| Research Article | ||
Open Vet J. 2023; 13(12): 1614-1622 Open Veterinary Journal, (2023), Vol. 13(12): 1614–1622 Original Research Comparative assessment of regulated methods and PCR in the diagnosis of trichophytosis in veterinary mycologyMynbay Umitzhanov1*, Botagoz Abdiramanova1, Aspen Abutalip1, Nurbol Bakirov1 and Saule Sarimbekova21Department of Biological Safety, Kazakh National Agrarian Research University, Almaty, Republic of Kazakhstan 2Department of Physiology, Morphology and Biochemistry by N.U. Bazanova, Kazakh National Agrarian Research University, Almaty, Republic of Kazakhstan *Corresponding Author: Mynbay Umitzhanov. Department of Biological Safety, Kazakh National Agrarian Research University, Almaty, Republic of Kazakhstan. Email: mynbayumitzhanov [at] yahoo.com Submitted: 30/08/2023 Accepted: 15/12/2023 Published: 31/12/2023 © 2023 Open Veterinary Journal
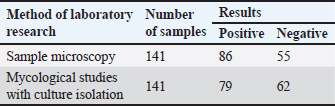
AbstractBackground: There is an increase in the incidence of human and animal infectious skin diseases of fungal etiology in the world. The main source of infecting the population has become agricultural and stray animals. Aim: The objective of this study was to examine the morphophysiological and microbiological characteristics of pathogenic fungi belonging to the species Trichophyton verrucosum. This species is known to cause diseases in both humans and livestock in Kazakhstan. In addition, the study aimed to assess the feasibility of using the polymerase chain reaction (PCR) method for detecting T. verrucosum. This assessment was conducted in comparison to the outcomes of conventional laboratory diagnostic tests commonly employed for trichophytosis. Methods: The research focused on analyzing 141 samples of pathological material obtained from calves in Almaty, Turkestan, and Kyzylorda regions. These calves exhibited clinical symptoms of skin disease. The study aimed to identify the causative agent using various techniques, including microscopic examination, microbiological methods involving the isolation of pure cultures, and PCR. Results: The detection of the causative agent of dermatophytosis using conventional methods was relatively low, 86% for the microscopic method, and 79% for the microbiological method with the isolation of the culture of the pathogen. Extraction and detection of the genetic material of the causative agent of the disease for PCR was carried out according to the method developed by the authors. The effectiveness of the PCR method was 97.9%, which is significantly higher (p < 0.05) compared with the diagnostic effectiveness of conventional methods. The PCR method using specific primers identified the causative agent in 98% of cases, which significantly (p < 0.05) exceeded the results obtained using conventional diagnostic methods. Accordingly, the PCR method had better sensitivity and specificity indicators. Conclusion: The conducted study recommends the method of PCR diagnosis of dermatophytosis for fast and reliable confirmation of the diagnosis of dermatophytosis in humans and animals in Kazakhstan. Keywords: Microscopy, Fungal cultivation, Prognostic predictive value, Dermatophytosis, Molecular detection. IntroductionDermatophytosis is a collective name for fungal diseases that affect a significant number of species of agricultural and domestic animals, as well as humans. They are caused by microscopic fungi of three main genera—Trichophyton, Epidermophyton, Nannizzia, Paraphyton, Lophophyton, Microsporum, and Arthroderma. The study by Ugochukwu et al. (2022) has concluded that of the three genera of dermatomycoses, Epidermophyton affects exclusively humans, whereas Microsporum and Trichophyton, in most cases, affect animals. Chowdhary et al. (2022) found that representatives of the genus Trichophyton are most often the causative agent of superficial skin diseases of animals and humans, the Indan strain of Trichophyton indotineae, which is resistant to antifungal drugs, has recently become especially widespread. Lysková et al. (2021) also indicated that most cases of a local outbreak of the disease in humans and animals in the Czech Republic in 2020 were caused by Trichophyton quinckeanum, which confirms cross-infection with one pathogen of different species of living organisms. According to Paryuni et al. (2020), there is an increase in the incidence of fungal skin diseases in the world. In particular, in Europe, the incidence of dermatophytosis in cats and dogs ranges from 20% to 30%, depending on the country. The increase in morbidity is facilitated by the fact that the pathogens of infection are very resistant to environmental factors, and can survive in keratinized skin, animal hair, and wool, maintaining their pathogenicity for several years. Accurate diagnosis of dermatophytosis is critical for prompt treatment and preventing spread but can be challenging due to the nonspecific symptoms and limitations of traditional diagnostic methods like microscopy and fungal culture (Paliy et al., 2023). Misdiagnosis allows infections to persist, leading to exacerbated symptoms, transmission to others, and potentially the emergence of antifungal resistance (Bulegenova et al., 2019). This highlights the need for more reliable diagnostic techniques. In recent years, dermatomycosis outbreaks have been increasingly reported in urban areas of Kazakhstan, primarily affecting stray cat and dog populations. This poses a significant public health concern, as animals serve as reservoirs for zoonotic transmission to humans, particularly children. Beyond the direct health impacts, dermatomycosis outbreaks can have detrimental economic effects stemming from treatment costs and productivity losses (Hajiyeva et al., 2022). Only in 2022, in Kazakhstan, according to the publications of news portals, fungal skin diseases in people were registered quite often. According to Forbes.kz, last year, 51 people were affected last year in Kostanay Region alone (Forbes, 2022). Similar reports were published last year on the 24.kz (2022) portal, where it was already reported about 76 infected people with dermatophytosis in Kyzylorda in the first half of 2022, as well as on the Liter.kz (2023) portal, with reference to the Ministry of Health of the Republic of Kazakhstan, about a 1.5-fold increase in the incidence of fungal skin diseases among people compared to the previous year in Almaty (2023). The greatest incidence of dermatophytosis was recorded among children under 14 years of age, 85% of all cases. The increase in morbidity in Kazakhstan can be traced only in an epidemiological way, while little attention is paid to the main source of dermatophytosis—agricultural and domestic animals (Kirimbayeva et al., 2023). This aspect of the spread of infection remains beyond statistics. In the region of Kazakhstan, the most common etiological agent identified for dermatophytosis is the Trichophyton verrucosum (Kukhar et al., 2019). Individuals affected by this fungal infection often experience a range of clinical symptoms, which kickstart with itching being the most noticeable initial sign, leading to an irresistible urge to scratch the affected area of the skin (Suleymanova, 2022). Following the itching, inflammation arises, causing the skin to become sore, which might result in discomfort or even pain. The skin may also exhibit redness or develop a rash, making the affected area visibly distinct. Small papular vesicles, which are small, raised, fluid-filled bumps, may emerge on the skin, further exacerbating the discomfort. In some instances, the rash may display a central clearing, where the center appears normal or clear surrounded by a ring of red, inflamed skin. The manifestation of these symptoms can vary significantly from one individual to another, and the severity of the infection also plays a crucial role in the presentation of symptoms, thereby making dermatophytosis a condition with a diverse symptomatic profile (Nussipov et al., 2017). In agricultural settings, there is an ongoing effort to vaccinate young animals against dermatophytosis as part of antiepizootic measures. However, in large urban areas, the presence of a significant population of stray cats and dogs is contributing to an increase in the occurrence of dermatophytosis among both animals and the human population. Consequently, a significant number of reports regarding outbreaks of trichophytic dermatophytosis in Kazakhstan are originating from major cities such as Almaty and Kostanay. The manuscript posits several key hypotheses regarding the diagnostics of dermatomycosis/dermatophytosis. First, it suggests that traditional diagnostic methods, specifically microscopic examination, and microbiological seeding, might fall short in their sensitivity and specificity. Moreover, while modern diagnostic techniques, such as MALDI -TOF and enzyme-linked immunosorbent assay (ELISA), have been introduced as alternatives, they too come with limitations and might not completely overcome the challenges associated with older methods. Central to the research is the assertion that the polymerase chain reaction (PCR) technique could potentially emerge as a more accurate, efficient, and rapid tool for diagnosing dermatomycosis/dermatophytosis (Tyliszczak et al., 2018). This study aimed to evaluate the accuracy of PCR for diagnosing dermatomycosis caused by T. verrucosum, compared to traditional microscopic examination and fungal culture techniques. While a promising method, limitations exist in that PCR reagents can be expensive and require skilled technicians. Furthermore, genetic mutations could impact primer binding, reducing detection sensitivity over time. Materials and MethodsSample collectionSamples were obtained from a total of 141 pathological materials collected from calves across individual farms in the Almaty, Turkestan, and Kyzylorda regions between 2018 and 2022. These samples were taken from the periphery of skin lesions that had not undergone topical drug treatment. Information including the date and place of collection, age of the animal, and the degree of lesion were recorded in parallel. The collected material was placed in test tubes with cotton plugs. Sample preparationA portion of the collected material was transferred to a slide using a preparation needle. Subsequently, 15%–20% alkaline caustic potassium solution was added, and the mixture was briefly heated until crystals formed around the drop. Following this, a drop of 50% glycerine solution was added, and a cover glass was placed over the preparation. The samples were then examined at magnifications of 10× and 40×. Microbiological analysisThe samples were inoculated on Saburo agar (Topan LLP, Uralsk), selective medium M188 (HiMedia, India), and M1026 with rice extract (HiMedia, India). To prevent the growth of saprophytic bacteria, penicillin antibiotics were added to the media at a dose of 60–120 μg/cm³. Particles of pathological material were transferred to the nutrient medium using a sterile loop and placed at a distance of 1–1.5 cm. Up to 10 seedings were performed for each sample. Incubation was conducted for 3–4 weeks at a temperature of 25°C–30°C, with periodic inspections. The type of pathogen was determined by assessing the shape, color, and pigmentation of colonies on the nutrient medium, followed by microscopic examination of the mycelium’s shape, spore placement, and other structural features of the vegetative form. DNA extractionDNA extraction was carried out via nucleosorption. A test tube containing the pathological material was mixed with 300 ml of lysis solution (5M guanidine thiocyanate, 1% Triton X100). After mixing, the reaction mixture was heated for 5 minutes at 65°C and centrifuged for 5 minutes at 10,000 × g to isolate DNA. The supernatant was transferred to a new test tube, and 25 ml of sorbent (silica gel) was added. After mixing and settling, the sorbent was precipitated by centrifugation, and the precipitate was dissolved in 300 ml of a washing solution (5M guanidine thiocyanate). Next, 950 ml of washing solution (70% ethyl alcohol) was added to the precipitate, and it was centrifuged for 30 seconds at 2,000 × g. The supernatant was removed, and the precipitate was dried in a thermostat for 5–10 minutes at 65°C. Subsequently, 50 ml of TE buffer for DNA elution was added to the test tube, mixed, and placed in a thermostat at 65°C for 5 minutes. The tubes were then centrifuged for 1 minute at 10,000 g, resulting in purified DNA ready for PCR. PCRPCR was conducted in a Tertsik amplifier using a kit with Taq DNA polymerase and an AS buffer from SibEnzyme. Initially, DNA denaturation was performed at 94°C for 2 minutes, followed by 25–30 amplification cycles. Each cycle included DNA denaturation at 94°C for 20 seconds, primer annealing at a temperature ranging from 58°C to 64°C for 20 seconds, and elongation at 72°C for 10–20 seconds. Amplification product analysis was carried out through agarose gel electrophoresis. Statistical analysisStatistical analysis of the PCR results was performed using TIBCO Statistica version 14.0.0.15. Specificity, sensitivity, diagnostic effectiveness, prognostic value, and both positive and negative likelihood ratios were calculated to assess the diagnostic accuracy and reliability of the test. Significance was determined. ResultsAll the collected samples were examined in parallel using two conventional diagnostic methods—microscopic phase-contrast examination and isolation on nutrient media to obtain pure cultures. The results were obtained, which are presented in Table 1. From the studies, 98 cases had their preliminary diagnoses validated, accounting for 69.5% of all samples assessed. Dermatophytes were detected in 98 samples of skin scrapings from symptomatic calves through conventional laboratory methods. Light (phase-contrast) microscopy identified fungal elements in 86 (87.8%) of these cases. Mycological studies using culture on nutrient media identified them in 79 (80.6%) instances. It is notable that while microscopy serves as a preliminary diagnostic tool, it was the sole confirmation of skin infection in 19 (19.4%) instances. Only 67 (68.4%) of the diagnoses were concurrently confirmed by both regulated methods, which suggests potential issues with the specificity and sensitivity of these procedures. Lack of fungal growth in certain samples could be attributed to nonviable fungal spores, possibly a result of prior treatments for nondermatophyte-related conditions. Microscopic examination of wool and hair revealed distinctive signs of fungal infections. Notably, a sheath of arthrospores was observed encircling the hair bulb. In addition, spore chains were discovered both around and within the hair shaft. Accompanying these spores, mycelial strands were identified both externally and internally in the wool, as well as within skin flakes. The diameter of these arthrospores in pathological samples extracted from calves diagnosed with dermatophytosis varied between 2.5 and 7 µm. Phase contrast microscopy of samples from smooth skin areas presented pronounced mycelial strands of varying lengths. These filaments predominantly exhibited a linear morphology, occasionally demonstrating branching and segmented patterns. Interestingly, atypical mycelial forms were observed in certain samples, particularly those sourced from calves in the Turkestan Region. These irregularities displayed the presence of hyphae and intricate thread networks, which might be indicative of a prolonged, chronic infection. Microscopic analysis also highlighted polymorphic spores with an inconsistent morphology, either dispersed freely or arranged in chain formations. While these specimens were recognized as indicative of dermatophytosis, ascertaining the precise pathogenic species from their morphological features posed challenges. Table 1. Results of studies of samples of pathological material obtained from calves in 2018–2022.
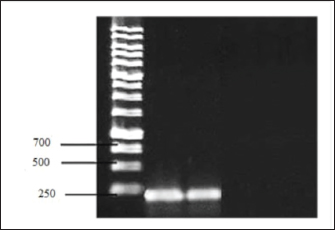
All tissue and wool samples underwent mycological examination irrespective of their microscopic analysis results. From these, 79 pure cultures of pathogenic fungi were derived. Mycological cultivation exhibited the emergence of slow-growing colonies characterized by a discoid configuration with a prominent leathery, wrinkled center of a greyish-yellow hue and a powdery whitish periphery. To identify the specific pathogen type from the colony culture, preparations were made and subjected to phase-contrast microscopy. This revealed an abundance of branching mycelial strands with accompanying spores. Furthermore, chlamydospore development was evident at the filament tips, manifesting in brush-like formations. Given the general observation that the pathogenic fungal species cultivated thrived at the body temperatures of the host animals, the cultivation temperature was marginally increased to 37°C. In addition, thiamine was introduced to the cultivation medium. These amendments to the mycological cultivation protocol not only facilitated faster growth of vegetative forms but also yielded colonies with a more consistent morphology. By assessing the microbiological and morphological features, including the structure of the micellar filaments, the arrangement and morphology of the spores, and the various attributes of colony growth, the fungi were classified as T. verrucosum. The extraction and identification of genetic material from animals exhibiting skin disease symptoms were executed following a method tailored for DNA extraction from skin samples, specifically designed for the PCR process. The extracted DNA content from pathological samples ranged between 2 and 37 ng/µl, adequately meeting the prerequisites for the PCR technique in terms of sample volume. The PCR approach centers on amplifying a specific gene fragment found in RNA, which encodes for chitin synthetase. Primers from the “SibEnzyme” diagnostic kit for dermatomycoses detection were employed. The 30 µl reaction mixture comprised 1 µl of the DNA obtained from the pathological material, 3 µl of a 10× Taq polymerase buffer, 1 µl of specific primers, and 1 µl of Taq polymerase. The “hot start” method was incorporated to optimize PCR and diminish nonspecific amplification. Herein, to minimize the chances of primers nonspecifically annealing to low-homologous nucleotide sequences, Taq polymerase was introduced post preheating to 94°C. Electrophoresis ran at a consistent 120 V voltage. A positive PCR result was signified by the detection of specific amplification products: target fragments of 231 bp that exhibited luminescent red-orange bands post-electrophoresis. For visualization, the agarose gel underwent ethidium bromide staining for a duration of 10–15 minutes. The electrophoresis outcomes were visualized in a UV chamber (Fig. 1). Utilizing the PCR technique alongside the SibEnzyme test kit—inclusive of Taq DNA polymerase and an AS buffer—facilitated the detection of genetic material in 96 of the 98 confirmed fungal infection cases, accounting for a 98% success rate. This notably surpassed the outcomes of traditional diagnostic assays. A comparative analysis of the discussed diagnostic approaches based on criteria such as predictive value, study duration, and test complexity revealed PCR’s superiority. While microscopic evaluation proved to be the swiftest and most cost-effective, it merely provides an initial diagnosis, necessitating further infectious agent isolation for validation. Comparing microbiological cultivation with PCR, the molecular genetic method emerges as distinctly advantageous. Conventional mycological examination can span 3–4 weeks for diagnosis confirmation, in contrast to mere hours via PCR. Given that dermatomycoses are zoonoses, this time factor is paramount. Furthermore, within this study’s scope, PCR’s efficacy in validating the preliminary diagnosis exceeded the standard fungi cultivation technique by 19%. To meticulously gauge the informativeness of the PCR method in detecting T. verrucosum dermatomycetes within pathological material—versus standard laboratory diagnostic procedures for calf fungal skin ailments—several statistical metrics were employed. This involved criteria grounded on the determination of sensitivity, specificity, diagnostic result efficacy, and the likelihood of obtaining a credible outcome. The ensuing statistical insights derived from this study’s sample are illustrated in Figure 2.
Fig. 1. Results of electrophoretic separation of PCR amplification products of a sample of pathological material from a sick animal.
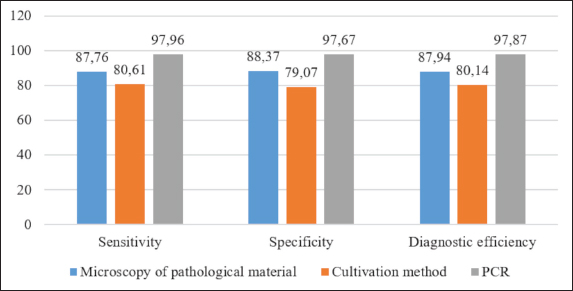
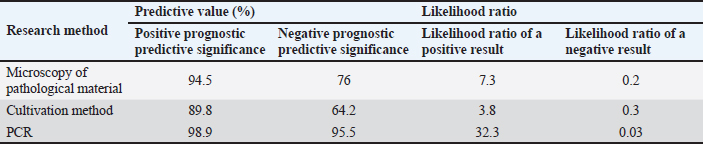
Fig. 2. Comparative assessment of sensitivity, specificity, and diagnostic effectiveness in the diagnosis of dermatophytosis. Indicators of specificity and sensitivity, diagnostic effectiveness, prognostic value, and likelihood ratio were calculated. The highest statistical results were observed for the PCR method. Thus, the proportion of truly positive cases of diseases in the controlled group of animals determined by PCR was 98% (p < 0.05), which is significantly higher in comparison with conventional diagnostic methods. Accordingly, the PCR specificity index significantly exceeded the results obtained by microscopy and cultivation and amounted to 97.7% (p < 0.05). However, a more important indicator in evidence-based medicine is diagnostic effectiveness (Derkach and Klymenko, 2023). Compared with other methods of laboratory diagnosis of dermatophytosis, which were used in the analysis of the material, PCR allowed with much greater accuracy—97.9% (p < 0.05) to determine the frequency of positive and negative cases of the disease in the controlled population. A statistical analysis of the comparative characteristics of diagnostic methods for dermatophytosis would not be complete without calculating predictive indicators that describe the test distribution of morbidity in the study population. The results of this analysis are shown in Table 2. The method of determining predictive value indicators consists of comparing the results obtained using the diagnostic method with the most reliable diagnostic method and calculating quantitative coefficients characterizing this comparison. Since none of the methods can be considered a reference for dermatophytosis, the comparison was made with the complex result of standard methods that are recommended for laboratory diagnostics of mycological skin diseases. As in the case of previous statistical results, the predictive coefficients turned out to be more reliable for the PCR than for other methods used. Thus, the probability of the presence of the disease with a positive PCR result for dermatophytosis caused by the pathogen T. verrucosum, estimated through a positive prognostic value, had the greatest value (98.9), as well as the value of the likelihood ratio of a positive result (32.3), determining the presence of a true disease with the lowest probability of obtaining a negative result (0.03). The results of the studies and their statistical analysis indicate that the PCR method allows the detection and identification of pathogens of dermatophytosis, in particular T. verrucosum, in various samples of pathological material. In addition, this method has significantly higher diagnostic efficiency, sensitivity, and specificity in the laboratory diagnosis of zooanthroponous dermatomycoses, compared with the microscopic and cultural methods. DiscussionThe increase in the incidence of fungal skin diseases in animals and humans has become a reality in the modern world. Outbreaks of infection among the population of several prosperous European countries require a revision of the principles of diagnosing such diseases to ensure prompt treatment and timely prevention. An increase in the number of stray animals in cities, where the epidemiological process is most widespread, contributes to the aggravation of the situation, since it is this group of animals that has been identified as the main source of infection. Considering the similarity of clinical signs of dermatophytosis with other skin diseases, as well as the increase in the number of cases of registration of atypical and latent forms of development of these diseases in animals, makes the search for modern express methods of diagnosis of mycoses an urgent area of research. The conventional mechanism of diagnosis for mycological skin diseases involves the use of two methods—microscopic examination of pathological material and microbiological, the purpose of which is to obtain a pure culture of the pathogen and its identification (Akhmetov and Chidunchi, 2015; Zhantlessova et al., 2022). If the first method allows, almost in a matter of minutes, to identify signs of mycological infection in animals and humans, then the duration of the microbiological method, even with the most optimistic calculations, lasts from 2 to 4 weeks. Table 2. Predictive performance indicators of the use of different methods in the diagnosis of dermatophytosis caused by T. verrucosum.
This indicates that almost every third animal from which pathological material was taken was not a patient with dermatophytosis. Such a high percentage of negative results is associated with similar symptoms of dermatophytosis with inflammatory and other skin lesions. Given the ability of dermatophytosis pathogens to cause diseases in humans, diagnostics should confirm or refute the preliminary diagnosis with a high degree of reliability in a short period of time. In further studies and statistical calculations, samples were used only after laboratory confirmation of the disease and isolation of the pathogen from the pathological material. Considering the results obtained as part of the research for the preparation of this paper, it can be stated that even experienced veterinary specialists who selected pathological material in a third of cases could not distinguish dermatophytosis from other skin diseases, since in 30.5% of samples of pathological material none of the conventional methods of pathogens of fungal etiology were isolated. This is conditioned by the fact that the effectiveness of conventional methods turned out to be low. The sensitivity of the microscopic examination method was 87.8%, and microbiological seeding was even lower—80.6%, which is not enough for zooanthroponotic diseases when there is a possibility of developing the disease among the population. Similar conclusions were made by Heckler et al. (2023), since late or incorrect diagnosis is dangerous with a potential health risk; therefore, the authors consider the use of modern diagnostic methods for accelerated diagnosis of dermatophytosis. According to Gosink (2022), conventional methods are characterized by a high percentage of false positive and false negative diagnoses. The morphological characteristics of the causative agent of cattle dermatophytosis T. verrucosum, and the physiological features of cultures isolated from samples of pathological material with fungal lesions of the skin and coat of calves were studied. Despite the presence of characteristic morphological signs of mycelium and spores of the pathogen, based on which their preliminary differentiation is possible, the latter remains a rather difficult task. This is conditioned by adaptive changes in the morphological structure of the pathogen due to the action of pharmacological drugs on it or the protective mechanisms of the body itself in chronic infection. The study by Gnat et al. (2019) indicates a change in the morphological structure of the causative agent of the disease with frequent passages, prolonged cultivation on “hungry” media, or other factors. Therefore, the authors point out that the phenotype of the pathogen under microscopy cannot be the basis for a diagnosis. In studies, when comparing microscopic and mycological methods, their effectiveness differed significantly. Thus, in the diagnosis of dermatophytosis caused by T. verrucosum, the informative value of the cultural method was characterized by the following indicators: sensitivity—80.6%, specificity—79.1%, and diagnostic efficiency—80.1%, which turned out to be significantly lower than the corresponding values obtained by microscopic examination of 87.8%, 88.4%, and 87.9%. A possible explanation for this pattern is the complexity of cultivating T. verrucosum, which is consistent with the study by Peano et al. (2008). Until recently, it was not possible to solve the problem of a fast and highly sensitive method of diagnosing pathogens of dermatophytosis. The applied laboratory methods and techniques, due to their low information content, did not allow them to be recommended as a replacement for the conventional system of diagnosis for fungal skin diseases. The search for modern methods for the diagnosis of dermatophytosis was conducted in different directions—this is the use of the most modern approaches using laser mass spectrometry (MALDI-TOF) technology and the easiest to carry out ELISA. However, each of these methods has its drawbacks. MALDI-TOF, in studies by Baumbach et al. (2021), did not reveal the correct identification of the causative agents of dermatophytosis according to the databases provided by the software. More optimistic results were obtained by Aruna and Ramalingappa (2022), when using the enzyme-linked immunoassay. Diagnostic sensitivity and specificity of the method were 93.8% and 93.3%, positive and negative prognostic values were also high and amounted to 93.8% and 90%, respectively, but a significant antigenic pool of pathogens and cross-reactions between different types of Trichophyton makes this method less informative. PCR may be the only promising method that can be used as a reference for the diagnosis of dermatophytosis in animals and humans. A comparative analysis of the results of laboratory methods used in this study showed that PCR was characterized by the highest diagnostic efficiency of 97.9% (p < 0.05) when diagnosing dermatophytosis in young cattle caused by the pathogen of the species T. verrucosum, while standard methods did not exceed 80%–88% efficiency. This is also confirmed by the high values of the sensitivity of the method (80%), its specificity (97.7%), and the prognostic value of a positive result (98.9%), which is with high statistical reliability (p < 0.05) higher than similar indicators obtained during conventional (regulated) research methods. Similar conclusions were obtained by Gräser and Saunte (2020), which indicate that molecular diagnostics are 30% more sensitive than microbiological methods. One of the main predictive indicators that allow classifying PCR as the most accurate diagnostic method is the likelihood ratio of a positive result (Iskakova et al., 2022). Statistical calculations of quantitative indicators obtained during the research in the framework of this study indicate that the probability of an accurate diagnosis in PCR is several times higher than when using conventional approaches in the diagnosis of skin mycoses. Moreover, the likelihood of obtaining a negative result with PCR (0.03) was revealed to be an order of magnitude lower than that of microscopic and mycological methods, 0.2 and 0.3, respectively. The results obtained are also confirmed in the study by Álvarez-Mosquera et al. (2018), in which high prognostic values were obtained for both positive and negative diagnoses (90.9% and 94.6%, respectively). Summing up the results of the study and preparing recommendations for subsequent work on the development of objective ideas about the extent of the spread of dermatophytosis, the authors suggest that the most appropriate method for monitoring morbidity among the cattle population is the use of molecular genetic approaches that are able to identify and establish the typification of the pathogen with a high degree of probability. Therefore, future studies will be aimed at conducting a clinical test of the PCR method for its implementation and regulation in laboratory practice for the detection and confirmation of the diagnosis of dermatophytosis caused by the causative agent T. verrucosum in Kazakhstan, since the research results indicate that this method is significantly more informative when searching for fungal infection in pathological material compared with microscopic and cultural methods. Summarising the results of the experiments and analyzing the results obtained, the authors recommend using the PCR method for widespread use in the laboratory diagnosis of dermatophytosis. This method is able to confirm or refute the diagnosis in a short period of time, which will allow us to move to a new level of the fight against dermatophytosis. Using the PCR method in both epidemiological and veterinary diagnostics will reduce the time it takes to confirm the disease and enable the implementation of preventive measures to prevent the spread of infection. ConclusionIn the comprehensive study on the diagnostic methods for dermatophytosis, findings underscore the limitations of conventional methods. Microscopic examination and microbiological cultivation, while historically significant, yielded a diagnostic effectiveness of no more than 88%. Conversely, the PCR method demonstrated superior diagnostic capabilities, with an efficacy rate of 97.9%. This method’s adaptability in extracting genetic material from skin and wool samples, combined with the precision of the primers used, ensures reliable detection of the T. verrucosum pathogen. However, the study has its limitations. The focused sample set, specifically from calves, might limit the generalization of findings across diverse animal populations or different fungal pathogens. Moreover, while PCR showed promising results, its practical application in varied settings, considering potential logistical or technical challenges, remains unexplored. Future research should delve into the clinical applicability of the PCR method across different animal species and geographies. Comparative studies with other emerging diagnostic technologies could further elucidate the most effective strategies. Understanding barriers to the widespread adoption of PCR, both technologically and regulatorily, especially in regions such as Kazakhstan, becomes paramount. This study acts as a foundation, emphasizing the transition from traditional to advanced molecular diagnostic methods, with the ultimate goal of timely and accurate detection of dermatophytosis to mitigate its spread and enhance animal health outcomes. AcknowledgmentsNone. Author contributionsA and B developed the project. C performed the experiment. D and E analyzed the results and wrote the manuscript. All authors reviewed the manuscript. Conflict of interestThe authors declare that there is no conflict of interest. FundingNo funding was received to assist with the preparation of this manuscript. Data availabilityTo access the data, kindly contact the corresponding author. References24.kz. 2022. The number of cases of dermatophytosis is growing in Kyzylorda. Available via https://24.kz/ru/news/social/item/549044-kolichestvo-sluchaev-dermatomikoza-rastjot-v-kyzylorde Akhmetov, K.K. and Chidunchi, I.Y. 2015. Structural organization of muscular elements of a skin-muscular sac of trematodes: literature survey. Int. J. Zool. Res. 11(1), 1–8. Álvarez-Mosquera, I., Hernáez, S., Sánchez, J., Suárez, M.D. and Cisterna, R. 2018. Diagnosis of superficial mycoses by a rapid and effective PCR method from samples of scales, nails and hair. Mycopathologia 183(5), 777–783. Aruna, G.L. and Ramalingappa, B. 2022. Development of indirect ELISA and its evaluation in comparison with KOH hydrolysis and fungal culture for the immuno diagnosis of Trichophyton rubrum and Trichophyton mentagrophytes infection in humans. Acta Trop. 235, 106590. Baumbach, C.M., Müller, S., Reuschel, M., Uhrlaß, S., Nenoff, P., Baums, C.G. and Schrödl, W. 2021. Identification of zoophilic dermatophytes using MALDI-TOF mass spectrometry. Front. Cell. Infect. Microbiol. 11, 631681. Bulegenova, M., Biyashev, K., Kirkimbaeva, Z., Biyashev, B., Ermagambetova, S., Oryntayev, K. and Altenov, A. 2019. The effect of the drug enterocol on the humoral factors of calf body resistance. Adv. Anim. Vet. Sci. 7(8), 674–680. Chowdhary, A., Singh, A., Kaur, A. and Khurana, A. 2022. The emergence and worldwide spread of the species Trichophyton indotineae causing difficult-to-treat dermatophytosis: a new challenge in the management of dermatophytosis. PLoS Pathog. 18(9), e1010795. Derkach, I. and Klymenko, S. 2023. Current state of scientific research and prospects for using basidiomycetes in veterinary medicine: a literature review. Ukr. J. Vet. Sci. 14(2), 57–75. Forbes. 2022. 51 people fell ill with ringworm in Kostanay region. Available via https://forbes.kz/news/2022/09/29/newsid_285527 Gnat, S., Nowakiewicz, A. and Zięba, P. 2019. Taxonomy of dermatophytes—the classification systems may change but the identification problems remain the same. Postępy Mikrobiologii Adv. Microbiol. 58(1), 49–58. Gosink, J. 2022. PCR allows rapid, differentiated dermatophytosis diagnostics. Available via https://clinlabint.com/pcr-allows-rapid-differentiated-dermatophytosis-diagnostics/ Gräser, Y. and Saunte, D.M.L. 2020. A hundred years of diagnosing superficial fungal infections: where do we come from, where are we now and where would we like to go? Acta Derm. Venereol. 100(9), 00111. Hajiyeva, N., Gafarov, I., Hajiyeva, A., Sultanova, N. and Panahova, T. 2022. Forecasting of atopic dermatitis in newborns. Indian J. Dermatol. 67(3), 311. Heckler, I., Sabalza, M., Bojmehrani, A., Venkataraman, I. and Thompson, C. 2023. The need for fast and accurate detection of dermatophytosis. Med. Mycol. 61(5), 037. Iskakova, A.N., Abitayeva, G.K., Abeev, A.B. and Sarmurzina, Z.S. 2022. Meta-analysis data of the accuracy of tests for meat adulteration by real-time PCR. Data Brief 41, 107972. Kirimbayeva, Z., Abutalip, A., Mussayeva, A., Kuzembekova, G. and Yegorova, N. 2023. Epizootological monitoring of some bacterial infectious diseases of animals on the territory of the Republic of Kazakhstan. Comp. Immunol. Microbiol. Infect. Dis. 102, 102061. Kukhar, Y., Kiyan, V., Smagulova, A. and Nikulina, A. 2019. Identification of dermatomycetes isolated from people and animals with dermatophytoses on the territory of Kazakhstan. Adv. Anim. Vet. Sci. 7(1), 21–27. Liter.kz. 2023. The incidence of ringworm in Almaty increased by 1.5 times in 2022. Available via https://liter.kz/v-1-5-raza-vyrosla-zabolevaemost-dermatomikozami-v-almaty-v-2022-godu-1675669781/ Lysková, P., Dobiáš, R., Čmoková, A., Kolařík, M., Hamal, P., Šmatláková, K., Hušek, J., Mencl, K., Mallátová, N., Poláčková, Z., Krnáčová, A., Palkovičová, K., Jablonská, D., Macháčová, J., Drlík, Z., Bázsóová, D., Jaworská, P., Svobodová, L. and Hubka, V. 2021. An outbreak of Trichophyton quinckeanum zoonotic infections in the Czech Republic transmitted from cats and dogs. J. Fungus 7(9), 684. Nussipov, Y., Markabayeva, A., Gianfaldoni, S., Tchernev, G., Wollina, U., Lotti, J., Roccia, M.G., Fioranelli, M. and Lotti, T. 2017. Clinical and epidemiological features of dermatophyte infections in Almaty, Kazakhstan. Macedonian J. Med. Sci. 5(4), 409–413. Paliy, A., Pavlichenko, O., Rodionova, K., Morozov, M. and Dankevych, N. 2023. Treatment of pets with the active substance dexpanthenol in wound processes. Sci. Horizons 26(3), 9–23. Paryuni, A.D., Indarjulianto, S. and Widyarini, S. 2020. Dermatophytosis in companion animals: a review. Vet. World 13(6), 1174–1181. Peano, A., Tizzani, P., Gallo, M.G., Molinar Min, A., Rambozzi, L. and Meneguz, P.G. 2008. Dermatophytosis due to Trichophyton verrucosum in a chamois (Rupicapra rupicapra). Eur. J. Wildl. Res. 54, 153–156. Suleymanova, T.H. 2022. Study of the antimicrobial and antifungal effects of new synthesis ethyl aromatic and heterocyclic derivatives. Azerb. Pharm. Pharmacother. J. 22(1), 61–65. Tyliszczak, B., Drabczyk, A. and Kudłacik–Kramarczyk, S. 2018. Smart, self-repair polymers based on acryloyl-6-aminocaproic acid and modified with magnetic nanoparticles—preparation and characterization. Int. J. Polym. Anal. Charact. 23(3), 226–235. Ugochukwu, I.C.I., Luca, I., Sani, N.A., Omeke, J.N., Anyanwu, M.U., Odigie, A.E., Onoja, R.I., Ocheja, O.B., Ugochukwu, M.O., Makanju, O.A. and Aneke, C.I. 2022. Important mycosis of wildlife: emphasis on etiology, epidemiology, diagnosis, and pathology—a review: PART 2. Animals 12(15), 1897. Zhantlessova, S., Savitskaya, I., Kistaubayeva, A., Ignatova, L., Talipova, A., Pogrebnjak, A. and Digel, I. 2022. Advanced “green” prebiotic composite of bacterial cellulose/pullulan based on synthetic biology-powered microbial coculture strategy. Polymers 14(15), 3224. | ||
| How to Cite this Article |
| Pubmed Style Umitzhanov M, Abdiramanova B, Abutalip A, Bakirov N, Sarimbekova S. Comparative assessment of regulated methods and PCR in the diagnosis of trichophytosis in veterinary mycology. Open Vet J. 2023; 13(12): 1614-1622. doi:10.5455/OVJ.2023.v13.i12.11 Web Style Umitzhanov M, Abdiramanova B, Abutalip A, Bakirov N, Sarimbekova S. Comparative assessment of regulated methods and PCR in the diagnosis of trichophytosis in veterinary mycology. https://www.openveterinaryjournal.com/?mno=167537 [Access: May 13, 2024]. doi:10.5455/OVJ.2023.v13.i12.11 AMA (American Medical Association) Style Umitzhanov M, Abdiramanova B, Abutalip A, Bakirov N, Sarimbekova S. Comparative assessment of regulated methods and PCR in the diagnosis of trichophytosis in veterinary mycology. Open Vet J. 2023; 13(12): 1614-1622. doi:10.5455/OVJ.2023.v13.i12.11 Vancouver/ICMJE Style Umitzhanov M, Abdiramanova B, Abutalip A, Bakirov N, Sarimbekova S. Comparative assessment of regulated methods and PCR in the diagnosis of trichophytosis in veterinary mycology. Open Vet J. (2023), [cited May 13, 2024]; 13(12): 1614-1622. doi:10.5455/OVJ.2023.v13.i12.11 Harvard Style Umitzhanov, M., Abdiramanova, . B., Abutalip, . A., Bakirov, . N. & Sarimbekova, . S. (2023) Comparative assessment of regulated methods and PCR in the diagnosis of trichophytosis in veterinary mycology. Open Vet J, 13 (12), 1614-1622. doi:10.5455/OVJ.2023.v13.i12.11 Turabian Style Umitzhanov, Mynbay, Botagoz Abdiramanova, Aspen Abutalip, Nurbol Bakirov, and Saule Sarimbekova. 2023. Comparative assessment of regulated methods and PCR in the diagnosis of trichophytosis in veterinary mycology. Open Veterinary Journal, 13 (12), 1614-1622. doi:10.5455/OVJ.2023.v13.i12.11 Chicago Style Umitzhanov, Mynbay, Botagoz Abdiramanova, Aspen Abutalip, Nurbol Bakirov, and Saule Sarimbekova. "Comparative assessment of regulated methods and PCR in the diagnosis of trichophytosis in veterinary mycology." Open Veterinary Journal 13 (2023), 1614-1622. doi:10.5455/OVJ.2023.v13.i12.11 MLA (The Modern Language Association) Style Umitzhanov, Mynbay, Botagoz Abdiramanova, Aspen Abutalip, Nurbol Bakirov, and Saule Sarimbekova. "Comparative assessment of regulated methods and PCR in the diagnosis of trichophytosis in veterinary mycology." Open Veterinary Journal 13.12 (2023), 1614-1622. Print. doi:10.5455/OVJ.2023.v13.i12.11 APA (American Psychological Association) Style Umitzhanov, M., Abdiramanova, . B., Abutalip, . A., Bakirov, . N. & Sarimbekova, . S. (2023) Comparative assessment of regulated methods and PCR in the diagnosis of trichophytosis in veterinary mycology. Open Veterinary Journal, 13 (12), 1614-1622. doi:10.5455/OVJ.2023.v13.i12.11 |