| Research Article | ||
Open Vet J. 2023; 13(12): 1669-1682 Open Veterinary Journal, (2023), Vol. 13(12): 1669–1682 Original Research Investigation of the effect of mutual vaccination with pest des petits ruminants and polyvalent foot and mouth disease vaccines on the immune response of sheepToka Yaser1*, Nabil Bkear2, Yassien Badr2, Ehab El-Sayed Ibrahim1 and Mohamed Hassan Khodeir11Agriculture Research Center (ARC), Veterinary Serum and Vaccine Research Institute (VSVRI), Cairo, Egypt 2Department of Infectious Diseases and Epidemics, Faculty of Veterinary Medicine, Damanhour University, Damanhour, Egypt *Corresponding Author: Toka Yaser. Agriculture Research Center (ARC), Veterinary Serum and Vaccine Research Institute (VSVRI), Cairo, Egypt. Email: totyyasser676 [at] gmail.com Submitted: 10/10/2023 Accepted: 27/11/2023 Published: 31/12/2023 © 2023 Open Veterinary Journal
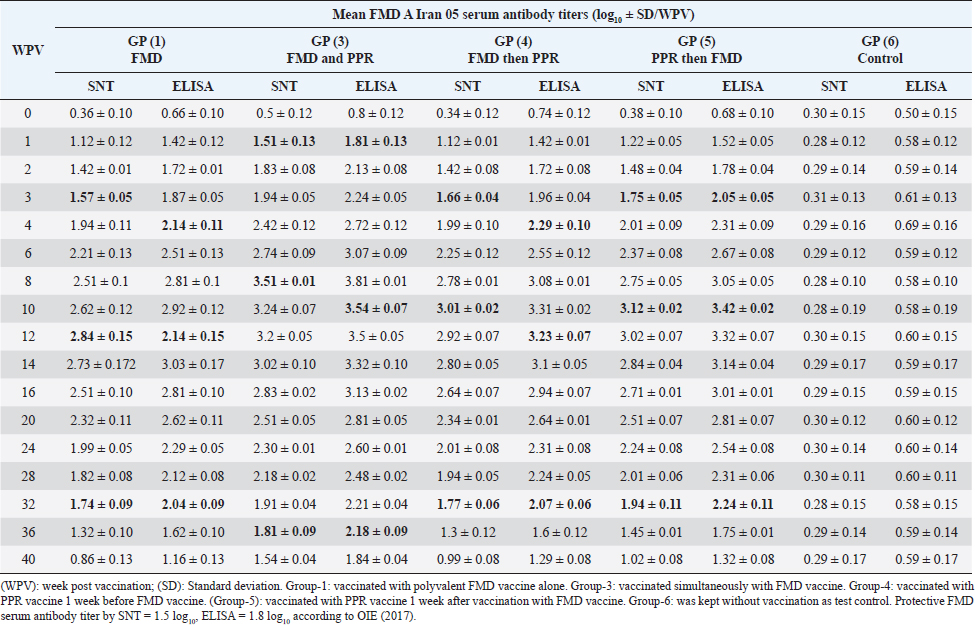
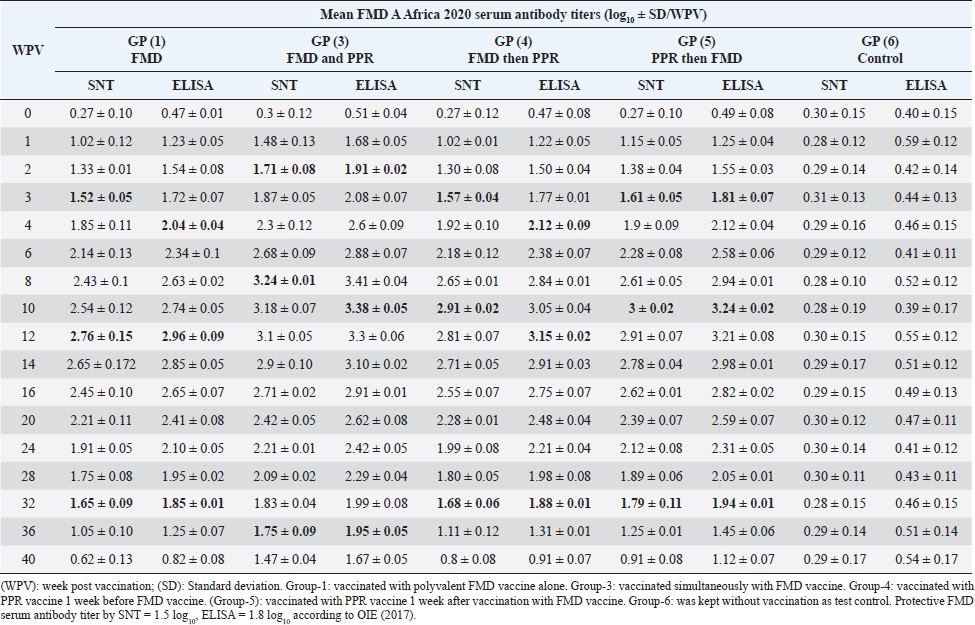
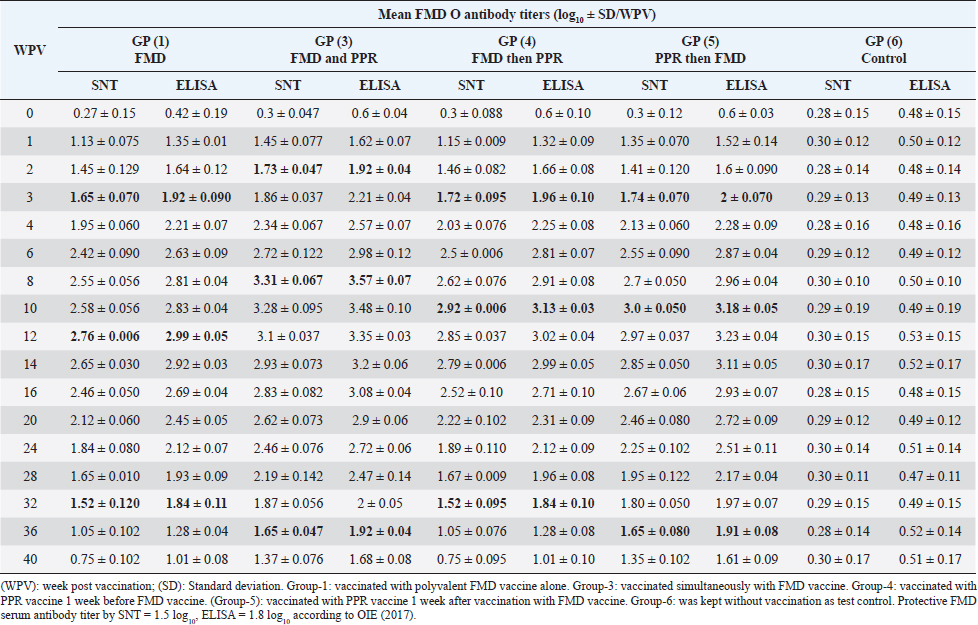
AbstractBackground: Pest des petits ruminants (PPRs) and foot and mouth disease (FMD) are two viral infectious diseases affecting sheep dramatically causing great economic losses. Therefore, attention should be directed toward their control, especially through the application of well-designed vaccination schedules with specific potent vaccines. Aim: Determination of the possibility of sheep vaccination with PPR and FMD vaccines in a mutual schedule. Methods: Different groups of sheep have vaccinated with live attenuated PPR vaccine and inactivated polyvalent FMD vaccine in a mutual manner (one before the other at weekly intervals or simultaneously) followed by monitoring of the induced immunity to both vaccines using serum neutralization test (SNT) and enzyme linked immune sorbent assay (ELISA). Results: SNT and ELISA revealed that there was no antagonizing effect of any vaccine on the immune response to the mutual vaccination of sheep to the other where the obtained antibody titers in single vaccinated sheep groups were similar to those in the simultaneous vaccinated group. Conclusion: Simultaneous vaccination of sheep with PPR and polyvalent FMD vaccine is of applicable benefit saving time, effort, and stress factors on the animals. Keywords: PPR, FMD, Vaccine, Serum neutralization, ELISA. IntroductionPest des petits ruminants (PPR) is an acute, highly contagious notifiable, and economically important transboundary viral disease of sheep and goats associated with high morbidity and mortality (Diallo et al., 2007). The disease was responsible for at least US$1.5 million in economic losses to the Iranian owners of sheep and goats (Bazarghani et al., 2006). This disease is caused by structurally, Morbillivirus as pleomorphic, enveloped particles (Gibbs et al., 1979). It causes severe clinical signs depending on the species, age, strain virulence, and secondary infectious agents. Such signs include fever, respiratory signs, off food, depression, erosive stomatitis, catarrhal inflammation of ocular and nasal mucous, profuse watery diarrhea, fetid and blood-stained and often end-stage bronchopneumonia due to bacterial complications and immunosuppression (Zahur et al., 2009; OIE, 2013 and Chowdhury et al., 2014). PPR infection was recorded in sheep flocks in the Giza governorate (Moatamadia village, Zenien, Saft El-Labn, and Kafrberak El-Khiam) (Safwat, 2015). PPR has reappeared in some Egyptian governorates (Mahmoud et al., 2017; Ahmed et al., 2021). PPR virus (PPRV) antibodies were detected in 63.7% of sheep and goats, in which goats were more sensitive to infection of the PPR virus than sheep, and the viral antigen detection increased in winter more than in other seasons. In addition, the results showed that the percentage of positivity increased in middle Egypt than in other localities of Egypt (Wafaa et al., 2021). Morbidity and mortality rates in PPRV endemic areas are lower compared to the rates observed in PPRV epidemics in nonendemic areas. Mortality is higher in young animals compared to adults in both endemic and epidemic settings, and goats tend to be more severely affected than sheep (Truong et al., 2014). PPR antibody levels reached protective levels within 21 days of sheep vaccination with live attenuated PPR vaccine, which continued up to 1 year (Baksi et al., 2018) where serum neutralization test (SNT) titer ≥8 was deemed to be protective (Santhosh et al., 2013). No untoward reactions were observed following the vaccination of sheep with live attenuated PPR vaccine and the immune response was uniform in all the age groups of sheep under study. All vaccinated animals developed high titer of antibodies (Saritha et al., 2015). Vaccinated goats with live attenuated PPR vaccines (Nigeria 75/1 and Sungri 96) by either the subcutaneous or intranasal route, and 28 days later challenged intranasally with virulent PPR virus, regardless of vaccination route, produced PPRV-specific antibodies post vaccination and were protected from clinical disease, and considered to have induced sterilizing immunity. (Mahapatra et al., 2020). Foot and mouth disease (FMD) is the most important disease of domestic livestock in the world in terms of economic impact due to its ability to cause losses of reduced milk yields, abortions, prenatal mortalities, premature culling, hindering trade of animals locally and internationally and restriction on movement of people which affect the tourism sector (James and Rushton, 2002). It affects cloven-hoofed animals, including farm animals and wildlife species, inflicting severe damage to the international trade and livestock industry (Di Giacomo et al., 2022). Perry and Rich (2007) reported that FMD is a highly contagious transboundary animal disease that is capable of spreading rapidly among susceptible animals and devastates livestock populations. The disease may fail to develop in approximately 25% of infected sheep while a further 20% may develop only a single observable lesion (Hughes et al., 2002). The clinical signs of FMD are mild with few lesions in sheep and goats and include mild oral lesions and lameness, fever, death of young animals, foot lesions along the coronary band or interdigital spaces and on the dental pad and agalactia in milking sheep and goats (Amas et al., 2003; El-Shehawy et al., 2004). Recently, the FMD virus (FMDV) has been classified in the order Picornavirades of the family Picornaviridae. In addition to the genus Aphthovirus, to which this type of animal viruses belongs, this family involved Bovine rhinitis B virus, Equine rhinitis A virus, and Bovine rhinitis A virus (Azeem et al., 2020; Veronika and Michael, 2020). The virus probably most often infects sheep and goats by direct contact. It was found that contact sheep with infected pigs had developed gross lesions consistent with FMD by 5 days postinfection. Sheep play an important role in the epidemiology and transmission of FMD. Moreover, FMD is suspected to have been transmitted to sheep in which infection is frequently sub-clinical. Therefore, it is important to identify animals that have been exposed to the virus and have developed antibodies. Such animals may become carriers and thus be a potential source of new outbreaks (Amas et al., 2003; El-Shehawy et al., 2004). It was stated that the clinical signs of FMD are mild with few lesions in sheep and goats and include mild oral lesions and lameness, fever, death of young animals, foot lesions along the coronary band or interdigital spaces and on the dental pad, and agalactia in milking sheep and goats (Queensland government, 2021). It was concluded that different serological techniques such as solid-phase competition enzyme linked immune sorbent assay (ELISA), virus neutralization test (VNT), and liquid-phase blocking ELISA can be used for the detection of antibodies against FMDV that appear in the serum of cattle after 4 days of infection and referred to the existence of previous or circulating infection. Furthermore, it can be applied to distinguish between immunized and infected animals by detection of structural and nonstructural proteins (Wong et al., 2020). This work was designed to determine to any extent PPR and FMD vaccines could be administrated to sheep in a mutual schedule on the needed time, i.e., the wanted time of vaccination; through investigation of the effect of one of them on the immune response of vaccinated sheep in addition to evaluated the benefit of each used vaccination schedule. Materials and MethodsFMD vaccinePolyvalent FMD montanide ISA206 inactivated vaccine was supplied by the FMD department, Veterinary Serum and Vaccine Research Institute (VSVRI), and used to vaccinate the experimental sheep using a dose of 1.5 ml/sheep. FMD virus strainsA locally isolated FMD virus serotypes O Pan-Asia-2; A Iran 05; A Africa 2020; A Africa G IV Egypt 2022; A Venezuela and SAT2/EGY/2012 of calves’ origin were typed and subtyped at the FMD Research Department, VSVRI, Abasia, Cairo and confirmed by the World Reference Laboratories, Pirbright, UK. These viral serotypes were stored at −70°C and were used for vaccine preparation and serological tests (SNT and ELISA). PPR virusThe Vero cells adapted PPRV Nigeria 75/1 vaccine strain was obtained from the reference laboratory PANVAC, Debre Zeit, Ethiopia; and used for vaccine production and serological assays by Rinderpest Vaccine Research Department, (VSVRI), Abasia, Cairo, Egypt. The virus titer was 106 TCID50/ml. PPR vaccineLive attenuated Vero cell PPR vaccine was supplied by RPRD and used for vaccination of the experimental sheep using a dose of 102.5 TCID50/sheep inoculated S/C. Experimental animalsThirty healthy native breed male sheep of 6–12 months old were found to be free from external and internal parasites as clinically examined and serologically negative against FMDV and PPR antibodies as proved by SNT and ELISA. They were housed under hygienic measures in separate pens, receiving balanced ration and adequate water. These sheep were subjected to mutual vaccination with FMD and PPR vaccines as follows where they were divided into six groups (five sheep/group): Group-1, vaccinated with the inactivated montanide adjuvanted polyvalent FMD vaccine Group-2, vaccinated with the live attenuated PPR vaccine Group-3, vaccinated simultaneously with the two vaccines Group-4, vaccinated with FMD vaccine 1 week after vaccination with PPR vaccine Group-5, vaccinated with PPR vaccine 1 week after vaccination with FMD vaccine. The used doses were 1.5 ml of FMD/sheep and 102.5TCID50/sheep of PPR vaccine inoculated subcutaneously in the neck side. Group-6 was kept without vaccination as a test control. Cell culturesBaby Hamster kidney cell line (BHK21) It was kindly supplied by the Animal Research Institute, Pirbright, UK, and propagated at the FMD department, VSVRI using minimum essential medium with Eagle’s salts supplemented with 10% newborn calf serum and used in SNT and virus titration. African green monkey kidney cells (Vero) A certified Vero cells were used for PPR vaccine preparation and performance of SNT. The cells were maintained at the department of RPVR, VSVRI, Abasia, Cairo. Blood samplesSerum samples were separated from whole blood samples after being kept at room temperature for 2 hours and centrifugated for 20 minutes at approximately 1,000×g. The clear obtained serum samples were stored at −80°C to avoid loss of bioactivity and contamination. Serum samples were collected from all sheep groups before and weekly after vaccination for 1 month then every 2 weeks up to 40 weeks post vaccination for monitoring of induced FMD and PPR antibody levels. Evaluation of sheep humeral immune response to PPR and FMD vaccinesSerum samples collected from all sheep’s groups were subjected to estimation of PPR and FMD antibody titers (serotypes O pan Asia, A Iran O5, SAT2/ EGY/2012 and SAT2/EGY/2018) by SNT using the micro titer technique (Ferreira, 1976) where FMD neutralizing antibody titer was expressed as log10/ml according to Reed and Muench (1938) while PPR neutralizing antibody titer was expressed as the reciprocal of the final serum dilution which neutralized and inhibited the CPE of 100 TCID50 of PPR virus according to Singh et al. (1967). On the other side, indirect ELISA was carried out as described by Voller et al. (1976). Statistical analysisThe obtained data were analyzed using analysis of variance in the SPSS-12 statistical software package. Multiple comparisons of means were made using Duncan’s multiple range tests at p ˂ 0.05%. The results represent the average of five replicates and are presented as the mean ± SE. Ethical approvalThe work was carried out according to DMU/VetMed-2023/040. ResultsRegarding FMD type O serum neutralizing antibody titers, it was found that they reached a protective level (1.73 ± 0.047) by the second week post vaccination (WPV) in the case of sheep simultaneous vaccination with PPR while in the case of polyvalent FMD single vaccination it was (1.65 ± 0.070) by the third WPV as well as in case of administration FMD vaccine 1 week before PPR vaccine (1.72 ± 0.095) and in case of vaccination with PPR vaccine 1 week before FMD (1.74 ± 0.070). These titers reached their peaks (3.31 ± 0.067, 2.76 ± 0.006, 2.92 ± 0.006, and 3.0 ± 0.050) by the 8th, 12th, 10th, and 10th-week post vaccination then began to decrease gradually by the 10th and 12th week to record nonprotective levels (1.37 ± 0.076 on the 40th week, 1.05 ± 0.102 on 36th week, 1.05 ± 0.076 on 36th week, and 1.35 ± 0.102 on the 40th week in sheep simultaneously vaccinated with the two vaccines; in single FMD vaccination, in FMD vaccination before PPR and PPR vaccination before FMD, respectively). The results of ELISA came parallel to the results of SNT with the values 1.92 ± 0.04, 1.92 ± 0.090, 1.96 ± 0.10, and 2 ± 0.070 by the second week and a third week post vaccination in both sheep groups vaccinated simultaneously; vaccinated with FMD before PPR and PPR vaccination before FMD, respectively) recording ELISA peaks 3.57 ± 0.07 by the 8th WPV, 2.99 ± 0.05 by the 12th week, and 3.13 ± 0.03 and 3.18 ± 0.05 by the 10th week post vaccination, respectively, to reach nonprotective levels 1.68 ± 0.08, 1.28 ± 0.04, 1.28 ± 0.08, and 1.61 ± 0.09 by the 40th, 36th, 36th, and 40th WPV, respectively, as shown in Table 1. FMD type A Iran serum neutralizing antibody titers reached a protective level (1.51 ± 0.13) by the first WPV in the case of sheep simultaneous vaccination with PPR, while in the case of polyvalent FMD single vaccination it was (1.57 ± 0.05) by the third WPV as well as in case of administration FMD vaccine 1 week before PPR vaccine (1.66 ± 0.04) and in case of vaccination with PPR vaccine 1 week before FMD (1.75 ± 0.05) at third WPV. These titers reached their peaks (3.51 ± 0.01, 2.84 ± 0.15, 3.01 ± 0.02, and 3.12 ± 0.02) by the 8th, 12th, 10th, and 10th WPV then began to decrease gradually by the 10th and 12th week to record nonprotective levels (1.54 ± 0.04 on the 40th week, 1.32 ± 0.10 on 36th week, 1.3 ± 0.12 and 1.45 ± 0.01 on 36th week in sheep simultaneously vaccinated with the two vaccines; in single FMD vaccination, in FMD vaccination before PPR and PPR vaccination before FMD, respectively). The results of ELISA came parallel to those of SNT with the values 1.92 ± 0.04 by the 1st WPV; 2.14 ± 0.11, 2.29 ± 0.10, and 2.05 ± 0.05 by the 4th week and 3rd week post vaccination in both sheep groups vaccinated simultaneously; vaccinated with FMD before PPR and PPR vaccination before FMD, respectively) recording ELISA peaks 3.54 ± 0.07 by the 10th WPV, 2.14 ± 0.15 by the 12th week, 3.23 ± 0.07 by the 12th week, and 3.42 ± 0.02 by the 10th week post vaccination, respectively, to reach nonprotective levels 1.84 ± 0.04, 1.62 ± 0.10, 1.6 ± 0.12, and 1.75 ± 0.01 by the 40th, 36th, 36th, and 36th WPV, respectively. These results are tabulated in Table 2. Among FMD type A Africa 2020 serum neutralizing antibody titers, it was found that they recorded a protective level (1.71 ± 0.08) by the second WPV in case of sheep simultaneous vaccination with PPR while in case of polyvalent FMD single vaccination, it was (1.57 ± 0.05) by the third WPV as well as in case of administration FMD vaccine 1 week before PPR vaccine (1.57 ± 0.04) and in case of vaccination with PPR vaccine 1 week before FMD (1.61 ± 0.05) at third WPV. These titers reached their peaks (3.24 ± 0.01, 2.76 ± 0.15, 2.91 ± 0.02, and 3 ± 0.02) by the 8th, 12th, 10th, and 10th WPV then began to decrease gradually by the 10th and 14th week to record nonprotective levels (1.47 ± 0.04 on the 40th week, 1.05 ± 0.10 on 36th week, 1.11 ± 0.12 and 1.25 ± 0.01 on 36th week in sheep simultaneously vaccinated with the two vaccines; in single FMD vaccination, in FMD vaccination before PPR and PPR vaccination before FMD, respectively). In a parallel manner to the results of SNT, ELISA results showed the values 1.91 ± 0.02 by the 2nd WPV, and 2.04 ± 0.04, 2.12 ± 0.09, and 1.81 ± 0.07 by the 4th week and 3rd week post vaccination in both sheep groups vaccinated simultaneously; vaccinated with FMD before PPR and PPR vaccination before FMD, respectively) recording ELISA peaks 3.38 ± 0.05 by the 10th WPV, 2.96 ± 0.09 by the 12th week, 3.15 ± 0.02 by the 12th week, and 3.24 ± 0.02 by the 12th week post vaccination, respectively, to reach nonprotective levels 1.67 ± 0.05, 1.25 ± 0.07, 1.31 ± 0.01, and 1.45 ± 0.06 by the 40th, 36th, 36th, and 36th WPV, respectively, as shown in Table 3. FMD type A Africa G IV Egypt 2022 serum neutralizing antibody titers were found with a protective level (1.62 ± 0.14) by the second WPV in the case of sheep simultaneous vaccination with PPR while in case of polyvalent FMD single vaccination it was (1.50 ± 0.1) by the third WPV as well as in case of administration FMD vaccine 1 week before PPR vaccine (1.6 ± 0.02) at third WPV and in case of vaccination with PPR vaccine 1 week before FMD (1.51 ± 0.05) at second WPV. These titers with their peaks 3.19 ± 0.31, 2.86 ± 0.18, 2.89 ± 0.12, and 3.1 ± 0.12 recorded by the 8th, 12th, 10th, and 10th WPV began to decrease gradually by the 10th and 14th week to record nonprotective levels (1.32 ± 0.08 on the 40th week, 1.17 ± 0.15 and 1.21 ± 0.12 on 36th week, 1.21 ± 0.09 on 40th week in sheep simultaneously vaccinated with the two vaccines; in single FMD vaccination, in FMD vaccination before PPR and PPR vaccination before FMD, respectively). Table 2. Mean FMD serotype A Iran 05 serum antibody titer in vaccinated sheep.
Table 3. Mean FMD serotype A Africa 2020 serum antibody titer in vaccinated sheep.
Table 4. Mean FMD serotype A Africa G IV Egypt 2022serum antibody titer in vaccinated sheep.
Table 5. Mean FMD serotype A Venezuela serum antibody titer in vaccinated sheep.
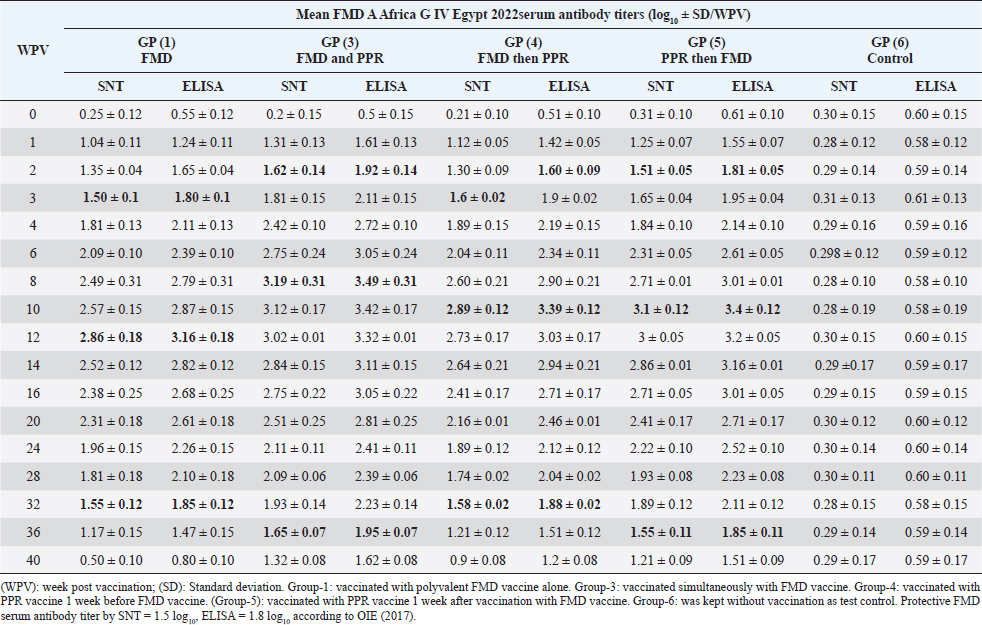
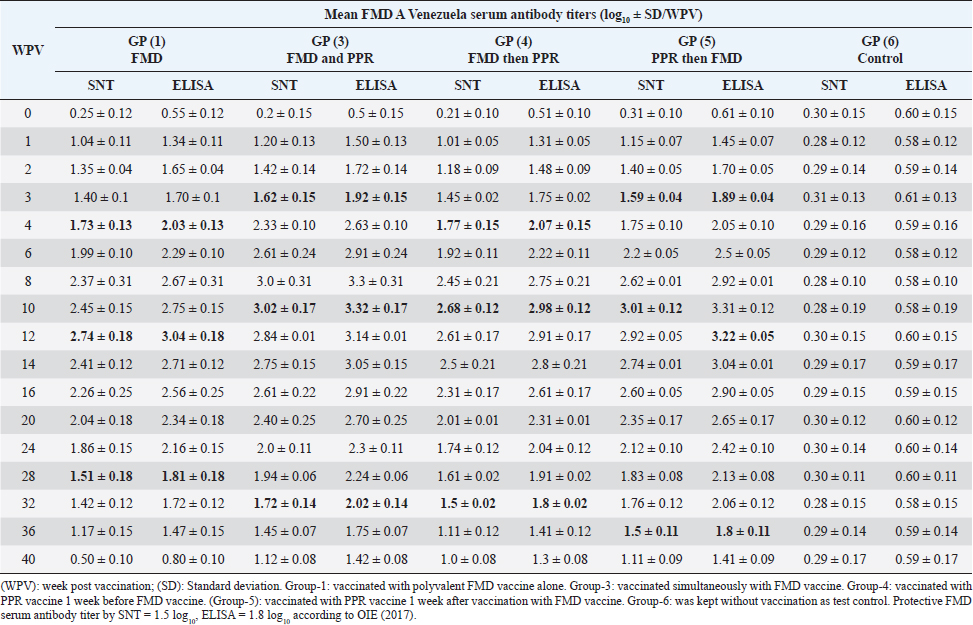
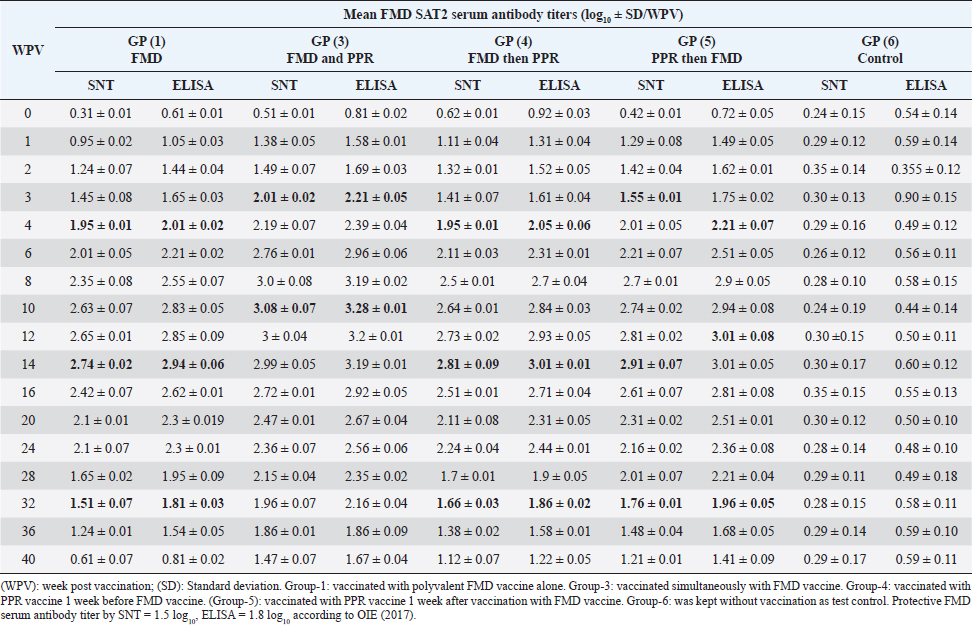
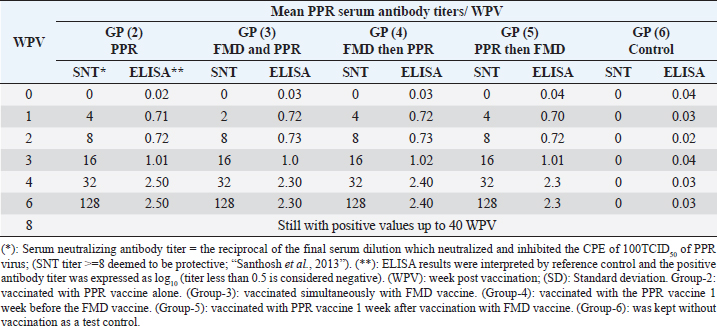
On the other side, the results of ELISA came in a similar manner as those of SNT with the values 1.92 ± 0.14 by the 2nd WPV, 1.80 ± 0.1, 1.9 ± 0.02, 1.81 ± 0.05 by the 4th week and 2nd week post vaccination in both sheep groups vaccinated simultaneously; vaccinated with FMD before PPR and PPR vaccination before FMD, respectively) recording ELISA peaks 3.49 ± 0.31 by the 8th WPV, 3.16 ± 0.18 by the 12th week, and 3.39 ± 0.12 by the 10th week and 3.4 ± 0.12 by the 10th week post vaccination, respectively, to reach nonprotective levels 1.62 ± 0.08, 1.47 ± 0.15, 1.51 ± 0.12, and 1.51 ± 0.09 by the 40th, 36th, 36th, and 40th WPV, respectively, as shown in Table 4. FMD type A Venezuela serum neutralizing antibody titers, reached a protective level (1.62 ± 0.15) by the thi rd WPV in the case of sheep simultaneous vaccination with PPR, while in the case of polyvalent FMD single vaccination, it was (1.73 ± 0.13) by the fourth WPV as well as in case of administration FMD vaccine 1 week before PPR vaccine (1.77 ± 0.15) at fourth WPV and in case of vaccination with PPR vaccine 1 week before FMD (1.59 ± 0.04) at thirdWPV. These titers reached their peaks (3.02 ± 0.17, 2.74 ± 0.18, 2.68 ± 0.12, and 3.01 ± 0.12) by the 10th, 12th, 10th, and 10th WPV then began to decrease gradually by the 12th and 14th week to record nonprotective levels (1.45 ± 0.07 on the 36th week, 1.42 ± 0.12 on 32th week, 1.11 ± 0.12 on 36th week, and 1.11 ± 0.09 on 40th week in sheep simultaneously vaccinated with the two vaccines; in single FMD vaccination, in FMD vaccination before PPR and PPR vaccination before FMD, respectively). FMD type A Venezuela ELISA results as those of SNT showed values 1.92 ± 0.15 by the 3rd WPV, 2.03 ± 0.13, 2.07 ± 0.15 by the 4th week, and 1.89 ± 0.04 3rd week post vaccination in both sheep groups vaccinated simultaneously; vaccinated with FMD before PPR and PPR vaccination before FMD, respectively) with peak values of 3.32 ± 0.17 by the 10th WPV, 3.04 ± 0.18 by the 12th week, 2.98 ± 0.12, and 3.31 ± 0.12 by the 10th week post vaccination, respectively, to reach nonprotective levels 1.75 ± 0.14, 1.72 ± 0.12, 1.41 ± 0.12, and 1.41 ± 0.09 by the 36th, 32th, 36th, and 40th WPV, respectively, as shown in Table 5. The exhibited FMD type SAT2 serum neutralizing antibody titers in different vaccinated sheep groups were within a protective level (2.01 ± 0.02) by the 3rd WPV in case of sheep simultaneous vaccination with PPR, while in case of polyvalent FMD single vaccination, it was (1.95 ± 0.01) by the 4th WPV as well as in case of administration FMD vaccine 1 week before PPR vaccine (1.95 ± 0.01) at 4th WPV and in case of vaccination with PPR vaccine 1 week before FMD (1.53 ± 0.01) at 3rd WPV. Peak SNT titers (3 ± 0.04, 2.74 ± 0.02, 2.81 ± 0.09, and 2.91 ± 0.07) were determined by the 12th, 14th, 14th, and 14th WPV then began to decrease gradually by the 14th and 16th week to record nonprotective levels (1.47 ± 0.08 on the 40th week, 1.24 ± 0.01, 1.38 ± 0.01, and 1.48 ± 0.04 on 36th week in sheep simultaneously vaccinated with the two vaccines; in single FMD vaccination, in FMD vaccination before PPR and PPR vaccination before FMD, respectively). In a similar way the results of ELISA as those of SNT showed protective values of 2.21 ± 0.05 by the 3rd WPV, 2.01 ± 0.02, 2.05 ± 0.06, 2.21 ± 0.07 by the 4th week and 2nd week post vaccination in both sheep groups vaccinated simultaneously; vaccinated with FMD before PPR and PPR vaccination before FMD, respectively) with peak values of 3.2 ± 0.01 by the 12th WPV, 2.94 ± 0.06, and 3.01 ± 0.01 by the 14th week, and 3.01 ± 0.08 by the 12th week post vaccination, respectively, reaching nonprotective levels (1.67 ± 0.04, 1.54 ± 0.05, 1.58 ± 0.01, and 1.68 ± 0.05) by the 40th, 36th, 36th, and 36th WPV, respectively (Table 6). Vaccination of different groups of sheep with live attenuated PPR vaccine revealed that all of them exhibited protective serum-neutralizing antibody titer (8) by the second week recording their peak (128) by the 6th week and still constant up to 40 WPV without any antagonizing effect of FMD vaccine. The results of indirect ELISA confirmed those of SNT indicating that all vaccinated sheep exhibited protective PPR-ELISA titers (0.70–0.72 log10) by the first week with peak titer 2.3–2.5 log10 on the 6th week which remained stable within protective level up to 40 weeks post vaccination as shown in Table 7. DiscussionThe main attention of vaccine researchers is often directed toward the determination of the most valuable vaccination schedule of livestock especially against devastating infectious viral diseases such as PPR and FMD. Depending on the fact that although sheep infected with the FMD virus usually do not show clear clinical signs, they play a very important role in the disease epidemiology where they act as carrier host representing a source of infection to the higher susceptible host (cattle and buffalo) (Ganter et al., 2001); it is a very important aim to aid the disease control through vaccination of sheep. In addition, the present work sheds light on the possibility of mutual vaccination of sheep with PPR and FMD vaccines. The study includes the vaccination of five groups of native male sheep (five sheep/group) subjected to single PPR and FMD vaccinations; one vaccine 1 week before the other and simultaneous vaccination followed by evaluation of their humeral immune response through the application of SNT and indirect ELISA of their serum samples on different intervals up to 40 weeks post vaccination. The present obtained SNT and indirect ELISA results (tabulated in Tables 1–6) revealed that the induced antibodies in vaccinated sheep against the five serotypes of FMD virus had the same behavior reached high protective levels by the second week post vaccination in the case of sheep simultaneous vaccination with PPR higher than those obtained in case of polyvalent FMD single vaccination as well as in case of administration FMD vaccine 1 week before PPR vaccine and in case of vaccination with PPR vaccine 1 week before FMD. These titers reached their peaks by the 8th, 12th, 10th, and 10th week post vaccination, respectively, then began to decrease gradually by the 10th and 12th week to record nonprotective levels on the 40th week in sheep simultaneously vaccinated with the two vaccines; in single FMD vaccination, in FMD vaccination before PPR and PPR vaccination before FMD, respectively). These findings appear to be supported by those of (Fatthia, 2003) who obtained similar results indicating that the immune response of vaccinated goats to the FMD Montanide ISA 206 vaccine persisted for 36 weeks and the protective FMD serum neutralizing antibody titer is not less than 1.5 log10 and ELISA titer 1.8 log10 with similar duration of immunity. In addition, the use of FMD virus quadrivalent double emulsion (Montanide ISA206) vaccines in sheep elicited a good immune quickly developed response and the animals maintained their neutralizing antibody titers at >3 log10 for a long duration (Patil et al., 2002). Table 1. Mean FMD serotype O serum antibody titer in vaccinated sheep.
Significantly at p ˂ 0.05%, it is clear that these findings indicate that the PPR vaccine enhances the immune response of vaccinated sheep to the FMD vaccine whether it was administrated 1 week before, after, or simultaneously with the FMD vaccine. Such enhancement induced a longer duration of protective levels of FMD antibody levels (40 weeks) which was 36 weeks in the case of a single FMD vaccination. In this respect, Mansoor et al. (2017) found that goats vaccinated with PPR and FMD vaccines had significantly (p < 0.05) higher antibody titers to two serotypes of FMD virus at 28-, 45-, and 60-day post immunization compared to goats vaccinated with FMD vaccine alone, while goats vaccinated with PPR vaccines alone or PPR and FMD vaccines exhibited similar antibody kinetics against PPR virus up till 60 days post vaccination. In addition, previous studies clarified that sheep and goats simultaneously vaccinated with FMD and PPR vaccines exhibited the same immune response without significant differences (Ibrahim et al., 2000; Abdel-Razek et al., 2006). It has been described that the PPR vaccine virus does not interfere with the immunogenicity of unrelated antigens (Rajak et al., 2005). The total antibody levels were significantly high at 28th, 45th, and 60th DPV for FMDsO and at the 45th and 60th DPV for FMDsA1 in the group that was vaccinated with FMD-vac + PPR-vaccine compared to FMD vaccine applied singly. This observation also supports the lack of interference by the PPR vaccinal virus when administered concurrently with the FMD vaccine. Table 6. Mean FMD serotype SAT2 serum antibody titer in vaccinated sheep.
Table 7. Mean PPR serum antibody titer in vaccinated sheep.
Regarding the induced PPR antibodies in the different vaccinated sheep, the results of SNT and EKISA (Table7) revealed that all animals exhibited protective antibody titers (8 by SNT and more than 0.5log10 by ELISA) by the second week with a peak titer (128) by the 6th week without change up to 40 weeks post vaccination without any antagonizing effect of FMD vaccine. It was stated that PPR SNT titer ≥8 was deemed to be protective (Santhosh et al., 2013). A single dose of a PPR vaccine contains ~103 TCID50 of Vero cell-attenuated PPRV and is believed to provide protective immunity in sheep and goats for about 4 years (Singh et al., 2009). In addition, the vaccine is considered quite safe without any significant immunosuppressive effect on the host (Rajak et al., 2005). It was noticed that the polyvalent FMD vaccine did not antagonize sheep immune response to the PPR vaccine as previously showed by Ibrahim et al. (2000); Abdel-Razek et al. (2006) and Mansoor et al. (2017) who showed that sheep vaccinated singly with PPR vaccine exhibited similar antibody levels as those vaccinated with FMD vaccine. ConclusionDepending on the present results we could conclude that vaccination of sheep with PPR and FMD vaccines does not antagonize animal immune response to any of them preferring the administration of the PPR vaccine simultaneously with FMD or 1 week after it to reach a higher level of FMD immunity with longer duration than in case of single FMD vaccination. In addition, and as vaccination is the main control strategy of FMD and PPR simultaneous vaccinations help to increase work speed and reduce labor and vaccination stress for the animals. AcknowledgmentsThe authors would like to thank the Departments of FMD Vaccine Research and PPR Vaccine Research; VSVRI, Abasia, Cairo, and the Department of Infectious Diseases and Epidemics, Faculty of Veterinary Medicine, Damanhur University, for their support. Conflict of interestThe authors declare that they have no competing interests. FundingThe study did not receive any external funds. Authors contributionsToka Yaser: The experimental researcher. Nabil Bkear: The academy supervisor. Yassien Badr: The scientific writing reviewer. Ehab El-Sayed Ibrahim: The supervisor on FMD research work. Mohamed Hassan Khodeir: The supervisor on PPR research work. Data availabilityAll data are provided in the manuscript. Any extra data needed can be provided on reasonable request from the corresponding author. ReferencesAbdel-Razek, B.A., Hussein, G.H., Manal, A.M., Amal, A.F. and Soad, M.S. 2006. Effect of simultaneous vaccination with goat pox and PPR vaccines on the immune response of goats. Benha. Vet. Med. J. 17, 255–263. Ahmed, S., Hosny, W.A., Mahmoud, M. and Mahmoud, M.A. 2021. Isolation and identification of peste des petits ruminants virus from goats in Egyptian governorates. Vet. World. 14(4), 926–932. Amas, S.F., Pacheco, J.M., Mason, P.W., Schneider, J.L., Alvarez, R.M., Clark, L.K. and Ragland, D. 2003. Procedures of preventing the transmission of FMDV to pigs and sheep by personnel in contact with infected pigs. Vet. Rec. 153, 137–140. Azeem, A., Rashid, I., Hassan, M.M., Asad, M., Kaukab, G., Tehseen, A. and Aamir, S. 2020. A review on foot and mouth disease in dairy animals, etiology, pathogenesis and clinical findings. Pure. Appl. Biol. 9(1), 821–832. Baksi, S., Dave, H., Rao, N., Malsaria, P., Khan, M.Q. and Pk, C. 2018. Evaluation of peste des petits ruminants (PPR) cell culture vaccine in goat and sheep in INDIA. Bangladesh. J. Vet. Med. 16(1), 59–63. Bazarghani, T.T., Charkhkar, S., Doroudi, J. and Bani Hassan, E. 2006. A review on peste des petits ruminants (PPR) with special reference to PPR in Iran. J. Vet. Med. B. Infect. Dis. Vet. Public. Health. 53(1), 17–18. Chowdhury, E.H., Bhuiyan, A.R., Rahman, M.M., Siddique, M.S.A. and Islam, M.R. 2014. Natural peste des petits ruminants virus infection in black Bengal goats: virological, pathological and immunohistochemical investigation. BMC. Vet. Res. 10, 1–10. Diallo, A., Minet, C., Le Goff, C., Berhe, G., Albina, E., Libeau, G. and Barrett, T. 2007. The threat of peste des petits ruminants: progress in vaccine development for disease control. Vaccine 25, 5591–5597. Di Giacomo, S., Bucafusco, D., Schammas, J.M., Pega, J., Miraglia, M.C., Barrionuevo, F., Capozzo, A.V. and Perez-Filgueira, D.M. 2022. Assessment on different vaccine formulation parameters in the protection against heterologous challenge with FMDV in cattle. Viruses 14, 1781. El-Shehawy, L.E., Tallat, A.A. and EL-Watany, H.M. 2004. Some studies on maternal immunity of FMD in sheep. J. Egypt. Vet. Med. Assoc. 64, 3. Fatthia, A.M. 2003. Vaccination of goats with FMD vaccines. MVSc Thesis, University of Alexandria, Alexandria, Egypt. Ferreira, M.E.V. 1976. Prubade microneutralization poraestudies de anticueropos de la fibre aftosa. In 13th Centropanamericano Fiebre Aftosa, (21/22), pp 17–24. Ganter, M., Grau nke, W.D., Steng, G. and Worbes, H. 2001. FMD in sheep and goats. PhD Thesis, University of London, London, UK. Gibbs, P.J., Taylor, W.P., Lawman, M.J. and Bryant, J. 1979. Classification of pest des petits ruminant’s virus as the fourth member of the genus Morbillivirus. Int. Virol. 11, 268–274. Hughes, G.J., Kitching, R.P. and Woolhouse, M.E.J. 2002. Dose dependent response of sheep inoculated intranasally with a type ‘O’ FMDV. J. Comp. Pathol. 127, 22–29. Ibrahim, I.M.I., Wafaa, E.D. and Daoud, A.M. 2000. Serological response of sheep vaccinated with combined PPR and FMD vaccine. Suez. Canal. Vet. Med. J. 11, 319–325. James, A.D. and Rushton, I. 2002. The economics of FMD. Rev. Sci. Tech. Off. Int. Epiz. 21(3), 637–644. Mahapatra, M., Selvaraj, M. and Parida, S. 2020. Comparison of immunogenicity and protective efficacy of PPR live attenuated vaccines (Nigeria 75/1 and Sungri 96) administered by intranasal and subcutaneous routes. Vaccines 8(2), 168. Mahmoud, M.A., Elbayoumy, M.K., Sedlky, D. and Ahmed, S. 2017. Serological investigation of some important RNA viruses affecting sheep and goats in Giza and Beni- Suef governorates in Egypt. Vet. World. 10(10), 1161–1166. Mansoor, M.K., Al-Rawahi, A.H., El-Tahir, H.A., Al-Faraei, B., Hussain, M.H., Asi, M.N., Al-Hussani, I. and Sabar, S. 2017. Concurrent vaccination of goats with foot and mouth disease (FMD) and peste des petits ruminants (PPR) booster vaccines. Trop. Anim. Health. Prod. 50, 1–3. OIE. 2013. Peste des petits ruminants, OIE terrestrial manual. Paris, France: OIE, pp: 1–14. OIE. 2017. Manual of diagnostic tests and vaccines for terrestrial animals. Paris, France: OIE. Patil, P.K., Bayry, J., Ramakrishna, C., Hugar, B., Misra, L.D. and Natarajan, C. 2002. Immune response of goats against FMD quadrivalent vaccine: comparison of double oil emulsion and aluminum hydroxide gel vaccine in eliciting immunity. Vaccine 20, 2781–2789. Perry, B.D. and Rich, K.M. 2007. Poverty impacts of foot-and-mouth disease and the poverty reduction implications of its control. Vet. Rec. 160, 238–241. Queensland government. 2021. Available via https://www.publications.qld.gov.au/dataset/c9f25be4-8c5f-4317-8901-b2f80f765381/resource/44a056ce-ee83-44ac-b45f-2e63e99592d4/download/fmd-a-guide-for-veterinarians.pdf Rajak, K.K., Sreenivasa, B.P., Hosamani, M., Singh, R.P., Singh, S.K., Singh, R.K. and Bandyopadhyay, S.K. 2005. Experimental studies on immunosuppressive effects of pest des petits ruminants (PPR) virus in goats. Comp. Immunol. Microbiol. Infect. Dis. 28, 287–296. Reed, L.J. and Muench, H. 1938. A simple method for estimating fifty percent (50%) end points. Am. J. Hyg. 27, 493–497. Safwat, M.S.M. 2015. Epidemiological studies on peste des petits ruminants. Giza, Egypt: Cairo University. Santhosh, A.K., Gomes, A.R., Hegde, R., Rathnamma, D., Veeregowda, B.M., Byregowda, S.M., Renukaprasad, C., Bhanuprakash, V., Prabhudas, K., Hegde, N.R. and Isloor, S. 2013. Comparative immunogenicity of two peste des petits ruminants (PPR) vaccines in South Indian sheep and goats under field conditions. Indian. J. Virol. 24(3), 373–379. Saritha, G., Shobhamani, B. and Nalini kumari, K. 2015. Evaluation of efficacy of PPR live attenuated vaccine. Int. J. Recent. Sci. Res. 6(8), 5578–5580. Singh, R.K., Balamurugan, V., Bhanuprakash, V., Sen, A., Saravanan, P. and Pal Yadav, M. 2009. Possible control and eradication of peste des petits ruminants from India: technical aspects. Vet. Ital. 45, 449–462. Singh, K.V., Osman, O.A., Thanaa, I.B. and Ivon, E. 1967. Colostrum transfer of rinderpest neutralizing antibodies to offspring of vaccinated dams. Can. J. Comp. Med. Vet. Sci. 31, 295–298. Truong, T., Boshra, H., Embury-Hyatt, C., Nfon, C., Gerdts, V., Tikoo, S., Babiuk, L., Kara, P., Chetty, T., Mather, A., Wallace, D. and Babiuk, S. 2014. Peste des petits ruminants virus tissue tropism and pathogenesis in sheep and goats following experimental infection. PLoS One 9, e87145. Veronika, D. and Michael, E. 2020. Cell culture propagation of foot-and-mouth disease virus: adaptive amino acid substitutions in structural proteins and their functional implications. Virus. Genes. 56, 1–15. Voller, A., Bidwell, D. and Bartlett, A. 1976. Enzyme immunosorbent assay for diagnosis of virus infection; manual of clinical microbiol. Washington, DC: American Society for Microbiology, pp: 506–512 Wafaa, A.H., Eman, M.B., Nibal, M.S., Nahed, K., Sozan, A.H., Sahar, I.M., Habashi, A.R., Mervat, M.M., Hanan, A.F., Essam Ibrahim, Momtaz, A.S. and AbdElhakm, M.M. 2021. Current situation of peste des petits ruminants (PPR) virus disease in some Egyptian Governorates in the period between years 2014 to 2019. Egypt. J. Anim. Health. 1(4), 35–48. Wong, C.L., Yong, C.Y., Ong, H.K., Ho, K.L. and Tan, W.S. 2020. Advances in the diagnosis of foot-and-mouth disease. Front. Vet. Sci. 7, 477. Zahur, A.B., Ullah, A., Irshad, H., Farooq, M.S., Hussain, M. and Jahangir, M. 2009. Epidemiological investigations of a peste des petits ruminants (PPR) outbreak in Afghan sheep in Pakistan. Pak. Vet. J. 29, 174–178. | ||
| How to Cite this Article |
| Pubmed Style Yaser T, Bkear N, Badr Y, Ibrahim EE, Khodeir MH. Investigation of the effect of mutual vaccination with pest des petits ruminants and polyvalent foot and mouth disease vaccines on the immune response of sheep. Open Vet J. 2023; 13(12): 1669-1682. doi:10.5455/OVJ.2023.v13.i12.16 Web Style Yaser T, Bkear N, Badr Y, Ibrahim EE, Khodeir MH. Investigation of the effect of mutual vaccination with pest des petits ruminants and polyvalent foot and mouth disease vaccines on the immune response of sheep. https://www.openveterinaryjournal.com/?mno=172039 [Access: July 01, 2025]. doi:10.5455/OVJ.2023.v13.i12.16 AMA (American Medical Association) Style Yaser T, Bkear N, Badr Y, Ibrahim EE, Khodeir MH. Investigation of the effect of mutual vaccination with pest des petits ruminants and polyvalent foot and mouth disease vaccines on the immune response of sheep. Open Vet J. 2023; 13(12): 1669-1682. doi:10.5455/OVJ.2023.v13.i12.16 Vancouver/ICMJE Style Yaser T, Bkear N, Badr Y, Ibrahim EE, Khodeir MH. Investigation of the effect of mutual vaccination with pest des petits ruminants and polyvalent foot and mouth disease vaccines on the immune response of sheep. Open Vet J. (2023), [cited July 01, 2025]; 13(12): 1669-1682. doi:10.5455/OVJ.2023.v13.i12.16 Harvard Style Yaser, T., Bkear, . N., Badr, . Y., Ibrahim, . E. E. & Khodeir, . M. H. (2023) Investigation of the effect of mutual vaccination with pest des petits ruminants and polyvalent foot and mouth disease vaccines on the immune response of sheep. Open Vet J, 13 (12), 1669-1682. doi:10.5455/OVJ.2023.v13.i12.16 Turabian Style Yaser, Toka, Nabil Bkear, Yassien Badr, Ehab El-sayed Ibrahim, and Mohamed Hassan Khodeir. 2023. Investigation of the effect of mutual vaccination with pest des petits ruminants and polyvalent foot and mouth disease vaccines on the immune response of sheep. Open Veterinary Journal, 13 (12), 1669-1682. doi:10.5455/OVJ.2023.v13.i12.16 Chicago Style Yaser, Toka, Nabil Bkear, Yassien Badr, Ehab El-sayed Ibrahim, and Mohamed Hassan Khodeir. "Investigation of the effect of mutual vaccination with pest des petits ruminants and polyvalent foot and mouth disease vaccines on the immune response of sheep." Open Veterinary Journal 13 (2023), 1669-1682. doi:10.5455/OVJ.2023.v13.i12.16 MLA (The Modern Language Association) Style Yaser, Toka, Nabil Bkear, Yassien Badr, Ehab El-sayed Ibrahim, and Mohamed Hassan Khodeir. "Investigation of the effect of mutual vaccination with pest des petits ruminants and polyvalent foot and mouth disease vaccines on the immune response of sheep." Open Veterinary Journal 13.12 (2023), 1669-1682. Print. doi:10.5455/OVJ.2023.v13.i12.16 APA (American Psychological Association) Style Yaser, T., Bkear, . N., Badr, . Y., Ibrahim, . E. E. & Khodeir, . M. H. (2023) Investigation of the effect of mutual vaccination with pest des petits ruminants and polyvalent foot and mouth disease vaccines on the immune response of sheep. Open Veterinary Journal, 13 (12), 1669-1682. doi:10.5455/OVJ.2023.v13.i12.16 |