| Research Article | ||
Open Vet J. 2023; 13(12): 1654-1668 Open Veterinary Journal, (2023), Vol. 13(12): 1654-1668 Original Research Plasma-activated water application for detoxification of aflatoxin B1, ochratoxin A, and fumonisin B1 in poultry feedsHiba Salahaldeen Alnaemi1*, Tamara Natiq Dawood2 and Qais Thanoon Algwari31Veterinary Public Health Department, Veterinary Medicine College, Mosul University, Mosul, Iraq 2Veterinary Public Health Department, Veterinary Medicine College, Baghdad University, Baghdad, Iraq 3Electronics Department, Electronic Engineering College, Nineveh University, Mosul, Iraq *Corresponding Author: Hiba Salahaldeen Alnaemi. Veterinary Public Health Department, Veterinary Medicine College, Mosul University, Mosul, Iraq. Email: hibaalnaemi6 [at] uomosul.edu.iq Submitted: 21/09/2023 Accepted: 15/12/2023 Published: 31/12/2023 © 2023 Open Veterinary Journal
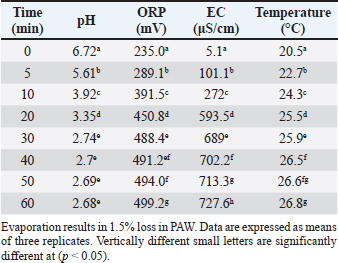
AbstractBackground: Plasma-activated water (PAW) is considered one of the emerging strategies that has been highlighted recently in the food industry for microbial decontamination and mycotoxin detoxification, due to its unique provisional characteristics. Aim: The effectiveness of PAW for aflatoxin B1 (AFB1), ochratoxin A (OTA), and fumonisin B1 (FB1) detoxification in naturally contaminated poultry feeds with its impacts on the feed quality were inspected. Methods: PAW-30 and PAW-60 were utilized for feed treatment for six time durations (5, 10, 15, 20, 40, and 60 minutes) each. The alterations in the physicochemical properties of PAW after different time durations of plasma inducement and treatment with and without feed samples were monitored. Competitive enzyme-linked immunosorbent assay (ELISA) was employed for estimation of mycotoxin levels and high performance liquid chromatography (HPLC) was utilized for results confirmation. Feed composition analyses with peroxide values (PVs) estimation were implemented according to standard analytical methods. Results: The physicochemical properties of PAW showed a significant decrease (p < 0.05) in pH value from 6.72 to 2.68 and a significant increase (p < 0.05) in oxidation–reduction potential (ORP), electrical conductivity (EC), and temperature from 235 mV, 5.1 μS/cm, and 20.5°C to 499.2 mV, 727.6 μS/cm, and 26.8°C, respectively, after 60 minutes of plasma inducement in a time-dependent manner. The mycotoxins decay kinetics after PAW application were illustrated. Mycotoxins degradation efficiency significantly increased (p < 0.05) with increasing water activation time. A significant increase (p < 0.05) in AFB1, OTA, and FB1 degradation levels was reported mainly during the first 10 minutes of treatment for AFB1 and the first 15 minutes for OTA and FB1 to record values of 28.33%, 32.14%, and 34.62% and 33.80%, 40.70%, and 43.38% after 60 minutes of feed exposure to PAW-30 and PAW-60, respectively. Significant differences (p < 0.05) between examined mycotoxins in their degradation levels were recorded, where FB1 exhibited the highest degradation levels. Generally, feed compositions were slightly affected by PAW and fats were still having good quality. Conclusion: The possibility of PAW for degrading more than a quarter to a third of the original quantity of targeted mycotoxins in poultry feeds after 10 minutes of treatment with a slight effect on feed quality. Keywords: Aflatoxin B1, Fumonisin B1, Ochratoxin A, Plasma-activated water, Poultry feeds. IntroductionAflatoxin B1 (AFB1), ochratoxin A (OTA), and fumonisin B1 (FB1), the secondary metabolites produced by different species of mycotoxigenic molds, were categorized within the most commonly encountered mycotoxins that constitute a great concern to human and animal health due to their toxigenic, carcinogenic, and mutagenic effects in addition to their economic impacts (Majeed and Khammas, 2010; Abbas et al., 2012; Jawad and Alwan, 2020; Awuchi et al., 2021). Mycotoxigenic molds exist throughout the environment, so, mycotoxins enter the food chain as a result to mold infection for human foods and animal feeds, especially crops (Ashiq, 2015; Khalifa et al., 2017; Mohamed and Al-Shamary, 2022; Minati and Mohammed-Ameen, 2023). Contamination of feeds with mycotoxins represents a major risk to animal health, in addition to their serious health threats to humans subjected to these mycotoxins indirectly through consuming foods of animal origin obtained from animals fed on contaminated feeds (Awuchi et al., 2021). Several research studies documented the occurrence of AFB1, OTA, and FB1 in animal feeds, particularly poultry feeds and their ingredients worldwide (Mokubedi et al., 2019; Kemboi et al., 2020; Rahim et al., 2020; Sadeeq, 2020; Zhao et al., 2021; Gueye et al., 2022; Sokolovic et al., 2022). Many strategies have been developed to rid or inactivate mycotoxins in the food chain, including physical, chemical, and biological strategies (Al-Naemey et al., 2008; Hassan, 2017; Marshall et al., 2020). Non-thermal physical technologies occupied growing attention recently as they are inexpensive, time-saving, have low side effects on food quality, and the capacity to be employed with a wide range of foods (Allai et al., 2023). Plasma-activated water (PAW), a non-thermal physical technology, has acquired great attention in recent years in food safety, as it is an eco-friendly and chemical-free technique without or with minimal negative impacts on food quality (Ma et al., 2015). PAW can be generated through plasma discharge over the water surface, in water, or in gas bubbles underwater. A series of convoluted processes such as excitation, ionization, and dissociation can originate as a consequence of the collision of high-energy electrons produced by a plasma discharge with water resulting in the production of an abundance of reactive oxygen and nitrogen species (RONS) (Thirumdas et al., 2018). Many chemical and physical operations, including molecule collision, mass transfer, sputtering, liquid evaporation, and ultra-violet radiation happen during reactive species transmission (Bruggeman et al., 2016). The characteristics of PAW generated depend on the chemical reactions that happen between reactive molecules generated by plasma and those present in the water leading to modification in pH, ORP, EC, and surface tension of activated water (Malajowicz et al., 2022). So, as with cold plasma (CP), the most prominent influence of PAW in mycotoxins degradation is related to reactive species generated in the gas phase which are transported to water through the plasma–water interface resulting in the induction of secondary reactive species in water. These reactive species react with the various active rings, double and triple bonds, and functional groups related to mycotoxins structure. Reactive species distribution in water results in uniform exposure to these reactive species and the capability to control their dosages (Zhou et al., 2020). Mycotoxins detoxification by PAW is primarily attributed to the reaction of reactive nitrogen species (RNS) with water as nitrates (NO3−), nitrites (NO2−), and peroxynitrate (OONO2−) to form nitric acid, nitrous acid, and peroxynitrous acid leading to production of a plentiful of hydrogen ions, in addition to generation of reactive oxygen species (ROS) as ozone (O3), hydrogen peroxide (H2O2), and hydroxyl radical (OH•) (Xiang et al., 2019). As PAW recently came into light, studies involving mycotoxins detoxification of foods using this technique were not investigated extensively. AFB1 detoxification by PAW was investigated only in the study performed by Xu et al. (2023) which referred to the efficacy of PAW in combination with cold atmospheric-pressure plasma (CAP) in AFB1 degradation. PAW efficiency in detoxification of deoxynivalenol (DON) was studied by other researchers, as in the study conducted by Chen et al. (2019), which reported a significant reduction in DON concentration in raw barley to reach degradation level of 22.5% in the first 5 minutes of treatment. Also, Qiu et al. (2022) recorded a DON degradation rate in wheat of about 58.78% after 24 h PAW treatment. Feizollahi et al. (2023) elucidated that PAW bubbles resulted in 57.3% DON degradation during the malting process of naturally infected barley. PAW efficacy in AFB1, OTA, and FB1 detoxification in feeds has not been studied yet, so the objectives of this study were to investigate the efficiency of PAW in AFB1, OTA, and FB1 detoxification from poultry feeds and to evaluate its impact on the nutritional components and PVs of the feeds. Materials and MethodsFeed samplesPelleted poultry feed samples were obtained from poultry feed plants and poultry farms in Nineveh Province, northern Iraq from April to September 2022. The samples were homogenized and quartered to obtain a 1 kg of representative samples. The samples were ground using a laboratory mill and sieved through NO. 18 mesh sieve. The samples that showed co-occurrence for AFB1, OTA, and FB1 at the highest levels were used for PAW application. CD systemThe corona discharge (CD) was used to create the PAW. CD system was comprised of many components including a DC power supply, a zero voltage switch circuit (ZVS) with a transformer, an air pump, an electrode assembly, and a sample treatment plate. The plasma was generated under normal atmospheric pressure conditions using a DC input voltage to the ZVS switch circuit with an output voltage up to 10 kV and a frequency of 100 Hz. The plasma was produced through CD between two stainless steel electrodes, and the discharge showed characteristics of a streamer type. For a plasma plume creation and avoiding spark, an air pump (Model RS-510, Bulgaria) was employed. The pump worked at a constant airflow rate of 2.5 liters per minute (lpm), and dry air was utilized as the gas for jet emission or as a carrier gas. PAW productionThe plasma was created above the deionized water surface in the gas and gas–liquid interphase at approximately 1.5 cm distance between the electrode tip and the water surface. One hundred milliliters of deionized water were put in a modified container of polypropylene equipped with a stainless steel base (8.3 × 8.3 × 8.3 cm) to permit CD generation with agitation every 5 minutes. The activated water by CD for 30 (PAW-30) and 60 (PAW-60) minutes was used instantly for feed samples treatment. The ground feed sample with PAW at a ratio of 1:2.5 w/v was put in an Erlenmeyer flask with a glass stopper and agitated using an orbital shaker. Treatments of the feed samples with PAW-30 and PAW-60 were achieved at six time durations (5, 10, 15, 20, 40, and 60 minutes). The control group was treated in a similar way with deionized water for the same time durations. After treatment, feed samples were put in a thin layer of about 0.1 cm and immediately dried in the oven (Memmert UM 400, Germany) at 40°C until reached moisture content of about 9.85%, the moisture content in the samples before treatment. The samples were analyzed immediately after treatments. All the experiments were implemented in triplicate. Physicochemical properties of PAWThe physicochemical properties of PAW including pH, ORP, EC, and temperature were estimated before (deionized water at zero time) and after plasma treatment for different activation times (5, 10, 20, 30, 40, 50, and 60 minutes). Also, pH, ORP, and EC of PAW-30 and PAW-60 with and without feed samples were estimated after different exposure time durations. The pH, ORP, and temperature were estimated using pH/mV/temperature bench top meter (PHB-213, USA). EC was measured using EC meter (ATO-CDTYM-DDS-11C, China). ELISA for AFB1, OTA, and FB1 quantitation in poultry feedsAFB1, OTA, and FB1 concentrations in poultry feed samples before and after PAW application were estimated using ELISA kits (Elabscience Biotechnology Inc., USA). The test kit is based on the competitive-ELISA for the detection of targeted mycotoxins in feed samples. Feed samples pretreatment was achieved according to the instructions of the kit manufacturer. Competitive ELISA procedures for AFB1, OTA, and FB1 were implemented according to the kit manufacturer instructions. The standards and samples were examined in duplicate. The optical density (OD) values were determined using a microplate reader (HumaReader HS, Germany) at 450 nm within 10 minutes from stopping reaction. Results confirmation by HPLC analysisChromatographic analysis was conducted on poultry feed samples that showed co-occurrence of AFB1, OTA, and FB1 at the highest levels for PAW treatment. Also, samples that exhibited the highest mycotoxin degradation levels after treatment were analyzed in order to confirm the ELISA results. HPLC analysis for AFB1 was performed according to Kim et al. (2001). AFB1 standard was obtained from Sigma-Aldrich (St. Louis, MO, USA). Acetonitrile-methanol (1:1 v/v) of HPLC-grade was used for preparing AFB1 stock solution. At first, AFB1 was extracted from ground poultry feed samples. Clean-up of samples was conducted using Sep-Pak® silica cartridges (Waters Corporation, Milford, MA, USA). Chloroform-acetone (9:1 v/v) was used for AFB1 elution. After derivatization, the lower phase of the derivatized solution was used for HPLC (Shimadzu Corporation, Japan). The column used was a Nucleodur® C18 column (4.6 × 250 mm, Germany). The fluorescence detector wavelength was adjusted at 360 nm excitation and 440 nm emission (Shimadzu Corporation, Japan). The mobile phase used was methanol-acetonitrile-water (17:17:70 v/v/v). The flow rate was adjusted at 1 ml/minute. OTA existence confirmation in poultry feed samples by HPLC was performed as recommended by Nesheim et al. (1992). OTA standard was purchased from Sigma-Aldrich (St. Louis, MO, USA). OTA stock solution was prepared in benzene-acetic acid (99:1 v/v). After mycotoxin extraction, cleanup of the bicarbonate extract was conducted by using Sep-Pak® C18 cartridge (Waters). Ethyl acetate-methanol-acetic acid (95:5:0.5) was used for OTA elution. OTA estimation was performed by HPLC with fluorescence detection adjusted at 333 nm for excitation and 460 nm for emission. The mobile phase was acetonitrile-water-acetic acid (99:99:2 v/v/v). The flow rate was adjusted at 1 ml/minute. FB1 standard was purchased from Sigma-Aldrich (St. Louis, MO, USA) and stock solution was prepared in acetonitrile-water (1:1 v/v). FB1 was extracted from poultry feed samples for HPLC analysis according to Shephard et al. (1990). After FB1 extraction, an aliquot was cleaned-up by strong anion extraction (SAX) cartridges (InertSep SAX, GL sciences, Japan). FB1 was eluted with acetic acid in methanol. HPLC injection was performed between one to 2 minutes after derivatization. The fluorescence detector was set at excitation and emission wavelengths of 335 and 440 nm, respectively. The mobile phase was methanol: 0.1 M sodium dihydrogen phosphate (80:20). The flow rate was set at 1 ml/minute. Mycotoxin degradation percentages and mycotoxin degradation kineticsAfter PAW application, mycotoxin degradation percentages in poultry feeds were measured according to the following equation: Mycotoxin degradation (%)=(1−Ct/C0) × 100 Where: Ct=the concentration of mycotoxin at time (t). C0=the initial concentration of mycotoxin at zero time. Mycotoxin degradation kinetics were modeled using exponential decay model (Bosch et al., 2017), which can be fit as follow: y=y0+A1*exp(-(x-x0)/t1) Where: y ≡ Ct; y0 ≡ C0; x ≡ t; A1, x0 and t1 are constants. Evaluation of the impact of PAW on the nutritional composition and PVs of the poultry feedsThe impacts of PAW on the nutritional composition and PVs of poultry feeds were inspected. Moisture in feed depending on water loss-on-drying at 135°C for 2 hours was estimated according to AOAC official method 930.15 (AOAC, 1996). Total carbohydrate was measured in accordance with the phenol–sulfuric acid method (DuBois et al., 1956). Crude fat was estimated by a Soxhlet system as per AOAC, method 920.39 (AOAC, 2006). Kjeldahl method was adopted for crude protein determination as stated by AOAC method 984.13 (AOAC, 1990). Ash was measured as described by AOAC Official method 942.05 (Thiex et al., 2012). Method 965.33 recommended by AOAC (AOAC, 2000) was employed to estimate the PVs in the feed fats. Statistical analysisStatistical analysis of the data was achieved using one way and two way analysis of variance procedure of the Sigma Stat for windows Version 3.10. Duncan ̓s Multiple Range Test was done for comparison of the means at p < 0.05 (Steel and Torri, 1960). Table 1. The physicochemical properties (pH, ORP, EC and Temperature) of PAW during different time durations of plasma inducement.
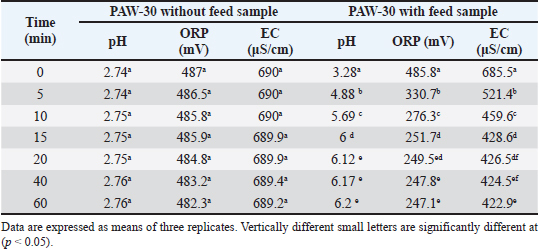
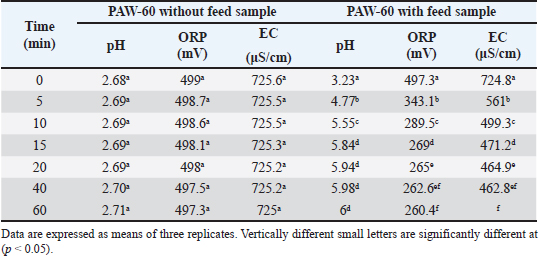
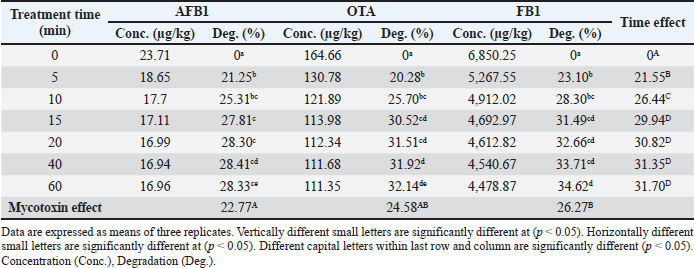
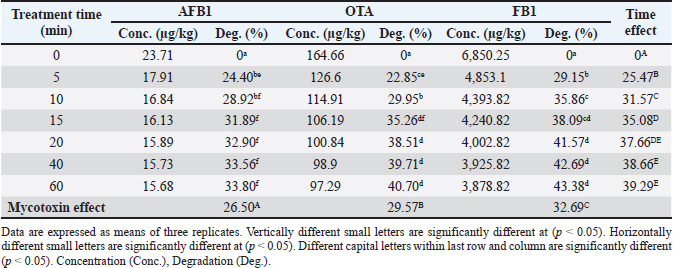
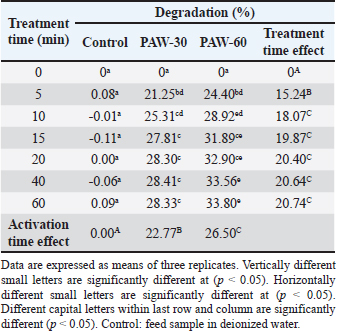
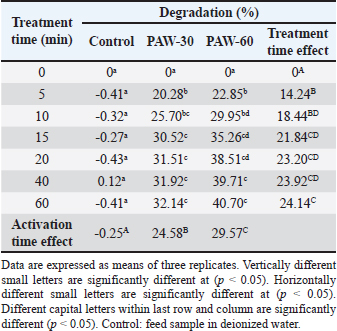
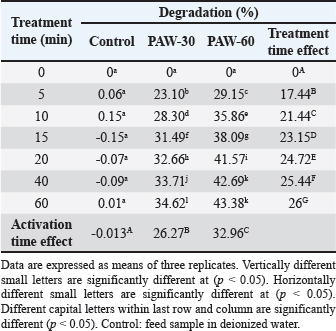
Ethical approvalNot needed for this study. ResultsResults related to detecting the alterations in the physicochemical properties of PAW (pH, ORP, EC, and temperature) after different time durations of plasma inducement revealed significant differences (p < 0.05) in their values particularly during the first 30 minutes of treatment. The pH value decreased significantly (p < 0.05) from 6.72 in untreated deionized water to reach value of 2.68 after 60 minutes of PAW inducement. While, the values of ORP, EC, and temperature increased significantly (p < 0.05) in PAW after 60 minutes of plasma inducement to reach values of 499.2 mV, 727.6 μS/cm, and 26.8°C comparable with their values in untreated water 235.0 mV, 5.1 μS/cm, and 20.5°C, respectively (Table 1). Exposing feed samples to PAW-30 and PAW-60 for different time durations till 60 minutes resulted in a significant increase (p < 0.05) in pH values with a significant decrease (p < 0.05) in ORP and EC values principally during the first-time durations. Conversely, PAW-30 and PAW-60 without feed samples showed insignificant differences (p < 0.05) in pH, ORP, and EC values after 60 minutes (Tables 2 and 3). Results concerning the degradation effects of PAW-30 and PAW-60 for AFB1, OTA, and FB1 in poultry feeds submitted a decrease in concentrations of mycotoxins from 23.71, 164.66, and 6,850.25 µg/kg in untreated samples to record values of 16.96, 111.35, and 4,478.87 µg/kg after PAW-30 exposure and 15.68, 97.29, and 3,878.82 µg/kg after PAW-60 exposure for 60 minutes, respectively. A significant increase (p < 0.05) in the degradation levels of studied mycotoxins chiefly during the first 10 minutes of treatment for AFB1 and first 15 minutes for OTA and FB1 was indicated to reach values of 28.33%, 32.14%, and 34.62% and 33.80%, 40.70% and 43.38% after 60 minutes of feed samples exposure to PAW-30 and PAW-60, respectively. Also, results showed significant differences (p < 0.05) between AFB1 and FB1 in their degradation levels after treatment with PAW-30 (22.77% and 26.27%, respectively) and between the three studied mycotoxins (AFB1, OTA, and FB1) after treatment with PAW-60 (26.50%, 29.57%, and 32.96%, respectively), where FB1 exhibited the highest degradation levels (Tables 4 and 5). Table 2. The physicochemical properties (pH, ORP and EC) of PAW-30 without and with feed samples after different time durations.
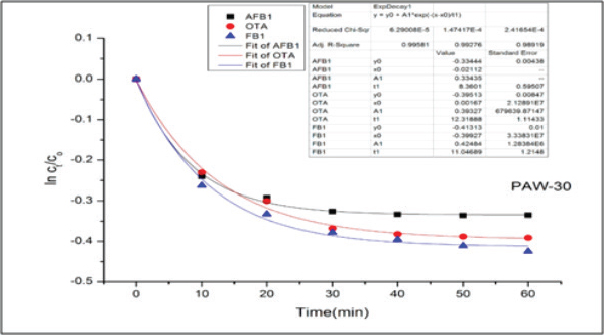
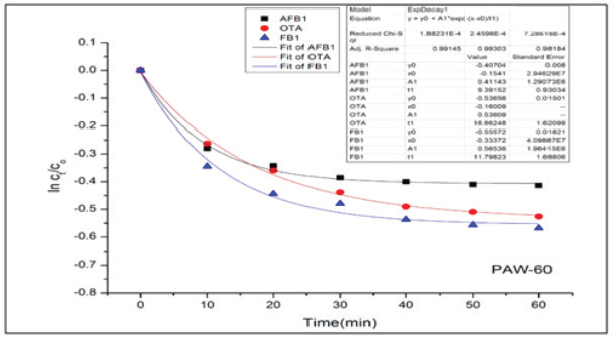
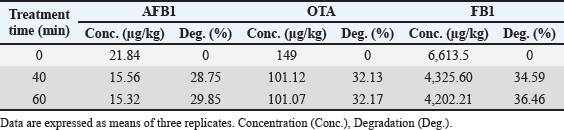
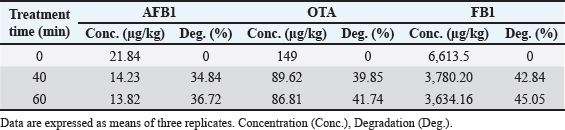
Results regarding the evaluation of the effect of increasing water activation time by plasma in mycotoxins degradation elucidated a significant increase (p < 0.05) in AFB1, OTA, and FB1 degradation levels with increasing water activation time (Tables 6–8). The degradation rate of mycotoxins following an exponential model was well fitted (Adj. R2 > 0.98) for AFB1, OTA, and FB1 for both PAW-30 and PAW-60 (Figs. 1 and 2). ELISA results substantiation was established by HPLC analysis for feed samples exposed to PAW treatment and samples with the highest degradation levels after treatment. According to HPLC analysis, results related to detecting AFB1, OTA, and FB1 in feed samples before and after PAW treatment displayed mean concentrations less than those estimated in ELISA with degradation levels higher than those recorded in ELISA. Results of HPLC analysis showed the co-existence of AFB1, OTA, and FB1 at mean concentrations of 21.84, 149, and 6,613.5 µg/kg, respectively. These concentrations decreased after 40 and 60 minutes of feed exposure to PAW-30 to reach 15.56, 101.12, and 4,325.60 µg/kg and 15.32, 101.07, and 4,202.21 µg/kg, respectively, and PAW-60 to reach 14.23, 89.62, and 3,780.20 µg/kg and 13.82, 86.81. and 3,634.16 µg/kg, respectively. The degradation levels of AFB1, OTA, and FB1 after 40 and 60 minutes of PAW-30 exposure were 28.75%, 32.13%, and 34.59% and 29.85%, 32.17%, and 36.46%, respectively, and PAW-60 exposure were 34.84%, 39.85%, and 42.84% and 36.72%, 41.74%, and 45.05%, respectively (Tables 9 and 10). Table 3. The physicochemical properties (pH, ORP, and EC) of PAW-60 without and with feed samples after different time durations.
Table 4. Effect of PAW-30 on AFB1, OTA and FB1 mean concentrations and degradation percentages in poultry feeds.
Table 5. Effect of PAW-60 on AFB1, OTA, and FB1 mean concentrations and degradation percentages in poultry feeds.
Table 6. Effect of water activation time by plasma on AFB1 degradation levels in poultry feed samples.
Table 7. Effect of water activation time by plasma on OTA degradation levels in poultry feed samples.
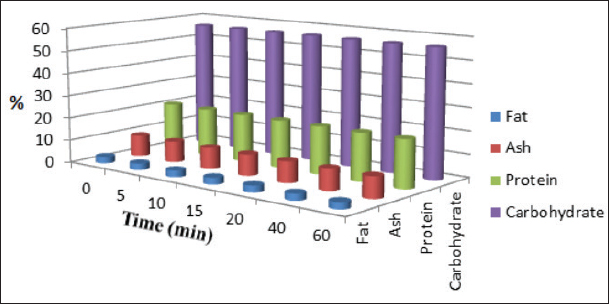
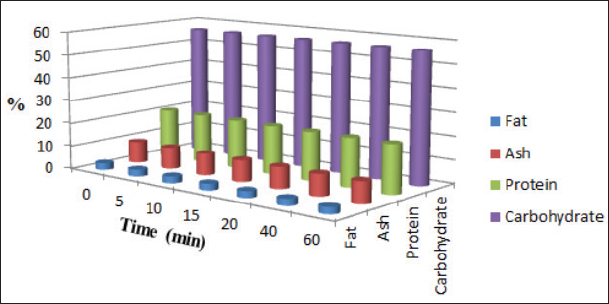
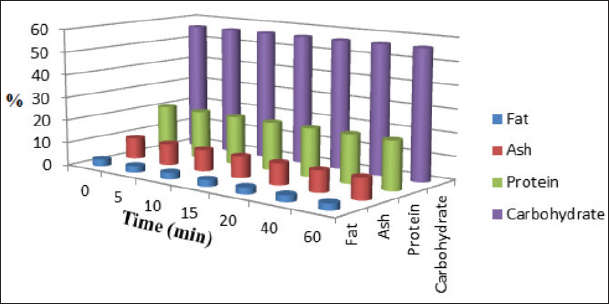
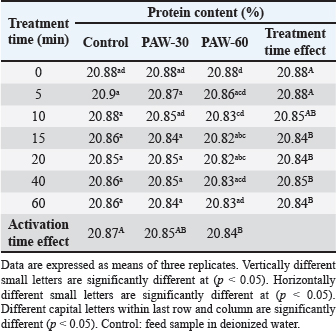
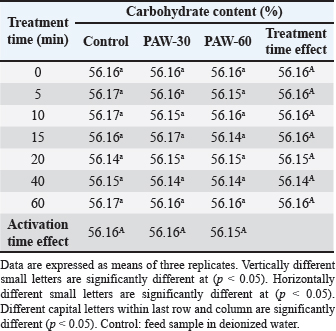
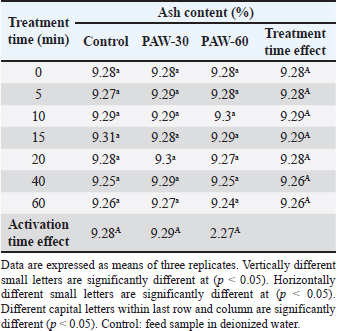
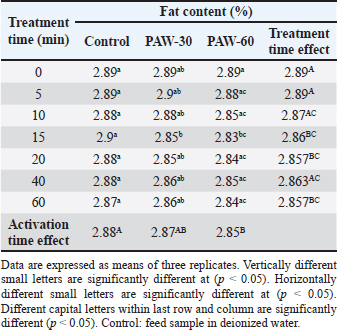
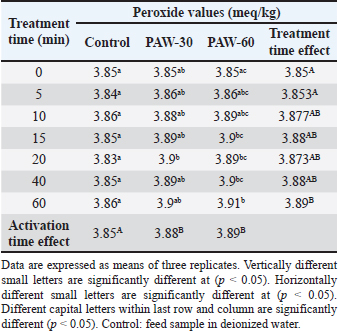
Results regarding PAW effect on feed components referred to insignificant differences (p < 0.05) in protein content of the control group and PAW-30 and PAW-60 treated feed samples with increasing exposure time, with the exception of feed samples treated with PAW-60 which showed a significant decrease (p < 0.05) in protein content after 15 and 20 minutes of treatment (20.82% for both) comparable with protein content in untreated ones (20.88%). Results pointed out that increasing water activation time by plasma to 60 minutes (PAW-60) resulted in a significant decrease (p < 0.05) in protein content of feed samples (20.84%) comparable with those treated with deionized water (20.87%) (Figs. 3–5, Table 11). Concerning the PAW effect on the carbohydrate and ash contents of poultry feed samples, results exhibited insignificant differences (p < 0.05) in their contents during the different feed exposure times and during the different water activation times (Figs. 3–5, Tables 12 and 13). Also, insignificant differences (p < 0.05) in poultry feed fats were demonstrated after different exposure times of feed to inactivated water (control), PAW-30, and PAW-60. Although feeds treated with PAW-60 for 15 minutes recorded a significant decrease (p < 0.05) in fat content (2.83%) in comparison with the untreated ones (2.89%). Regarding the effect of water activation time on fat content, results presented a significant decrease (p < 0.05) in fat content of feed samples treated with PAW-60 (2.85%) comparable with the control group (2.88%) (Figs. 3–5, Table 14). After PAW treatment, oxidative quality evaluation of poultry feed fats was conducted depending on the estimation of PVs. Results showed no significant differences (p < 0.05) in PVs after feed exposure for different time durations to inactivated water, PAW-30 and PAW-60, with the exception of feed samples treated with PAW-60 for 60 minutes which displayed a significant increase in PV (3.91 meq/kg) comparing with the untreated ones (3.85 meq/kg). Likewise, a significant increase in PVs in samples treated with water activated for 30 and 60 minutes (3.88 and 3.89 meq/kg, respectively) was recorded comparable with those treated with inactivated water (3.85 meq/kg) (Table 15). Table 8. Effect of water activation time by plasma on FB1 degradation levels in poultry feed samples.
DiscussionPAW is a promising alternative strategy to conventional food sanitizers and has been recently researched for mycotoxins detoxification. PAW provides the maximal exposure of the whole surfaces of treated foods to reactive species, which may pave the way for large-scale application on a wide variety of agricultural products in large quantities (Herianto et al., 2021). Results demonstrated significant changes (p < 0.05) in the physicochemical properties of PAW represented by a significant decrease (p < 0.05) in pH value with a significant increase (p < 0.05) in ORP, EC, and temperature after different time durations of plasma inducement substantially during the first 30 minutes of treatment. These results were in consensus with other research studies, although the variations in the levels of these properties in the produced PAW (Ma et al., 2015; Chen et al., 2019; Feizollahi et al., 2023). The values of the aforementioned physicochemical properties are related directly to the forms and levels of the dissolved RONS in addition to the active ions in PAW. Several parameters control the forms and levels of the generated reactive species during water activation by plasma, resulting in the variations in the physicochemical properties of the generated PAW. These parameters include gas type, gas flow rate, plasma generating system, electrode configuration, discharge time, applied voltage, power, liquid type, the distance between the electrode, and the water surface, in addition to electron temperature (Thirumdas et al., 2018). PAW in general has a lower pH value and higher ORP and EC values than inactivated water (Malajowicz et al., 2022). Low pH values refer to the presence of high concentrations of dissolved RNS, such as NO2− and NO3− (Xiang et al., 2019). High ORP and EC values indicate high concentrations of dissolved ROS and active ions, respectively (Ma et al., 2015; Xiang et al., 2019). The increment in PAW temperature which was reported in our study was definitely inadequate to participate in mycotoxins degradation, where the optimum degradation conditions for mycotoxins in the food matrix amount to 210.85°C/54.71 minutes (Gbashi et al., 2019).
Fig. 1. Degradation kinetics of AFB1, OTA and FB1 in poultry feeds after PAW-30 application for different time durations (n=3).
Fig. 2. Degradation kinetics of AFB1, OTA, and FB1 in poultry feeds after PAW-60 application for different time durations (n=3). Table 9. HPLC analysis of poultry feed samples that showed the highest co-occurrence and highest degradation levels of AFB1, OTA, and FB1 after PAW-30 treatment.
Table 10. HPLC analysis of poultry feed samples that showed the highest co-occurrence and highest degradation levels of AFB1, OTA, and FB1 after PAW-60 treatment.
Feed samples treatment for different time durations with PAW-30 and PAW-60 till 60 minutes showed a significant increase (p < 0.05) in pH values with a significant decrease (p < 0.05) in ORP and EC values particularly during the first-time durations. These alterations may be attributed to the reaction of feed components including mycotoxins with RONS which result in the depletion or reduction in the levels of these accumulated reactive species. Similar findings were mentioned by Chen et al. (2019).
Fig. 3. Effect of deionized water (control) treatment on the nutritional composition (%) of poultry feeds (n=3).
Fig. 4. Effect of PAW-30 treatment on the nutritional composition (%) of poultry feeds (n=3). Our results elucidated a significant increase (p < 0.05) in the degradation levels of studied mycotoxins mainly during the first 10 minutes of treatment for AFB1 and the first 15 minutes for OTA and FB1 to amount values of 28.33%, 32.14%, and 34.62% and 33.80%, 40.70%, and 43.38% after 60 minutes of feed exposure to PAW-30 and PAW-60, respectively. AFB1 and OTA regulatory limits set by the European Commission (EC) are 20 and 100 µg/kg, respectively in poultry feeds (EC, 2006; EC, 2011). In accordance with these limits and to initial mycotoxin levels measured in our study, results indicated that PAW-30 and PAW-60 application for 5 minutes was adequate to degrade AFB1 to levels under this limit. While, OTA required 40 minutes of exposure to PAW-60 to reach the legal limit. Concerning FB1, samples used for PAW treatment which exhibited the highest levels were within the allowable limits for FB1+ FB2 in poultry feeds (20 000 µg/kg), where no maximum limit for FB1 alone was established in poultry feeds by the EC (2006). Only one study investigated the efficacy of PAW in AFB1 detoxification, while there are no studies related to studying its effect on OTA and FB1 detoxification. Xu et al. (2023) referred to the efficiency of employing CAP in combination with PAW for AFB1 degradation, as CAP effectively degraded AFB1 while PAW resulted in additional degradation but with comparatively long time treatment. The researchers attributed AFB1 degradation in PAW to the singlet oxygen (1O2) as the major reactive species that result in the methyl group destruction on the benzene side chain and oxidizing the terminal furan ring double bond in AFB1 molecule. Results were in agreement to a certain extent with the results obtained by Chen et al. (2019) which indicated that PAW resulted in a significant reduction in DON levels in raw barley mainly during the first 5 minutes of treatment. The crucial role of RONS in mycotoxins detoxification may be clarified when comparing the results of pH, ORP, and EC values estimation during feed treatment with the results of mycotoxins degradation, where they significantly altered during the first time durations of feed treatment with PAW-30 and PAW-60.
Fig. 5. Effect of PAW-60 treatment on the nutritional composition (%) of poultry feeds (n=3). Table 11. Effect of PAW application on the protein contentof poultry feeds.
Table 12. Effect of PAW application on the carbohydrate content of poultry feeds.
Significant differences (p < 0.05) were recorded between AFB1 and FB1 in their degradation levels after exposure to PAW-30 and between the three studied mycotoxins (AFB1, OTA, and FB1) after exposure to PAW-60, as FB1 exhibited the highest degradation levels. Bosch et al. (2017) mentioned that mycotoxins’ degradation rate may be influenced by the chemical structure. Mycotoxins with long aliphatic chains as FB1 are degraded rapidly after CP treatment compared with those with a compact structure of condensed aromatic rings, while mycotoxins with mixed structures of aliphatic chains and condensed rings had intermediate degradation rates. As mycotoxins degradation by PAW is dominated by the activity of reactive species generated as with CP so these differences in mycotoxin degradation may be attributed to the differences in the chemical structures of studied mycotoxins. Table 13. Effect of PAW application on the ash content of poultry feeds.
Table 14. Effect of PAW application on the fat content of poultry feeds.
Table 15. Effect of PAW application on the peroxide values of poultry feed fats.
AFB1, OTA, and FB1 degradation levels increased significantly (p < 0.05) with increasing water activation time by plasma, where PAW efficiency increases in a manner dependent on increasing activation time (Ma et al., 2015; Guo et al., 2022). HPLC analysis, for ELISA results confirmation, pointed out that AFB1, OTA, and FB1 were detected in feed samples before and after PAW treatment at mean concentrations less than those measured in ELISA with degradation levels higher than those reported in ELISA. Results were in line with the results mentioned by Alshawabkeh et al. (2015) which indicated that AFB1 levels measured by HPLC were lower than those detected by ELISA. Nesic et al. (2017) recorded an aflatoxin mean concentration by ELISA (7.47 ppb) higher than that recorded by HPLC (6.23 ppb), while ochratoxin was found at a mean concentration by ELISA (5.80 ppb) lower than that reported by HPLC (6.47 ppb). Feed composition analysis presented insignificant differences (p < 0.05) in protein content of feed samples treated with inactivated water, PAW-30, and PAW-60 with exposure time increment, except feed samples treated with PAW-60 after 15 and 20 minutes of treatment displayed a significant decrease (p < 0.05) in protein content (20.82% for both) comparable with the untreated ones (20.88%). Results were in concurrence with those explained by Astorga et al. (2022) which referred to an insignificant reduction (p < 0.05) in the protein content of beef meat after treatment with PAW-30. Xinyu et al. (2020) indicated that protein oxidation and protein structure were better retarded by PAW. While Herianto et al. (2021) referred to the occurrence of conformational changes in protein which may be induced as a result of a specific bond breakdown and side chain modulation by PAW ROS. Furthermore, results clarified that increasing water activation time by plasma to 60 minutes (PAW-60) led to a significant decrease (p < 0.05) in protein content of feed samples (20.84%) in comparison with those treated with deionized water (20.87%). Increasing the water activation time by plasma leads to the generation of higher concentrations of reactive species in PAW (Lee et al., 2023), which may adversely affect proteins. Results showed insignificant differences (p < 0.05) in carbohydrate content of PAW-treated feed samples during the different feed exposure times and during the different water activation times. Insignificant differences in carbohydrate content after PAW treatment were reported in other studies (Choi et al., 2019; Zheng et al., 2019; Xiang et al., 2020). Also, poultry feed fats exhibited insignificant differences (p < 0.05) in their content after different exposure times of feed to PAW-30 and PAW-60, with the exception of feeds treated with PAW-60 for 15 minutes showed a significant decrease (p < 0.05) in fat content (2.83%) in comparison with the untreated ones (2.89%). An insignificant increase (p < 0.05) in PVs was stated after feed exposure for different time durations to PAW-30 and PAW-60, except feed samples treated with PAW-60 for 60 minutes showed a significant increase in PV (3.91 meq/kg) in comparison with the untreated ones (3.85 meq/kg). The fats and oils are classified according to their oxidative quality into four categories. If PV < 5 meq/kg this means there is no oxidation, while if it ranges between 5 and 10 meq/kg there are first signs of oxidation, values between 10 and 20 meq/kg indicate the presence of oxidation and if PV > 20 meq/kg there is a strong oxidation (Wealleans et al., 2021). Our results recorded an increase in PVs after PAW treatment, however, feed fats were still having good quality, where PVs were less than 5 meq/kg. Also, an increment in PV to 1.33, 1.57, 3.68, and 4.20 meq/kg was reported by Jung et al. (2015) after 0, 7, 14, and 21 days of PAW-cured sausage storage, although this increment was followed by a decrease in value to 3.67 meq/kg after storage to 28 days. Nguyen (2022) referred to the insignificant modification in the fatty acids composition, in addition to a slight reduction in the cholesterol with a significant increase in its oxidized products in mussel samples treated with PAW. Lipid peroxidation in food treated with PAW is attributed to the interactions between ROS and food cells (Zhao et al., 2021). On the other hand, Astorga et al. (2022) presented a reduction in lipid oxidation extent in beef meat after PAW treatment, which may be attributed to protein denaturation by ROS that results in lipid oxidation limitation (Nguyen, 2022). In general, the efficiency of PAW in mycotoxins detoxification and its effect on food quality depends on many parameters such as the form of plasma generated, and the processing parameters, in addition to mycotoxin and food matrix type (Chen et al., 2022). ConclusionsThe present study showed the possibility of PAW, as an eco-friendly non-thermal technology, for degrading more than a quarter to a third of the original quantity of AFB1, OTA, and FB1 in poultry feeds naturally contaminated after 10 minutes of treatment. Increasing water activation time by plasma resulted in an increase in the ORP and EC values and a decrease in pH value as indicators for RONS generation which play the most important role in mycotoxins detoxification by PAW. So, the more time of water activation, the more mycotoxin degradation efficacy. In accordance with mycotoxin levels estimated in our study, results clarified that 5 minutes of poultry feeds exposure to PAW-30 and PAW-60 was sufficient to reach to AFB1 permissible limit and 40 minutes exposure to PAW-60 to reach to OTA permissible limit. FB1 demonstrated the highest degradation level, then OTA, whereas AFB1 exhibited the lowest degradation level. In general, feed components were slightly affected by PAW. The feed fats were still fresh and of good quality. AcknowledgmentsThe authors are grateful to the Veterinary Medicine College, Baghdad University for support and providing facilities. Thanks are also extended to the General Science Department, Basic Education College, Mosul University for PAW production by corona discharge system and mycotoxins estimation with ELISA reader. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis research did not receive any external funding. Authors contributionsHiba Salahaldeen Alnaemi proposed the study idea. All authors contributed to the study design. Hiba Salahaldeen Alnaemi performed the experiments. Qais Thanoon Algwari supervised the PAW experiments. Hiba Salahaldeen Alnaemi, Tamara Natiq Dawood and Qais Thanoon Algwari analyzed the data. Hiba Salahaldeen Alnaemi wrote the first draft of the manuscript. Tamara Natiq Dawood and Qais Thanoon Algwari supervised and revised the manuscript writing. All the authors reviewed and approved the final version of the manuscript. Data availabilityThe data supporting the findings of this research are available from the corresponding author upon reasonable request. ReferencesAbbas, D.A., Faraj, M.K. and Abed, A.R. 2012. Some biolochemical and histopathological effects of different oral doses ochratoxin A in male rats. Iraqi J. Vet. Med. 36(0E), 182–189. Allai, F.M., Azad, A.A., Mir, N.A. and Gul, K. 2023. Recent advances in non-thermal processing technologies for enhancing shelf life and improving food safety. Appl. Food Res. 3(1), 100258. Al-Naemey, H.M.M., Ja`afar, N.S. and Omran, H.A. 2008. Study of using Thymbra spicata leaves to reduce the toxic immunosuppressive effect of aflatoxin in broilers. Iraqi J. Vet. Med. 32(1), 140–147. Alshawabkeh, K., Alkhalaileh, N.I., Abdelqader, A., Al-Fataftah, A.A. and Herzallah, S.M. 2015. Occurrence of aflatoxin B1 in poultry feed and feed ingredients in Jordan using ELISA and HPLC. American-Eurasian J. Toxicol. Sci. 7 (4), 316–320. AOAC (Association of Official Analytical Chemists). 1990. Protein (crude) determination in animal feed: copper catalyst kjeldahl method 984.13. 15th ed. Official Methods of Analysis of AOAC International, Gaithersburg. AOAC (Association of Official Analytical Chemists). 1996. Moisture in animal feed, method 930.15. 16th ed. Official Methods of Analysis of AOAC International, Gaithersburg. AOAC (Association of Official Analytical Chemists). 2000. AOAC official method 965.33: Peroxide value. In Official method of analysis of AOAC International. Ed., Horwitz, W. 17th ed. Gaithersburg, Md, USA: AOAC International, p: 12. AOAC (Association of Official Analytical Chemists). 2006. Official Methods of Analysis of AOAC International, 18th ed. Ashiq, S. 2015. Natural occurrence of mycotoxins in food and feed: Pakistan perspective. Comprehensive Rev. Food Sci. Food Safety. 14, 159–175. Astorga, J.B., Hadinoto, K., Cullen, P., Prescott, S. and Trujillo, F.J. 2022. Effect of plasma activated water on the nutritional composition, storage quality and microbial safety of beef. LWT- Food Sci. Tech. 154, 112794. Awuchi, C.G., Ondari, E.N., Ogbonna, C.U., Upadhyay, A.K., Baran, K., Okpala, C.O., Korzeniowska, M. and Guine, R.P. 2021. Mycotoxins affecting animals, foods, humans, and plants: types, occurrence, toxicities, action mechanisms, prevention, and detoxification strategies—A revisit. Foods. 10(6), 1279. Bosch, L., Pfohl, K., Avramidis, G., Wieneke, S., Viöl, W. and Karlovsky, P. 2017. Plasma-based degradation of mycotoxins produced by Fusarium, Aspergillus and Alternaria species. Toxins (Basel). 9(3), 97. Bruggeman, P.J., Kushner, M.J., Locke, B.R., Gardeniers, J.G.E., Graham, W.G., Graves, D.B., Hofman-Caris, R.C.H.M., Maric, D., Reid, J.P., Ceriani, E., Fernandez Rivas, D., Foster, J.E., Garrick, S.C., Gorbanev, Y., Hamaguchi, S., Iza, F., Jablonowski, H., Klimova, E., Kolb, J., Krcma, F., Lukes, P., Machala, Z., Marinov, I., Mariotti, D., Mededovic Thagard, S., Minakata, D., Neyts, E.C., Pawlat, J., Petrovic, Z., Pflieger, R., Reuter, S., Schram, D.C., Schröter, S., Shiraiwa, M., Tarabová, B., Tsai, P.A., Verlet, J.R.R., von Woedtke, T., Wilson, K.R., Yasui, K. and Zvereva, G. 2016. Plasma–liquid interactions: a review and roadmap. Plasma Sources Sci. Technol. 25, 053002. Chen, D., Chen, P., Cheng, Y., Peng, P., Liu, J., Ma, Y., Liu, Y. and Ruan, R. 2019. Deoxynivalenol decontamination in raw and germinating barley treated by plasma-activated water and intense pulsed light. Food Bioprocess Tech. 12, 246–254. Chen, H., Arcega, R., Herianto, S., Hou, C. and Lin, C. 2022. Mycotoxin decontamination of foods using nonthermal plasma and plasma-activated water. Mycotoxins and food safety—recent advances. IntechOpen. Available at: http://dx.doi.org/10.5772/intechopen.95720. Choi, E.J., Park, H.W., Kim, S.B., Ryu, S., Lim, J., Hong, E.J., Byeon, Y.S. and Chun, H.H. 2019. Sequential application of plasma-activated water and mild heating improves microbiological quality of ready-to-use shredded salted Chinese cabbage (Brassica pekinensis L.). Food Control. 98, 501–509. DuBois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A. and Smith, F. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356. European Commission (EC). 2006. Commission Recommendation 2006/576/EC of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. O J L 229, 23.8. 2006, pp: 7–9. European Commission (EC). 2011. Commission Regulation (EU) 2011/574 of 16 June 2011 Amending Annex I to Directive 2002/32/EC of the European Parliament and of the Council as regards maximum levels for nitrite, melamine, ambrosia spp. and carry-over of certain coccidiostats and histomonostats and consolidating. O J L 159, 17.6. 2011, pp: 7–24. Feizollahi, E., Basu, U., Fredua-Agyeman, R., Jeganathan, B., Tonoyan, L., Strelkov, S.E., Vasanthan, T., Siraki, A.G. and Roopesh, M.S. 2023. Effect of plasma-activated water bubbles on Fusarium graminearum, deoxynivalenol, and germination of naturally infected barley during steeping. Toxins. 15(2), 124. Gbashi, S., Madal, N.E., De Saeger, S., De Boevre, M. and Njobeh, P.B. 2019. Numerical optimization of temperature-time degradation of multiple mycotoxins. Food Chem. Toxicol. 125, 289–304. Gueye, R., Sandefo, V., Beye, B., Faye, E., Diop, A., Sarr, S., Ndiaye, B. and Diop, Y. 2022. Assessment of poultry feed contamination level by aflatoxin B1: Quantification by two chromatographic analysis methods. Food Nutr. Sci. 13, 950–961. Guo, J., Wang, J., Xie, H., Jiang, J., Li, C., Li, W., Li, L., Liu, X. and Lin, F. 2022. Inactivation effects of plasma-activated water on Fusarium graminearum. Food Control. 134, 108683. Hassan, F.F. 2017. Detection of aflatoxin B1 in some canned foods and reduction of toxin by ultraviolet radiation. Iraqi J. Sci. 58(4C), 2343–2349. Herianto, S., Hou, C.Y., Lin, C.M. and Chen, H.L. 2021. Nonthermal plasma-activated water: a comprehensive review of this new tool for enhanced food safety and quality. Compr. Rev. Food Sci. Food Safety. 20(1), 583–626. Jawad, B.J. and ALwan, M.J. 2020. Influence of mycotoxins on immune responses against Salmonella typhimurium infection of broiler chickens. Iraqi J. Agric. Sci. 51(6), 1716–1725. Jung, S., Kim, H.J., Park, S., Yong, H.I., Choe, J.H., Jeon, H.J., Choe, W. and Jo, C. 2015. The use of atmospheric pressure plasma-treated water as a source of nitrite for emulsion-type sausage. Meat Sci. 108, 132–137. Kemboi, D.C., Ochieng, P.E., Antonissen, G., Croubels, S., Scippo, M.L., Okoth, S., Kangethe, E.K., Faas, J., Doupovec, B., Lindahl, J.F. and Gathumbi, J.K. 2020. Multi-mycotoxin occurrence in dairy cattle and poultry feeds and feed ingredients from Machakos Town, Kenya. Toxins. 12(12), 762. Khalifa, R.R., Aljarah, N.S. and Matny, O.N. 2017. Detection and investigation of Aspergillus niger and ochratoxin A in walnut and peanut. Iraqi J. Agric. Sci. 48(5), 1223–1230. Kim, E.K., Shon, D.H., Yoo, J.Y., Ryu, D., Lee, C. and Kim, Y.B. 2001. Natural occurrence of aflatoxins in Korean meju. Food Addit. Contam. 18(2), 151–156. Lee, G., Choi, S., Yoo, M., Chang, H. and Lee, N. 2023. Effects of plasma-activated water treatment on the inactivation of microorganisms present on cherry tomatoes and in used wash solution. Foods. 12(13), 2461. Ma, R.N., Wang, G.M., Tian, Y., Wang, K., Zhang, J. and Fang, J. 2015. Non-thermal plasma-activated water inactivation of food-borne pathogen on fresh produce. J. Hazard Mater. 300, 643–651. Majeed, S.H.A. and Khammas, E.J. 2010. Aflatoxin in chicken’s feed and its effects on apoptosis. Iraqi J. Vet. Med. 34(1), 29–43. Malajowicz, J., Khachatryan, K. and Kozłowska, M. 2022. Properties of water activated with low-temperature plasma in the context of microbial activity. Beverages. 8(4), 63. Marshall, H., Meneely, J.P., Quinn, B., Zhao, Y., Bourke, P., Gilmore, B.F., Zhang, G. and Elliott, C.T. 2020. Novel decontamination approaches and their potential application for post-harvest aflatoxin control. Trends Food Sci. Technol. 106, 489–496. Minati, M.H. and Mohammed-Ameen, M.K. 2023. First report of three kinds of mycotoxins dioxynivalenol, nivalenol and fumonisin B2 in seeds of seven wheat cultivars in Iraq. Iraqi J. Vet. Med. 43(1), 43–49. Mohamed, A.M. and Al-Shamary, E.I. 2022. Isolation and identification of aflatoxin B1 producing fungi from stored wheat in some silos of Baghdad. Iraqi J. Agric. Sci. 53(6), 1427–1436. Mokubedi, S.M., Phoku, J.Z., Changwa, R.N., Gbashi, S. and Njobeh, P.B. 2019. Analysis of mycotoxins contamination in poultry feeds manufactured in selected provinces of South Africa using UHPLC-MS/MS. Toxins. 11(8), 452. Nesheim, S., Stack, M.E., Trucksess, M.W., Eppley, R.M. and Krogh, P. 1992. Rapid solvent-efficient method for liquid chromatographic determination of ochratoxin A in corn, barley, and kidney: collaborative study. J. AOAC Int. 75 (3), 481–487. Nesic, K.D., Pisinov, B.P., Jaksic, S.M., Tasic, A.М., Savic, B.M. and Pavlovic, N.J. 2017. Comparison of ELISA and HPLC methods for the detection of mycotoxins by analysing proficiency test results. Matica Srpska J. Nat. Sci. Novi Sad. 133, 79–93. Nguyen, H.M. 2022. Effects of plasma activated water (PAW) on oxidation of lipids in seafood, Ph.D. Dissertation, Department of agricultural, food and environmental Sciences University Politechnica, Romania. p. 32. Qiu, Y., Chen, X., Zhang, J., Ding, Y. and Lyu, F. 2022. Effects of tempering with plasma activated water on the degradation of deoxynivalenol and quality properties of wheat. Food Res. Int. 162 (Pt B), 112070. Rahim, H.M., Othman, D.H., Majeed, R.K., Saeed, N.M. and Ismaeel, D.O. 2020. Mycotoxins in poultry feed and feed ingredients in Sulaymaniyah, Kurdistan region of Iraq. Bas. J. Vet. Res. 19(2), 210–222. Sadeeq, E.T. 2020. Some mycotoxins contamination in animal feed and feedstuff ingredients in Duhok Province. J. Uni. Duhok. 23(2), 182–188. Shephard, G.S., Sydenham, E.W., Thiel, P.G. and Gelderblom, W.C. 1990. Quantitative determination of fumonisins B1 and B2 by high-performance liquid chromatography with fluorescence detection. J. Liq. Chromatogr. 13(10), 2077–2087. Sokolovic, M., Berendika, M., Zelenika, T.A., Simpraga, B., Krstulovic, F. 2022. Determination of fumonisins in grains and poultry feedstuffs in Croatia: a 16-year study. Toxins. 14(7), 444. Steel, R.G. and Torri, J.H. 1960. Principles and procedures of statistics. New York, Toronto, London: McGraw- Hill Book Company. Thiex, N., Novotny, L. and Crawford, A. 2012. Determination of ash in animal feed: AOAC official method 942.05 revisited. J. AOAC Int. 95(5), 1392–1397. Thirumdas, R., Kothakota, A., Annapure, U., Siliveru, K., Blundell, R., Gatt, R. and Valdramidis, V.P. 2018. Plasma activated water (PAW): chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 77, 21–31. Wealleans, A.L., Bierinckx, K., Witters, E., di Benedetto, M. and Wiseman, J. 2021. Assessment of the quality, oxidative status and dietary energy value of lipids used in non-ruminant animal nutrition. J. Sci. Food Agric. 101(10), 4266–4277. Xiang, Q., Xiufang, L., Shengnan, L., Yunfang, M., Chunqing, X. and Yanhong, B. 2019. Effect of plasma-activated water on microbial quality and physicochemical characteristics of mung bean sprouts. Innov. Food Sci. Emerg. Technol. 52, 49–56. Xiang, Q., Zhang, R., Fan, L., Ma, Y., Wu, D., Li, K. and Bai, Y.A. 2020. Microbial inactivation and quality of grapes treated by plasma-activated water combined with mild heat. LWT. 126, 109336. Xinyu, L., Xiang, Q., Cullen, P.J., Su, Y., Chen, S., Ye, X., Liu, D. and Ding, T. 2020. Plasma-activated water (PAW) and slightly acidic electrolyzed water (SAEW) as beef thawing media for enhancing microbiological safety. LWT. 117, 108649. Xu, H., Fang, C. and Huang, Q. 2023. Achieving improved efficiency for removal of aflatoxin B1 by combination use of cold atmospheric-pressure plasma and plasma-activated water. J. Water Process Eng. 54, 104004. Zhao, L., Zhang, L., Xu, Z., Liu, X., Chen, L., Dai, J., Karrow, N.A. and Sun, L. 2021. Occurrence of aflatoxin B1, deoxynivalenol and zearalenone in feeds in China during 2018–2020. J. Animal Sci. Biotechnol. 12, 74. Zhao, Y.M., Oliveira, M., Burgess, C.M., Cropotova, J., Rustad, T., Sun, D.W. and Tiwari, B.K. 2021. Combined effects of ultrasound, plasma-activated water, and peracetic acid on decontamination of mackerel fillets. LWT. 150(2), 111957. Zheng, Y., Wu, S., Dang, J., Wang, S., Liu, Z., Fang, J. and Zhang, J. 2019. Reduction of phoxim pesticide residues from grapes by atmospheric pressure non-thermal air plasma activated water. J. hazardous materials. 377, 98–105. Zhou, R.W., Zhou, R.S., Wang, P.Y., Xian, Y.B., Mai-Prochnow, A., Lu, X.P., Cullen, P.J., Ostrikov, K. and Bazaka, K. 2020. Plasma-activated water: generation, origin of reactive species and biological applications. J. Phys. D Appl. Phys. 53 (30), 303001. | ||
| How to Cite this Article |
| Pubmed Style Alnaemi HS, Dawood TN, Algwari QT. Plasma-activated water application for detoxification of aflatoxin B1, ochratoxin A, and fumonisin B1 in poultry feeds. Open Vet J. 2023; 13(12): 1654-1668. doi:10.5455/OVJ.2023.v13.i12.15 Web Style Alnaemi HS, Dawood TN, Algwari QT. Plasma-activated water application for detoxification of aflatoxin B1, ochratoxin A, and fumonisin B1 in poultry feeds. https://www.openveterinaryjournal.com/?mno=170300 [Access: May 13, 2024]. doi:10.5455/OVJ.2023.v13.i12.15 AMA (American Medical Association) Style Alnaemi HS, Dawood TN, Algwari QT. Plasma-activated water application for detoxification of aflatoxin B1, ochratoxin A, and fumonisin B1 in poultry feeds. Open Vet J. 2023; 13(12): 1654-1668. doi:10.5455/OVJ.2023.v13.i12.15 Vancouver/ICMJE Style Alnaemi HS, Dawood TN, Algwari QT. Plasma-activated water application for detoxification of aflatoxin B1, ochratoxin A, and fumonisin B1 in poultry feeds. Open Vet J. (2023), [cited May 13, 2024]; 13(12): 1654-1668. doi:10.5455/OVJ.2023.v13.i12.15 Harvard Style Alnaemi, H. S., Dawood, . T. N. & Algwari, . Q. T. (2023) Plasma-activated water application for detoxification of aflatoxin B1, ochratoxin A, and fumonisin B1 in poultry feeds. Open Vet J, 13 (12), 1654-1668. doi:10.5455/OVJ.2023.v13.i12.15 Turabian Style Alnaemi, Hiba Salahaldeen, Tamara Natiq Dawood, and Qais Thanoon Algwari. 2023. Plasma-activated water application for detoxification of aflatoxin B1, ochratoxin A, and fumonisin B1 in poultry feeds. Open Veterinary Journal, 13 (12), 1654-1668. doi:10.5455/OVJ.2023.v13.i12.15 Chicago Style Alnaemi, Hiba Salahaldeen, Tamara Natiq Dawood, and Qais Thanoon Algwari. "Plasma-activated water application for detoxification of aflatoxin B1, ochratoxin A, and fumonisin B1 in poultry feeds." Open Veterinary Journal 13 (2023), 1654-1668. doi:10.5455/OVJ.2023.v13.i12.15 MLA (The Modern Language Association) Style Alnaemi, Hiba Salahaldeen, Tamara Natiq Dawood, and Qais Thanoon Algwari. "Plasma-activated water application for detoxification of aflatoxin B1, ochratoxin A, and fumonisin B1 in poultry feeds." Open Veterinary Journal 13.12 (2023), 1654-1668. Print. doi:10.5455/OVJ.2023.v13.i12.15 APA (American Psychological Association) Style Alnaemi, H. S., Dawood, . T. N. & Algwari, . Q. T. (2023) Plasma-activated water application for detoxification of aflatoxin B1, ochratoxin A, and fumonisin B1 in poultry feeds. Open Veterinary Journal, 13 (12), 1654-1668. doi:10.5455/OVJ.2023.v13.i12.15 |