on adult and larvae stages are validated by FAO and can serve as an orientation diagnosis of the resistance profile developed in field conditions. An alternative was proposed as a modification of the larval immersion test (LIT), where syringes were used seeking to reduce the work necessary to perform the original technique, resulting in the syringe immersion test (SIT). Aim: The aim of this study was to expand the SIT for the characterization of sensitivity to Macrocyclic Lactones (MLs) in R. microplus and provide information on field strain sensitivity of R. microplus larvae. Methods: Log-logistic dose-response model for Ivermectin (IVM), Doramectin (DRM), and Moxidectin (MOX) were performed using concentrations ranging from 0.01 to 20.0 ppm (n=6, 3 replicates per level on each drug). Larvae sensitivity results were determined after 24 hours of incubation at 27°C/90% RH, counting live/dead larvae. The final model will be decided as the best fit according to the model selection AIC criteria for each drug. Pharmacodynamic parameters [lower limit, slope, and effective dose at different levels (ED20, ED50, ED80, and ED95)] and its 95% confidence interval were considered for drug comparison. Results: Dose-response models were fitted for IVM, DRM, and MOX. MOX had the lowest ED50 of the three drugs, implying that MOX is of higher potency (two folds) when compared to IVM and DRM on R. microplus larvae using SIT. DRM had a different slope compared to IVM and MOX (p < 0.05), while IVM and MOX showed a similar slope (p > 0.05). Conclusion: This study allowed us to standardize the technique for larvae immersion for each ML, granting a new tool for in vitro test as a screening technique for tick sensitivity. Keywords: Drugs, Ectoparasiticides, Pharmacodynamics, Resistance, Ticks. IntroductionRhipicephalus microplus (R. microplus) is one of the most destructive ectoparasites of livestock in tropical and subtropical areas. They are responsible for severe economic losses through both the direct effects of blood feeding and indirectly as vectors of pathogens (Eckstein et al., 2015; Molento, 2020). Annual losses due to tick infestation of R. microplus were estimated to be US$3.24 billion (Grisi et al., 2014) and US$573.61 million (Rodriguez-Vivas et al., 2017) for Brazil and Mexico, respectively. As stated by Rajput et al. (2006), ticks must be controlled to meet the world’s needs for animal protein. The main methods of control can be classified into chemical methods (application of acaricides) and non-chemical methods (application of biological products and biological control). Biological control can be established using natural predators, such as the cattle egret (Bubulcus ibis) or parasites (Escherichia colli, Cedecea lapagei, and Enterobacter agglomerans) (Gonzalez, 1975; Brum, 1988) or even applying fungi as Metarhizium anisopliae, as discussed by Da Costa et al. (2002). Another control method is based on the application of natural products made from plant extracts (Guerra, 1985), especially when considering nontarget organisms and environmental effects (Panella et al., 2005; Dietrich et al., 2006). Currently, chemical treatments are almost the only available resource for the control of this parasite (Fiel and Nari, 2013), with several disadvantages due to the high cost of products and damage caused by residues (for nontarget organisms and the environments) (Pruett, 1999). Cattle tick control has traditionally been based on the application of acaricide strategies by dip bath, spraying, pour-on, or injection (Cruz et al., 2021). Incorrect dilutions, inappropriate application, overdosing, and/or persistent use are among the main factors that contribute to the emergence of acaricide resistance in ticks (Obaid et al., 2022), with growing concern for residues in milk and meat subproducts, affecting the public health through the contamination of the food chain (Camargo-Mathías, 2018). The predominant contributing factors in the development of resistance may include misuse of drugs (Bianchi et al., 2003) and use of the wrong concentration of acaricide (Dolan, 1999), leading to the failure of the tick control program (Pegram et al., 2000). There are different methodologies to determine the efficacy and efficiency (FAO, 2004; Holdsworth et al., 2022—Appendix A) of acaricides against R. microplus. For the in-vitro techniques, both adult engorged females [adult immersion test, AIT (Whitnall and Bradford, 1947)] or larvae can be used [larval package test, LPT (Stone and Haydock, 1962); larval immersion test, LIT (Shaw, 1966)]. According to Klafke et al. (2012), LIT is a technique based on the immersion of tick larvae in different solutions to later let dry and be placed on a packet of filter paper folded by the middle and then closed on the sides with metal clips, showing similarities with the LPT. The LPT presented lower sensitivity than LIT, which is a more sensitive test, detecting resistant phenotypes in a population even when present at a low frequency, assisting in the early diagnosis of resistance in the field. Sindhu et al. (2012) proposed a modification of the LIT technique using syringes to reduce the labor required to perform the original technique, leading to the emergence of the syringe immersion test (SIT). Farias et al. (2016) compared the SIT to the original LIT, concluding that the SIT proposed by Sindhu et al. (2012) presented various advantages such as: a) reduction of syringe preparation time, b) reduction of the physical space needed to store the larvae during the test, c) reduction of the risk of environmental and operator contamination with the solutions used (due to lower volume and improvement in the drying process), and d) reduction of the volume of solution used. Macrocyclic lactones (MLs) are divided into two main groups: avermectins and milbemycins. The avermectins used as ectoparasiticides are ivermectin (IVM), abamectin, and doramectin (DRM); among the milbemycins are milbemycin and moxidectin (MOX). These drugs act as high-affinity agonists on the α-subunit of chloride-selective ion channels present in the parasite. In previous experiments using the Sao Gabriel strain, Martins and Furlong (2001) demonstrated cross-resistance between DRM, MOX, and IVM. It is necessary to have a sensitivity diagnostic tool that allows obtaining results in the shortest possible time from a low number of adult ticks or from larvae of R. microplus populations of the different establishments that wish to establish or update their control or eradication plan in the field. The aim of this study was to expand the SIT for the characterization of Mozo strain sensitivity to ML and provide information on the field strain sensitivity of R. microplus larvae between IVM, DRM, and MOX. Materials and MethodsChemicalsIVM (Lot 49450511) and DRM (Lot 07492109) were donated by Compañia Cibeles S.A. (Uruguay). MOX (Lot MX-A2007025) was obtained from Laboratorio Pasteur S.A. (Uruguay). All other reagents used in this work were from SIGMA Chemical Company. Stock solutions were prepared in acetone [IVM (2,000 ppm), DRM (2,000 ppm), and MOX (100 ppm)]. R. microplus larvaeMozo strain In Uruguay, the R. microplus strain Mozo is used as the standard susceptible strain by the regulatory authorities. The larvae are obtained after the incubation of teleogynous ticks according to the specifications indicated by Drummond et al. (1973). In brief, adult female ticks are conditioned in Petri dishes and incubated with controlled temperature and humidity (27°C and 90%). After 14 days of incubation, the xenogyns are removed, and a new 25-day incubation period is continued for the hatching of viable larvae. Once hatching has occurred, 14–16 days are waited to begin larval testing. This point allows the comparison of different populations of larvae synchronized at the same time of development in terms of vitality and survival time. Field strain Larvae were obtained from three engorged ticks from each of four different animals and stored individually (n=12) to assess for drug sensitivity variance among the field strain. Adult ticks, eggs, and larvae were managed by applying the same protocols as Mozo strain. The farm manager reported reduced efficacy when using IVM for R. microplus control. Syringe assembly for SIT 2.5 mL syringes were used, to which the pivot was cut, and a 120 µm filter mesh was adjusted. The syringes are connected to the vacuum pump, and by adding tips, only viable larvae (those that show active movement) are captured. Once the syringes are loaded, holes are made to allow air entry at the time of immersion. The plunger is placed, covering the holes to prevent larvae from escaping. Larvae immersion The immersion of larvae was based on the publication of Chaparro-Gutiérrez et al. (2020). Syringes loaded with larvae are subjected to immersion for 5 minutes, in the different Drug:Diluent solution prepared from each stock solution. Corresponding drug dilutions were prepared daily according to the methodology published by FAO (2004). The diluent was used as a control solution, formulated from 1% acetone, and 0.02% Triton-X in distilled water. After the immersion time, syringes are removed, dried on drying paper, and placed in a flow hood for 1 hour prior to incubation for 24 hours. Incubation conditions are similar to those established for adult ticks (27°C and 90% relative humidity). After 24 hours, larval mortality was determined by counting both live and dead larvae. Larvae that were paralyzed or that moved only their appendages, but were unable to walk, were considered dead. Mozo strain For IVM and DRM, the final concentrations used to adjust the dose-response model were in the range of 20–0.155 ppm, and in the case of MOX, the final concentrations were between 1 and 0.01 ppm. Larvae immersion was tested in triplicate. A total of six dose-response curves were adjusted for every drug. Field strain From the models fitted to the Mozo strain, the field strain was tested at the ED50, ED80, and ED95 concentrations estimated for IVM, DRM, and MOX. Pharmacodynamic function Larvae mortality and log-transformed concentration data were used to assess the pharmacodynamic profile for increasing acaricides concentrations (four-parameter log-logistic model) (Ritz et al., 2015). The maximum effect (Emax) for each curve was set to 1 (100% mortality), being that the maximum possible mortality cannot exceed 100%. The estimated values for the lower limit of efficacy, slope (indicating the sensitivity of the technique), ED50 (as an estimation of the potency), and ED95 (considered as a discriminating dose) are shown along with their standard error. Statistical significance was determined with a 95% confidence interval. Statistical analysisThe dose-response models were fitted for the Mozo strain using R software (R Core Team, 2023) and the drc package (Ritz et al., 2015). The ratio for the pharmacodynamic parameters (lower, slope, and ED50) was calculated between IVM, DRM, and MOX. Kruskal-Wallis tests were performed for the field strain at ED50, ED80, and ED95 for each drug. All tests were performed with a statistical significance set to 95%. Ethical approvalNot needed for this study. ResultsDose-response fittingModel fitting for larval mortality of each drug (IVM, DRM, and MOX) and the pharmacodynamic parameters are shown in Figure 1 and Table 1, respectively. For each drug, dose-response models are summarized, considering the six adjusted curves as one. Pharmacodynamic parameters comparisonThe ratio for the pharmacodynamic parameters (lower limit, slope, and ED50) is shown in Table 2. The ED50 ratio between the drugs included in this study showed a significant difference between IVM and DRM when compared to MOX; a similar situation appears when comparing the lower limit. ED50 ratio between IVM and DRM versus MOX is higher than 1, meaning that MOX has the lowest ED50 of the three drugs, implying that MOX is of higher potency (two folds) when compared to IVM and DRM on R. microplus larvae, using SIT. In contrast, DRM has a different slope compared to IVM and MOX (p < 0.05), while IVM and MOX showed a similar slope (p > 0.05). This would indicate that all drugs achieve maximal efficacy, with DRM having the lowest sensitivity relative to IVM and MOX in modifying its response from minimal to maximal efficacy. SIT tested on field populationsIndividual efficacy results for each field tick, and Mozo strain by SIT using IVM, DRM, or MOX in field populations are shown in Figure 2. At the three levels studied (ED50, ED80, and ED95), all field ticks showed individual efficacies lower than those expected with the reference strain (Mozo). Although with ED95, the response was clearly lower than expected (all values were lower than 50% efficacy), it is here where a greater dispersion of individual response was visualized with a range of efficacy variation from two-fold (IVM and DRM) to ten-fold (MOX) between ticks. For the ED50 and ED80 levels, the low sensitivity of the field strains to each drug did not allow for distinguishing differences in their efficacies (all samples were below 20% mortality). DiscussionSIT is presented as an alternative to diagnose R. microplus susceptibility to MLs. Sensitivity tests performed on adult ticks (AIT) have logistical disadvantages compared to techniques performed on larvae (Spickett et al., 1983). Among the disadvantages of using adult individuals, Jonsson et al. (2007) stated that these tests require a larger number of individuals to increase the number of replicates and increase the predictive value of the test. Obtaining a sufficient number of adult ticks to perform the sensitivity diagnosis could be difficult in certain epidemiological periods or particular situations of infestation within a farm. Another disadvantage that occurs when working with adult ticks is that the vitality of the individuals is affected by the time elapsed from capture to implementation of in-vitro assays (Klafke et al., 2012); the opposite case occurs when using larvae, where there is a time window between the capture of adults ticks in the field and larvae hatching from eggs. Using a biological matrix such as R. microplus larvae, we were able to increase the number of replicates per individual obtained to include in the analysis, ensuring, on the one hand, a greater number of replicates and, on the other, evaluating the response in the offspring of the ticks already present in the animals. Both aspects are relevant for obtaining reliable and predictive results in the use of drugs for parasite control.
Fig. 1. Dose-response model fitting for R. microplus Mozo strain larvae mortality against IVM, DRM, and MOX using SIT Table 1. Pharmacodynamic parameters for dose-response model on R. microplus Mozo strain larvae against IVM, DRM, and MOX using SIT.
Table 2. Pharmacodynamic parameters ratio [lower limit, slope, and effective dose 50 (ED50)] between dose-response models on R. microplus Mozo larvae against IVM, DRM, and MOX using the SIT.
Differential aspects to consider when performing larval sensitivity techniques (LIT vs. SIT) are the immersion time and larval handling. Regarding the immersion time, Sabatini et al. (2001) stated that the toxicity of adult females to IVM is positively influenced by the immersion time. Klafke et al. (2012) evaluated different immersion times for AIT (1, 5, and 30 minutes); for the mentioned authors, the advantage of using 30 minutes of immersion lies in the lower use of active ingredients, although the results obtained present a higher variability, probably due to the lower number of replicates compared to lower immersion times. For techniques using larvae (LPT and LIT) Klafke et al. (2012) compared both, obtaining dissimilar results for the parameters of lethal concentration 50 (LC50), where the toxicity of IVM using LPT was lower than for LIT. The LC50 of LPT was 90 times higher than the LC50 obtained by LIT (1236 vs. 16 ppm, LPT and LIT, respectively). Using larval immersion techniques, the whole individual is exposed to the concentration, ensuring drug entry via cuticular and joint routes, potentially increasing drug entry to the parasite, which could explain the higher sensitivity of the immersion techniques. In our model for IVM, after fitting the values to the log-normal scale for the six dose-response curves studied, the ED50 ranged from 0.47 to 0.53, while the slope was between 1.26 and 1.49. In comparison with that reported by Klafke et al. (2012) for LPT and LIT, our results obtained with SIT are markedly below those obtained by different techniques. This allows us to indicate that SIT could be an alternative technique that would provide greater sensitivity for the early detection of field strains resistant to IVM. Regarding larval manipulation, vigorous shaking of larvae during LIT is described by Klafke et al. (2012), along with constant manipulation of individuals. Employing SIT avoids the mechanical effect of vigorous shaking, ensuring complete immersion of larvae within the syringe while reducing larval manipulation, both potential sources of variation found in LPT and LIT. In our assay, using the same IVM-sensitive Mozo strain, we expanded the range of concentrations needed for the construction of dose-response curves to obtain a better characterization of the response (20–0.156 ppm). Our study extends the validation of the SIT technique to other MLs (DRM and MOX). Regarding DRM and MOX, in-vitro tests on R. microplus intermediate life stages are scarce, being mainly used for field efficacy tests based on commercial formulations. The efficacy profiles of these acaricides are focused on field studies on adult forms of the cattle tick. Brito et al. (2011) worked with different families of acaricides on field strains (106 commercial establishments). In the case of MLs, they compared in-vitro efficacy on adult ticks of the genus R. microplus, using commercial formulations diluted in distilled water until the desired concentrations were achieved (10 ppm for the AIT technique), obtaining efficacy results above 95% for IVM, DRM, and MXD, for all field strains evaluated. Potency (ED50) ratio analysis between avermectins (IVM and DRM) showed no differences against R. microplus larvae (p > 0.05). However, when analyzing the data for the 95% effective dose (ED95), a marked difference between the concentration needed to achieve an ED95 is evident, where DRM requires lower concentrations to achieve the mentioned effect in a sensitive population. This may be due to the differences in the estimated values for the slope of the dose-response curve for the three endectocidal molecules. DRM presents a steeper (lower) slope compared to IVM (1.07 for DRM and 1.37 for IVM), reflecting differences in the speed of changes for R. microplus larvae of the Mozo strain when the SIT technique is used, where changes in the concentrations of both DRM and IVM generate increases in larval mortality but DRM does so gradually, requiring higher concentrations to reach the larval mortality levels of IVM. On the other hand, MOX presents a similar slope to IVM but with higher potency (ED50MOX < ED50IVM=ED50DRM), achieving the same level of effect at lower concentrations. A possible explanation could be that MOX has a higher lipophilicity than IVM (logPMOX=6; logPIVM=4.8) (Ménez et al., 2012a), which would favor the penetration of the acaricide upon contact with the larvae during immersion, resulting in higher mortality at lower concentrations. Prichard et al. (2012) stated the structural differences between avermectins (IVM and DRM) and milbemycins (MOX) and how they impact both pharmacokinetic and pharmacodynamic behavior, where the interaction of MOX with glutamate-mediated chloride receptors (mechanism of action of MLs) differs from IVM, suggesting differential involvement of other amino-gated chloride channels with the actions of avermectins and MOX. Within the variety of ligand-mediated chloride receptors, at some receptors, IVM was more potent than MOX, whereas at other receptors, MOX was more potent than IVM (Prichard et al., 2012).
Fig. 2. Individual efficacy results for R. microplus on Mozo strain and field population larvae, by SIT on IVM, DRM, and MOX. When using field strains, larval mortality against IVM, DRM, and MOX was found below the mortality thresholds when compared with the Mozo strain, either when comparing by drug or by ED level within each drug (ED50 vs. ED80 vs. ED95), indicating the possibility of a field strain developing resistance genes against MLs. Acaricide resistance may be metabolic (increasing the detoxification ability of the acaricide), structural alterations in the exoskeleton (reducing the penetration of the acaricides), or molecular (target-site mutation) (Pohl et al., 2012). R. microplus shows widespread resistance against MLs, particularly against IVM, leading to a concern about the possibility of cross-resistance between MLs, given their similar molecular structures and mechanisms of action (Ferreira et al., 2022). The emergence of resistance to any avermectin reduces efficacy against all avermectins, while milbemycins maintain high efficacy against avermectin-resistant populations (Ferreira et al., 2022), being that resistance to milbemycins develops more slowly than to avermectins (Ranjan et al., 2002). Although, in our work, we only evaluated toxicological response, results are indicative of cross-resistance between MLs, considering that the ticks came from animals that received IVM as a parasitological control drug. Similar conclusions were made by other authors (Pohl et al., 2012) regarding the presence of cross-resistance between IVM and MOX in field strains from commercial farms where MOX had never been used for ecto or endoparasite control. ConclusionThe SIT for R. microplus larvae is presented as an alternative for the determination of the sensitivity of tick strains in cattle against IVM, DRM, and MOX, being a new monitoring tool for the sensitivity of R. microplus to MLs in integrated parasite control programs. AcknowledgmentsLucia Vidal for her collaboration in the initial stages of the development of the laboratory methodology, to Laboratorios Compañia Cibeles S.A. and Pasteur S.A. (Uruguay) for the donation of the active principles used in the studies. Departamento de Parasitologia, DILAVE (Ministerio de Ganadería Agricultura y Pesca, Uruguay) for providing the Mozo strain. Conflict of interestThe authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Authors contributionsGS and DR designed this study and performed the data analysis. JC performed the laboratory tests. GS and DR wrote and edited the manuscript. JC edited the manuscript. GS and DR finalized the manuscript, and all the authors approved the final version. FundingThis work was supported by Universidad de la República, Uruguay. Data availabilityThe data that support the findings of this study are available from the authors upon reasonable request. ReferencesBianchi, M.W., Barre, N. and Messad, S. 2003. Factors related to cattle infestation level and resistance to acaricides in Boophilus microplus tick populations in New Caledonia. Vet. Parasitol. 112, 75–89. Brito, L.G., Barbieri, F.S., Rocha, R.B., Oliveira, M.C. and Ribeiro, E.S. 2011. Evaluation of the efficacy of acaricides used to control the cattle tick, Rhipicephalus microplus, in dairy herds raised in the Brazilian Southwestern Amazon. Vet. Med. Int. 2011, 806093. Brum, J.G.W. 1988. Infecção em teleóginas de Boophilus microplus (Acari: Ixodidae) por Cedecea lapagei. 200f. Tese (Doutorado em Ciências)—Instituto de Biologia, Universidade Federal Rural do Rio de Janeiro, Rio de Janeiro. Camargo-Mathías, M.I. 2018. Inside ticks: morphophysiology, toxicology and therapeutic perspectives [online]. São Paulo: Editora Unesp, pp: 199. Chaparro-Gutiérrez, J.J., Villar, D., and Schaeffer, D.J. 2020. Interpretation of the Larval immersion test with ivermectin in populations of the cattle tick Rhipicephalus (Boophilus) microplus from Colombian farms. Ticks Tick-borne Dis. 11(2), 101323. Cruz, R.R., García, D.I.D., Silva, S.L., and Domínguez, F.R. 2021. Integrated management of the cattle tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) and the Acaricide resistance mitigation. In Insecticides—Impact and Benefits of Its Use for Humanity, Ed Reboledo, R. [Internet]. IntechOpen; 2022. Available from: http://dx.doi.org/10.5772/intechopen.95712, pp: 207-222 Da Costa, G.L., Sarquis, M.I., De Moraes, A.M. and Bittencourt, V.R. 2002. Isolation of Beauveria bassiana and Metarhizium anisopliae var. anisopliae from Boophilus microplus tick (Canestrini, 1887), in Rio de Janeiro State, Brazil. Mycopathologia 154, 207–209. Dietrich, G., Dolan, M.C., Peralta-Cruz, J., Schmidt, J., Piesman, J., Eisen, R.J. and Karchesy, J.J. 2006. Repellent activity of fractioned compounds from Chamaecyparis nootkatensis essential oil against nymphal Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol. 43(5), 957–961. Dolan, T.T. 1999. Dogma and misunderstanding in East Coast Fever. Trop. Med. Int. Health 4, A3–A11. Drummond, R.O., Ernst, S.E., Trevino, J.L., Gladney, W.J. and Graham, O.H. 1973. Boophilus annulatus and B. Microplus: Laboratory tests of Insecticides. J. Econ. Entomol. 66(1), 130–133. Eckstein, C., Lopes, L., Romero Nicolino, R., Oliveira, C.S. and Haddad, J. 2015. Economic impacts of parasitic diseases in cattle. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 10(51), 1–10. FAO. 2004. Module 1. Ticks: acaricide resistance: diagnosis, management and prevention. Available at: https://www.fao.org/3/ag014e/ag014e.pdf Farias, J.A., Gulias Gomes, C.C., Minho, A.P., Domingues, R., Macke Franck, B., Leitzke Granada, R. and Pereira de Souza, A. 2016. Comparison of three Larval immersion tests in syringe to evaluate acaricidal activity of chemical solutions. Ciências Agrárias, Londrina 37(5), 3205–3208. Ferreira, L.C., Lima, E.F., Silva, A.L., Oliveira, C.S., Filho, G.M., Sousa, L.C., Klafke, G.M., Feitosa, T.F. and Vilela, V.L. 2022. Cross-resistance between macrocyclic lactones in populations of Rhipicephalus microplus in Brazil’s Semiarid Region. Exp. Appl. Acarol. 87(1), 109–117. Fiel, C. and Nari, A. 2013. Enfermedades parasitarias de importancia clínica y productiva en rumiantes. Ed Nari and Fiel, Montevideo, Uruguay: Editorial Agropecuaria Hemisferio Sur. Gonzalez, J.C. 1975. O Controle do Carrapato Bovino. Porto Alegre: Sulina, pp: 104. Grisi, L., Leite, R.C., Martins, J.R., Barros, A.T., Andreotti, R., Cançado, P.H., León, A.A., Pereira, J.B. and Villela, H.S. 2014. Reassessment of the potential economic impact of cattle parasites in Brazil. Rev. Bras. Parasitol. Vet. 23, 150–156. Guerra, M.S. 1985. Receituário caseiro: alternativas para o controle de pragas e doenças de plantas cultivadas e de seus produtos. Brasília, EMBRATER (EMBRATER. Informações Técnicas, 7), pp: 166. Holdsworth, P., Rehbein, S., Jonsson, N.N., Peter, R., Vercruysse, J. and Fourie, J. 2022. World Association for the Advancement of Veterinary Parasitology (WAAVP) second edition: Guideline for evaluating the efficacy of parasiticides against ectoparasites of ruminants. Vet. Parasitol. 302, 109613. Jonsson, N.N., Miller, R.J. and Robertson, J.L. 2007. Critical evaluation of the modified-adult immersion test with discriminating dose bioassay for Boophilus microplus using American and Australian isolates. Vet. Parasitol. 146(3–4), 307–315. Klafke, G.M., Castro-Janer, E., Mendes, M.C., Namindome, A. and Schumaker, T.T.S. 2012. Applicability of in vitro bioassays for the diagnosis of ivermectin resistance in Rhipicephalus microplus (Acari: Ixodidae). Vet. Parasitol. 184(2–4), 212–220. Martins, J.R. and Furlong, J. 2001. Avermectin resistance of the cattle tick Boophilus microplus in Brazil. Vet. Rec. 149(2), 64. Ménez, C., Sutra, J.F., Prichard, R. and Lespine, A. 2012. Relative neurotoxicity of ivermectin and moxidectin in Mdr1ab (-/-) mice and effects on mammalian GABA(A) channel activity. PLOS Neglect. Trop. D. 6(11), e1883. Molento, M. 2020. Avaliação seletiva de bovinos para o controle do carrapato. Brasilia, Brasil.: Ministério da Agricultura, Pecuária e Abastecimento. Available via https://www.gov.br/agricultura/pt-br/assuntos/producao-animal/arquivos-publicacoes-bem-estar-animal/CARRAPATOS2.pdf [Accessed July 21, 2023]. Obaid, M.K., Islam, N., Alouffi, A., Khan, A.Z., da Silva Vaz, I., Tanaka, T. and Ali, A. 2022. Acaricides resistance in ticks: Selection, diagnosis, mechanisms, and mitigation. Front. Cell. Infect. Microbiol. 12, 941831. Panella, N.A., Dolan, M.C., Karchesy, J.J., Xiong, Y., Peralta-Cruz, J., Khasawneh, M., Montenieri, J.A. and Maupin, G.O. 2005. Use of novel compounds for pest control: insecticidal and acaricidal activity of essential oil components from heartwood of Alaska yellow cedar. J. Med. Entomol. 42(3), 352–358. Pegram, R.G., Wilson, D.D. and Hansen J.W. 2000 Past and present national tick control programs. Why they succeed or fail? Ann. NY Acad. Sci. 916, 546–554. Pohl, P.C., Klafke, G.M., Júnior, J.R., Martins, J.R., da Silva Vaz, I., Jr. and Masuda, A. 2012. ABC transporters as a multidrug detoxification mechanism in Rhipicephalus (Boophilus) microplus. Parasitol. Res. 111(6), 2345–2351. Prichard, R., Ménez, C. and Lespine, A. 2012 Moxidectin and the avermectins: consanguinity but not identity. Int. J. Parasitol. Drugs Drug Resist. 2, 134–153. Pruett, J.H. 1999. Immunological control of arthropods ectoparasites—a review. Int. J. Parasitol. 29, 25–32. R Core Team. 2023. R: a language and environment for statistical computing; R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org/ Rajput, Z.I., Hu, S.H., Chen, W.J., Arijo, A.G. and Xiao, C.W. 2006. Importance of ticks and their chemical and immunological control in livestock. J. Zhejiang University Sci. B. 7(11), 912–921. Ranjan, S., Wang, G.T., Hirschlein, C. and Simkins K.L. 2002 Selection for resistance to macrocyclic lactones by Haemonchus contortus in sheep. Vet. Parasitol. 103, 109–117. Ritz, C., Baty, F., Streibig, J.C. and Gerhard, D. 2015. Dose-response analysis using R. PLoS ONE 10(12), e0146021. Rodriguez-Vivas, R.I., Grisi, L., Pérez de León, A.A., Silva Villela, H., Torres-Acosta, J.F.J., Fragoso Sánchez, H., Romero Salas, D., Rosario Cruz, R., Saldierna, F. and García-Carrasco, D. 2017. Potential economic impact assessment for cattle parasites in Mexico review. Rev. Mex. Cienc. Pec. 8(1), 61–74. Sabatini, G.A., Kemp, D.H., Hughes, S., Nari, A. and Hansen, J., 2001. Test to determine LC50 and discriminating doses for macrocyclic lactones against the cattle tick Boophilus microplus. Vet. Parasitol. 95, 53–62. Shaw, R.D. 1966. Culture of an organophosphorus-resistant strain of Boophilus microplus (Can.) and an assessment of its resistance spectrum. Bull. Entomol. Res. 56, 389–405. Sindhu, Z.U., Jonsson, N.N. and Iqbal, Z. 2012. Syringe test (modified larval immersion test): a new bioassay for testing acaricidal activity of plant extracts against Rhipicephalus microplus. Vet. Parasitol. 188(3–4), 362–367. Spickett, A.M. and Nari Henrioud, A.J. 1983. The efficacy of the Drummond adult test on Boophilus microplus females (Acarina: Ixodidae) subjected to various periods of cold storage prior to organophosphate testing. Onderstepoort J. Vet. Res. 50(3), 197–198. Stone, B.F. and Haydock, P., 1962. A method for measuring the acaricide susceptibility of the cattle tick Boophilus microplus (Can.). Bull. Entomol. Res. 53, 563–578. Whitnall, A.B. and Bradford, B., 1947. An arsenic resistant tick and its control with gammexane dips. Bull. Entomol. Res. 38, 353–372. | ||
| How to Cite this Article |
| Pubmed Style Robaina D, Caballero J, Suárez G. Syringe immersion test as in vitro bioassay against Rhipicephalus microplus: Macrocyclic lactones dose-response relationship. Open Vet J. 2023; 13(10): 1259-1267. doi:10.5455/OVJ.2023.v13.i10.4 Web Style Robaina D, Caballero J, Suárez G. Syringe immersion test as in vitro bioassay against Rhipicephalus microplus: Macrocyclic lactones dose-response relationship. https://www.openveterinaryjournal.com/?mno=162433 [Access: September 01, 2024]. doi:10.5455/OVJ.2023.v13.i10.4 AMA (American Medical Association) Style Robaina D, Caballero J, Suárez G. Syringe immersion test as in vitro bioassay against Rhipicephalus microplus: Macrocyclic lactones dose-response relationship. Open Vet J. 2023; 13(10): 1259-1267. doi:10.5455/OVJ.2023.v13.i10.4 Vancouver/ICMJE Style Robaina D, Caballero J, Suárez G. Syringe immersion test as in vitro bioassay against Rhipicephalus microplus: Macrocyclic lactones dose-response relationship. Open Vet J. (2023), [cited September 01, 2024]; 13(10): 1259-1267. doi:10.5455/OVJ.2023.v13.i10.4 Harvard Style Robaina, D., Caballero, . J. & Suárez, . G. (2023) Syringe immersion test as in vitro bioassay against Rhipicephalus microplus: Macrocyclic lactones dose-response relationship. Open Vet J, 13 (10), 1259-1267. doi:10.5455/OVJ.2023.v13.i10.4 Turabian Style Robaina, Diego, Jessica Caballero, and Gonzalo Suárez. 2023. Syringe immersion test as in vitro bioassay against Rhipicephalus microplus: Macrocyclic lactones dose-response relationship. Open Veterinary Journal, 13 (10), 1259-1267. doi:10.5455/OVJ.2023.v13.i10.4 Chicago Style Robaina, Diego, Jessica Caballero, and Gonzalo Suárez. "Syringe immersion test as in vitro bioassay against Rhipicephalus microplus: Macrocyclic lactones dose-response relationship." Open Veterinary Journal 13 (2023), 1259-1267. doi:10.5455/OVJ.2023.v13.i10.4 MLA (The Modern Language Association) Style Robaina, Diego, Jessica Caballero, and Gonzalo Suárez. "Syringe immersion test as in vitro bioassay against Rhipicephalus microplus: Macrocyclic lactones dose-response relationship." Open Veterinary Journal 13.10 (2023), 1259-1267. Print. doi:10.5455/OVJ.2023.v13.i10.4 APA (American Psychological Association) Style Robaina, D., Caballero, . J. & Suárez, . G. (2023) Syringe immersion test as in vitro bioassay against Rhipicephalus microplus: Macrocyclic lactones dose-response relationship. Open Veterinary Journal, 13 (10), 1259-1267. doi:10.5455/OVJ.2023.v13.i10.4 |