| Review Article | ||
Open Vet J. 2023; 13(12): 1517-1535 Open Veterinary Journal, (2023), Vol. 13(12): 1517-1535 Review Article Host immune responses against African swine fever virus: Insights and challenges for vaccine developmentFredmoore L. Orosco1,2,3*1Virology and Vaccine Institute of the Philippines Program, Department of Science and Technology, Industrial Technology Development Institute, Taguig, Philippines 2S&T Fellows Program, Department of Science and Technology, Taguig, Philippines 3Department of Biology, College of Arts and Sciences, University of the Philippines Manila, Manila, Philippines *Corresponding Author: Fredmoore L. Orosco. Virology and Vaccine Institute of the Philippines Program, Industrial Technology Development Institute, Department of Science and Technology, Taguig, Philippines. Email: orosco.fredmoore [at] gmail.com Submitted: 08/09/2023 Accepted: 22/11/2023 Published: 31/12/2023 © 2023 Open Veterinary Journal
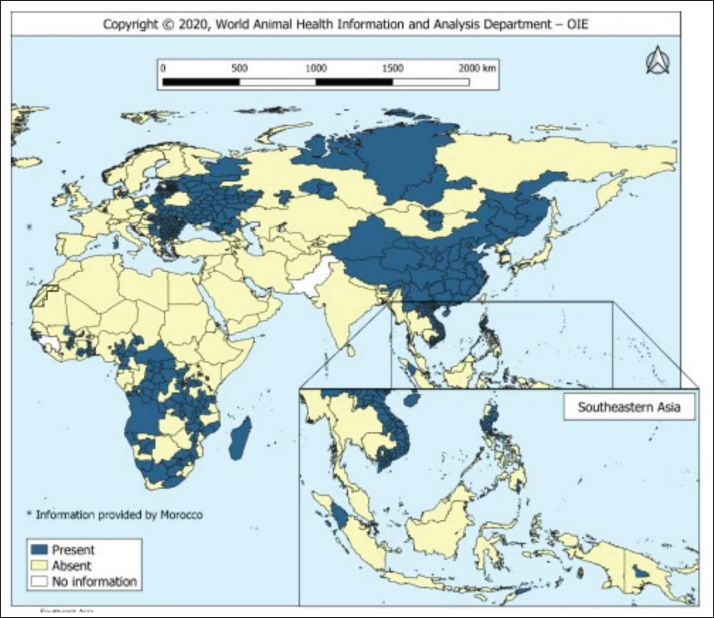
AbstractThe African swine fever virus (ASFV) poses a serious threat to global swine populations, underscoring the urgent need for effective preventive strategies. This comprehensive review investigates the intricate interplay between innate, cellular, and humoral immunity against ASFV, with a focus on their relevance to vaccine development. By delving into immunopathogenesis and immunological challenges, this review article aims to provide a holistic perspective on the complexities of ASFV infections and immune evasion. Key findings underscore the critical role of innate immune recognition in shaping subsequent adaptive immune defenses, potential protective antigens, and the multifaceted nature of ASFV-specific antibodies and cytotoxic T-cell responses. Despite advancements, the unique attributes of ASFV present hurdles in the development of a successful vaccine. In conclusion, this review examines the current state of ASFV immune responses and offers insights into future research directions, fostering the development of effective interventions against this devastating pathogen. Keywords: African swine fever virus, Immune response, Swine industry, Vaccine development. IntroductionOne hundred years ago, the emergence of African swine fever (ASF) in Kenya devastated domestic pigs (Sus scrofa domesticus) (Costard et al., 2013). ASF is attributed to ASF virus (ASFV), an enveloped icosahedral arbovirus with a double-stranded DNA structure that displays genomic similarities to poxviruses, housing approximately 160 genes sequenced in its genome. ASF’s incubation period of ASF spans a variable timeframe contingent on factors such as virus virulence, host attributes, viral load, and route of infection (Guinat et al., 2016). Clinically, ASF symptoms resemble those of other hemorrhagic illnesses and manifest in diverse forms, including peracute, acute, subacute, and chronic. In cases of peracute manifestation induced by potent ASFV strains, pig mortality can increase to 100% within a mere four (dpi), often without discernible lesions (Gallardo et al., 2015). In addition to domestic pigs, ASFV carriers include warthogs (Phacochoerus africanus), bush pigs (Potamochoerus porcus), and parasitizing ticks (Ornithodoros moubata spp.), potentially serving as reservoirs that sustain infections for over five years, contributing to the endemicity of ASF within regions (Penrith, 2020). Notably, ASFV persistence within ticks for extended periods, such as the documented four-year presence in unoccupied domestic pig premises in Madagascar, highlights the virus’s resilience (Jori et al., 2023). The presence of ASF since its first documentation in Kenya in 1921 has led to its dissemination across African and non-African countries (Fig. 1), affirming its endemic nature and persistent threat to the swine populations across approximately 32 African countries (Penrith, 2013). ASFV primarily targets immune cells of myeloid lineage, such as dendritic cells, macrophages, and monocytes (Sánchez et al., 2012). The virus enters host cells through either clathrin-mediated endocytosis or macropinocytosis (Duan et al., 2022). Intracellularly, ASFV possesses distinct layers: a large DNA genome at the core, encased by a core shell, inner lipid envelope, and icosahedral capsid. As ASFV buds through its plasma membrane, it acquires an external envelope. Although both extracellular and intracellular virions are infectious, the precise role of the outer envelope is still uncertain (Wang et al., 2019). The ASFV genome size varies significantly (170–194 kb) across geographical isolates and encompasses over 150 open reading frames (ORFs) (Dixon et al., 2013). ASFV genes are categorized into immediate-early, early, intermediate, and late classes (Cackett et al., 2020).
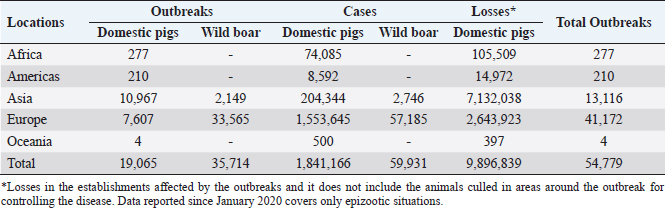
Fig. 1. Global distribution of ASFV (OIE, 2020). Vaccination serves as a potent strategy against viral infections; however, the development of an effective vaccine against ASFV remains elusive (Karger et al., 2019; Orosco, 2023a). The challenge lies in the incomplete understanding of the host immune responses triggered by ASFV (Dixon et al., 2019). Complex evasion tactics and genetic variability hinder the development of an effective ASF vaccine (Correia et al., 2013; Orosco, 2023a). The ongoing epidemics in Asian nations and the resurgence of ASF in Europe (Table 1) underscores the necessity for an ASF vaccine. Fortunately, dedicated efforts are underway to explore anti-ASFV immune responses and identify potential vaccine antigens. This review aims to comprehensively examine the innate, cellular, and humoral immune responses against ASFV, relevant to the development of a safe and effective vaccine against the pathogen. The immunopathogenesis and immunological challenges in ASFV vaccine development are also discussed. African swine fever virus (ASFV): An overviewASFV is a large enveloped DNA virus and represents the only member of the Asfarviridae family and Asfivirus genus (Arias et al., 2018). The genetic makeup of the virus encodes 150–165 proteins that are indispensable for the evasion of host immunity and virus replication (Dixon et al., 2013). A comprehensive proteomic map of ASFV has been outlined previously (Alejo et al., 2018; Mahedi et al., 2023). Notably, both structural and infection-related proteins play roles in regulating host immune evasion mechanisms. ASFV enters the host cells through cell surface receptors, often following an infection route through the tonsils proximate to the lymph nodes (Reis et al., 2017). Table 1. The number of outbreaks and losses caused by ASF in different regions (2016–2022) (OIE, 2022).
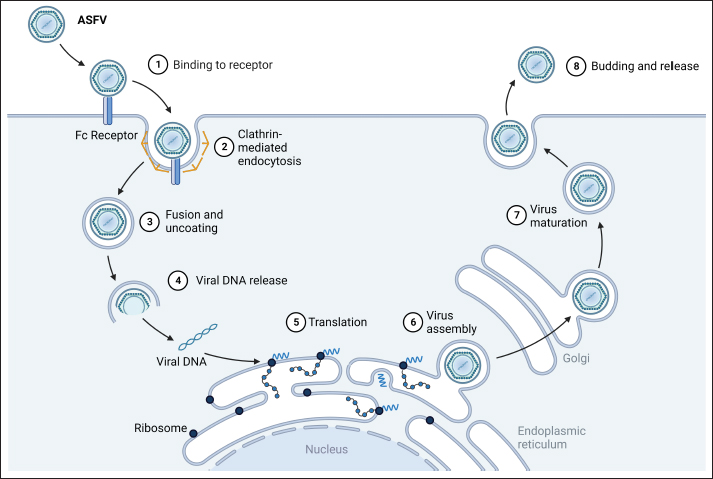
Subsequent to viremia, ASFV translocates to tissue organs, with primary transmission occurring through direct contact with infected pigs, contaminated fomites, or swills (Guinat et al., 2016; Orosco et al., 2023b). Ornithodoros ticks acquire the virus while feeding on infected pigs; within them, the virus undergoes replication in the gut tissues and subsequently migrates to the salivary glands. These infected ticks act as vectors and can transfer the virus from one pig to another through bites. ASFV’s entry of ASFV into pig cells occurs via endocytosis (Fig. 2), encompassing both clathrin-mediated and receptor-mediated pathways (Galindo and Alonso, 2017). In new herds, ASF presents with extensive mortality, marked by elevated fever, reduced appetite, limited mobility, and congregating pigs. In severe cases, death can precede the emergence of other clinical indications for up to four days. The onset of clinical symptoms can be influenced by factors such as incubation period, viral genotype, exposure route, environmental circumstances, and animal breed (Jori and Bastos, 2009). Less severe cases of ASF are characterized by petechial hemorrhage, mucoid diarrhea, and skin reddening near the ears, abdomen, and limbs (Pikalo et al., 2019). Post-mortem examinations revealed hemorrhages within internal organs, including the liver, lungs, intestines, kidneys, heart, and lymph nodes. The spleen has an increased size and abnormally darkened appearance (Sánchez-Cordón et al., 2018; Orosco et al., 2023c). The contributions of free-living hosts, scavenging pigs, and pigs recovering from ASF to the epidemiology of the disease remain uncertain, although several investigations suggested their potential roles in virus transmission (Probst et al., 2019). Immunopathogenesis of ASFVASF is distinguished by profound leukopenia, particularly lymphopenia, and a widespread immunodeficiency (Patil et al., 2020). Initial pig infection stems from the oral-nasal route or bites from infected ticks. The virus reproduces within regional lymph nodes or tonsils (Pikalo et al., 2019) and subsequently disseminates via the blood and lymph to secondary replication sites within 2–3 days (Colgrove et al., 1969; Orosco, 2024a) before migrating to other organs, enabling replication across diverse cell types (Heuschele, 1967). Macrophages and monocytes are the primary targets of ASFV (Dixon et al., 2019). Although ASFV is a DNA virus, its replication occurs in the cytoplasm, not within the nucleus (Coelho and Leitão, 2020). Infected monocyte-macrophages exhibit enlargement and nuclear chromatin margination and contain an intracytoplasmic juxtanuclear inclusion body. These bodies reveal viral factories by transmission electron microscopy. Virus replication leads to necrosis in host cells, and virions are released via budding (Salguero, 2020). ASF-triggered monocyte-macrophage destruction has been linked to ASFV-induced apoptosis or necrosis (Afe et al., 2023). The ASFV genome encompasses genes that influence programmed cell death, with both inhibitory (i.e., A179L, A224L, DP71L, and EP153R) and inductive roles (i.e., A199L and E183L) (Netherton et al., 2019a). Certain genes can enhance the survival of infected cells whereas apoptosis is believed to be an improbable cause of cell death within the infected monocyte-macrophage subset (Dixon et al., 2017). ASF is distinguished by the extensive degradation of lymphoid organs, including the lymph nodes, spleen, tonsils, and thymus (Sánchez-Cordón et al., 2021). In acute ASFV infection, notable proportions of T and B lymphocytes, along with macrophages, undergo cell death (Schäfer et al., 2022). Replication of the virus within monocyte-macrophages triggers its activation, leading to increased secretion of proinflammatory cytokines during the early stages of the disease (Gómez-Villamandos et al., 2013). The heightened expression of proinflammatory cytokines recognized as a “cytokine storm,” underpins the considerable lymphocyte apoptosis in proximity to activated/infected monocyte-macrophages within tissues (Machuka et al., 2022).
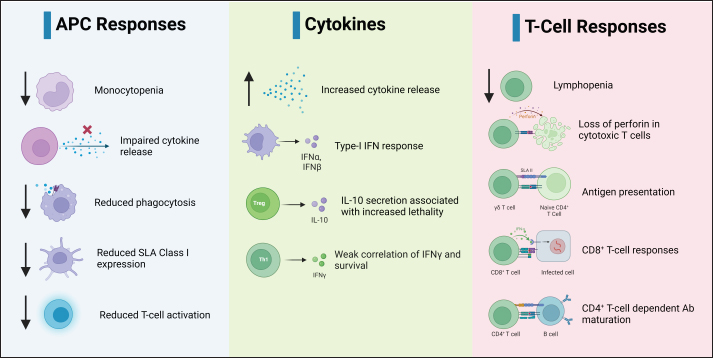
Fig. 2. Replication cycle of ASFV. Innate immunity against ASFVInnate immune sensing The innate immunity constitutes the initial defense against infection (Riera Romo et al., 2016). Pathogens carry distinctive conserved elements termed pathogen-associated molecular patterns (PAMPs), which are recognized by pattern recognition receptors (PRRs) in the cytoplasm or on cell surfaces (Roers et al., 2016). This recognition triggers innate immunity, culminating in the induction of proinflammatory cytokines and type I interferons (IFNs) to coordinate innate immunity (Iwasaki and Medzhitov, 2015), as illustrated in Figure 3. Among these, IFNs, particularly type I IFNs, play a pivotal role in antiviral defense (Ivashkiv and Donlin, 2014). Specific IFN-stimulated genes, including IFN-induced transmembrane (IFITM) genes and myxovirus resistance (Mx), which contribute to anti-ASFV defense, have been described previously (Muñoz-Moreno et al., 2016). ASFV primarily multiplies within mononuclear-phagocytic cells, which is pivotal for instigating innate and adaptive host immunity (Sánchez et al., 2012). Consequently, cytoplasmic PRRs, such as cyclic AMP-GMP synthase (cGAS), Toll-like receptors (TLRs), RIG-I-like receptors (RLRs) and/or stimulators of interferon genes (STING), have a crucial role in the detection of ASFV infections (Iwasaki and Medzhitov, 2015). ASFV also produces proteins capable of subduing TLR and cGAS recognition, thereby evading antiviral immunity (García-Belmonte et al., 2019). In contrast to the virulent strain Armenia/07, attenuated ASFV NH/P68 promptly triggers the cGAS-STING-IRF3 cascade, leading to early STING phosphorylation and trafficking through cGAMP mediation. Following TBK1 and IRF3 activation, substantial IFN-β production occurs during NH/P68 infection, whereas virulent ASFV impedes IFN synthesis, a pivotal link between innate and adaptive immunity (García-Belmonte et al., 2019). Detailed insights into IFNs’ regulatory mechanisms in ASFV infection across in vitro and in vivo investigations have been reviewed previously (Dixon et al., 2019). ASFV’s interference of ASFV with innate immunity begins by evading PRR recognition (Dixon et al., 2019). As such, the I329L protein resembles TRIF in its C-terminal region and acts as a type I transmembrane protein, curtailing dsRNA-triggered IRF3 and NF-κB activation, which are the central components of innate immunity. This suggests that the viral modulation gene could potentially target TRIF, a key MyD88-independent adaptor. In addition, ASFV’s A238L protein binds the p65 subunit of NF-κB, thereby inhibiting its functionality (Hong et al., 2022).
Fig. 3. Host immune responses against ASFV infection. Apoptosis and autophagy Viruses often employ diverse tactics to modulate apoptosis and autophagy as natural antiviral responses (Leonardi et al., 2021). ASFV inhibits apoptosis in infected macrophages to support viral replication and the dissemination of new virions (Dixon et al., 2019). ASFV produces several proteins, A179L, A224L, and DP71L, which govern host autophagy and apoptosis. As such, A179L inhibits autophagy and apoptosis by binding to Beclin-1. The apoptosis inhibitor A224L suppresses caspase 3 activation and amplifies NF-κB, curtailing apoptosis via anti-apoptotic genes, such as Bcl-2 and IAP (Shi et al., 2021). DP71L hampers CHOP induction in vitro, indicating that ASFV encodes analogous functional genes (Zhang et al., 2010). Although the specifics of apoptosis inhibition remain unclear, ASFV’s effects on autophagy are less explored, with limited knowledge beyond A179L inhibiting starvation-induced autophagosome formation and LC3B presence at ASFV replication sites upon overexpression (Banjara et al., 2019). A comprehensive investigation of the factors affecting apoptosis in uninfected lymphocytes and macrophages during acute ASFV stages holds the potential for vaccine development (Gómez-Villamandos et al., 2013; Orosco, 2024b). Cytokines and chemokines Cytokines critically regulate innate immune and inflammatory responses, including IFNs, chemotactic factors, interleukins (ILs), and tumor necrosis factors (TNFs) (Schwartz et al., 2016). Macrophages, which is ASFV’s primary target, engage in pivotal interactions (Fig. 3). In vitro studies indicate that the attenuated NHV strain of ASFV triggers higher IL-12p40, TNF-α, and IFN-α levels than the virulent strain (Schäfer et al., 2022). Proinflammatory cytokines increase in ASFV-infected pigs, peaking at 7 dpi, underlining their role in ASFV pathogenesis (Zakaryan et al., 2015). In acute ASFV infections, these cytokines promote lymphocyte apoptosis (Salguero, 2020). Detailed analysis of ASFV interactions with macrophage and monocytes revealed that field isolates such as 22653/14 replicate more efficiently in monocytes and moMΦ, while inducing lower IL-1α, IL-1β, and IL-18release than avirulent BA71V (Franzoni et al., 2017). A subsequent investigation on porcine monocyte-derived macrophages demonstrated attenuated strain NH/P68-infected moM1 secreting higher levels of IL-18, IL-1β, and IL-1a than the virulent strain 22653/14, suggesting compromised immune response development due to ASFV 22653/14 covert macrophage replication (Franzoni et al., 2020). Transcriptome analysis revealed that TNF family cytokines, along with upregulated IL-17F and IFNs and downregulated IL-10, significantly contribute to ASF pathogenesis and tissue inflammation (Zhu et al., 2019). Infection of porcine monocyte-derived DCs (moDCs) with ASFV strains leads to efficient replication, functional modulation, and reduced cytokine responses, potentially hindering protective immune activation (Franzoni et al., 2018). Cellular immunity against ASFVNumerous studies have underscored the crucial role of T-cell responses in combatting ASFV infections. While extensive research exists on human and murine T cell responses, understanding of porcine T cell immunity, especially against non-zoonotic diseases such as ASF, remains limited. In this section, existing insights and gaps in understanding porcine T cell immunity to ASFV infections and vaccination are discussed. Lymphocyte activationEvidence indicates that attenuated ASFV infection in pigs results in survival and subsequent protection against virulent strain challenges of the same genotype (Acton and Reis e Sousa, 2016). Attenuated ASFV infection triggers ASFV-specific CTLs and memory T cells (Fig. 3), as shown by lymphocyte proliferation assays, highlighting the protective immune responses (Lokhandwala et al., 2016). Anti-CD4 and anti-CD8 mAbs hinder ASFV-specific T-cell proliferation differently, depending on the viral state, underscoring the complexity of immune interactions (Mair et al., 2014). Certain ASFV antigens that offer protection without antibodies emphasize the significance of T-cells in eliminating infected cells (Acton and Reis e Sousa, 2016). Beyond T cells, NK cells and γδ T cells are also activated upon ASFV infection; however, their roles in protection and potential cross-protection through T-helper cells require further verification (Takamatsu et al., 2013). αβ T CellsAll mammalian T cells share hallmark features, such as CD3 expression and TCR, enabling antigen recognition and cellular activation. The TCR comprises α and β chains or γ and δ chains in αβ and γδ T cells, respectively. In pigs, the prevalent CD3+ T cell subset comprises αβ T cells, encompassing CD4+ T helper (Th) cells, CD8α+ CTLs, and a distinctive CD4+/CD8α+ double-positive (DP) T cell population, serving as effector and memory cells (Schäfer et al., 2022). This section explores the characteristics and roles of these subsets in combating ASFV infections. CD8α+ αβ T cells and cytotoxic responsesCD4–/CD8α+ αβ T cells, commonly identified as CTLs, recognize antigens presented by SLA class I molecules, thereby responding to intracellular antigens (Fig. 3). Their function involves eliminating the infected cells to hinder the propagation of intracellular pathogens, including viruses. The initial indications of T-cell involvement surfaced in the early 1980s, as leukocytes from pigs infected with attenuated ASFV strains displayed specific cytotoxicity against infected cells (Pabst, 2020). Notably, animals infected with low-virulence ASFV strains exhibit persistent cytotoxic responses and the establishment of T cell memory up to two years post-infection, in contrast to virulent ASFV-infected animals (Schäfer et al., 2022). Extensive investigations characterized CTL responses in pigs that survived low-virulence ASFV infection (Galindo and Alonso, 2017). Utilizing ASFV-immune pig PBMCs, they demonstrated specific lysis through CTL assays, employing NHV-infected macrophages as targets. This process showed specific CTL activity, with reduced lysis observed when SLA-mismatched PBMCs were used or when SLA I was blocked with a specific antibody. Moreover, the depletion of CD8α+ cells significantly reduced specific lysis in CD8α-depleted samples, establishing the role of CD8α+ CTLs in conferring protective immunity (Galindo and Alonso, 2017). These ex vivo analyses marked the initial description of protective immune responses mediated by CD8α+ CTLs. A study highlighting the essential role of CD8α+ T cells in immune protection was conducted (Sánchez-Cordón et al., 2018). They utilized low-virulence OURT88/3-infected animals, which cleared the infection and subsequently depleted surviving animals of CD8α+ lymphocytes using specific antibodies. Upon challenge with the highly virulent OURT88/1, depleted animals succumbed to infection, whereas nondepleted animals survived with mild clinical signs. Although various porcine lymphocyte subsets express CD8α, the exact contributors to immunity remain unclear. Attempts to specifically deplete CTLs using a different antibody (clone PPT22) resulted in partial depletion in one out of ten animals, and this particular animal succumbed to the challenge. Although this study focused on memory responses, it provided definitive in vivo evidence of the crucial role of CD8α + T cells in immune memory against ASFV (Sánchez-Cordón et al., 2018). Recent investigations of cytotoxic responses involved domestic pigs and wild boars during distinct ASFV infections at up to 10 dpi. Moderate virulent Estonia2014 infection induced CD8α+ CTL frequency increases 5–10 dpi in infected domestic pigs, paralleled by similar responses in wild boars. In addition, wild boars displayed T-bet-dependent differentiation at 10 dpi, indicating antiviral Th1 response initiation (Oura et al., 2005). Highly virulent Armenia2007 infection prompted DP T cell dominance in both subspecies, with CD8α+ CTLs showing no variation until 7 dpi (Schäfer et al., 2021). An intriguing and consistently reproducible discovery emerged from multiple independent trials: a marked reduction in the expression of the key lytic effector protein perforin within various cytotoxic effector populations at 4–5 dpi during both moderately and highly virulent ASFV infections. This phenomenon was particularly pronounced in domestic pigs infected with the Armenia2007 strain, and to a lesser degree after the Estonia2014 infection. While wild boars also exhibited reduced perforin expression, the effect was generally milder than that in domestic pigs (Hühr et al., 2020). However, the cause of this observation remains unclear. In addition to perforin, CTLs employ other cytotoxic molecules, including the TNF-related apoptosis-inducing ligand (TRAIL) or Fas ligand (FasL or CD95L/CD178), to trigger death receptor-mediated apoptosis instead of direct cell lysis (Schäfer et al., 2021). It is plausible that ASFV-infected CTLs switch to apoptosis induction via FasL or TRAIL, which can lead to a complete loss of perforin expression (Prager and Watzl, 2019). This adaptation might be advantageous, as certain ASFV strains encode a viral homolog (A179L) of the anti-apoptotic Bcl-2 protein (Meiraz et al., 2009), which most effectively counteracts perforin-mediated killing (Sutton et al., 1997). Reduced perforin in domestic pigs could signify a shift toward death receptor-mediated killing, whereas the response in wild boar might have been caused by the virus. Liver-based antiviral perforin responses have been linked to substantial tissue damage (Welz et al., 2018), offering a potential explanation for the observed Kupffer cell degeneration in wild boars and broader differences in disease outcomes (Sehl et al., 2020). Another hypothesis involves perforin consumption, possibly due to T cell exhaustion or hyperinflammatory responses. The immediate secretion of synthesized perforin, which is not detectable by the mAb employed (Hersperger et al., 2008), suggests proinflammatory and polyclonal activation. Intense inflammatory cues and high antigen burdens during acute ASFV infections can lead to continuous T-cell activation, culminating in T-cell exhaustion (Erickson et al., 2015). However, the exact mechanism requires further investigation. CD4+ and CD4+/CD8α+ αβ T cellsPorcine CD4+ T cells encompass CD4+/CD8α– αβ Th cells, which play roles in antibody production, immune response orchestration, and affinity maturation (Fig. 3). Unlike humans and murine T cells, activated or memory CD4+ T cells express CD8α (Pabst, 2020). This phenomenon has been observed across species, including monkeys, chickens, and dogs (Overgaard et al., 2015). This chapter explores CD4+/CD8α+ DP T cells that contribute to immune processes. Despite the focus on CTL responses, emerging studies have shed light on CD4+ Th cell responses during ASFV infection. Early investigations have demonstrated CD4+ T cell proliferation in response to live or UV-inactivated ASFV, highlighting their participation (Bosch-Camós et al., 2022). Blocking experiments indicated CD4+ Th cell involvement, although discerning CD4+/CD8α– from DP T cells is challenging (Bosch-Camós et al., 2022). Functional relevance was observed when attenuated BA71 ASFV-immunized PBMCs showed reduced antibody production upon CD4+ Th cell depletion, thus emphasizing their significance (Lopera-Madrid et al., 2017). It is noteworthy that the depletion experiments conducted by Oura et al. likely affected DP T cells formed during primary ASFV infection, making it challenging to distinguish the repercussions of depleting CD4–/CD8α+ or DP T cells (Oura et al., 2005). This, along with other studies demonstrating DP T-cell development after ASFV infection, underscores their role in secondary responses to ASFV. While investigations into DP T cell function during immunity generally focus on memory responses due to their gradual development over infection, comparative studies involving highly virulent Armenia2007 and moderately virulent Estonia2014 ASFV infections examined primary T cell responses (Schäfer et al., 2021). Domestic pigs infected with Armenia2007 exhibited a substantial increase in DP T cells at 5–7 dpi. Notably, these cells did not proliferate, as indicated by the absence of Ki-67 expression. The inverse correlation with observed CD4+ T cell levels suggested the differentiation of DP T cells from CD4+/CD8α– Th cells. Conversely, in wild boars, DP T cells underwent extensive proliferation without a significant increase during the study. These cells could have spread to unexamined tissues or undergone apoptosis during antiviral responses (Schäfer et al., 2021). Estonia2018, a moderately virulent strain, triggered increased DP T cell frequencies in the livers, lungs, and spleens of domestic pigs and the livers and lungs of wild boar. Notably, DP T cell proliferation is exclusive to the spleen in infected domestic pigs (Sauter-Louis et al., 2021). Interestingly, perforin loss observed in CTLs was not mirrored in the DP T cells. As proliferating DP T cells were solely identified in the spleen, it was inferred that during moderately virulent ASFV infection, DP T cells likely assume orchestrating, rather than cytotoxic, roles (Sauter-Louis et al., 2021). This contrasts with prior evidence of perforin increase in DP T cells after infection with the low-virulence OURT88/3 strain, although this study employed different infection strains and was conducted with only two pigs. Collectively, these investigations underscore the substantial contribution of DP T cells, which serve as both memory cells guarding against recurrent infections and evolving effector cells during primary ASFV infections. Regulatory T-cell responsesRegulatory T cells (Tregs) share the CD3+/CD4+/CD25+/FoxP3+ phenotype with other mammals and merit dedicated discussion because of their distinctive immunoregulatory role (Żabińska et al., 2016). Tregs are a subset of CD4+ αβ T cells that secrete IL-10, an immunomodulatory cytokine with dual effects, suppressing antigen presentation and proinflammatory Th1 cytokines (Fig. 3), while enhancing antibody production and B cell responses (Catalán et al., 2021). The comprehension of Treg responses in ASFV-infected pigs remains incomplete, as infected animals typically succumb or require euthanasia before Treg responses manifest. However, insights from ASFV and other viruses suggest that Tregs play a pivotal role in modulating porcine antiviral response. For instance, porcine Tregs were linked to viral persistence during porcine reproductive and respiratory syndrome virus (PRRSV) infection via IL-10 secretion, which dampens Th1-mediated antiviral responses (Silva-Campa et al., 2012). Notably, several ASFV vaccination studies observed elevated IL-10 levels in nonresponsive pigs that eventually succumbed to challenge infections, accompanied by increased IFNγ levels, which is a potential sign of skewed immune responses in moribund animals (Sánchez-Cordón et al., 2020). Numerous infection investigations have highlighted the detrimental link between IL-10 secretion and survival outcomes. For instance, Cabezón et al. conducted a study involving wild boars exposed to classical swine fever virus (CSFV) immediately after birth, followed by infection with the moderately virulent E75 ASFV strain. All CSFV-infected animals succumbed to infection within 6–7 dpi, whereas CSFV-negative animals secreted detectable IL-10 levels. Interestingly, some of these IL-10-producing wild boar survived, indicating a complex relationship between survival, infection, and IL-10 (Cabezón et al., 2017). A separate investigation demonstrated that vaccinated wild boars showed no increase in IL-10 levels after contact with shedding ASFV-infected pigs. In contrast, unvaccinated controls display elevated IL-10 levels and later succumb to ASFV infection (Barroso-Arévalo et al., 2021). Similarly, Reis et al. noted increased IL-10 levels in animals fatally challenged with the virulent OURT88/1 ASF strain, following immunization with an I329L deletion mutant derived from the attenuated OURT88/3 strain. These observations collectively suggest that elevated IL-10 levels might indicate a system unable to recover rather than a coordinated immune response (Reis et al., 2020). Consequently, previous investigations in wild boars and domestic pigs have identified increased and early Treg frequencies in wild boars during moderately and highly virulent ASFV infections, aligning with higher lethality and disease severity. However, cytokine measurements were not performed in those studies (Schäfer et al., 2019). Conversely, Post et al. (2017) presented a contrasting perspective, associating elevated IL-10 levels with survival during moderately virulent ASFV infections. However, the relatively modest IL-10 concentrations and notable age and quantity distinctions between survivors and nonsurvivors raise questions about the robustness of these findings, suggesting that age-related disparities might play a role. γδ T cellsIn contrast to the conventional TCR-MHC interaction that activates αβ T cells, γδ TCR is believed to function akin to PRRs (Chien et al., 2014), with viral proteins identified as ligands of γδ TCRs (Halary et al., 2005). Unlike humans or mice, pigs possess abundant circulating γδ T cells, which are grouped into terminally differentiated effector (CD2+/CD8α+), activated (CD2+/CD8α–), and naïve (CD2–/CD8α–) subsets based on CD2 and CD8α expression (Sedlak et al., 2014). Given their functional flexibility and substantial numbers, the role of γδ T cells in ASFV infection has been explored. A recent investigation involving domestic pigs revealed that increased γδ T cell frequencies correlated with enhanced survival after low- or high-dose infection with moderately virulent Netherlands’86 ASFV (Post et al. 2017). Previous studies have indicated that porcine γδ T cells can present antigens to other T cells (Takamatsu et al., 2002) and restore ASFV-specific proliferation without professional APCs (Takamatsu et al., 2006). Thus, it is possible that the observed protection stems from heightened antigen presentation by γδ T cells, compensating for compromised presentation by APCs in early ASFV infection. However, the small survivor cohorts and the most pronounced impact in correlation with age suggest that survival is primarily influenced by immune maturity and age rather than specific cell frequencies (Post et al., 2017). The protective role of γδ T cell responses faces challenges from studies on ASFV infections in wild boars. These studies consistently reveal a γδ T cell-dominated response with more severe clinical manifestations and higher fatality rates following experimental infection, in contrast to domestic pigs (Zani et al., 2018). Highly virulent ASFV Armenia2007 infection prompts heightened γδ T cell levels or CD8α+ effector cell differentiation in the spleen, gastrohepatic lymph nodes, and blood of wild boar, distinct from domestic pigs (Hühr et al., 2020). Disparities between the two species were more pronounced during trials with the moderately virulent ASFV Estonia2018 strain. Notably, domestic pigs exhibit minimal γδ T cell responses, while wild boars display significant γδ T cell bias and elevated CD8α+ γδ T cell frequencies at 4–7 dpi (Schäfer et al., 2021). This trend is most pronounced in infected wild boar livers, correlating with severe Kupffer cell degeneration at 7–10 dpi (Sehl et al., 2020). Increased T-bet+ T cells are also exclusive to infected wild boar (Schäfer et al., 2021). These findings, in contrast to prior studies, suggest a potentially pathogenic γδ T cell-driven immune response, potentially contributing to the higher fatality rate upon Estonia2018 infection (Sehl et al., 2020). The divergent outcomes of ASFV infection in domestic pigs and wild boars may also stem from intrinsic differences in their immune responses, an area that has not yet been extensively explored. Nonconventional T cellsOther T cell subsets exhibit distinct characteristics that differentiate them from the conventional αβ and γδ T cell populations. An extensively studied example is the invariant natural killer T (iNKT) cells. These cells share typical T-cell traits, including thymic development, CD3 and TCR expression, and antigen-specific responses upon activation. However, they also possess unique attributes that separate them from each other. iNKT cells show an antigen-experienced phenotype post-thymic egress, and their distinctive invariant TCR, with limited variability, recognizes a narrow range of antigens, typically glyco- and phospholipids or α-galactosylceramide (αGC) in research contexts, bound to MHC-related CD1d. Furthermore, cytokines, including type I IFN, IL-18, and IL-12, can trigger iNKT cell responses (Kohlgruber et al., 2016). Despite some exploration of porcine iNKT cells (Thierry et al., 2012), their role in ASFV infection remains largely unexplored. Early studies have suggested the involvement of NKT cell-like lymphocytes in ASFV responses, as these cells expanded following in vitro culture of naïve PBMCs with SLA-matched ASFV-infected cells (Denyer et al., 2006). Subsequent investigations using a murine CD1d tetramer loaded with the αGC analog PBS57 confirmed the presence of iNKT cells in ASFV infection. In vivo studies demonstrated a significant increase in iNKT cell frequency in the gastrohepatic lymph nodes, liver, peripheral blood, and lungs of domestic pigs infected with the highly virulent Armenia2007 strain (Schäfer et al., 2019). Notably, although in vivo responses were evident, no activation was observed when porcine PBMCs were exposed to Armenia2007 in vitro because of the potential immune escape mechanisms. Conversely, during moderately virulent Estonia2014 infection, an upsurge in ICOS+ iNKT cells in the blood and spleen was identified (Schäfer et al., 2021), a marker associated with highly activated iNKT cells in pigs and mice. Collectively, these findings suggest the participation of porcine iNKT cells in antiviral responses, potentially linked to their robust IFNγ production capacity. However, further investigation is required to fully comprehend the scope and influence of iNKT cell responses (Kohlgruber et al., 2016). In addition to iNKT cells, mucosal-associated invariant T (MAIT) cells represent another subset of nonconventional T cells. These cells have been thoroughly studied in various species, including mice and humans, and have been implicated in antiviral responses against multiple viruses (Garner et al., 2018). However, in pigs, only one study has reported the presence of cells expressing transcripts of the invariant MAIT cell TCR chain TRAV1-TRAJ33 (Xiao et al., 2019). However, comprehensive cellular identification and analysis of their roles in response to infectious diseases in pigs remain unclear. Humoral immunity against ASFVPassive administration of convalescent swine sera has demonstrated the potential for protection against virulent ASFV challenge, providing significant insight into the development of an efficacious ASFV vaccine (Wang et al., 2019; Orosco, 2024c). The generation of specific antibodies targeting p72 and p54 proteins during infection has been shown to neutralize approximately 10% more virus infectivity than antibodies derived from pigs immunized with recombinant proteins. These antibodies, specifically anti-p72 and anti-p54, exert inhibitory effects on virus binding to cells, whereas anti-p30 antibodies impede virus entry. Notably, antibodies raised against the dynein-binding domain of p54 have exhibited substantial neutralization of virus infectivity in vitro (Escribano et al., 2013). On the other hand, antibodies interfering with viral spread can disrupt various entry mechanisms, including macropinocytosis and clathrin-mediated endocytosis (Sánchez-Vizcaíno et al., 2012). While vaccination with antibodies against p30 and p54 proteins displayed some disease course reduction upon challenge with the E75 isolate, complete immune protection against various virulent ASFV isolates remains elusive. Antibody responses targeting structural (A151R, A104R, K78R, B602L, and EP153R) and function-undefined proteins (CP312R, C44L, E184L, K205R, and K145R) were reported in recovered domestic swine (Lokhandwala et al., 2019). Immunization with recombinant adenovirus cocktails confirmed the immunogenicity of CD2v, B602L, K205R, CP530R, and A104R but failed to provide protection against virulent ASFV strains, suggesting the potential for antibody-dependent enhancement (ADE) of disease (Lokhandwala et al., 2017). In summary, ASFV infection triggers both innate and adaptive immune reactions (Fig. 3). However, uncontrolled innate responses and compromised adaptive immunity can result in local and systemic tissue damage. While no definitive neutralizing antibodies have been identified for ASFV protection, the presence of anti-ASFV antibodies and CTLs appears to be crucial for defense. A comprehensive understanding of host immune responses and distinct cellular subsets is necessary to elucidate the immune responses at play during ASFV infection. The insight gained from discerning the specific CTL response and SLA-I sequencing, as well as the variations in protection seen with subunit vaccines, has implications for future vaccine designs. Immunological challenges in ASFV vaccine developmentThe challenges in developing an ASF vaccine arise primarily from the intricate nature of the virus and gaps in our understanding of immune protection mechanisms and key antigens (Turlewicz-Podbielska et al., 2021). This section discusses the various attributes of ASFV and extrapolates their potential implications for vaccine design, drawing insights from immunological research on other viruses. Infectious and abundant extracellular and intracellular virionsSimilar to poxviruses, ASFV displays infectivity in both the intracellular and extracellular forms (McFadden, 2005). Infectious intracellular ASFV virions of various strains have been observed within the host cells at different time points, accompanied by extracellular virions (Canter et al., 2022). In contrast to poxviruses, in which extracellular virions constitute a small fraction of the total, ASFV extracellular virions constitute a significant portion of the virus population (Payne, 1986). While poxvirus studies have identified factors affecting extracellular virion release, such as A33 and A34 proteins, the mechanisms of ASFV have been less explored (Su et al., 2010). ASFV extracellular virions encompass both enveloped virions released directly from the cell membrane and cell-associated enveloped virions (CEVs) that bud with actin tail propulsion (Mucker et al., 2020). Intracellular ASFVs include immature and mature virions (Rodríguez et al., 2004). Antibodies against intracellular virion surface proteins, such as p54, p72, and p30/32 have demonstrated partial protection and neutralization against ASFV (Barderas et al., 2001). Given the diverse infectious forms, both intracellular and extracellular ASFV virions contribute to pathogenesis, posing challenges for vaccine development. This complexity may explain the limited success of vaccine trials targeting intracellular virion antigens (Neilan et al., 2004). Extremely glycosylated CD2v and C-type lectinASFV CD2v and C-type lectin proteins exhibit significant glycosylation, which is a common feature of viral envelope proteins synthesized in the Golgi complex and ER (Watanabe et al., 2019). CD2v is recognized for its hemadsorption activity in infected cells and its presence in mature virions (Rodríguez et al. 1993). The exact location of ASFV’s C-type lectin of ASFV is uncertain, as it was not detected in mass spectrometric analyses of extracellular virions, potentially due to its low abundance (Alejo et al., 2018). Although vaccinia virus C-type lectins are outer envelope components, ASFVs might be below detection levels, such as C-type lectins in CEVs (Mucker et al., 2020). Both CD2v and C-type lectins are markedly glycosylated, with CD2v possessing 14 potential N-linked glycosylation sites in its ectodomain and C-type lectin with eight such sites (Goatley and Dixon, 2011). Their glycosylation density, approximately one site per 13 amino acids, surpasses that of other ASFV structural proteins and poxvirus outer envelope proteins (Goatley and Dixon, 2011). Glycans constitute a considerable proportion of CD2v and C-type lectins with molecular weights of approximately 55% and 65%, respectively (Goatley and Dixon, 2011). Deletion of the CD2v and C-type lectin genes in attenuated ASFVs has shown reduced or abolished protection in pigs (Petrovan et al., 2022). However, these highly glycosylated proteins present challenges for vaccine development owing to glycan heterogeneity, weak carbohydrate-protein binding affinities, and immune tolerance to self-like glycans (Crispin and Doores, 2015). This “glycan shield” negatively affects immune responses and peptide loading onto MHC molecules (Li et al., 2021). Inducing neutralizing antibodies against highly glycosylated proteins, such as HIV Env spike or ASFV CD2v and C-type lectin, presents substantial challenges (Li et al., 2021). Very low estimated surface densities of most envelope proteinsUsing mass spectrometry, a study found CD2v in two of three purified ASFV extracellular virion samples, whereas C-type lectin was not detected. CD2v’s abundance among ASFV structural proteins was notably low, in contrast to all five outer envelope proteins of the vaccinia virus (Alejo et al., 2018). The density of antigens on surfaces significantly affects antigenicity and immunogenicity. HIV, with its sparse spike surface density, evades the immune response (Amitai et al., 2018). CD2v’s estimated surface density is low (0.03 protein per 100 nm², whereas neutralizing antibody-inducing inner membrane proteins have higher densities, such as p12 (0.94), p30/p32 (0.13), and p54 (0.33) (Alejo et al., 2018). Although these estimations might be influenced by factors such as tryptic sites, solubility, and modifications, the low surface densities of p30, CD2v, p54, and possibly C-type lectins could dampen antibody avidity and immunogenicity (Goatley and Dixon, 2011). CD2v might have the majority of its expression within infected cells, impacting its surface density, and its cleavage into glycosylated and nonglycosylated fragments further contributes (Goatley and Dixon, 2011). Hyperimmune sera recognize recombinant CD2v, but not convalescent sera from recovered pigs, hinting at a low CD2v presence on virus particles (Ruiz-Gonzalvo et al., 1996). The interaction of C-type lectin with MHC-I proteins and the inhibition of MHC-I exocytosis could limit its expression on the cytoplasmic membrane (Hurtado et al., 2011). ASFV infection enhance by naïve seraNaïve sera can amplify ASFV infection compared to the culture medium, indicating that certain serum components enhance infectivity (Canter et al., 2022). Enveloped viruses, including dengue, Ebola, HIV, and poxviruses, exploit phosphatidylserine (PtdSer)-binding serum proteins as bridging molecules for TAM receptors on macrophages, promoting clathrin-mediated endocytosis (Chua et al., 2019). PtdSer, normally exposed during apoptosis, becomes an “eat me” signal for macrophages to engulf dying cells (Lemke, 2019). Viral infection activates caspase-3, exposing PtdSer on infected cell surfaces, a phenomenon termed apoptotic mimicry (Amara and Mercer, 2015). Exposing PtdSer to the plasma membrane of infected cells is crucial for ASFV budding, paralleling other enveloped viruses (Canter et al., 2022). Monocyte-derived macrophages are notably more susceptible to ASFV infection compared to monocytes, likely because of their heightened phagocytic activity (Franzoni et al., 2017). PtdSer on the ASFV envelope possibly contributes to macrophage preference and explains the sustained infectivity of CD2v or C-type lectin knockout ASFV (Canter et al., 2022). The role of PtdSer in ASFV infection, if confirmed, could pose a significant challenge for ASF vaccine development. Unknown but likely numerous virus receptorsASFV’s preference of ASFV for macrophage infection implies restricted expression of macrophage-specific receptors as the key entry point (Gaudreault and Richt, 2019). Clathrin-mediated endocytosis and macropinocytosis are both used in pig macrophage infections (Hernáez et al., 2016). Elevated SWC9 (possibly CD80) expression renders monocytic cells susceptible to ASFV infection (McCullough et al., 1999). While the CD163 antibody inhibits ASFV infection and binding (Sánchez-Torres et al., 2003), CD163 alone is not sufficient for infection (Lithgow et al., 2014), as CD163-knockout pigs still succumb to infection (Popescu et al., 2017). Therefore, other receptors are likely to be involved (Sánchez et al., 2017). CD2v binding to CD58 and C-type lectin glycosylation suggest its involvement. N-linked glycans in viral spike proteins are crucial for infectivity (Li et al., 2020), and CD2v and C-type lectin glycans may interact with host glycan-binding proteins. Macrophages possess these binding proteins (Park et al., 2021), although neither CD2v nor C-type lectins are vital for ASFV replication (Galindo et al., 2000), implying the presence of additional receptors. PtdSer receptors, observed in the apoptotic mimicry of enveloped viruses, may also facilitate ASFV infection (Chua et al., 2019). Host membrane adhesive proteins, such as ITGα4β7 and CD54, have similarly increased infectivity in HIV (Burnie and Guzzo, 2019). Host membrane proteins, such as ITGαVβ1, ITGα3β1, and CD9, are found in ASFV extracellular virions, suggesting that they might enhance ASFV infectivity through receptor binding. The abundant presence of PtdSer receptors and GBPs in macrophages likely explains ASFV macrophage-targeting. A possible immune-mediated enhancement in vaccine challenge studies has been noted, yet Fc receptors have not been implicated (Alcami and Viñuela, 1991). The involvement of poorly immunogenic PtdSer and host proteins could be a key hurdle in ASF vaccine development. Antibodies cannot completely neutralize ASFVVirus neutralization by antibodies is a key defense mechanism; however, ASFV-specific antibodies exhibit only partial neutralization effects (Gómez-Puertas and Escribano, 1997). Even at high antibody concentrations, 5%–20% of virions remain non-neutralized (Pérez-Núñez et al., 2019). Antibodies against p54 and p72 hinder attachment, while ASFV p30 antibodies impede internalization (Gómez-Puertas et al., 1996). Inoculation with CD2v-expressing baculovirus yielded neutralizing activity, suggesting that p72, p54, p30, and CD2v are protective antigens (Ruiz-Gonzalvo et al., 1996). However, antibodies to p12, despite inhibiting infection, lack neutralization potential (Carrascosa et al., 1995). The neutralizing activity of C-type lectins p22 and p17 is unknown. Extracellular virions of the vaccinia virus resist neutralization more than intracellular virions (Benhnia et al., 2013), and both extracellular and intracellular ASFV virions evade complete neutralization (Canter et al., 2022). The mechanism underlying incomplete neutralization remains unclear. Preincubation with non-neutralizing sera inhibits ASFV-neutralizing serum effects (Gómez-Puertas and Escribano, 1997), aligning with the enhancing impact of naïve serum. Strikingly, PtdIns removal from ASFV decreased neutralization, whereas adding PtdIns enhanced it (Gómez-Puertas et al., 1997). As both PtdSer and PtdIns are anionic phospholipids, their distribution imbalance could affect the membrane curvature, which is essential for ASFV budding. Excessive PtdIns may hinder PtdSer-receptor binding, blocking apoptotic mimicry. This scenario could render ASFV more susceptible to antibody neutralization. Low antigen density, high glycosylation, host membrane proteins, and apoptotic mimicry on the envelope collectively contribute to incomplete neutralization of ASFV. Viral proteins control apoptosis and/or inhibit MHC-I expressionASFV infection induces apoptosis in host cells (Galindo et al., 2012). Various ASFV proteins, including CD2v, MGF360, MGF505, and E183L, exhibit proapoptotic activity (Gao et al., 2021). Conversely, ASFV also produces anti-apoptotic proteins such as A224L, A179L, DP71L, and EP153R (Wang et al., 2020). Moreover, pEP153R reduces the expression of MHC-I molecules in infected cell membranes (Hurtado et al., 2011). ASFV proteins blocking MHC-I expression and apoptosis may compromise cytotoxicity mediated by antigen-specific T-cells, NK cells, or antibodies. This interference could weaken the effectiveness of the vaccine-induced immune response. Understudied protective immune mechanismsBoth cellular and humoral-mediated immunity have been investigated for their contributions to protective immunity. The significance of antibodies in safeguarding against ASFV infection has been comprehensively reviewed (Escribano et al., 2013). Similar to the protective effect of passively transferred antibodies in mice against the vaccinia virus (Lustig et al., 2009), the protective role of antibodies has been demonstrated in pigs through the passive transfer of serum or colostrum antibodies from convalescent animals (Onisk et al., 1994). Although ASFV cannot be completely neutralized by hyperimmune sera from convalescent or protected pigs in in vitro assays, antibodies can induce cytotoxicity in ASFV-infected cells through antibody-dependent cellular cytotoxicity (ADCC) and complement-mediated cytotoxicity (CDC) in vitro. Although not reported, antibody-dependent cellular phagocytosis (ADCP) might also contribute to ASFV clearance. Despite these antibody activities, subunit vaccines containing potentially protective antigens from both intracellular and extracellular virions, such as p30, p54, p72, and CD2v, failed to provide full protection in pigs, despite the presence of antigen-specific antibodies with some neutralizing activity (Sunwoo et al., 2019). Cytotoxicity mediated by cytotoxic lymphocytes was reported using 51Cr release assays in vitro (Alonso et al., 1997). Immunity against ASFV has been associated with increased NK cell activity (Leitão et al., 2001) and cytotoxicity of CD8+ T cells (Takamatsu et al., 2013). Depletion of CD8+ lymphocytes abolished the protection against virulent ASFV strain (Oura et al., 2005). Pigs immunized with plasmid DNA containing ASFV antigen genes to selectively activate T cells displayed partial protection and elevated CD8+ cell counts in the blood after day 3 of infection compared with control pigs (Argilaguet et al., 2012). Cross-protection conferred by attenuated ASFV correlates with cell-mediated immunity parameters measured through T-cell epitope assays, lymphocyte proliferation, and IFN-γ ELISPOT (Bosch-Camós et al., 2021). T effector cells induced by MHC epitopes of ASFV T cell epitope assays CD2v, x p30, p72, and pp62have been identified in infected, vaccinated, and/or protected pigs (Sun et al., 2021). Although pigs vaccinated with a combination of multiple Ad5-vectored ASFV genes exhibited immune recall responses in IFN-γ ELISPOT assays, they did not protect against severe diseases (Lokhandwala et al., 2017). Notably, ASFV T cell epitopes were identified through systematic screening using IFN-γ ELISPOT assays, and Ad5 vectors carrying genes containing dominant T cell epitopes reduced viremia but did not prevent severe disease in vaccinated pigs (Netherton et al., 2019b). Thus, achieving robust pig protection through cell-mediated immunity remains challenging. It is important to note that a demonstration of cell-mediated cytotoxicity that prevents or reduces the release of infectious ASFV virions from infected cells has yet to be established. ConclusionUnraveling the intricate interplay between innate, cellular, and humoral immune responses against ASFV has provided crucial insights into the elusive quest for an effective vaccine against this pathogen. The immunopathogenesis of ASFV infection highlights the delicate balance between host immunity and viral evasion. While the innate response primes the immune landscape, adaptive immunity orchestrates defense mechanisms through both cell- and antibody-mediated pathways. Despite the significant progress, several research gaps remain. The intricate glycosylation pattern of ASFV envelope proteins, exemplified by CD2v and C-type lectins, poses a challenge for vaccine design due to potential immune evasion. Further studies investigating the impact of antigen density, glycan shielding, and apoptotic mimicry on immune recognition are essential. The dual role of apoptosis in viral propagation and immune evasion necessitates comprehensive investigations of ASFV protein interactions and their influence on cytotoxicity. While the significance of neutralizing antibodies has been established, the incomplete neutralization of ASFV virions presents another difficult problem. Understanding the mechanisms underlying this phenomenon, including the potential role of host membrane proteins and phospholipids, is a pivotal research frontier. In addition, deciphering the contribution of antigen-specific T cells to ASFV clearance and protection remains a critical knowledge gap. Addressing these research gaps is key to advancing ASFV vaccine development. Innovative strategies can exploit the potential of nanoparticle-based immunogens, allowing controlled antigen presentation to enhance immune responses. The emerging field of synthetic biology may enable the rational design of antigens with improved immunogenicity. Moreover, leveraging cutting-edge techniques such as single-cell sequencing and systems immunology will reveal intricate immune dynamics during ASFV infection, facilitating the identification of correlates of protection. Therefore, the journey toward an effective ASFV vaccine is characterized by multifaceted challenges and opportunities. The intricate arms race between viral immune evasion strategies and host immune responses underscores the need for innovative approaches that harness the full potential of innate, cellular, and humoral immunities. By addressing current gaps and capitalizing on emerging technologies, we are poised to unlock new avenues in ASFV vaccine development, thereby safeguarding the swine industry against this devastating pathogen. AcknowledgmentsThe author would like to thank the DOST S&T Fellows Program, the Philippine Council for Agriculture, Aquatic and Natural Resources Research and Development (DOST-PCAARRD) for funding this research project, and the Industrial Technology Development Institute (DOST-ITDI) for hosting this research project. Conflict of interestThe author declares that there are no conflicts of interest. FundingThis study was funded by the Philippine Council for Agriculture, Aquatic and Natural Resources Research and Development of the Department of Science and Technology (DOST-PCAARRD). Data availabilityAll data are available in the published manuscript. ReferencesActon, S.E. and Reis e Sousa, C. 2016. Dendritic cells in remodeling of lymph nodes during immune responses. Immunol. Rev. 271, 221–229. Afe, A.E., Shen, Z.-J., Guo, X., Zhou, R. and Li, K. 2023. African swine fever virus interaction with host innate immune factors. Viruses 15, 1220. Alcami, A. and Viñuela, E. 1991. Fc receptors do not mediate African swine fever virus replication in macrophages. Virology 181, 756–759. Alejo, A., Matamoros, T., Guerra, M. and Andrés, G. 2018. A Proteomic atlas of the African swine fever virus particle. J. Virol. 92, e01293–18. Alonso, F., Domı́nguez, J., Viñuela, E. and Revilla, Y. 1997. African swine fever virus-specific cytotoxic T lymphocytes recognize the 32 kDa immediate early protein (vp32). Virus Res. 49, 123–130. Amara, A. and Mercer, J. 2015. Viral apoptotic mimicry. Nat. Rev. Microbiol. 13, 461–469. Amitai, A., Chakraborty, A. K. and Kardar, M. 2018. The low spike density of HIV may have evolved because of the effects of T helper cell depletion on affinity maturation. PLoS Comput. Biol. 14, e1006408. Argilaguet, J.M., Pérez-Martín, E., Nofrarías, M., Gallardo, C., Accensi, F., Lacasta, A., Mora, M., Ballester, M., Galindo-Cardiel, I., López-Soria, S., Escribano, J.M., Reche, P.A. and Rodríguez, F. 2012. DNA vaccination partially protects against African swine fever virus lethal challenge in the absence of antibodies. PloS One 7, e40942. Arias, M., Jurado, C., Gallardo, C., Fernández-Pinero, J. and Sánchez-Vizcaíno, J.M. 2018. Gaps in African swine fever: analysis and priorities. Transbound. Emerg. Dis. 65 Suppl 1, 235–247. Banjara, S., Shimmon, G.L., Dixon, L.K., Netherton, C.L., Hinds, M.G. and Kvansakul, M. 2019. Crystal structure of African swine fever virus A179L with the autophagy regulator beclin. Viruses 11, 789. Barderas, M.G., Rodríguez, F., Gómez-Puertas, P., Avilés, M., Beitia, F., Alonso, C. and Escribano, J.M. 2001. Antigenic and immunogenic properties of a chimera of two immunodominant African swine fever virus proteins. Arch. Virol. 146, 1681–1691. Barroso-Arévalo, S., Barasona, J.A., Cadenas-Fernández, E. and Sánchez-Vizcaíno, J.M. 2021. The role of interleukine-10 and interferon-γ as potential markers of the evolution of African swine fever virus infection in wild boar. Pathog. Basel Switz. 10, 757. Benhnia, M.R.-E.-I., Maybeno, M., Blum, D., Aguilar-Sino, R., Matho, M., Meng, X., Head, S., Felgner, P.L., Zajonc, D.M., Koriazova, L., Kato, S., Burton, D.R., Xiang, Y., Crowe, J.E., Peters, B. and Crotty, S. 2013. Unusual features of vaccinia virus extracellular virion form neutralization resistance revealed in human antibody responses to the smallpox vaccine. J. Virol. 87, 1569–1585. Bosch-Camós, L., Alonso, U., Esteve-Codina, A., Chang, C.-Y., Martín-Mur, B., Accensi, F., Muñoz, M., Navas, M.J., Dabad, M., Vidal, E., Pina-Pedrero, S., Pleguezuelos, P., Caratù, G., Salas, M.L., Liu, L., Bataklieva, S., Gavrilov, B., Rodríguez, F. and Argilaguet, J. 2022. Cross-protection against African swine fever virus upon intranasal vaccination is associated with an adaptive-innate immune crosstalk. PLOS Pathog. 18, e1010931. Bosch-Camós, L., López, E., Collado, J., Navas, M.J., Blanco-Fuertes, M., Pina-Pedrero, S., Accensi, F., Salas, M.L., Mundt, E., Nikolin, V. and Rodríguez, F. 2021. M448R and MGF505-7R: Two African swine fever virus antigens commonly recognized by ASFV-specific T-cells and with protective potential. Vaccines 9, 508. Burnie, J. and Guzzo, C. 2019. The incorporation of host proteins into the external HIV-1 envelope. Viruses 11, 85. Cabezón, O., Muñoz-González, S., Colom-Cadena, A., Pérez-Simó, M., Rosell, R., Lavín, S., Marco, I., Fraile, L., de la Riva, P.M., Rodríguez, F., Domínguez, J. and Ganges, L. 2017. African swine fever virus infection in Classical swine fever subclinically infected wild boars. BMC Vet. Res. 13, 227. Cackett, G., Matelska, D., Sýkora, M., Portugal, R., Malecki, M., Bähler, J., Dixon, L. and Werner, F. 2020. The African swine fever virus transcriptome. J. Virol. 94; 10.1128/jvi.00119-20. Canter, J.A., Aponte, T., Ramirez-Medina, E., Pruitt, S., Gladue, D.P., Borca, M.V. and Zhu, J.J. 2022. Serum neutralizing and enhancing effects on African swine fever virus infectivity in adherent pig PBMC. Viruses 14, 1249. Carrascosa, A.L., Sastre, I. and Viñuela, E. 1995. Production and purification of recombinant African swine fever virus attachment protein p12. J. Biotechnol. 40, 73–86. Catalán, D., Mansilla, M.A., Ferrier, A., Soto, L., Oleinika, K., Aguillón, J.C. and Aravena, O. 2021. Immunosuppressive mechanisms of regulatory B cells. Front. Immunol. 12. Chien, Y., Meyer, C. and Bonneville, M. 2014. γδ T cells: First line of defense and beyond. Annu. Rev. Immunol. 32, 121–155. Chua, B.A., Ngo, J.A., Situ, K. and Morizono, K. 2019. Roles of phosphatidylserine exposed on the viral envelope and cell membrane in HIV-1 replication. Cell Commun. Signal. CCS 17, 132. Coelho, J. and Leitão, A. 2020. The African swine fever virus (asfv) topoisomerase ii as a target for viral prevention and control. Vaccines 8, 312. Colgrove, G.S., Haelterman, E.O. and Coggins, L. 1969. Pathogenesis of African swine fever in young pigs. Am. J. Vet. Res. 30, 1343–1359. Correia, S., Ventura, S. and Parkhouse, R.M. 2013. Identification and utility of innate immune system evasion mechanisms of ASFV. Virus Res. 173, 87–100. Costard, S., Mur, L., Lubroth, J., Sanchez-Vizcaino, J.M. and Pfeiffer, D.U. 2013. Epidemiology of African swine fever virus. Virus Res. 173, 191–197. Crispin, M. and Doores, K.J. 2015. Targeting host-derived glycans on enveloped viruses for antibody-based vaccine design. Curr. Opin. Virol. 11, 63–69. Denyer, M.S., Wileman, T.E., Stirling, C.M.A., Zuber, B. and Takamatsu, H.-H. 2006. Perforin expression can define CD8 positive lymphocyte subsets in pigs allowing phenotypic and functional analysis of Natural Killer, Cytotoxic T, Natural Killer T and MHC un-restricted cytotoxic T-cells. Vet. Immunol. Immunopathol. 110, 279–292. Dixon, L.K., Chapman, D.A.G., Netherton, C.L. and Upton, C. 2013. African swine fever virus replication and genomics. Virus Res. 173, 3–14. Dixon, L.K., Islam, M., Nash, R. and Reis, A.L. 2019. African swine fever virus evasion of host defences. Virus Res. 266, 25–33. Dixon, L.K., Sánchez-Cordón, P.J., Galindo, I. and Alonso, C. 2017. Investigations of pro- and anti-apoptotic factors affecting African swine fever virus replication and pathogenesis. Viruses 9, 241. Duan, X., Ru, Y., Yang, W., Ren, J., Hao, R., Qin, X., Li, D. and Zheng, H. 2022. Research progress on the proteins involved in African swine fever virus infection and replication. Front. Immunol. 13, 947180. Erickson, J.J., Lu, P., Wen, S., Hastings, A.K., Gilchuk, P., Joyce, S., Shyr, Y. and Williams, J.V. 2015. Acute viral respiratory infection rapidly induces a CD8+ T cell exhaustion-like phenotype. J. Immunol. Baltim. Md 1950 195, 4319–4330. Escribano, J.M., Galindo, I. and Alonso, C. 2013. Antibody-mediated neutralization of African swine fever virus: Myths and facts. Virus Res. 173, 101–109. Franzoni, G., Graham, S.P., Giudici, S.D., Bonelli, P., Pilo, G., Anfossi, A.G., Pittau, M., Nicolussi, P.S., Laddomada, A. and Oggiano, A. 2017. Characterization of the interaction of African swine fever virus with monocytes and derived macrophage subsets. Vet. Microbiol. 198, 88–98. Franzoni, G., Graham, S.P., Sanna, G., Angioi, P., Fiori, M.S., Anfossi, A., Amadori, M., Dei Giudici, S. and Oggiano, A. 2018. Interaction of porcine monocyte-derived dendritic cells with African swine fever viruses of diverse virulence. Vet. Microbiol. 216, 190–197. Franzoni, G., Razzuoli, E., Dei Giudici, S., Carta, T., Galleri, G., Zinellu, S., Ledda, M., Angioi, P., Modesto, P., Graham, S.P. and Oggiano, A. 2020. Comparison of macrophage responses to African swine fever viruses reveals that the NH/P68 strain is associated with enhanced sensitivity to Type I IFN and cytokine responses from classically activated macrophages. Pathogens 9, 209. Galindo, I., Almazán, F., Bustos, M.J., Viñuela, E. and Carrascosa, A.L. 2000. African swine fever virus EP153R open reading frame encodes a glycoprotein involved in the hemadsorption of infected cells. Virology 266, 340–351. Galindo, I. and Alonso, C. 2017. African swine fever virus: A review. Viruses 9, 103. Galindo, I., Hernáez, B., Muñoz-Moreno, R., Cuesta-Geijo, M.A., Dalmau-Mena, I. and Alonso, C. 2012. The ATF6 branch of unfolded protein response and apoptosis are activated to promote African swine fever virus infection. Cell Death Dis. 3, e341. Gallardo, M.C., Reoyo, A. de la T., Fernández-Pinero, J., Iglesias, I., Muñoz, M.J. and Arias, M.L. 2015. African swine fever: a global view of the current challenge. Porc. Health Manag. 1, 21. Gao, Q., Yang, Y., Quan, W., Zheng, J., Luo, Y., Wang, H., Chen, X., Huang, Z., Chen, X., Xu, R., Zhang, G. and Gong, L. 2021. The African swine fever virus with MGF360 and MGF505 deleted reduces the apoptosis of porcine alveolar macrophages by inhibiting the NF-κB signaling pathway and interleukin-1β. Vaccines 9, 1371. García-Belmonte, R., Pérez-Núñez, D., Pittau, M., Richt, J.A. and Revilla, Y. 2019. African swine fever virus Armenia/07 virulent strain controls interferon beta production through the cGAS-STING pathway. J. Virol. 93; 10.1128/jvi.02298-18. Garner, L.C., Klenerman, P. and Provine, N.M. 2018. Insights into mucosal-associated invariant T cell biology from studies of invariant natural killer T cells. Front. Immunol. 9, 1478. Gaudreault, N.N. and Richt, J.A. 2019. Subunit vaccine approaches for African swine fever virus. Vaccines 7, 56. Goatley, L.C. and Dixon, L.K. 2011. Processing and localization of the African swine fever virus CD2v transmembrane protein. J. Virol. 85, 3294–3305. Gómez-Puertas, P. and Escribano, J.M. 1997. Blocking antibodies inhibit complete African swine fever virus neutralization. Virus Res. 49, 115–122. Gómez-Puertas, P., Oviedo, J.M., Rodríguez, F., Coll, J. and Escribano, J.M. 1997. Neutralization susceptibility of African swine fever virus is dependent on the phospholipid composition of viral particles. Virology 228, 180–189. Gómez-Puertas, P., Rodríguez, F., Oviedo, J.M., Ramiro-Ibáñez, F., Ruiz-Gonzalvo, F., Alonso, C. and Escribano, J.M. 1996. Neutralizing antibodies to different proteins of African swine fever virus inhibit both virus attachment and internalization. J. Virol. 70, 5689–5694. Gómez-Villamandos, J.C., Bautista, M.J., Sánchez-Cordón, P.J. and Carrasco, L. 2013. Pathology of African swine fever: the role of monocyte-macrophage. Virus Res. 173, 140–149. Guinat, C., Gogin, A., Blome, S., Keil, G., Pollin, R., Pfeiffer, D.U. and Dixon, L. 2016. Transmission routes of African swine fever virus to domestic pigs: current knowledge and future research directions. Vet. Rec. 178, 262–267. Halary, F., Pitard, V., Dlubek, D., Krzysiek, R., de la Salle, H., Merville, P., Dromer, C., Emilie, D., Moreau, J.-F. and Déchanet-Merville, J. 2005. Shared reactivity of Vδ2neg γδ T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J. Exp. Med. 201, 1567–1578. Hernáez, B., Guerra, M., Salas, M.L. and Andrés, G. 2016. African swine fever virus undergoes outer envelope disruption, capsid disassembly and inner envelope fusion before core release from multivesicular endosomes. PLOS Pathog. 12, e1005595. Hersperger, A.R., Makedonas, G. and Betts, M.R. 2008. Flow cytometric detection of perforin upregulation in human CD8 T cells. Cytometry A 73A, 1050–1057. Heuschele, W.P. 1967. Studies on the pathogenesis of African swine fever I. Quantitative studies on the sequential development of virus in pig tissues. Arch. Für Gesamte Virusforsch. 21, 349–356. Hong, J., Chi, X., Yuan, X., Wen, F., Rai, K.R., Wu, L., Song, Z., Wang, S., Guo, G. and Chen, J.-L. 2022. I226R protein of African swine fever virus is a suppressor of innate antiviral responses. Viruses 14, 575. Hühr, J., Schäfer, A., Schwaiger, T., Zani, L., Sehl, J., Mettenleiter, T.C., Blome, S. and Blohm, U. 2020. Impaired T-cell responses in domestic pigs and wild boar upon infection with a highly virulent African swine fever virus strain. Transbound. Emerg. Dis. 67, 3016–3032. Hurtado, C., Bustos, M.J., Granja, A.G., de León, P., Sabina, P., López-Viñas, E., Gómez-Puertas, P., Revilla, Y. and Carrascosa, A.L. 2011. The African swine fever virus lectin EP153R modulates the surface membrane expression of MHC class I antigens. Arch. Virol. 156, 219–234. Ivashkiv, L.B. and Donlin, L.T. 2014. Regulation of type I interferon responses. Nat. Rev. Immunol. 14, 36–49. Iwasaki, A. and Medzhitov, R. 2015. Control of adaptive immunity by the innate immune system. Nat. Immunol. 16, 343–353. Jori, F. and Bastos, A.D.S. 2009. Role of wild suids in the epidemiology of African swine fever. EcoHealth 6, 296–310. Jori, F., Bastos, A., Boinas, F., Van Heerden, J., Heath, L., Jourdan-Pineau, H., Martinez-Lopez, B., Pereira de Oliveira, R., Pollet, T., Quembo, C., Rea, K., Simulundu, E., Taraveau, F. and Penrith, M.-L. 2023. An updated review of Ornithodoros ticks as reservoirs of African swine fever in Sub-Saharan Africa and Madagascar. Pathogens 12, 469. Karger, A., Pérez-Núñez, D., Urquiza, J., Hinojar, P., Alonso, C., Freitas, F.B., Revilla, Y., Le Potier, M.-F. and Montoya, M. 2019. An update on African swine fever virology. Viruses 11, 864. Kohlgruber, A.C., Donado, C.A., LaMarche, N.M., Brenner, M.B. and Brennan, P.J. 2016. Activation strategies for invariant natural killer T cells. Immunogenetics 68, 649–663. Leitão, A., Cartaxeiro, C., Coelho, R., Cruz, B., Parkhouse, R.M.E., Portugal, F.C., Vigário, J.D. and Martins, C.L.V. 2001. The non-haemadsorbing African swine fever virus isolate ASFV/NH/P68 provides a model for defining the protective anti-virus immune response. J. Gen. Virol. 82, 513–523. Lemke, G. 2019. How macrophages deal with death. Nat. Rev. Immunol. 19, 539–549. Leonardi, L., Sibéril, S., Alifano, M., Cremer, I. and Joubert, P.-E. 2021. Autophagy modulation by viral infections influences tumor development. Front. Oncol. 11. Li, Y., Liu, D., Wang, Y., Su, W., Liu, G. and Dong, W. 2021. The importance of glycans of viral and host proteins in enveloped virus infection. Front. Immunol. 12. Li, Q., Wu, J., Nie, J., Zhang, L., Hao, H., Liu, S., Zhao, C., Zhang, Q., Liu, H., Nie, L., Qin, H., Wang, M., Lu, Q., Li, X., Sun, Q., Liu, J., Zhang, L., Li, X., Huang, W. and Wang, Y. 2020. The impact of mutations in SARS-CoV-2 spike on viral infectivity and antigenicity. Cell 182, 1284–1294.e9. Lithgow, P., Takamatsu, H., Werling, D., Dixon, L. and Chapman, D. 2014. Correlation of cell surface marker expression with African swine fever virus infection. Vet. Microbiol. 168, 413–419. Lokhandwala, S., Petrovan, V., Popescu, L., Sangewar, N., Elijah, C., Stoian, A., Olcha, M., Ennen, L., Bray, J., Bishop, R.P., Waghela, S.D., Sheahan, M., Rowland, R.R.R. and Mwangi, W. 2019. Adenovirus-vectored African swine fever virus antigen cocktails are immunogenic but not protective against intranasal challenge with Georgia 2007/1 isolate. Vet. Microbiol. 235, 10–20. Lokhandwala, S., Waghela, S.D., Bray, J., Martin, C.L., Sangewar, N., Charendoff, C., Shetti, R., Ashley, C., Chen, C.-H., Berghman, L.R., Mwangi, D., Dominowski, P.J., Foss, D.L., Rai, S., Vora, S., Gabbert, L., Burrage, T.G., Brake, D., Neilan, J. and Mwangi, W. 2016. Induction of robust immune responses in swine by using a cocktail of adenovirus-vectored African Swine fever virus antigens. Clin. Vaccine Immunol. CVI 23, 888–900. Lokhandwala, S., Waghela, S.D., Bray, J., Sangewar, N., Charendoff, C., Martin, C.L., Hassan, W.S., Koynarski, T., Gabbert, L., Burrage, T.G., Brake, D., Neilan, J. and Mwangi, W. 2017. Adenovirus-vectored novel African swine fever virus antigens elicit robust immune responses in swine. PLOS ONE 12, e0177007. Lopera-Madrid, J., Osorio, J.E., He, Y., Xiang, Z., Adams, L.G., Laughlin, R.C., Mwangi, W., Subramanya, S., Neilan, J., Brake, D., Burrage, T.G., Brown, W.C., Clavijo, A. and Bounpheng, M.A. 2017. Safety and immunogenicity of mammalian cell derived and modified Vaccinia Ankara vectored African swine fever subunit antigens in swine. Vet. Immunol. Immunopathol. 185, 20–33. Lustig, S., Maik-Rachline, G., Paran, N., Melamed, S., Israely, T., Erez, N., Orr, N., Reuveny, S., Ordentlich, A., Laub, O., Shafferman, A. and Velan, B. 2009. Effective post-exposure protection against lethal orthopoxviruses infection by vaccinia immune globulin involves induction of adaptive immune response. Vaccine 27, 1691–1699. Machuka, E.M., Juma, J., Muigai, A.W.T., Amimo, J.O., Pelle, R. and Abworo, E.O. 2022. Transcriptome profile of spleen tissues from locally-adapted Kenyan pigs (Sus scrofa) experimentally infected with three varying doses of a highly virulent African swine fever virus genotype IX isolate: Ken12/busia.1 (ken-1033). BMC Genomics 23, 522. Mahedi, M.R.A., Rawat, A., Rabbi, F., Babu, K.S., Tasayco, E.S., Areche, F.O., Pacovilca-Alejo, O.V., Flores, D.D.C., Aguilar, S.V., Orosco, F.L., Syrmos, N., Mudhafar, M., Afrin, S. and Rahman, M.M. 2023. Understanding the global transmission and demographic distribution of Nipah virus (NiV). Res. J. Pharm. Technol. 16, 3588–3594. Mair, K.H., Sedlak, C., Käser, T., Pasternak, A., Levast, B., Gerner, W., Saalmüller, A., Summerfield, A., Gerdts, V., Wilson, H.L. and Meurens, F. 2014. The porcine innate immune system: an update. Dev. Comp. Immunol. 45, 321–343. McCullough, K.C., Basta, S., Knötig, S., Gerber, H., Schaffner, R., Kim, Y.B., Saalmüller, A. and Summerfield, A. 1999. Intermediate stages in monocyte-macrophage differentiation modulate phenotype and susceptibility to virus infection. Immunology 98, 203–212. McFadden, G. 2005. Poxvirus tropism. Nat. Rev. Microbiol. 3, 201–213. Meiraz, A., Garber, O.G., Harari, S., Hassin, D. and Berke, G. 2009. Switch from perforin-expressing to perforin-deficient CD8+ T cells accounts for two distinct types of effector cytotoxic T lymphocytes in vivo. Immunology 128, 69–82. Mucker, E.M., Lindquist, M. and Hooper, J.W. 2020. Particle-specific neutralizing activity of a monoclonal antibody targeting the poxvirus A33 protein reveals differences between cell associated and extracellular enveloped virions. Virology 544, 42–54. Muñoz-Moreno, R., Cuesta-Geijo, M.Á., Martínez-Romero, C., Barrado-Gil, L., Galindo, I., García-Sastre, A. and Alonso, C. 2016. Antiviral role of IFITM proteins in African swine fever virus infection. PLOS ONE 11, e0154366. Neilan, J.G., Zsak, L., Lu, Z., Burrage, T.G., Kutish, G.F. and Rock, D.L. 2004. Neutralizing antibodies to African swine fever virus proteins p30, p54, and p72 are not sufficient for antibody-mediated protection. Virology 319, 337–342. Netherton, C.L., Connell, S., Benfield, C.T.O. and Dixon, L.K. 2019a. The genetics of life and death: virus-host interactions underpinning resistance to African swine fever, a viral hemorrhagic disease. Front. Genet. 10. Netherton, C.L., Goatley, L.C., Reis, A.L., Portugal, R., Nash, R.H., Morgan, S.B., Gault, L., Nieto, R., Norlin, V., Gallardo, C., Ho, C.-S., Sánchez-Cordón, P.J., Taylor, G. and Dixon, L.K. 2019b. Identification and immunogenicity of African swine fever virus antigens. Front. Immunol. 10. OIE, 2020. Global disease monitoring reports. World Organization for Animal Health, pp: 1-3. OIE, 2022. Global situation of African swine fever. World Organization for Animal Health, pp: 1-2. Onisk, D.V., Borca, M.V., Kutish, G., Kramer, E., Irusta, P. and Rock, D.L. 1994. Passively transferred African swine fever virus antibodies protect swine against lethal infection. Virology 198, 350–354. Orosco, F.L. 2023a. Current progress in diagnostics, therapeutics, and vaccines for African swine fever virus. Vet. Integr. Sci. 21, 751–781. Orosco, F. 2023b. Advancing the frontiers: revolutionary control and prevention paradigms against Nipah virus. Open Vet. J. 13, 1056–1056. Orosco, F.L. 2023c. Breaking the chains: advancements in antiviral strategies to combat Nipah virus infections. Int. J. One Health, 122–133. Orosco, F.L. 2024a. Immune evasion mechanisms of porcine epidemic diarrhea virus: a comprehensive review. Vet. Integr. Sci. 22, 171–192. Orosco, F.L. 2024b. Recent advances in peptide-based nanovaccines for re-emerging and emerging infectious diseases. J. Adv. Biotechnol. Exp. Ther. 7(1), 106–117. Orosco, F.L., and Espiritu, L.M. 2024c. Navigating the landscape of adjuvants for subunit vaccines: recent advances and future perspectives. Int. J. Appl. Pharm. 16(1). Oura, C.A.L., Denyer, M., Takamatsu, H. and Parkhouse, R.M.E. 2005. In vivo depletion of CD8+ T lymphocytes abrogates protective immunity to African swine fever virus. J. Gen. Virol. 86, 2445–2450. Overgaard, N.H., Jung, J.-W., Steptoe, R.J. and Wells, J.W. 2015. CD4+/CD8+ double-positive T cells: more than just a developmental stage? J. Leukoc. Biol. 97, 31–38. Pabst, R. 2020. The pig as a model for immunology research. Cell Tissue Res. 380, 287–304. Park, D.D., Chen, J., Kudelka, M.R., Jia, N., Haller, C.A., Kosaraju, R., Premji, A.M., Galizzi, M., Nairn, A.V., Moremen, K.W., Cummings, R.D. and Chaikof, E.L. 2021. Resident and elicited murine macrophages differ in expression of their glycomes and glycan-binding proteins. Cell Chem. Biol. 28, 567–582.e4. Patil, S.S., Suresh, K.P., Vashist, V., Prajapati, A., Pattnaik, B. and Roy, P. 2020. African swine fever: a permanent threat to Indian pigs. Vet. World 13, 2275–2285. Payne, L.G. 1986. The existence of an envelope on extracellular cowpox virus and its antigenic relationship to the vaccinia envelope. Arch. Virol. 90, 125–133. Penrith, M.-L. 2013. History of “swine fever” in Southern Africa. J. S. Afr. Vet. Assoc. 84, 6. Penrith, M.L. 2020. Current status of African swine fever. CABI Agric. Biosci. 1, 11. Pérez-Núñez, D., Sunwoo, S.-Y., Sánchez, E.G., Haley, N., García-Belmonte, R., Nogal, M., Morozov, I., Madden, D., Gaudreault, N.N., Mur, L., Shivanna, V., Richt, J.A. and Revilla, Y. 2019. Evaluation of a viral DNA-protein immunization strategy against African swine fever in domestic pigs. Vet. Immunol. Immunopathol. 208, 34–43. Petrovan, V., Rathakrishnan, A., Islam, M., Goatley, L.C., Moffat, K., Sanchez-Cordon, P.J., Reis, A.L. and Dixon, L.K. 2022. Role of African swine fever virus proteins EP153R and EP402R in reducing viral persistence in blood and virulence in pigs infected with BeninΔDP148R. J. Virol. 96, e0134021. Pikalo, J., Zani, L., Hühr, J., Beer, M. and Blome, S. 2019. Pathogenesis of African swine fever in domestic pigs and European wild boar—lessons learned from recent animal trials. Virus Res. 271, 197614. Popescu, L., Gaudreault, N.N., Whitworth, K.M., Murgia, M.V., Nietfeld, J.C., Mileham, A., Samuel, M., Wells, K.D., Prather, R.S. and Rowland, R.R.R. 2017. Genetically edited pigs lacking CD163 show no resistance following infection with the African swine fever virus isolate, Georgia 2007/1. Virology 501, 102–106. Post, J., Weesendorp, E., Montoya, M. and Loeffen, W.L. 2017. Influence of age and dose of African swine fever virus infections on clinical outcome and blood parameters in pigs. Viral Immunol. 30, 58–69. Prager, I. and Watzl, C. 2019. Mechanisms of natural killer cell-mediated cellular cytotoxicity. J. Leukoc. Biol. 105, 1319–1329. Probst, C., Gethmann, J., Amler, S., Globig, A., Knoll, B. and Conraths, F.J. 2019. The potential role of scavengers in spreading African swine fever among wild boar. Sci. Rep. 9, 11450. Reis, A.L., Goatley, L.C., Jabbar, T., Lopez, E., Rathakrishnan, A. and Dixon, L.K. 2020. Deletion of the gene for the type I interferon inhibitor I329L from the attenuated African swine fever virus OURT88/3 strain reduces protection induced in pigs. Vaccines 8, 262. Reis, A.L., Netherton, C. and Dixon, L.K. 2017. Unraveling the armor of a killer: evasion of host defenses by African swine fever virus. J. Virol. 91, e02338-16. Riera Romo, M., Pérez-Martínez, D. and Castillo Ferrer, C. 2016. Innate immunity in vertebrates: an overview. Immunology 148, 125–139. Rodríguez, J.M., García-Escudero, R., Salas, M.L. and Andrés, G. 2004. African swine fever virus structural protein p54 is essential for the recruitment of envelope precursors to assembly sites. J. Virol. 78, 4299–1313. Rodríguez, J.M., Yáñez, R.J., Almazán, F., Viñuela, E. and Rodriguez, J.F. 1993. African swine fever virus encodes a CD2 homolog responsible for the adhesion of erythrocytes to infected cells. J. Virol. 67, 5312–5320. Roers, A., Hiller, B. and Hornung, V. 2016. Recognition of endogenous nucleic acids by the innate immune system. Immunity 44, 739–754. Ruiz-Gonzalvo, F., Rodríguez, F. and Escribano, J.M. 1996. Functional and immunological properties of the baculovirus-expressed hemagglutinin of African swine fever virus. Virology 218, 285–289. Salguero, F.J. 2020. Comparative pathology and pathogenesis of African swine fever infection in swine. Front. Vet. Sci. 7. Sánchez, E.G., Pérez-Núñez, D. and Revilla, Y. 2017. Mechanisms of entry and endosomal pathway of African swine fever virus. Vaccines 5, 42. Sánchez, E.G., Quintas, A., Pérez-Núñez, D., Nogal, M., Barroso, S., Carrascosa, Á.L. and Revilla, Y. 2012. African swine fever virus uses macropinocytosis to enter host cells. PLOS Pathog. 8, e1002754. Sánchez-Cordón, P.J., Floyd, T., Hicks, D., Crooke, H.R., McCleary, S., McCarthy, R.R., Strong, R., Dixon, L.K., Neimanis, A., Wikström-Lassa, E., Gavier-Widén, D. and Núñez, A. 2021. Evaluation of lesions and viral antigen distribution in domestic pigs inoculated intranasally with African swine fever virus Ken05/Tk1 (Genotype X). Pathogens 10, 768. Sánchez-Cordón, P.J., Jabbar, T., Chapman, D., Dixon, L.K. and Montoya, M. 2020. Absence of long-term protection in domestic pigs immunized with attenuated African swine fever virus isolate OURT88/3 or BeninΔMGF correlates with increased levels of regulatory T cells and interleukin-10. J. Virol. 94, e00350-20. Sánchez-Cordón, P.J., Montoya, M., Reis, A.L. and Dixon, L.K. 2018. African swine fever: a re-emerging viral disease threatening the global pig industry. Vet. J. Lond. Engl. 233, 41–48. Sánchez-Torres, C., Gómez-Puertas, P., Gómez-del-Moral, M., Alonso, F., Escribano, J.M., Ezquerra, A. and Domínguez, J. 2003. Expression of porcine CD163 on monocytes/macrophages correlates with permissiveness to African swine fever infection. Arch. Virol. 148, 2307–2323. Sánchez-Vizcaíno, J.M., Mur, L. and Martínez-López, B. 2012. African swine fever: an epidemiological update. Transbound. Emerg. Dis. 59, 27–35. Sauter-Louis, C., Conraths, F.J., Probst, C., Blohm, U., Schulz, K., Sehl, J., Fischer, M., Forth, J.H., Zani, L., Depner, K., Mettenleiter, T.C., Beer, M. and Blome, S. 2021. African swine fever in wild boar in Europe—a review. Viruses 13, 1717. Schäfer, A., Franzoni, G., Netherton, C.L., Hartmann, L., Blome, S. and Blohm, U. 2022. Adaptive cellular immunity against African swine fever virus infections. Pathogens 11. Schäfer, A., Hühr, J., Schwaiger, T., Dorhoi, A., Mettenleiter, T.C., Blome, S., Schröder, C. and Blohm, U. 2019. Porcine invariant natural killer T cells: functional profiling and dynamics in steady state and viral infections. Front. Immunol. 10, 1380. Schäfer, A., Zani, L., Pikalo, J., Hühr, J., Sehl, J., Mettenleiter, T.C., Breithaupt, A., Blome, S. and Blohm, U. 2021. T-cell responses in domestic pigs and wild boar upon infection with the moderately virulent African swine fever virus strain “Estonia2014.” Transbound. Emerg. Dis. 68, 2733–2749. Schwartz, D.M., Bonelli, M., Gadina, M. and O’Shea, J.J. 2016. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat. Rev. Rheumatol. 12, 25–36. Sedlak, C., Patzl, M., Saalmüller, A. and Gerner, W. 2014. CD2 and CD8α define porcine γδ T cells with distinct cytokine production profiles. Dev. Comp. Immunol. 45, 97–106. Sehl, J., Pikalo, J., Schäfer, A., Franzke, K., Pannhorst, K., Elnagar, A., Blohm, U., Blome, S. and Breithaupt, A. 2020. Comparative pathology of domestic pigs and wild boar infected with the moderately virulent African swine fever virus strain “Estonia 2014.” Pathogens 9, 662. Shi, J., Liu, W., Zhang, M., Sun, J. and Xu, X. 2021. The A179L gene of African swine fever virus suppresses virus-induced apoptosis but enhances necroptosis. Viruses 13, 2490. Silva-Campa, E., Mata-Haro, V., Mateu, E. and Hernández, J. 2012. Porcine reproductive and respiratory syndrome virus induces CD4+CD8+CD25+Foxp3+ regulatory T cells (Tregs). Virology 430, 73–80. Su, H.-P., Singh, K., Gittis, A.G. and Garboczi, D.N. 2010. The structure of the poxvirus A33 protein reveals a dimer of unique C-type lectin-like domains. J. Virol. 84, 2502–2510. Sun, W., Zhang, H., Fan, W., He, L., Chen, T., Zhou, X., Qi, Y., Sun, L., Hu, R., Luo, T., Liu, W. and Li, J. 2021. Evaluation of cellular immunity with ASFV infection by swine leukocyte antigen (SLA)—peptide tetramers. Viruses 13, 2264. Sunwoo, S.-Y., Pérez-Núñez, D., Morozov, I., Sánchez, E.G., Gaudreault, N.N., Trujillo, J.D., Mur, L., Nogal, M., Madden, D., Urbaniak, K., Kim, I.J., Ma, W., Revilla, Y. and Richt, J.A. 2019. DNA-protein vaccination strategy does not protect from challenge with African swine fever virus Armenia 2007 strain. Vaccines 7, 12. Sutton, V.R., Vaux, D.L. and Trapani, J.A. 1997. Bcl-2 prevents apoptosis induced by perforin and granzyme B, but not that mediated by whole cytotoxic lymphocytes. J. Immunol. 158, 5783–5790. Takamatsu, H.-H., Denyer, M.S., Lacasta, A., Stirling, C.M.A., Argilaguet, J.M., Netherton, C.L., Oura, C.A.L., Martins, C. and Rodríguez, F. 2013. Cellular immunity in ASFV responses. Virus Res. 173, 110–121. Takamatsu, H.-H., Denyer, M.S., Stirling, C., Cox, S., Aggarwal, N., Dash, P., Wileman, T.E. and Barnett, P.V. 2006. Porcine gammadelta T cells: Possible roles on the innate and adaptive immune responses following virus infection. Vet. Immunol. Immunopathol. 112, 49–61. Takamatsu, H.-H., Denyer, M.S. and Wileman, T.E. 2002. A sub-population of circulating porcine γδ T cells can act as professional antigen presenting cells. Vet. Immunol. Immunopathol. 87, 223–224. Thierry, A., Robin, A., Giraud, S., Minouflet, S., Barra, A., Bridoux, F., Hauet, T., Touchard, G., Herbelin, A. and Gombert, J.-M. 2012. Identification of invariant natural killer T cells in porcine peripheral blood. Vet. Immunol. Immunopathol. 149, 272–279. Turlewicz-Podbielska, H., Kuriga, A., Niemyjski, R., Tarasiuk, G. and Pomorska-Mól, M. 2021. African swine fever virus as a difficult opponent in the fight for a vaccine—current data. Viruses 13, 1212. Wang, J., Jin, S.X., Sun, H. and Chen, H. 2020. Insights into African swine fever virus immunoevasion strategies. J. Integr. Agric. 19, 11–22. Wang, N., Zhao, D., Wang, J., Zhang, Y., Wang, M., Gao, Y., Li, F., Wang, J., Bu, Z., Rao, Z. and Wang, X. 2019. Architecture of African swine fever virus and implications for viral assembly. Science 366, 640–644. Watanabe, Y., Bowden, T.A., Wilson, I.A. and Crispin, M. 2019. Exploitation of glycosylation in enveloped virus pathobiology. Biochim. Biophys. Acta BBA—Gen. Subj. 1863, 1480–1497. Welz, M., Eickhoff, S., Abdullah, Z., Trebicka, J., Gartlan, K.H., Spicer, J.A., Demetris, A.J., Akhlaghi, H., Anton, M., Manske, K., Zehn, D., Nieswandt, B., Kurts, C., Trapani, J.A., Knolle, P., Wohlleber, D. and Kastenmüller, W. 2018. Perforin inhibition protects from lethal endothelial damage during fulminant viral hepatitis. Nat. Commun. 9, 4805. Xiao, X., Li, K., Ma, X., Liu, B., He, X., Yang, S., Wang, W., Jiang, B. and Cai, J. 2019. Mucosal-associated invariant T cells expressing the TRAV1-TRAJ33 chain are present in pigs. Front. Immunol. 10, 2070. Żabińska, M., Krajewska, M., Kościelska-Kasprzak, K., Jakuszko, K., Bartoszek, D., Myszka, M. and Klinger, M. 2016. CD4+CD25+CD127− and CD4+CD25+Foxp3+ regulatory T cell subsets in mediating autoimmune reactivity in systemic lupus erythematosus patients. Arch. Immunol. Ther. Exp. (Warsz.) 64, 399–407. Zakaryan, H., Cholakyans, V., Simonyan, L., Misakyan, A., Karalova, E., Chavushyan, A. and Karalyan, Z. 2015. A study of lymphoid organs and serum proinflammatory cytokines in pigs infected with African swine fever virus genotype II. Arch. Virol. 160, 1407–1414. Zani, L., Forth, J.H., Forth, L., Nurmoja, I., Leidenberger, S., Henke, J., Carlson, J., Breidenstein, C., Viltrop, A., Höper, D., Sauter-Louis, C., Beer, M. and Blome, S. 2018. Deletion at the 5′-end of Estonian ASFV strains associated with an attenuated phenotype. Sci. Rep. 8, 6510. Zhang, F., Moon, A., Childs, K., Goodbourn, S. and Dixon, L. K. 2010. The African swine fever virus DP71L protein recruits the protein phosphatase 1 catalytic subunit to dephosphorylate eIF2alpha and inhibits CHOP induction but is dispensable for these activities during virus infection. J. Virol. 84, 10681–10689. Zhu, J.J., Ramanathan, P., Bishop, E.A., O’Donnell, V., Gladue, D.P. and Borca, M.V. 2019. Mechanisms of African swine fever virus pathogenesis and immune evasion inferred from gene expression changes in infected swine macrophages. PLOS ONE 14, e0223955. | ||
| How to Cite this Article |
| Pubmed Style Fredmoore Legaspi Orosco. Host immune responses against African swine fever virus: Insights and challenges for vaccine development. Open Vet J. 2023; 13(12): 1517-1535. doi:10.5455/OVJ.2023.v13.i12.2 Web Style Fredmoore Legaspi Orosco. Host immune responses against African swine fever virus: Insights and challenges for vaccine development. https://www.openveterinaryjournal.com/?mno=168751 [Access: June 30, 2025]. doi:10.5455/OVJ.2023.v13.i12.2 AMA (American Medical Association) Style Fredmoore Legaspi Orosco. Host immune responses against African swine fever virus: Insights and challenges for vaccine development. Open Vet J. 2023; 13(12): 1517-1535. doi:10.5455/OVJ.2023.v13.i12.2 Vancouver/ICMJE Style Fredmoore Legaspi Orosco. Host immune responses against African swine fever virus: Insights and challenges for vaccine development. Open Vet J. (2023), [cited June 30, 2025]; 13(12): 1517-1535. doi:10.5455/OVJ.2023.v13.i12.2 Harvard Style Fredmoore Legaspi Orosco (2023) Host immune responses against African swine fever virus: Insights and challenges for vaccine development. Open Vet J, 13 (12), 1517-1535. doi:10.5455/OVJ.2023.v13.i12.2 Turabian Style Fredmoore Legaspi Orosco. 2023. Host immune responses against African swine fever virus: Insights and challenges for vaccine development. Open Veterinary Journal, 13 (12), 1517-1535. doi:10.5455/OVJ.2023.v13.i12.2 Chicago Style Fredmoore Legaspi Orosco. "Host immune responses against African swine fever virus: Insights and challenges for vaccine development." Open Veterinary Journal 13 (2023), 1517-1535. doi:10.5455/OVJ.2023.v13.i12.2 MLA (The Modern Language Association) Style Fredmoore Legaspi Orosco. "Host immune responses against African swine fever virus: Insights and challenges for vaccine development." Open Veterinary Journal 13.12 (2023), 1517-1535. Print. doi:10.5455/OVJ.2023.v13.i12.2 APA (American Psychological Association) Style Fredmoore Legaspi Orosco (2023) Host immune responses against African swine fever virus: Insights and challenges for vaccine development. Open Veterinary Journal, 13 (12), 1517-1535. doi:10.5455/OVJ.2023.v13.i12.2 |