| Research Article | ||
Open Vet J. 2023; 13(12): 1597-1606 Open Veterinary Journal, (2023), Vol. 13(12): 1597–1606 Original Research Secretome of bovine umbilical vein endothelial cells promote wound healing regeneration on the second degree rat model burn injuryMavheena Moghan1, Paarvaishnee Naidu1, Nadhrah Binti Zulkifly1, Yulfia Nelymalik Selan2, Erif Maha Nugraha Setyawan3, Sugi Winarsih4 and Dwi Liliek Kusindarta1*1Department of Anatomy, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia 2Department of Anatomy, Faculty of Medicine and Veterinary Medicine, Nusa Cendana University, Kupang, Indonesia 3Department of Obstetrics and Gynecology, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia 4Department of Agriculture, Food, and Fisheries of Sleman Regency, Yogyakarta, Indonesia *Corresponding Author: Dwi Liliek Kusindarta. Department of Anatomy, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia. Email: indarta [at] ugm.ac.id Submitted: 21/08/2023 Accepted: 17/11/2023 Published: 31/12/2023 © 2023 Open Veterinary Journal
ABSTRACTBackground: Burn injuries are an alarming indicator of the sensitivity of human tissue when confronted with high temperatures or chemicals. The current treatment for burn wounds needs to be improved and more extensive in scope. Significant research advances concerning the therapeutic potential of secretomes over the past two decades have expanded the range of therapies that utilize secretomes to encompass populations other than stem cells. Aim: This study details how the secretome extracted from the bovine umbilical vein endothelial cell (BUVEC) promotes the healing of burn injuries. Methods: The 48 rats were divided into four groups, namely the control group with povidone-iodine, the 5% BUVEC-conditioned medium (CM) cream group, the 10% BUVEC-CM cream group, the 15% BUVEC-CM cream group. Animals induced type II burns under anesthesia. Treatment is carried out topically, two times a day. Every day the wound was measured. The animals were put to sleep for samples on days 5, 13, 21, and 19. Samples in the form of skins were soaked in 4% paraformaldehyde and processed with paraffin-embedded for tissue preparations. The research results were processed using two way ANOVA. Results: The study showed that on day 5, wound closure occurred, whereas in the povidone-iodine group, macroscopically, the wound closed faster. Epithelial repair, increased fibroblasts and collagen, and blood vessel formation greatly increased in the 15% BUVEC-CM group on days 13, 21, and 29. Conclusion: Taken together, BUVEC secretome promoted fibroblast regeneration, collagen formation, re-epithelialization, and hair follicle regeneration on the burn injury wound healing. Keywords: Secretome, BUVEC, Burn wound, Rat model, Remodeling. IntroductionBurn injuries are still regarded as one of the most serious medical emergencies afflicting people of all ages and genders (Tian et al., 2018). Every year, 11 million people worldwide suffer from burn injuries, which equates to at least 30,000 burn victims per day and up to 180,000 deaths annually. 90% of burns still occur in low- and middle-income regions, even though burn injuries are declining in wealthy nations. Meanwhile, burn injuries typically exhibit a two-peaked age distribution, with significant rates among minors and middle-aged workers, burn wounds in the senior community are linked to an astonishingly high fatality rate. Consequently, burns are a significant worldwide health problem linked to significant sickness, death, and incapacitating mental and financial effects that last a lifetime (Kareem et al., 2021). Recently, the purpose of burn injury treatment is to restore the damaged epidermis’s original structure and normal function. Depending on the extent of the damage, various levels of burn wound care are addressed e.g., the prevention of infection, surgical treatment, long-term or short-term wound covering, and reduction of scarring, ultimately deciding the burn patient’s survival and prognosis (Stone et al., 2018). Severe burns cause injury to the structure and function of the skin, in addition to the disappearance of cell ancestors required for skin rejuvenation and repair. The surgical treatment of burn injuries involves removing dead tissue to prevent infection in the burn wound, followed by using skin grafts extracted from healthy donor areas to cover the wound in a final and permanent manner. Nevertheless, autogenous skin grafts are only sometimes viable, especially when confronted with extensive burns, a restricted supply of donor sites, or a high bacterial burden on the wound surface (Ozhathil et al., 2021). In such cases, injuries are temporarily covered with allografts from deceased human skin, porcine skin xenografts, or dermal analogs until a final covering is possible with autologous skin transplants or artificial skin replacements. Synthetic substitutes for skin act as temporary frameworks for cellular infiltration, growth, and wound neovascularization (Vyas and Vasconez, 2014). Cultivated epithelial autografts (CEA) comprised of self-generated keratinocytes in a fibrin mesh and self-produced bilayered skin substitutes made from self-generated fibroblasts and keratinocytes are the most notable skin substitutes. Even though both of these products provide self-generated alternatives to split-thickness autografts, their delicacy, high costs, and lengthy production times prevent their clinical application. Current wound-covering materials are either ineffective, susceptible to time-consuming, immune rejection, acellular, or costly, necessitating the development of alternative materials. Burns can result from a range of causes, such as radiation, low temperatures, high temperatures, chemicals, and electricity. Treating burns is to reinstate the affected epidermis’s original physical structure and functional processes. The extent of the burn injury determines the appropriate level of care required, which includes preventing infection, performing surgical procedures, providing temporary or permanent wound protection, and minimizing scarring. These factors ultimately impact the burn patient’s survival and prognosis (Stone et al., 2018). Severe burns cause injury to the structure and function of the skin, as well as the loss of cell ancestors, required for skin repair and regeneration. The surgical treatment of burn wounds involves removing dead tissue to prevent infection, followed by definitive wound coverage with skin grafts extracted from healthy donor sites. However, using one’s own skin grafts is only sometimes a viable option, especially in cases of extensive burns with limited donor site options or a high bacterial burden on the wound bed. Circulating endothelial ancestors have been proposed as a valuable source for producing fully functional endothelial cells (ECs) and as potential candidates for treating a variety of clinical conditions, similar to stem cells (Castelli et al., 2016). It has been demonstrated that conditioned media and secretomes have therapeutic effects comparable to the paracrine effects of stem cells and endothelial progenitor cells (EPCs). Since it can restore deteriorated tissue, stem cell therapy is widely used for wound healing and regenerative disease therapy, particularly of mesenchymal origin. However, there is a risk of rejection with cell-based therapies, and cells can proliferate uncontrollably. These circumstances prompted the creation of cell-free treatments. Secretome derived from HUVEC is one of the cell-free therapeutic methods that has a similar therapeutic impact as the paracrine effects of the original cells, while also reducing the likelihood of rejection and HLA negativity (Zhang and Duan, 2018). The secretome of HUVEC shares the same characteristics as their ECs. Secretome plays a crucial role both in paracrine and autocrine cell communication. The paracrine impact of HUVEC may enhance EC generation from cord blood (CB) EPCs and adipose-derived stem cells (ADS). HUVEC models have investigated the molecular and signaling pathways implicated in blood vessel formation (Zhang and Duan, 2018). The HUVEC secretome contains an abundance of growth factor components and cytokines. Secretom HUVEC can boost fibroblast proliferation, migration, and collagen synthesis (Fallah et al., 2019; Zhang et al., 2020) in addition to enhancing the proliferation and differentiation of CB EPCs and delaying their senescence (Castelli et al., 2016). The secretome was in high request as a treatment for wound recovery and degenerative diseases at the time (Kim et al., 2017; Zhao et al., 2020). Current burn injury treatment limitations necessitate circulating endothelial progenitor cells and their derivatives as a conditioned medium (CM) to accelerate the healing of burn injuries. Although umbilical mesenchymal stem cells from humans, rats, and birds have been obtained and characterized (Svoradova et al., 2021), reports on large livestock, primarily bovine, are uncommon. This research seeks to determine how the secretome obtained from the bovine umbilical vein EC (BUVEC) aids in healing burn injury. Materials and MethodsBUVECs isolationBUVEC is obtained from the umbilical cord of a cow around the Special Region of Yogyakarta, which gives birth normally without a retained placenta. The umbilical vein was rinsed with Dulbecco’s phosphate-buffered saline (DPBS) (Capricorn, Ebsdorfergrund, Germany). Collagenase type II 0.025% solution (Langenselbold, Germany) in Hank’s balanced saline solution (HBBS) (Capricorn, Ebsdorfergrund, Germany) was deposited in the cattle’s lumen of the umbilical vein and incubated for 30 minutes at 37°C to release ECs. The collagenase type II solution cells were collected and centrifuged at 2400 rpm for 5 minutes. Pellets containing BUVECs were grown in Dulbecco’s modified Eagle medium (DMEM) (Gibco, Langenselbold, Germany) culture medium enriched with 10% fetal bovine serum (FBS) (Capricorn, Ebsdorfergrund, Germany), 2% penicillin/streptomycin (Capricorn, Ebsdorfergrund, Germany), and 0.5% amphotericin (Gibco, Langenselbold, Germany) and incubated at 37°C with 5% CO2. BUVECs-CM cream preparationBUVEC was grown in culture media, and after reaching 80% confluence, the medium was collected to be called BUVEC-CM. BUVEC-CM cream is made by mixing vaseline album cream (0.83 g/ml) and CM (1 g/ml). BUVEC-CM 5%, 10%, and 15% cream was administered each morning with a spatula. Experimental animal designThis research calculated the sample size using “Power and Sample Size Calculation” application version 3.2.6 (Informer Technologies, Inc.). In previous studies, the average wound closure treated with MSC-CM was 67.03%, while that of the controls was 52.18% (Laksmitawati et al., 2022). With an alpha of 0.01, a power of 0.90, an SD of 8.38, and a difference of 15%, the required sample is 11 tails plus one tail for reserve. A total of 48 Wistar rats (Rattus norvegicus) aged an average of 2–3 months weighing 200–300 g are obtained. All rats are acclimated individually in 40 × 20 × 18 cm cages at 24°C for 1 week, provided a 12 h dark/light cycle with ad libitum water and feed. The rats were arbitrarily separated into four groups, each group consisting of 12 rats. Group 1 will be treated with a povidone-iodine. Group 2 is treated with 5% BUVEC-CM cream, Group 3 10% BUVEC-CM cream, and Group 4 15% BUVEC-CM cream. The animals will be treated and observed for 5, 13, 21, and 29 days. On the first day, the burn wound will be administered, and the wound progress will be examined once daily. The animals will be euthanized on days 5, 13, 21, and 29, and skin samples will be taken. Euthanization was carried out with a combination of ketamine 10% at 100 mg/kg BW and 2% xylazine at a dose of 13 mg/kg intramuscularly. Burn wound inductionThe rats’ back hair was shaved and anesthetized with ketamine 0.6 mg/kg and xylazine 0.4 mg/kg intramuscularly. The shaved area is then disinfected with povidone-iodine. The electric cutter was 1 cm in diameter with a temperature of 80°C and placed on the rat’s back for 10 seconds with a little pressure. One injury was caused for each rat and treated with cream once daily, according to each group. Hematoxylin and eosin stainingSkin samples were fixed in 10% formalin for more than 24 hours, paraffin-embedded starting with samples dehydrated using graded ethanol, cleaned in xylene (KgaA), then embedded in paraffin (Leica Biosystems). The formalin fixed paraffin-embedded (FFPE) sample was sliced to 5 μm with a rotary microtome (Yamato RV 240). The slides were deparaffinized with xylol, and then rehydrated using absolute alcohol and graded alcohol. The tissue slides were stained using hematoxylin-eosin (Bio-Optica, Milan, Italy). The slides were observed using a light microscope (Olympus BX51, Tokyo, Japan). Images were taken with Optilab software (Optilab, Yogyakarta, Indonesia). Masson trichromeThe slides were deparaffinized with xylol, and then rehydrated using absolute alcohol and graded alcohol. Masson’s trichrome staining procedure is performed according to the Masson’s trichrome kit (Bio-Optica, Milan, Italy). Drop each slide as much as three drops of Weigert’s iron hematoxylin A and Weigert’s iron hematoxylin B solutions, and let stand for 15 minutes. The remaining dye on the slide was removed, and a solution of the picric acid alcoholic stable was added and let stand for 10 minutes. Wash with distilled water, add ponceau acid fuchsin solution, and leave for 5 minutes. Wash with distilled water, add phosphomolybdic acid solution, and leave for 10 minutes. The last solution, the light green solution, is dripped and waited for 10 minutes. Dehydration was carried out with graded alcohol and absolute alcohol, clearing with xylol, mounting with Balsama Canada, and coverslip. The slides were observed under a light microscope (Olympus BX51, Tokyo, Japan). Images were taken with Optilab software (Optilab, Yogyakarta, Indonesia). Enzyme-linked immunosorbent assayAn ELISA procedure was performed using human vascular endothelial growth factor (VEGF) and FGF (Fine Test, Wuhan, China) to determine the presence of VEGF and FGF. The procedure begins by washing the plate twice with washing buffer. A total of 100 μl of sample in BUVEC-CM was added to the wells in triplicate and incubated for 90 minutes with temperature 37°C. Samples were aspirated and washed three times. 100 μl of Biotin-labeled antibody was added to each well and incubated for 60 minutes. Aspirate and wash the plate three times. A working solution, HRP-strepvidin conjugate (SABC), as much as 100 μl was added to each well and incubated for 30 minutes. Aspirate and wash the plate five times. 90 µl of TMB was added to each well and incubated for 15–30 minutes. Stop the reaction with stop solution 50 μl. The absorbance of the sample is read as soon as possible at a wavelength of 450 nm. Analysis of dataQualitative analysis was carried out on macroscopic and microscopic examination of burns. The diameter of the wound was observed daily, and the wound closure area was measured using a caliper. Parameters observed include the density of fibroblasts, blood vessels, and collagens were counted using an image raster. Statistical data analyses of wound closure were calculated with two-way analysis of variance p < 0.05 was presumed to signify statistical significance. Statistical analysis was executed using GraphPad Prism 8 (La Jolla, CA). Ethical approvalAll trials were conducted with the consent of the Ethics Committee of the Faculty of Veterinary Medicine, Gadjah Mada University, Yogyakarta, Indonesia (with approval: 011/EC-FKH/Ex./2023). ResultsBUVEC-CM improves woundOur result showed that on day 5, the burn wounds were still inflamed. In the povidone-iodine group, the wound size reduced faster than in BUVEC-CM treated groups (Fig. 1). On day 13, povidone-iodine and BUVEC-CM groups showed crust formation, and the burn wound dried up at the same rate as BUVEC-CM treated groups. The progress of the wound closure from day 21 for BUVEC-CM treated groups was much better than the povidone-iodine groups, and the wound crust was peeled. But at day 29, BUVEC-CM healed slightly faster than povidone-iodine, but the difference is almost unnoticeable because wound closure is almost complete (Fig. 2). BUVEC-CM improves epithelization and hair follicles growthEpithelial recovery in each week, including day 5, 13, 21, and 29 of the non-treated skin, showed that the epidermis remains intact, and sebaceous glands are still visible. Epidermis, dermis, and hypodermis can be differentiated and are visibly intact. Muscle layers are also visible. Hair follicles are evenly distributed and visible. In epithelial recovery day 5 of povidone-iodine group and group 2 treated with BUVEC-CM, 5% showed a significant difference where epidermis in povidone-iodine was less intact in the BUVEC-CM 5% group, but both had presence of fibroblast (Fig. 3A). This shows that reepithelization was faster on day 5 for BUVEC-CM 5%. Day 13 shows more recovery progress—epithelial recovery is better in BUVEC-CM 5%, 10%, and 15% groups than povidone-iodine group (Fig. 3B). Epidermis and dermis are more intact in BUVEC-CM-treated groups. Moreover, epithelial recovery on day 21 in wounds treated in BUVEC-CM 5%, BUVEC-CM 10%, and BUVEC-CM 15% showed more hair follicle growth wrapped in sebaceous glands compared to povidone–iodine groups (Fig. 3C). Furthermore, epithelial recovery on day 29 shows almost fully complete wound closure and good reepithelization ratio, especially in BUVEC-CM 15% treated group compared to povidone–iodine, BUVEC-CM 5%, and BUVEC-CM 10% groups as shown in (Fig. 3D).
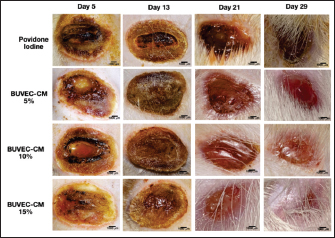
Fig. 1. Macroscopic picture of the healing process of type II burns treated with povidone-iodine, BUVEC-CM cream 5%, BUVEC-CM cream 10%, BUVEC-CM cream 15%. The macroscopic image shows that on the fifth day, the burn wound began to close and got smaller on the 29th day but had not closed completely.
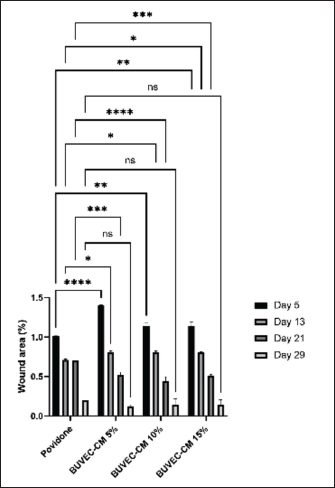
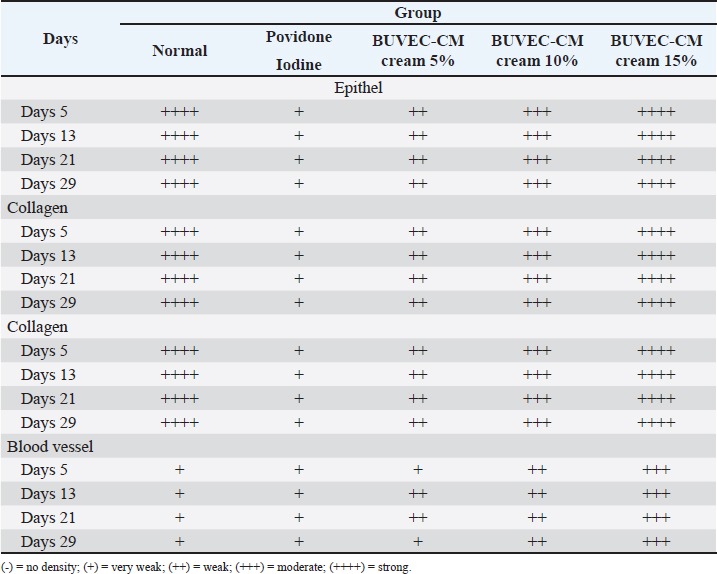
Fig. 2. Graph of wound area based on each treated group, including povidone-iodine, BUVEC-CM 5%, BUVECCM 10%, and BUVEC-CM 15%. On day 5, povidone and BUVEC-CM 5% show the highest significance. On day 13, povidone-iodine and BUVEC-CM 10% show less significance. On day 21, there was huge significance in BUVEC-CM 10%. *p < 0.1, **p < 0.01, ***p < 0.001, ****p < 0.0001. ns=non-significant. BUVEC-CM increased fibroblast density, collagen density, and blood vessel formationFibroblast proliferation (Fig. 4), blood vessel formation on days 5, 13, 21, 29 (Fig. 5A–D), and collagen formation on days 5, 13, 21, 29 (Fig. 6A–D) are important in wound healing. On days 5 and 13, BUVEC-CM 15% treated groups showed a higher fibroblast and collagen density than BUVEC-CM 5%, BUVEC-CM 10%, and povidone–iodine group (Table 1).
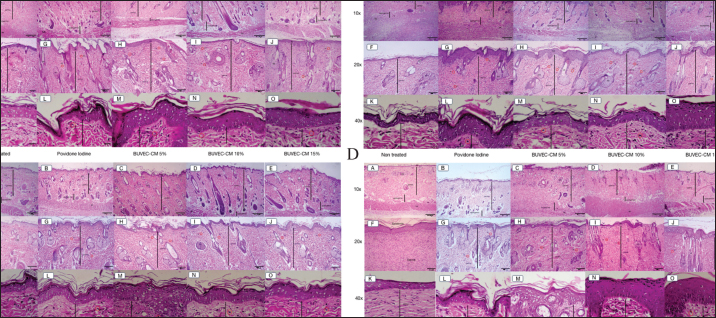
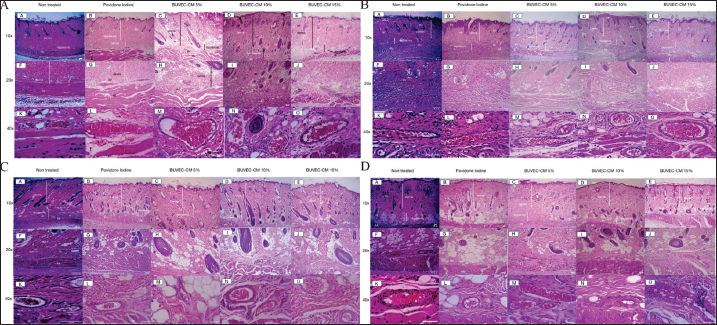
Fig. 3. Microscopic images of the epithelialization process of the healing process of type II burns treated with povidone-iodine, BUVEC-CM cream 5%, BUVEC-CM cream 10%, BUVEC-CM cream 15% on day 5 (3A), -13 (3B), -21 (3C), and -29 (3D) in 10×, 20×, and 40× magnification. Microscopic images show that on all days, epithelial formation in the 5%, 10%, and 15% BUVEC-CM cream group was faster than the povidone-iodine group, with the fastest epithelial formation occurring in the 15% BUVEC-CM group. EP=epidermis; SG=sebaceous glands; ML=muscle layer; HF=hair follicle; BV=blood vessel; Fl (red circle)=fibroblast.
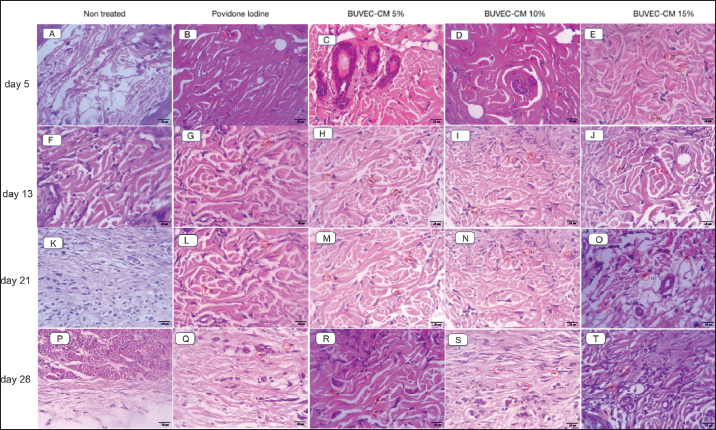
Fig. 4. Microscopic image of fibroblast density from the healing process of type II burns treated with povidone-iodine, BUVECCM cream 5%, BUVEC-CM cream 10%, BUVEC-CM cream 15% on day 5, -13 (4A), -21, and -29 (4B) in 40× magnification. In the microscopic image, it can be seen that in the 5%, 10%, and 15% BUVEC-CM cream group, fibroblast formation was faster than the povidone-iodine group, with the fastest fibroblast formation found in the 15% BUVEC-CM group. Fl (red circle)=fibroblast.
Fig. 5. Microscopic image of blood vessel formation from the healing process of type II burns treated with povidone-iodine, BUVEC-CM cream 5%, BUVEC-CM cream 10%, BUVEC-CM cream 15% on day 5 (5A), -13 (5B), -21 (5C), and -29 (5D) in 10×, 20×, and 40× magnification. In the microscopic image, it can be seen that in the 5%, 10%, and 15% BUVEC-CM cream group, blood vessels were faster than in the povidone-iodine group, with the fastest blood vessel formation found in the 15% BUVEC-CM group. EP=epidermis; ML=muscle layer; HF=hair follicle; SG=sebaceous glands; BV=blood vessel.
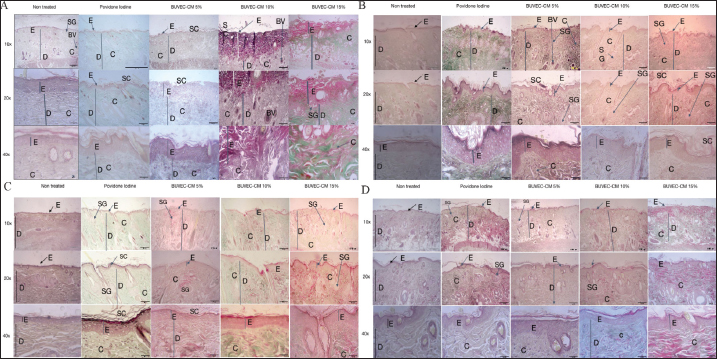
Fig. 6. Microscopic image of collagen density from the healing process of type II burns treated with povidone-iodine, BUVEC-CM cream 5%, BUVEC-CM cream 10%, BUVEC-CM cream 15% in 10×, 20×, and 40× magnification. In the microscopic image, it can be seen that in the 5%, 10%, and 15% BUVEC-CM cream group, collagen formation was faster than in the povidone-iodine group, with the fastest collagen formation occurring in the 15% BUVEC-CM group. E=epidermis; D=dermis; C=collagen; SG=sebaceous glands; BV=blood vessel; SC=scab.
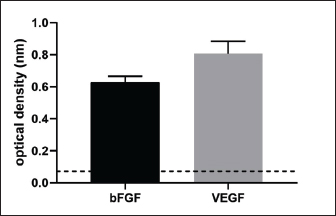
Fig. 7. Detection of VEGF and bFGF in BUVEC-CM using ELISA. VEGF and bFGF are contained in BUVEC-CMVEGF and bFGF were found in BUVEC-CM which were detected using ELISA. This conclusion was obtained from the optical density of VEGF and bFGF, respectively, 0.63 ± 0.03 and 0.8 ± 0.06 (Fig. 7). DiscussionBurn injuries are a sobering reminder of the vulnerability of human tissue when exposed to intense heat or chemicals (Jeschke et al., 2020). Victims may experience excruciating pain, impaired mobility, scarring, or disfigurement—all of which are visible reminders of their traumatic incident—while also confronting emotional challenges associated with self-image and social acceptance. Burn wounds necessitate prompt medical attention in order to prevent potential complications such as infection or hypovolemic shock caused by fluid loss due to damaged blood vessels in the injured area. Currently, the treatment for burn wounds is still not satisfying and with many limitations (Kadduora et al., 2017). In the last two decades, significant research advancements concerning the therapeutic potential of secretomes have broadened the scope of therapies based on secretomes to include populations other than stem cells. The term “secretome” refers to a collection of molecules secreted or excreted by living cells, including chemokines, growth factors, free nucleic acids, cytokines, extracellular vesicles, and lipids (Műzes and Sipos, 2022). The BUVEC secretome is a medium conditioned by bovine ECs. This investigation focused on the efficacy of BUVEC secretomes in healing burns in rat models. Table 1. Epithelial growth, fibroblast density, collagen density, and blood formation of burn wound treated by povidone iodine, BUVEC-CM cream 5%, BUVEC-CM cream 10%, BUVEC-CM cream 15%.
This investigation examined a level two burn wound, in which second-degree burns extend deeper into the dermis, resulting in blister formation. Compared to the use of povidone–iodine, the administration of secretome at stratified dosages of 5%, 10%, and 15% resulted in faster wound healing. Burns treated with secretome exhibited flawless re-epithelialization without scarring, uniform hair coverage, increased collagen, fibroblasts, and new blood vessels, leading to an increase in angiogenesis. According to previous research, the BUVEC secretome is abundant in amino acids, proteins, and cytokines, contributing to its functional efficacy. In addition, it is believed that the BUVEC secretome, like the secretome obtained from human umbilical vein ECs, contains numerous growth factors and antimicrobial peptides (Kusindarta and Wihadmadyatami, 2021; Larasati et al., 2022). This information corresponds to the composition of CM or secretome from HUVEC. Luminex assays revealed the presence of a number of growth factors and cytokines in the HUVEC secretome: bone morphogenetic protein-9, angiopoietin-2, interleukin-8, endoglin, endothelin-1, leptin, heparin-binding epidermal growth factor, VEGF-A,-C,-D, fibroblast growth factor-1 and -2, hepatocyte growth factor (Fromer et al., 2018). Due to their capacity to regulate communication within cells by facilitating the movement of lipids, proteins, or RNAs to specific cells, the secretome is highly particular interest. The secretome of endothelial progenitor cells seems to enhance the survival, growth, and formation of tubules in ECs by upregulating the expression of VEGF and endothelial nitric oxide synthase (Alwjwaj et al., 2021). In addition, endothelial progenitor cells-secretome has been shown that the secretome of endothelial progenitor cells promotes angiogenesis in both laboratory settings and living organisms (Maki et al., 2018). Tissue regeneration is a straightforward straight procedure in which growth factors induce cell growth, contributing to a series of modifications involving soluble agents, blood cells, the formation of extracellular matrix, and the multiplication of parenchymal cells (Plikus et al., 2021). The wound healing process will begin with an inflammatory reaction, cell growth, and the production of extracellular matrix components, will conclude with a remodeling phase. During this research, all groups developed scars. The purpose of scar formation is to seal the wound in order to prevent debris and bacteria from entering and to retain moisture trapped beneath the scar. Once the new layer of skin below the scab is fully developed, the scars will separate from the incision because the new tissue will push collagen, elongate the fibrin, or break down the collagen using enzymes produced by skin cells and white blood cells (Gonzalez et al., 2016). This occurrence can account for the large variation in daily lesion reduction percentages. During a vascular inflammation reaction, the damaged blood vessels constrict and the leaked blood clots, thereby maintaining the integrity of the blood vessel. The clotting process involves the activation and clustering of blood platelets and thrombocytes within a fibrin mesh, dependent on the influence of distinct elements (Periayah et al., 2017). Besides restoring balance and creating a shield against microbial intrusion, the fibrin mesh arranges the temporary structure necessary for cellular movement, which restores the skin’s protective barrier function and maintains its wholeness. Furthermore, this encourages cellular movement toward the lesion’s microenvironment and stimulates the growth of fibroblasts (Potekaev et al., 2021). In addition, normal dermal fibroblast responses to injury are essential for wound healing. The immediate reaction of dermal fibroblasts close to wound sites is known to be proliferation, followed by migration into the wound bed (Rognoni et al., 2018). The proliferation and migration rates of fibroblasts derived from fetal tissue were higher than those derived from adult tissue (Castillo et al., 2023). After treatment with BUVEC secretome, the migration, and proliferation of fibroblasts were notably enhanced in our study. Additionally, BUVEC secretome improved the viability and migration of dermal fibroblasts in a dose-dependent manner. This indicated a correlation between the quantity of BUVEC secretome and fibroblasts’ proliferation and migration rate. The collagen results indicated that burn wounds treated with secretome contained more collagen than the control and povidone–iodine groups. This phenomenon may be explained by the fact that fibroplasia initiates the creation of granulation tissue, which is defined by the multiplication of fibroblasts. These principal factors aid in the development of the freshly generated structure (Diller and Tabor, 2022). Collagen is the primary element of a fully developed connecting tissue lesion. Fibroblasts that produce collagen are enlisted from the dermis at the periphery of the wound to produce this protein. Establishing a complete basal membrane between the epidermis and dermis is crucial for restoring the skin’s integrity and functionality (Tracy et al., 2016). On the parameter of hair growth, the secretome group demonstrated quicker hair growth and hair follicle than the povidone–iodine group. These findings are comparable to those of other investigations on the impact of secretome on the treatment of alopecia in humans and rodents, indicating that secretome can promote hair growth. These investigations propose that the growth factors found in secretomes are responsible for hair growth (Salhab et al., 2022). Similarly to BUVEC secretome, this study’s results may be because secretome theoretically contains the maximum concentration of growth factors. Furthermore, when wound healing reaches the remodeling phase, there will be a decrease in angiogenesis and blood flow, causing the secretom group to display the least neovascularization. Certain growth factors can theoretically stimulate angiogenesis during the proliferation phase, between days 3 and 10 (Johnson and Wilgus, 2014). Consequently, if the wound recovery is still in the proliferation phase, the secretome group should exhibit the most neovascularization compared to the povidone iodine or untreated groups. In addition, the re-epithelization ratio of the BUVEC secretom group is superior to that of the povidone-iodine group. Keratinocytes and progenitor cells from oil glands or hair follicles are crucial to re-epithelization. This result may be because of the existence of cytokines and growth factors in secretom, producing enhanced stem cell and keratinocyte migration, as demonstrated by separate research on using secretom, resulting in enhanced fibroblast and keratinocyte migration. Recent research has demonstrated that BUVEC secretom promotes primary burn wound healing and regeneration via paracrine effect. This suggests that BUVEC secretome contains a variety of factors that stimulate fibroblast, keratinocyte, collagen, proliferation, migration, and differentiation, thereby enhancing the regeneration and functional recovery of burn injuries. ConclusionIn conclusion, the BUVEC secretome released substances with greater therapeutic potential for rat wound skin injury. According to our findings, BUVEC secretome promoted reepithelialization, fibroblast regeneration, collagen formation, and angiogenesis. In addition, BUVEC secretom promotes wound closure, hair follicle construction, and improves burn wound healing quality. Using BUVEC secretome as an alternative cell-free treatment strategy for burn wound healing avoids the ethical issues associated with cell transplantation and the danger of tumor formation, and it has the same function as cell-based therapy. AcknowledgmentsThe authors wish to thank Universitas Gadjah Mada for the Final Project Recognition Grant (RTA), with the grant number 5075/UN1.P.II/Dit-Lit/PT.01.01/2023 to Dwi Liliek Kusindarta. Conflict of interestThe authors declare that there is no conflict of interest. Author contributionConceptualization: DLK. Methodology: DLK, YNS. Validation: DLK, EMNS. Formal analysis: MM, PN, NBZ, DLK. Investigation: MM, PN, NBZ. Resources: EMNS, SW. Data curation: DLK, SW. Writing original draft preparation: MM, PN, NBZ. Writing review and editing: DLK, YNS, EMNS. Visualization: YNS. Supervision: DLK. Project administration: DLK. Funding acquisition: DLK. FundingThe “Recognition of Final Assignment” program, Research Directorate, Gadjah Mada University, funded this research. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesAlwjwaj, M., Rais Reskiawan, A.K. and Bayraktutan, U. 2021. The secretome of endothelial progenitor cells: a potential therapeutic strategy for ischemic stroke. Neural. Regen. Res. 16(8), 1483–1489. Castelli, G., Parolini, I., Cerio, A.M., D’Angiò, A., Pasquini, L., Carollo, M., Sargiacomo, M., Testa, U. and Pelosi, E. 2016. Conditioned medium from human umbilical vein endothelial cells markedly improves the proliferation and differentiation of circulating endothelial progenitors. Blood Cells Mol. Dis. 61, 58–65. Castillo, V., Díaz-Astudillo, P., Corrales-Orovio, R., San Martín, S. and Egaña, J.T. 2023. Comprehensive characterization of tissues derived from animals at different regenerative stages: a comparative analysis between fetal and adult mouse skin. Cells 12(9), 1215. Diller, R.B. and Tabor, A.J. 2022. The role of the extracellular matrix (ECM) in wound healing: a review. Biomimetics 7(3), 87. Fallah, A., Sadeghinia, A., Kahroba, H., Samadi, A., Heidari, H.R., Bradaran, B., Zeinali, S., Molavi, O., 2019. Therapeutic targeting of angiogenesis molecular pathways in angiogenesis-dependent diseases. Biomed. Pharmacother. 110, 775–785. Fromer, M.W., Chang, S., Hagaman, A.L.R., Koko, K.R., Nolan, R.S., Zhang, P., Brown, S.A., Carpenter, J.P. and Caputo, F.J. 2018. The endothelial cell secretome as a novel treatment to prime adipose-derived stem cells for improved wound healing in diabetes. J. Vasc. Surg. 68, 234–244. Gonzalez, A.C.D.O., Andrade, Z.D.A., Costa, T.F. and Medrado, A.R.A.P. 2016. Wound healing—a literature review. An. Bras. Dermatol. 91(5), 614–620. Jeschke, M.G., van Baar, M.E., Choudhry, M.A., Chung, K.K., Gibran, N.S. and Logsetty, S. 2020. Burn injury. Nat. Rev. Dis. Primers 6(11), 1–25. Johnson, K.E. and Wilgus, T.A. 2014. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv. Wound Care (New Rochelle) 3, 647–661. Kaddoura, I., Abu-Sittah, G., Ibrahim, A., Karamanoukian, R. and Papazian, N. 2017. Burn injury: review of pathophysiology and therapeutic modalities in major burns. Ann. Burns Fire Disasters. 30(2), 95-102. Kareem, N.A., Aijaz, A. and Jeschke, M.G. 2021. Stem cell therapy for burns: story so far. Biologics 15, 379–397. Kim, Y.J., mi Yoo, S., Park, H.H., Lim, H.J., Kim, Y.L., Lee, S., Seo, K.W. and Kang, K.S. 2017. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulates rejuvenation of human skin. Biochem. Biophys. Res. Commun. 493, 1102–1108. Kusindarta, D.L. and Wihadmadyatami, H. 2021. Conditioned medium derived from bovine umbilical mesenchymal stem cells as an alternative source of cell-free therapy. Vet. World. 14(10), 2588–2599. Laksmitawati, D.R., Noor, S.U., Sumiyati, Y., Hartanto, A., Widowati, W. and Pratami, D.K. 2022. The effect of mesenchymal stem cell-conditioned medium gel on burn wound healing in rat. Vet. World 15, 841–847. Larasati, V.A., Lembang, G.V., Tjahjono, Y., Winarsih, S., Ana, I.D., Wihadmadyatami, H. and Kusindarta, D.L. 2022. In vitro neuroprotective effect of the bovine umbilical vein endothelial cell conditioned medium mediated by downregulation of IL-1β, caspase-3, and caspase-9 expression. Vet. Sci. 9(2), 48. Maki, T., Morancho, A., Segundo, P.M.S., Hayakawa, K., Takase, H., Liang, A.C., Gabriel-Salazar, M., Medina-Gutiérrez, E., Washida, K., Montaner, J., Lok, J., Lo, E.H., Arai, K. and Rosell, A. 2018. Endothelial progenitor cell secretome and oligovascular repair in a mouse model of prolonged cerebral hypoperfusion. Stroke 49, 1003–1010. Műzes, G. and Sipos, F. 2022. Mesenchymal stem cell-derived secretome: a potential therapeutic option for autoimmune and immune-mediated inflammatory diseases. Cells 11(15), 2300. Ozhathil, D.K., Tay, M.W., Wolf, S.E. and Branski, L.K. 2021. A narrative review of the history of skin grafting in burn care. Medicina (Lithuania) 57(4), 380. Periayah, M.H., Halim, A.S., Zaharil, A. and Saad, M. 2017. Mechanism action of platelets and crucial blood coagulation pathways in hemostasis, Int. J. Hematol. Oncol. Stem Cell Res. 11(4), 319–327. Plikus, M.V., Wang, X., Sinha, S., Forte, E., Thompson, S.M., Herzog, E.L., Driskell, R.R., Rosenthal, N., Biernaskie, J. and Horsley, V. 2021. Fibroblasts: origins, definitions, and functions in health and disease. Cell 184(15), 3852–3872. Potekaev, N.N., Borzykh, O.B., Medvedev, G.V., Pushkin, D.V., Petrova, M.M., Petrov, A.V., Dmitrenko, D.V., Karpova, E.I., Demina, O.M. and Shnayder, N.A. 2021. The role of extracellular matrix in skin wound healing. J. Clin. Med. 10(24), 5947. Rognoni, E., Pisco, A.O., Hiratsuka, T., Sipilä, K.H., Belmonte, J.M., Mobasseri, S.A., Philippeos, C., Dilão, R. and Watt, F.M. 2018. Fibroblast state switching orchestrates dermal maturation and wound healing. Mol. Syst. Biol. 14(8), e8174. Salhab, O., Khayat, L. and Alaaeddine, N. 2022. Stem cell secretome as a mechanism for restoring hair loss due to stress, particularly alopecia areata: narrative review. J. Biomed. Sci. 29(1), 77. Stone, R., Natesan, S., Kowalczewski, C.J., Mangum, L.H., Clay, N.E., Clohessy, R.M., Carlsson, A.H., Tassin, D.H., Chan, R.K., Rizzo, J.A. and Christy, R.J. 2018. Advancements in regenerative strategies through the continuum of burn care. Front Pharmacol. 9, 672. Svoradova, A., Zmrhal, V., Venusova, E. and Slama, P. 2021. Chicken mesenchymal stem cells and their applications: a mini review. Animals 11(7), 1883. Tian, H., Wang, L., Xie, W., Shen, C., Guo, G., Liu, J., Han, C., Ren, L., Liang, Y., Tang, Y., Wang, Y., Yin, M., Zhang, J. and Huang, Y. 2018. Epidemiologic and clinical characteristics of severe burn patients: results of a retrospective multicenter study in China, 2011–2015. Burns Trauma 6, 14. Tracy, L.E., Minasian, R.A. and Caterson, E.J. 2016. Extracellular matrix and dermal fibroblast function in the healing wound. Adv. Wound Care (New Rochelle) 5(3), 119–136. Vyas, K.S. and Vasconez, H.C. 2014. Wound healing: biologics, skin substitutes, biomembranes and scaffolds. Healthcare (Switzerland) 2(3), 356–400. Zhang, S. and Duan, E. 2018. Fighting against skin aging: the way from bench to bedside. Cell Transplant. 27(5), 729–738. Zhang, Y., Bi, J., Huang, J., Tang, Y., Du, S. and Li, P. 2020. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomedicine 15, 6917–6934. Zhao, D., Yu, Z., Li, Y., Wang, Y., Li, Q. and Han, D. 2020. GelMA combined with sustained release of HUVECs derived exosomes for promoting cutaneous wound healing and facilitating skin regeneration. J. Mol. Histol. 51, 251–263. | ||
| How to Cite this Article |
| Pubmed Style Moghan M, Naidu P, Zulkifly Nb, Selan YN, Setyawan EMN, Winarsih S, Kusindarta DL. Secretome of bovine umbilical vein endothelial cells promote wound healing regeneration on the second degree rat model burn injury. Open Vet J. 2023; 13(12): 1597-1606. doi:10.5455/OVJ.2023.v13.i12.9 Web Style Moghan M, Naidu P, Zulkifly Nb, Selan YN, Setyawan EMN, Winarsih S, Kusindarta DL. Secretome of bovine umbilical vein endothelial cells promote wound healing regeneration on the second degree rat model burn injury. https://www.openveterinaryjournal.com/?mno=165991 [Access: May 13, 2024]. doi:10.5455/OVJ.2023.v13.i12.9 AMA (American Medical Association) Style Moghan M, Naidu P, Zulkifly Nb, Selan YN, Setyawan EMN, Winarsih S, Kusindarta DL. Secretome of bovine umbilical vein endothelial cells promote wound healing regeneration on the second degree rat model burn injury. Open Vet J. 2023; 13(12): 1597-1606. doi:10.5455/OVJ.2023.v13.i12.9 Vancouver/ICMJE Style Moghan M, Naidu P, Zulkifly Nb, Selan YN, Setyawan EMN, Winarsih S, Kusindarta DL. Secretome of bovine umbilical vein endothelial cells promote wound healing regeneration on the second degree rat model burn injury. Open Vet J. (2023), [cited May 13, 2024]; 13(12): 1597-1606. doi:10.5455/OVJ.2023.v13.i12.9 Harvard Style Moghan, M., Naidu, . P., Zulkifly, . N. b., Selan, . Y. N., Setyawan, . E. M. N., Winarsih, . S. & Kusindarta, . D. L. (2023) Secretome of bovine umbilical vein endothelial cells promote wound healing regeneration on the second degree rat model burn injury. Open Vet J, 13 (12), 1597-1606. doi:10.5455/OVJ.2023.v13.i12.9 Turabian Style Moghan, Mavheena, Paarvaishnee Naidu, Nadhrah binti Zulkifly, Yulfia Nelymalik Selan, Erif Maha Nugraha Setyawan, Sugi Winarsih, and Dwi Liliek Kusindarta. 2023. Secretome of bovine umbilical vein endothelial cells promote wound healing regeneration on the second degree rat model burn injury. Open Veterinary Journal, 13 (12), 1597-1606. doi:10.5455/OVJ.2023.v13.i12.9 Chicago Style Moghan, Mavheena, Paarvaishnee Naidu, Nadhrah binti Zulkifly, Yulfia Nelymalik Selan, Erif Maha Nugraha Setyawan, Sugi Winarsih, and Dwi Liliek Kusindarta. "Secretome of bovine umbilical vein endothelial cells promote wound healing regeneration on the second degree rat model burn injury." Open Veterinary Journal 13 (2023), 1597-1606. doi:10.5455/OVJ.2023.v13.i12.9 MLA (The Modern Language Association) Style Moghan, Mavheena, Paarvaishnee Naidu, Nadhrah binti Zulkifly, Yulfia Nelymalik Selan, Erif Maha Nugraha Setyawan, Sugi Winarsih, and Dwi Liliek Kusindarta. "Secretome of bovine umbilical vein endothelial cells promote wound healing regeneration on the second degree rat model burn injury." Open Veterinary Journal 13.12 (2023), 1597-1606. Print. doi:10.5455/OVJ.2023.v13.i12.9 APA (American Psychological Association) Style Moghan, M., Naidu, . P., Zulkifly, . N. b., Selan, . Y. N., Setyawan, . E. M. N., Winarsih, . S. & Kusindarta, . D. L. (2023) Secretome of bovine umbilical vein endothelial cells promote wound healing regeneration on the second degree rat model burn injury. Open Veterinary Journal, 13 (12), 1597-1606. doi:10.5455/OVJ.2023.v13.i12.9 |