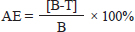| Research Article | ||
Open Vet J. 2023; 13(12): 1645-1653 Open Veterinary Journal, (2023), Vol. 13(12): 1645–1653 Original Research In vitro and in vivo action of turmeric oil (Curcuma longa L.) against Argulus spp. in goldfish (Carassius auratus)Banthita Saengsitthisak1, Wasana Chaisri2,3, Raktham Mektrirat4, Terdsak Yano2 and Surachai Pikulkaew2,3*1Faculty of Pharmacy, Payap University, Chiang Mai, Thailand 2Department of Food Animal Clinic, Faculty of Veterinary Medicine, Chiang Mai University, Chiang Mai, Thailand 3Research Center of Producing and Development of Products and Innovations for Animal Health and Production, Chiang Mai University, Chiang Mai, Thailand 4Department of Veterinary Biosciences and Public Health, Faculty of Veterinary Medicine, Chiang Mai University, Chiang Mai, Thailand *Corresponding Author: Surachai Pikulkaew. Department of Food Animal Clinic, Faculty of Veterinary Medicine, Chiang Mai University, Chiang Mai, Thailand. Email: surachai.pikul [at] cmu.ac.th; surapikulkaew [at] gmail.com Submitted: 20/09/2023 Accepted: 27/11/2023 Published: 31/12/2023 © 2023 Open Veterinary Journal
AbstractBackground: Argulus is a common and widespread ectoparasite that causes major parasitic diseases and is a virus and bacteria carrier in the ornamental fish trade. Aim: The purpose of this study is to determine what types of phytochemicals are present in the essential oil extracted from turmeric rhizome (Curcuma longa L.) and to assess the efficacy of turmeric oil in eliminating Argulus spp. infestations in goldfish (Carassius auratus). Methods: The chemical composition and quantity of the major substances in essential oils from fresh turmeric rhizome were detected by gas chromatography/mass spectrometry (GC-MS). The antiparasitic effect of turmeric oils on Argulus spp. was tested at 12.5, 25, 50, 100, and 200 ppm and compared to 0.25 ppm Neguvon® (the positive control). The percentage of Argulus spp. killed, the percentage of the mean mortality rate, and the effectiveness of each test were evaluated. Results: By using GC-MS analysis, it was possible to identify the primary phytochemical component of turmeric essential oil as b-turmerone. The results obtained from the in vitro test indicated that there was a correlation between the concentration of turmeric essential oil and the average mortality rate of fish lice. The mean mortality of fish louse exposed to 200 ppm turmeric essential oil was higher than the mean mortality of fish louse exposed to Neguvon® (p < 0.05). In an in vivo study, the effectiveness of 12.5 ppm turmeric essential oil against parasites was 44.44%, 55.46%, and 62.83% at 24, 48, and 72 hours, respectively. Conclusion: In summary, the efficacy of turmeric essential oil against fish louse has been shown both in vitro and in vivo studies. Keywords: Turmeric, Curcuma longa L., antiparasitic, Argulus spp. goldfish, Carassius auratus. IntroductionThe aquaculture industry encompasses significant global economic value and can generate substantial revenues for fish growers. Nevertheless, aquaculture operators will encounter a significant challenge in the form of diminished fish production and substantial economic losses resulting from a heightened mortality rate associated with parasites and disease outbreaks in fish farming (Tavares-Dias and Martin, 2017; Ananda Raja et al., 2020, 2022, 2023). Ectoparasites are the predominant parasites affecting fish, as they are known to infest nearly the whole culture system. Argulus spp., also referred to as fish lice, are ectoparasitic crustaceans with a broad distribution in aquatic environments. These organisms have the ability to parasitize and develop on a variety of host species (Steckler and Yanong, 2012; Aalberg et al., 2016; Alom et al., 2019; Ananda Raja et al., 2020, 2022). The Argulus spp. species engage in feeding and predatory behavior by damaging the epidermis of their host, introducing a toxic substance, and sucking blood, resulting in the development of argulosis disease (Stec kler and Yanong, 2012; Wafer et al., 2015; Aalberg et al., 2016; Alom et al., 2019; Ananda Raja et al., 2020, 2022). The presence of fish lice has emerged as a significant threat to the health of hosts, resulting in various adverse effects such as reduced growth, underweight conditions, hindered mating capabilities, heightened susceptibility to secondary bacterial or fungal infections, and severe skin damage in cases of heavy infestation. The resulting stress experienced by fish can lead to substantial morbidity and mortality rates among the affected animals (Steckler and Yanong, 2012; Wafer et al., 2015; Aalberg et al., 2016; Alom et al., 2019). Various methods have been employed for the control and treatment of Argulus spp. (Anan da Raja et al., 2020, 2022; Thakur et al., 2022). Malachite-green/formalin mixture, trichlorfon, emamectin benzoate, and organophosphate are the main chemicals used to reduce the number of parasites and/or treat diseases in fish; however, these chemicals could lead to negative situations for animals and their surroundings, which prompted drug resistance and negatively impacted the environment (Hakalahti-Sirén et al., 2008; Das et al., 2018; Ananda Raja et al., 2020, 2022; Thakur et al., 2022; Ananda Raja et al., 2023). Therefore, alternative antiparasitic treatments derived from medicinal plants, such as plant extracts and essential oils, may be preferable to conventional chemical treatments due to their efficacy, biodegradability, environmental friendliness, and cost-effectiveness (Kumar et al., 2012). In recent years, a greater emphasis has been placed on the use of medicinal plants in fish aquaculture for the prevention and treatment of a variety of health disorders (Kumar et al., 2012; Thakur et al., 2022). Curcuma longa L. (C. longa), also referred to as turmeric, is a botanical species belonging to the Zingiberaceae family (Kim e t al., 2013; Mitsuwan et al., 2020). The rhizome of turmeric is known to contain a variety of phytochemical components, making it a frequently utilized component for medicinal applications (Kim et al., 2013; Mitsuwan et al., 2020; Kumar et al., 2022). Extensive in vitro and in vivo tests have been performed with essential oils and extracts, which have been found to exhibit a wide range of pharmacological effects. These effects include antibacterial properties (Méndez et al., 2016; Singh et al., 2017), antioxidant activity (Hefnawy et al., 2016), antimalarial activity (Martinez-Correa et al., 2017), antiviral activity (Sornpet et al., 2017), as well as anti-inflammatory and anti-tumor effects (Araújo and Leon, 2001). In addition, the insecticidal and repellent properties of turmeric rhizome extract were well-known and utilized against insect invaders (Pitasawat et al., 2003). The purp ose of this study was to evaluate the phytochemical compounds in the essential oil from turmeric rhizome using gas chromatography/mass spectrometry (GC-MS) and to determine the antiparasitic activity of turmeric oil against Argulus spp. parasites that infested goldfish (Carassius auratus). Materials and MethodsEssential oils used in the experiment were obtained from C. longa L. cultivated in Chiang Mai province, Thailand. The fresh rhizomes of turmeric were cut and chopped into small pieces. Then, turmeric essential oil extraction was carried out by the hydro-distillation method in Clevenger’s apparatus (Hashimot o et al., 2016; Wesołowska et al., 2019; Jaiswal and Naik, 2021). The extracted essential oils were kept in a light-resistant, tightly sealed container and maintained at 4°C before use. The chemical compositions in the extracted turmeric essential oil were analyzed by GC-MS using an HP 6890 gas chromatograph coupled with an HP 5973 Mass Selective Detector (Agilent Technologies, Foster City, CA). Samples of 1 µl were injected in the electron impact mode. The chemical compounds in the sample were separated on a 30 m long capillary column (HP-5MS), 0.25 mm in diameter, with a 0.25 µm thick stationary phase film [(5% phenyl)-methylpolysiloxane]. The flow rate of helium through the column was kept at 1.0 ml·min−1. The total running time for a sample was about 50 minutes. The relative percentage of the essential oil constituents was evaluated from the total peak area of total ion chromatogram (TIC) by apparatus software (Hashimoto et al., 2016; Wesołowska et al., 2019; Jaiswal and Naik, 2021). For the preparation of stock solution and working test solution, the extracted essential oils were diluted in the organic solvent dimethyl sulfoxide (DMSO) for the preparation of stock solutions following Hashimoto et al. (2016). The stock solution had a concentration of 33,000 mg l−1 and was composed of 1 g of turmeric essential oils prepared in 29 ml of DMSO in a proportion of 1:29. The prepared essential oil stock solution was diluted in DMSO to obtain 12.5, 25, 50, 100, and 200 mg l−1, which were the different working test solutions in the experiments. The control solutions were water (negative control), Neguvon® (positive control), and 2% DMSO (vehicle control). Fish used in this experiment were goldfish (C. auratus) with an average weight of 12.56 ± 2.40 g, which were obtained from ornamental fish shops located in Chiang Mai, Thailand, for in vitro and in vivo studies. These fish were stocked and acclimatized under optimum physicochemical conditions for a period of 14 days, and commercial goldfish pellets were fed daily at 2% of body weight. For experimental design, Argulus-infested goldfish were picked up from ornamental fish shops located in Chiang Mai, Thailand. The fish management followed the standard operating procedure of the Aquatic Animal Medicine Laboratory of the Veterinary Faculty at Chiang Mai University. The eggs of Argulus were collected when they were inspected. The Argulus eggs were kept in an incubator at 28°C (Sahoo et al., 2013). Ten days after hatching, the Argulus larvae were chosen and used to create an artificial Argulus infection in healthy goldfish. This was done to test how well turmeric essential oil kills Argulus spp. parasites (Kumar et al., 2012; Mitsuwan et al., 2020). For in vitro test, the lice (Argulus spp.) were mildly picked up from the infested fish with a plastic tip, and actively moving parasites were collected into a petri dish with the help of a small hairbrush. During this period, fish were anesthetized with 50 mg l−1 of MS-222, and their behaviors and responses were also closely observed (Sneddon, 2012). The actively moving parasites were measured by vernier calipers and included the same size organism for further testing. The chosen lice were split into 8 groups of 10 live lice each and put in a Petri dish with 20 ml of different concentrations of working solution in triplicate. The working solution concentrations were 12.5, 25, 50, 100, and 200 mg l−1, which were higher than the concentrations in the control solution groups. The number of organisms killed at 30, 60, 90, 120, and every 1 hour until 24 hours determined the antiparasitic action of various turmeric essential oil concentrations. Parasitic death was considered when the organism did not move after 5 minutes of observation and with a gentle touch with a dressing forceps on some parasites with an incomplete physical appearance (Fig. 1). The monitoring was processed under a stereoscopic microscope (Kumar et al., 2012; Mitsuwan et al., 2020). For in vivo test, the toxicity concentration of turmeric essential oil was analyzed to determine the safety of the experimental solution for the animal. To determine the safety dose of the working solution for in vivo testing, find tests with 12.5, 25, 50, 100, and 200 mg l−1. The healthy goldfish was subjected to bath treatment with a diluted turmeric essential oil solution at each concentration as described above. During this period, the behaviors and responses of the animals were closely monitored and recorded. The stressed behaviors and responses of fish (such as dyspnea, anxiety, and slow breathing) were observed, and the immediate discontinuation of the experiment was performed. A safe dose of the working solution was selected for further study. The moderately infested parasite goldfish were sampled for bath treatment with a working solution and control solution, which were divided into four groups (nine infested fish per group). One Argulus-infested f ish was put into the tank, which contained 1,000 ml of solution as given below: Group I Argulus-infested fish exposed to purified water; Group II Argulus-infested fish treated with 0.25 mg l−1 of Neguvon®; Group III Argulus-infested fish exposed to 2% DMSO; Group IV Argulus-infested fish treated with 12.5 mg l−1 of turmeric essential oil. Parasite mortality was observed and recorded during the study. All experimental animals were observed. The effectiveness of each treatment was determined by comparing the average number of surviving parasites in each treatment group to the control group after a 72-hour period. The antiparasitic efficacy of all groups was calculated using the calculation method described by Wang et al. (2009).
AE=antiparasitic efficacy; B=the mean number of surviving Argulus in negative control; and T=Mean number of surviving Argulus in the treatment. The linear mixed model was performed to analyze the variance of the mortality rate of parasites and antiparasitic efficacy for in vitro and in vivo tests, respectively. Either in vitro and in vivo, the treatment and time were assigned as the fixed effects in the model. Tukey’s post hoc test was selected to examine differences among treatments and time. A statistical significance level of 0.05 was considered. Analysis of the data was done using the lme4 package (Bates et al., 2015) in the R statistical program R Core Team (2020).
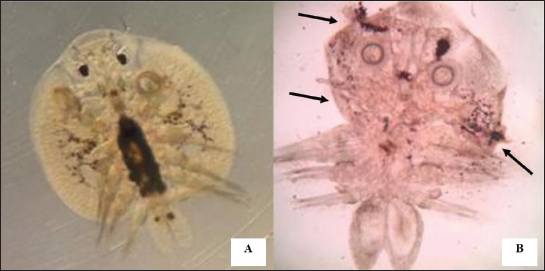
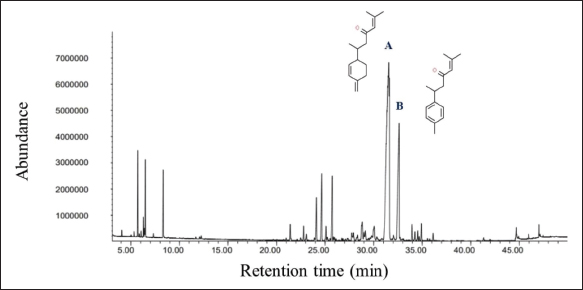
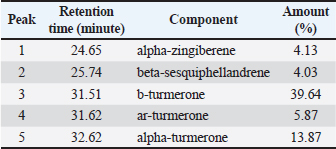
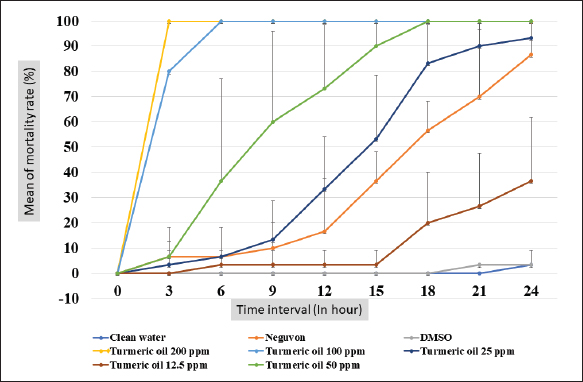
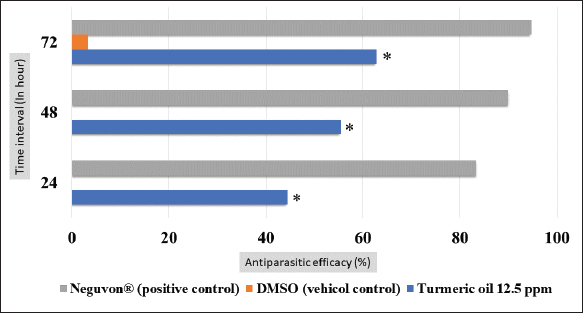
Fig. 1. Feature of a fish parasite in goldfish under stereoscopic microscopy. (A) Lived organism with mobility. (B) No movement of dead organisms with incomplete physical appearance (arrows). Ethical approvalThe Animal Care and Committee of the Faculty of Veterinary Medicine, Chiang Mai University (FVM-ACUC) (Process number: R17/2557), approved the investigation for use in animal studies. ResultsPlant material and chemical analysis of the essential oilThe extraction yield was 0.7% of the weight of the raw turmeric rhizome. Observed against a white background, turmeric essential oil possessed a fluid consistency and a pale yellow color. In addition, the extracted oil was liquid at ambient temperature. The GC-MS study found that the extracted essential oil of turmeric had a wide range of chemical components. A comprehensive number of components were identified in the extracted oil. The GC-MS chromatogram presented in Figure 2 exhibits a substantial number of peaks, each characterized by distinct chemical constituents. The extracted essential oil contained b-turmerone (39.64%) and alpha-turmerone (13.87%) as the primary chemical components, as indicated in Table 1. In addition, the compounds ar-turmerone, alpha-zingiberene, and beta-sesquiphellandrene were identified, with respective concentrations of 5.87%, 4.13%, and 4.03%. In vitro testThe results of effectiveness testing of different concentrations of turmeric oil, which were 12.5, 25, 50, 100, and 200 ppm, and control vehicles against the Argulus spp. parasite are presented in Figure 3. In the in vitro test, 200 pp m of turmeric oil performed the highest antiparasitic activity of up to 100% and killed Argulus spp. in 180 minutes. Furthermore, 50 and 100 ppm of essential oil revealed an antiparasitic efficacy of up to 100% and killed in 1,080 and 360 minutes, respectively. However, during observation time, the mean mortality rate (%) in the 12.5 and 25 ppm turmeric oil groups was 36.66 ± 25.16 and 93.33 ± 5.77, respectively. In addition, the antiparasitic efficacy of Neguvon® (the positive control) group showed 86.66 ± 23.09 in 1,440 minutes. Finally, after 24 hours, the results of the clean water group and the DMSO group were 3.33 ± 5.57. In vivo testThe toxicity of turmeric essential oil was determined at 12.5, 25, 50, 100, and 200 ppm. We found the safe dose of the working solution to be 12.5 ppm. At this concentration, there was a significantly higher antiparasitic effect than in the negative control and the vehicle control (p < 0.05). Thus, this concentration was selected to apply in the next experiment. In vivo antiparasitic efficac y testing demonstrated on infected fish that soaking with 12.5 ppm of turmeric essential oil, dimethyl sulfoxide, and Neguvon® resulted in the results shown in Figure 4. During the observation period, the Neguvon® group achieved the highest percentage decrease in Argulus spp. infection in treatment, 94.69 + 0.003. However, 12.5 ppm of essential oil presented 62.83 ± 0.004 of antiparasitic efficacy (%). DiscussionCurcuma longa L., commonly known as turmeric, was native to tropical South Asia but is now generally harvested in tropical regions of the world. It was botanically related to the Zingiberaceae family (Kim et al., 2013; Setyaningsih et al., 2019; Mitsuwan et al., 2020; Kumar et al., 2022). The role of essential oil from the rhizome of turmeric performed various pharmacological properties in terms of antibacterial, antiviral, and antiparasitic activities for treating disease in humans and animals (Méndez et al., 2016; Martinez-Correa et al., 2017; Singh et al., 2017; Sornpet et al., 2017). The percentage yield in essential oil extraction in this study was 0.7% of the weight of fresh turmeric rhizome. Similarly, some studies in India have reported that C. longa L. essential oil in fresh rhizomes yields between 0.37% and 0.8% in different agroclimatic zones (Sandeep et al., 2016), and between 0.61% and 1.45% in the northern part of the country (Garg et al., 1999). Furthermore, Pino et al. (2018), who studied in Amazonian Ecuador, revealed the percentage yield of essential oil in fresh rhizomes was 0.8%. On the other hand, the percentage yield of essential oil that was extracted from dry turmeric rhizome in the USA varied from 1.5% to 5.0% (Li et al., 2011). Moreover, the researchers in Brazil reported a percentage yield of essential oil from dry rhizomes between 3.0% and 5.16% (Guimarães et al., 2020). To date, the chemical compositions in turmeric oil have been investigated and generally contain primarily phenolic compounds and terpenoids, including diarylheptanoids (including those commonly known as curcuminoids), monoterpenes, sesquiterpenes, diterpenes, triterpenoids, alkaloids, and sterols. This present research detected turmerone (59.38%) as the major compound in the extracted oil from fresh rhizomes. Our results were in agreement with previous studies by Pino et al. (2018), who reported that turmerone (58.9%) was the most represented class of volatiles from rhizomes grown in Amazonian Ecuador, including ar-turmerone (45.5%) and alpha-turmerone (13.4%). Similar results were performed by Jaiswal and Naik (2021), Guimarães et al. (2020), Intirach et al. (2012), and Devkota and Rajbhandari (2016), whose data indicated that 58%, 50.05%, 49.21%, and 25.93% of turmerone were revealed as the major constituents, respectively. Nevertheless, the previous research presented the other compounds as the major components in the essential oil, including from fresh rhizomes in Brazil (11% zingiberene) (Gonçalves et al., 2019), from plants cultivated in Korea (27.70%–36.75% alpha-zingiberene) (Hwang et al., 2016), from raw materials grown in Sri Lanka (18.2% alpha-phellandrene) (Herath et al., 2017), and from herbs in Nigeria (13.9% beta-bisabolene) (Usman et al., 2009). In numerous previous studies presented, several factors could interfere with the percentage yield of essential oils and the chemical variation of essential oils in plants, such as temperature, humidity, altitude, ultraviolet radiation, soil and nutrient conditions, seasonality, plant age, methods of collection, drying, and part of the plant (Bowes and Zheljazkov, 2004; Yavari et al., 2010; Tavares et al., 2013; Guimarães et al., 2020).
Fig. 2. GC-MS chromatograph for essential oil isolated from Curcuma longa L. Chemical structures of the two major constituents identified from the turmeric essential oil, including (A) b-turmerone and (B) ar-turmerone. Table 1. Chemical composition of essential oil from Curcuma longa L. obtained by GC-MS analysis. The turmeric oil was extracted from the rhizomes of Curcuma longa L. by simultaneous hydro-distillation and analyzed by GC-MS. RT: Retention time.
Fig. 3. In vitro mortality of Argulus spp. treated with different concentrations of turmeric oil compared to controls (negative, positive, and vehicle control).
Fig. 4. In vivo mortality of Argulus spp. treated with 12.5 ppm of turmeric oil compared to positive and negative controls (* indicate significance at p < 0.05). This research also determined the antiparasitic efficacy of the extracted oil against fish lice in goldfish. We discovered turmeric oil had the ability to fight organisms. In vitro studies found a relationship between the concentration of turmeric oil and the mortality rate of Argulus spp. The increasing concentration has shown a rising mortality rate for parasites. At 200 mg l−1, essential oil performed the h ighest anti-parasitic activity. However, an in vivo test revealed that 12.5 mg l−1 of the testing solution was a suitable dose to treat infested animals without toxic effects. These results were in agreement with the study of the insecticidal and repellent efficacy of extracted oil from turmeric rhizome used against insect pests, as well as Tavares et al. (2013), who detected high percentages of ar-turmerone in nonpolar extracts and essential oils that presented anti-repellent activity to Sitophilus zeamais and Spodoptera frugiperda (Tavares et al., 2013), 50% and 100% of S. zeamais were killed by 0.1% of the active substance in 15 days and by 1% of ar-turmerone in 7 days, respectively. Moreover, in the case of S. frugiperda, they reported that 1% of the active compound eliminated 58.3% of S. frugiperda and reduced the development of this insect (Tavares et al., 2013). However, in the present study, to ascertain the acute toxicity in the fish model, which was evaluated by only monitoring the behaviors and responses of the animals, further experimentation and analysis of the biochemical and histopathological data should be done to understand the mechanism of the acute toxicity of turmeric oil (Ramesha et al., 2016; Bhartia and Rasool, 2021). During our investigation, we observed that fish infested with Argulus and exposed to a 2% DMSO solution, which served as the vehicle control for the in vivo test, did not exhibit parasite mortality within the first 72 hours. However, we did observe a mortality rate among the fish parasites after the 72-hour point. This finding was caused by the fact that Argulus spp. could not complete its lifecycle in the absence of a host (Steckler and Yanong, 2012; Thakur et al., 2022); the use of 2% DMSO had no effect on this result. Furthermore, when Intirach et al. (2012) investigated the larvicidal effects of turmeric oil against Anopheles cracens, they noticed that 100 mg l−1 of the extracted oil demonstrated promising efficacy with 100% larval mortality (Intirach et al., 2012). In addition, the results of the investigation indicated that the duration to eliminate fish lice in the in vitro test was less than in the in vivo test. The difference between in vitro and in vivo tests could be described by the treatment in the in vitro experiment: the Argulus were separated from the host with no protection, so the organisms were directly contacted with a chemical solution. However, under in vivo conditions, the essential oil was obstructed from approaching parasites by the scales and fins of the host, and the covered mucous on the fish body and lice, produced by the immune response of the fish, might also interfere with the contact between Argulus spp. and chemical reagent (Mitsuwan et al., 2020). On the other hand, the mechanism of antiparasitic activity of turmerone oil was not clarified, but it could be explained by Blenau et al. (2012), who reported antiparasitic mechanism action of volatile substances from herbal plants presented through inhibited and stimulated various substances and receptors such as octamine, tyramine, acetylcholine, and Gamma-aminobutyric acid (Blenau et al., 2012). In addition, the toxicity effect of essential oil in our study might be justified by the research of Kostyukovsky et al. (2002). High concentrations of essential oil from medical plants performed insecticidal and repellent activity by inhibiting the acetylcholinesterase enzyme but also could eliminate insects and vertebrate hosts, likely through organophosphates such as Neguvon® (Kostyukovsky et al., 2002). Due to this, it was important to acquire an appropriate dose of turmeric essential oil to fight against fish lice. Argulus spp. infesting fish caused an increase in diseased animals and a rise in the mortality rate of hosts, which was a serious problem and led to economic loss. The efficacy of antiparasitic treatment and acute toxicity using turmeric essential oil to cure diseased fish was confirmed by this present study. The primary active compounds in the extracted oil were also discovered. These might encourage the use of natural substances as an alternative way for the control and treatment of disease in aquaculture that have safety, low adverse effects, are decomposable, and have eco-friendly properties. Drug resistance from the overuse of hazardous chemicals, which is a serious global problem, might be solved. ConclusionAntiparasitic activity in numerous traditional medicinal plants has been investigated. However, the efficacy of turmeric essential oil against Argulus spp. infected fish is infrequently studied. Our research demonstrates that turmeric oil has a high potential for eradicating Argulus spp. Thus, the essential oil extracted from this plant can be used as an alternative treatment to the traditional chemicals that were responsible for a variety of ecological and health issues, such as persistent hazards in the environment, adverse effects on animal and human health, and a particular resistance to medications and chemicals. Further research is required to determine the mechanisms of action underlying the antiparasitic and toxic effects of turmeric oil’s active ingredients. In addition, a pharmaceutical preparation of turmeric oil could be created that is simple to use in fish aquaculture and has a precise dosage. AcknowledgmentsThe authors are thankful to the Faculty of Veterinary Medicine and the Research Center for Producing and Development of Products and Innovations for Animal Health and Production, Chiang Mai University, for the facility and instrument support. Conflict of interestThe authors declare that they have no competing interests. FundingThis research work was partially supported by Chiang Mai University, Thailand. Authors contributionsBS and SP are project administration and conceptualization methodology. BS, WC, RM, and SP performed investigation, and validation, BS, TY, and SP performed data curation, formal analysis, and visualization. BS wrote the original draft preparation. BS and SP are reviewing, editing, and approval of the final draft. All authors have read and agreed to the published version of the manuscript. Data availabilityThe data that supports the findings of this study are available from the corresponding author, upon reasonable request. ReferencesAalberg, K., Koščová, L., Šmiga, Ľ., Košuth, P., Koščo, J., Oros, M., Barčák, D. and Lazar, P.A. 2016. Study of fish lice (Argulus Sp.) infection in freshwater food fish. Folia. Vet. 60(3), 54–59. Alom, M.Z., Yasmin, M.S., Rahman, M.A. and Khan, S. 2019. Status, occurrence, intensity and impact of Argulosis in different brood stock ponds. MOJ. Eco. Environ. Sci. 4(5), 225‒229. Ananda Raja, R., Patil, P.K., Avunje, S., Aravind, R.P., Alavandi, S.V. and Vijayan, K.K. 2020. Biosafety, withdrawal and efficacy of anti-parasitic drug emamectin benzoate in Asian Seabass (Lates calcarifer). Aquaculture 525, 735335. Ananda Raja, R., Patil, P.K., Avunje, S., Kumaran, M., Periyakaruppan, A., Kondusamy, A., De, D., Jithendran, K.P., Alavandi, S.V. and Vijayan, K.K., 2023. Natural infestation of an anchor worm, Lernaea sp. in cage culture of Asian Seabass, Lates calcarifer juveniles and its control using an anti-parasitic drug, emamectin benzoate. J. Parasit. Dis. 47(2), 306–318. Ananda Raja, R., Patil, P.K., Avunje, S., Kumaran, M., Solanki, H.G., Jithendram, K.P., Alavandi, S.V. and Vijayan K.K. 2022. Efficacy of emamectin benzoate in controlling natural infestations of ectoparasites in economically important fish species of India. Aquaculture 551, 737940. Araújo, C.A.C. and Leon, L.L. 2001. Biological activities of Curcuma longa L. Mem. Inst. Oswaldo. Cruz. 96(5), 723–728. Bates, D., Mächler, M., Bolker, B.M. and Walker, S.C. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67(1), 48. Bhartia, S. and Rasool, F. 2021. Analysis of the biochemical and histopathological impact of a mild dose of commercial malathion on Channa punctatus (Bloch) fish. Toxicol. Rep. 8, 443–455 Blenau, W., Rademacher, E. and Baumann, A. 2012. Plant essential oils and formamidines as insecticides/acaricides: what are the molecular targets? Apidologie 43(3), 334–347. Bowes, K.M. and Zheljazkov, V.D. 2004. Factors affecting yields and essential oil quality of Ocimum sanctum L. and Ocimum basilicum L. Cultivars. J. Am. Soc. Hortic. Sci. 129(6), 789–794. Das, P., Mohanty, J., Badhe, M.R., Sahoo, P.K., Sardar, K.K. and Parija, S.C. 2018. Development of a western blot method for detection of fish ectoparasite Argulus siamensis antigens. J. Immunoass. Immunochem. 39(4), 439–450. Devkota, L. and Rajbhandari, M. 2016. Composition of essential oils in turmeric rhizome. Nepal. J. Sci. Technol. 16(1), 87–94. Garg, S.N., Bansal, R.P., Gupta, M.M. and Sushil, K. 1999. Variation in the rhizome essential oil and curcumin contents and oil quality in the land races of turmeric Curcuma longa of North Indian Plains. Flavour. Fragr. J. 14(5), 315–318. Gonçalves, G.M.S., Barros, P.P., Silva, G.H. and Fedes, G.R. 2019. The essential oil of curcuma longa rhizomes as an antimicrobial and its composition by gas chromatography/mass spectrometry. Rev. Ciênc. Méd. 28(1), 1–10. Guimarães, A.F., Andrade Vinhas, A.C., Ferraz Gomes, A., Humberto Souza, L. and Krepsky, P.B. 2020. Essential oil of Curcuma longa L. rhizomes chemical composition, yield variation and stability. Quim. Nova. 43(7), 909–913. Hakalahti-Sirén, T., Mikheev, V.N. and Valtonen, E.T. 2008. Control of freshwater fish louse Argulus coregoni: a step towards an integrated management strategy. Dis. Aquat. Organ. 82(1), 67–77. Hashimoto, G.S.O., Neto, F.M., Ruiz, M.L., Acchile, M., Chagas, E.C., Chaves, F.C.M. and Martins, M.L. 2016. Essential oils of Lippia sidoides and Mentha piperita against monogenean parasites and their influence on the hematology of Nile tilapia. Aquaculture 450, 182–186 Hefnawy, H.T., El-Shourbagy, G.A. and Ramadan, M.F. 2016. Phenolic extracts of carrot, grape leaf and turmeric powder: antioxidant potential and application in biscuits. J. Food. Meas. Charact. 10, 576–583. Herath, H.M.I.C., Wijayasiriwardene, T.D.C.M.K. and Premakumara, G.A.S. 2017. Comparative GC-MS analysis of all Curcuma species grown in Sri Lanka by multivariate test. Ruhuna. J. Sci. 8(2), 103–111. Hwang, K.W., Son, D., Jo, H.W., Kim, C.H., Seong, K.C. and Moon, J.K. 2016. Levels of curcuminoid and essential oil compositions in turmerics (Curcuma longa L.) grown in Korea. Appl. Biol. Chem. 59(2), 209–215. Intirach, J., Junkum, A., Tuetun, B., Choochote, W., Chaithong, U., Jitpakdi, A., Riyong, D., Champakaew, D. and Pitasawat, B. 2012. Chemical constituents and combined larvicidal effects of selected essential oils against Anopheles cracens (Diptera: Culicidae). Psyche. A. J. Entomol. (N.Y.), 591616. Jaiswal, S.G. and Naik, S.N. 2021. Turmeric oil: composition, extraction, potential health benefits and other useful applications. Avicenna. J. Med. Biochem. 9(2), 93–106. Kim, D.K., Lillehoj, H.S., Lee, S.H., Jang, S.I., Lillehoj, E.P. and Bravo, D. 2013. Dietary Curcuma longa enhances resistance against Eimeria maxima and Eimeria tenella infections in chickens. Poult. Sci. 92(10), 2635–2643. Kostyukovsky, M., Rafaeli, A., Gileadi, C., Demchenko, N. and Shaaya, E. 2002. Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants: possible mode of action against insect pests. Pest. Manag. Sci. 58(11), 1101–1106. Kumar, V., Das, B.K., Swain, H.S., Chowdhury, H., Roy, S., Bera, A.K., Das, R., Parida, S.N., Dhar, S., Jana, A.K. and Behera, B.K. 2022. Outbreak of Ichthyophthirius multifiliis associated with Aeromonas hydrophila in Pangasianodon hypophthalmus: the role of turmeric oil in enhancing immunity and inducing resistance against co-infection. Front. Immunol. 13(9), 1–19. Kumar, A., Raman, R.P., Kumar, K., Pandey, P.K., Kumar, V., Mohanty, S. and Kumar, S. 2012. Antiparasitic efficacy of piperine against Argulus Spp. on Carassius auratus (Linn. 1758): in vitro and in vivo study. Parasitol. Res. 111(5), 2071–2076. Li, S., Yuan, W., Deng, G., Wang, P., Yang, P. and Aggarwal, B.B. 2011. Chemical composition and product quality control of turmeric (Curcuma longa L.). Pharm. Crop. 5(1), 28–54. Martinez-Correa, H.A., Paula, J.T., Kayano, A.C.A., Queiroga, C.L., Magalhães, P.M., Costa, F.T.M. and Cabral, F.A. 2017. Composition and antimalarial activity of extracts of Curcuma longa L. obtained by a combination of extraction processes using supercritical CO2, ethanol and water as solvents. J. Supercrit. Fluids. 119(1), 122–129. Méndez, N.A., Angulo, O.A. and Martínez, I.I.C. 2016. Actividad antibacteriana in vitro de Curcuma longa (Zingiberaceae) frente a bacterias nosocomiales en Montería, Colombia. Rev. Biol. Trop. 64(9), 1201–1208. Mitsuwan, W., Sangkanu, S., Romyasamit, C., Kaewjai, C., Jimoh, T.O., Pereira, M.L., Siyadatpanah, A., Kayesth, S., Nawaz, M., Rahmatullah, M., Butler, M.S., Wilairatana, P., Wiart, C. and Nissapatorn, V. 2020. Curcuma longa rhizome extract and curcumin reduce the adhesion of Acanthamoeba triangularis trophozoites and cysts in polystyrene plastic surface and contact lens. Int. J. Parasitol. Drugs. Drug. Resist. 14(7), 218–229. Pino, J.A., Fon-Fay, F.M., Pérez, J.C., Falco, A.S., Hernández, I., Rodeiro, I. and Fernández, M.D. 2018. Chemical composition and biological activities of essential oil from turmeric (Curcuma longa L.) rhizomes grown in Amazonian Ecuador. Rev. CENIC. Cienc. Quím. (En línea). 49(1), 1–8. Pitasawat, B., Choochote, W., Tuetun, B., Tippawangkosol, P., Kanjanapothi, D., Jilpakdi, A. and Riyong, D. 2003. Repellency of aromatic turmeric Curcuma aromatica under laboratory and field conditions. J. Vector. Ecol. 28(2), 234–240. R Core Team. 2020. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available via https://www.R-project.org/ (Accessed 01 July 2023). Ramesha, M., Anitha, S., Poopal, R.K. and Shobana, C. 2016. Evaluation of acute and sub lethal effects of chloroquine (C18H26CIN3) on certain enzymological and histopathological biomarker responses of a freshwater fish Cyprinus carpio. Toxicol. Rep. 5, 18–27. Sahoo, P.K., Mohanty, J., Hemaprasanth, Kar, B., Mohanty, B.R., Garnayak, S.K. and Jena, J.K. 2013. Egg laying strategies and effect of temperature on egg development of Argulus siamensis. J. Parasit. Dis. 37(2),158–162. Sandeep, I.S., Kuanar, A., Akbar, A., Kar, B., Das, S., Mishra, A., Sial, P., Naik, P.K., Nayak, S. and Mohanty, S. 2016. Agroclimatic zone based metabolic profiling of turmeric (Curcuma longa L.) for phytochemical yield optimization. Ind. Crops. Prod. 85, 229–240. Setyaningsih, E., Kismiyati. and Subekti, S. 2019. The Effect of noni fruits (Morinda citrifolia) with different ripeness stages against the total erythrocytes and leukocytes of comet goldfish (Carassius auratus) infested by Argulus. IOP. Conf. Ser. Earth. Environ. Sci. 236(1), 1–6. Singh, N., Gupta, S. and Rathore, V. 2017. Comparative antimicrobial study of ethanolic extract of leaf and rhizome of Curcuma longa Linn. Pharmacogn. J. 9(2), 208–212. Sneddon, L.U. 2012. Clinical anesthesia and analgesia in fish. J. Exot. Pet. Med. 21(1), 32–43. Sornpet, B., Potha, T., Tragoolpua, Y. and Pringproa, K. 2017. Antiviral activity of five asian medicinal plant crude extracts against highly pathogenic H5N1 avian influenza virus. Asian. Pac. J. Trop. Med. 10(9), 871–876. Steckler, N. and Yanong, R.P.E. 2012. Argulus (Fish louse) infections in fish. EDIS 184, 1–4. Tavares, W.S., Freitas, S.S., Grazziotti, G.H., Parente, L.M.L., Lião, L.M. and Zanuncio, J.C. 2013. Ar-turmerone from Curcuma longa (Zingiberaceae) rhizomes and effects on Sitophilus zeamais (Coleoptera: Curculionidae) and Spodoptera frugiperda (Lepidoptera: Noctuidae). Ind. Crops. Prod. 46, 158–164. Tavares-Dias, M. and Martins, M.L. 2017. An overall estimation of losses caused by diseases in the Brazilian fish farms. J. Parasit. Dis. 41(4), 913–918. Thakur, K., Sharma, A., Sharma, D., Brar, B., Choudhary, K., Sharma, A.K., Mahajan, D., Kumar, R., Kumar, S. and Kumar, R. 2022. An insight into the interaction between Argulus siamensis and Labeo rohita offers future therapeutic strategy to combat Argulosis. Aquac. Int. 31(3), 1–15. Usman, L.A., Hamid, A.A., George, O.C., Ameen, O.M., Muhammad, N.O., Zubair, M.F. and Lawal, A. 2009. Chemical composition of rhizome essential oil of Curcuma longa L. growing in North Central Nigeria. World. J. Chem. 4(2), 178–181. Wafer, L.N., Whitney, J.C. and Jensen, V.B. 2015. Fish lice (Argulus japonicus) in goldfish (Carassius auratus). Comp. Med. 65(2), 93–95. Wang, G.X., Han, J., Feng, T.T., Li, F.Y. and Zhu, B. 2009. Bioassay-guided isolation and identification of active compounds from fructus arctii against Dactylogyrus intermedius (Monogenea) in goldfish (Carassius auratus). Parasitol. Res. 106(1), 247–255. Wesołowska, A., Jadczak, P., Kulpa, D. and Przewodowski, W. 2019. Gas chromatography-mass spectrometry (GC-MS) analysis of essential oils from AgNPs and AuNPs elicited Lavandula angustifolia in vitro cultures. Molecules 24(3), 606. Yavari, A., Nazeri, V., Sefidkon, F. and Hassani, M.E. 2010. Natural product communications oil variability of Thymus migricus. Nat. Prod. Commun. 5(6), 943–948. | ||
| How to Cite this Article |
| Pubmed Style Saengsitthisak �, Chaisri W, Mektrirat R, Yano T, Pikulkaew S. In vitro and in vivo action of turmeric oil (Curcuma longa L.) against Argulus spp. in goldfish (Carassius auratus). Open Vet J. 2023; 13(12): 1645-1653. doi:10.5455/OVJ.2023.v13.i12.14 Web Style Saengsitthisak �, Chaisri W, Mektrirat R, Yano T, Pikulkaew S. In vitro and in vivo action of turmeric oil (Curcuma longa L.) against Argulus spp. in goldfish (Carassius auratus). https://www.openveterinaryjournal.com/?mno=170034 [Access: July 01, 2025]. doi:10.5455/OVJ.2023.v13.i12.14 AMA (American Medical Association) Style Saengsitthisak �, Chaisri W, Mektrirat R, Yano T, Pikulkaew S. In vitro and in vivo action of turmeric oil (Curcuma longa L.) against Argulus spp. in goldfish (Carassius auratus). Open Vet J. 2023; 13(12): 1645-1653. doi:10.5455/OVJ.2023.v13.i12.14 Vancouver/ICMJE Style Saengsitthisak �, Chaisri W, Mektrirat R, Yano T, Pikulkaew S. In vitro and in vivo action of turmeric oil (Curcuma longa L.) against Argulus spp. in goldfish (Carassius auratus). Open Vet J. (2023), [cited July 01, 2025]; 13(12): 1645-1653. doi:10.5455/OVJ.2023.v13.i12.14 Harvard Style Saengsitthisak, �., Chaisri, . W., Mektrirat, . R., Yano, . T. & Pikulkaew, . S. (2023) In vitro and in vivo action of turmeric oil (Curcuma longa L.) against Argulus spp. in goldfish (Carassius auratus). Open Vet J, 13 (12), 1645-1653. doi:10.5455/OVJ.2023.v13.i12.14 Turabian Style Saengsitthisak, ฺฺbanthita, Wasana Chaisri, Raktham Mektrirat, Terdsak Yano, and Surachai Pikulkaew. 2023. In vitro and in vivo action of turmeric oil (Curcuma longa L.) against Argulus spp. in goldfish (Carassius auratus). Open Veterinary Journal, 13 (12), 1645-1653. doi:10.5455/OVJ.2023.v13.i12.14 Chicago Style Saengsitthisak, ฺฺbanthita, Wasana Chaisri, Raktham Mektrirat, Terdsak Yano, and Surachai Pikulkaew. "In vitro and in vivo action of turmeric oil (Curcuma longa L.) against Argulus spp. in goldfish (Carassius auratus)." Open Veterinary Journal 13 (2023), 1645-1653. doi:10.5455/OVJ.2023.v13.i12.14 MLA (The Modern Language Association) Style Saengsitthisak, ฺฺbanthita, Wasana Chaisri, Raktham Mektrirat, Terdsak Yano, and Surachai Pikulkaew. "In vitro and in vivo action of turmeric oil (Curcuma longa L.) against Argulus spp. in goldfish (Carassius auratus)." Open Veterinary Journal 13.12 (2023), 1645-1653. Print. doi:10.5455/OVJ.2023.v13.i12.14 APA (American Psychological Association) Style Saengsitthisak, �., Chaisri, . W., Mektrirat, . R., Yano, . T. & Pikulkaew, . S. (2023) In vitro and in vivo action of turmeric oil (Curcuma longa L.) against Argulus spp. in goldfish (Carassius auratus). Open Veterinary Journal, 13 (12), 1645-1653. doi:10.5455/OVJ.2023.v13.i12.14 |