| Case Report | ||
Open Vet J. 2023; 13(12): 1769-1775 Open Veterinary Journal, (2023), Vol. 13(12): 1769-1775 Case Report Analysis for the diagnostic accuracy of PCR detection of Macrorhabdus in companion birdsShoko Kanno1 and Yusuke Matsumoto2*1Blue Bird Veterinary Clinic, Matsuyama, Japan 2Transboundary Animal Diseases Research Center, Joint Faculty of Veterinary Medicine, Kagoshima University, Kagoshima, Japan *Corresponding Author: Yusuke Matsumoto. Transboundary Animal Diseases Research Center, Joint Faculty of Veterinary Medicine, Kagoshima University, Kagoshima, Japan. Email: ymatsu [at] vet.kagoshima-u.ac.jp Submitted: 18/10/2023 Accepted: 15/12/2023 Published: 31/12/2023 © 2023 Open Veterinary Journal
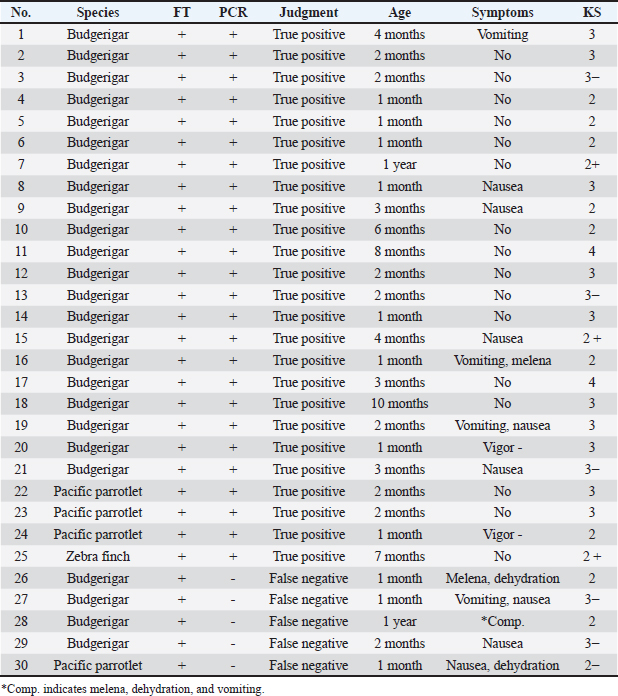
AbstractBackground: Macrorhabdus ornithogaster, a yeast-like fungus, has the potential to infect various bird species, including companion birds. Although birds infected with M. ornithogaster may often remain asymptomatic, the infection can develop into chronic wasting gastritis and even progress to gastric cancer, highlighting the importance of early detection of M. ornithogaster infection. Despite direct fecal examination being a commonly used diagnostic method, the polymerase chain reaction (PCR) is anticipated to offer a higher detection rate. However, the actual diagnostic accuracy of the PCR for M. ornithogaster remains unknown. Case Description: Ninety fecal samples collected from companion birds that visited or were admitted to a hospital, regardless of their stage of Macrorhabdus diagnosis or treatment, were subjected to PCR testing. An accuracy analysis was then performed, considering symptomatology, direct fecal testing (FT), and sequencing. The PCR test had a sensitivity of 83.33%, specificity of 95.00%, false negative rate of 16.67%, false positive rate of 5.00%, positive predictive value of 89.29%, negative predictive value of 91.94%, prevalence of 33.33%, positive likelihood ratio of 16.67, negative likelihood ratio of 0.18, and diagnostic odds ratio of 95.00. Conclusion: The findings suggest that the PCR for Macrorhabdus possesses high diagnostic accuracy, with the ability to accurately identify uninfected individuals as negative. While the direct fecal examination is appropriate for routine primary screening, in cases where M. ornithogaster is not detected by FT, the PCR may provide a more accurate and definitive diagnosis due to its high specificity. Keywords: Companion birds, Diagnostic accuracy, Direct fecal examination, Macrorhabdus ornithogaster, PCR. IntroductionMacrorhabdus ornithogaster is classified as an anamorphic ascomycetous yeast that is characterized by an elongated shape, spanning 2–3 × 20–80 μm in size (Tomaszewski et al., 2003; Kojima et al., 2022). The M. ornithogaster infects the stomachs of various bird species, including companion birds such as budgies and canaries, and domestic poultry such as chickens and ostriches (Van Herck et al., 1984; Martins et al., 2006; Behnke and Fletcher, 2011; Hanafusa et al., 2013; Lanzarot et al., 2013; Arabkhazaeli et al., 2016; Püstow and Krautwald-Junghanns, 2017; Rinder et al., 2017; Kojima et al., 2022). The pathogen is localized in the intermediate zone linking the proventriculus and ventriculus, where it grows on the luminal surface and possibly penetrates koilin (Van Herck et al., 1984; Hanafusa et al., 2013). Although Macrorhabdus infections may often remain asymptomatic, it has been implicated in chronic fatal wasting disease in companion birds, and when it is onset, gastritis symptoms persist even after eradication, leading to nausea, vomiting, undigested stools, stomach bleeding, diarrhea, and grinding, and in some cases develop into gastric cancer (Speer et al., 2004; Hannafusa et al., 2007; Powers et al., 2019; Kojima et al., 2022). Therefore, early diagnosis is an essential component of clinical care for birds. The fecal examination such as wet-mount test and fecal Gram staining (FGS) is a commonly used diagnostic test for Macrorhabdosis due to its cost-effectiveness and simplicity (Baron et al., 2021). In contrast, the polymerase chain reaction (PCR) test for Macrorhabdus is relatively underutilized due to its expense, outsourcing requirements, and time-consuming nature, as fecal examination alone can often provide a definitive diagnosis. However, a study comparing the diagnostic accuracy of two methods—FGS and PCR test of total fecal swab samples in a captive group of budgies—reported a higher detection rate for the PCR test (Sullivan et al., 2017). This suggests that relying solely on direct fecal examination may result in missed cases. While there is no available information on the diagnostic accuracy of the Macrorhabdus PCR test, it is unclear how PCR testing should be appropriately utilized in clinical practice. Typically, the diagnostic accuracy of an indicator test is evaluated by comparing it to the results of the most accurate diagnostic test available (White et al., 2011). However, this methodology cannot be applied to the PCR test for Macrorhabdus as it is likely the most accurate test currently available, with a reported higher detection rate than FGS. In practice, it can be challenging to accurately confirm the presence or absence of Macrorhabdus in patients. Thus, repeated direct fecal tests (FTs) should be performed to confirm the presence or absence of clinical signs. If the PCR test is positive despite negative direct fecal examination results, sequencing tests should be conducted. In addition, when the results of the first determination are inconclusive, repeated specimen collections are necessary to determine whether the patient is infected with M. ornithogaster according to the original reference standards. Based on these considerations, the diagnostic accuracy and usefulness of the PCR test for Macrorhabdus should be analyzed and discussed. Case DetailsThe group that was able to detect Macrorhabdus by direct fecal examination was designated as group (A) defined as the group that detected Macrorhabdus by direct fecal examination (#1–30), group (B) defined as the group that detected Macrorhabdus by direct fecal examination but did not detect it after treatment was designated (#31–60), and group (C) defined as the group that did not detect Macrorhabdus by direct fecal examination and had no symptoms (#61–90). The groups (A) to (C) consisted of several species of birds that visited or were admitted to a single hospital, including budgerigar (Melopsittacus undulatus), pacific parrotlet (Forpus coelestis), zebra finch (Taeniopygia guttata), java sparrow (Lonchura oryzivora), cockatiel (Nymphicus hollandicus), barred parakeet (Bolborhynchus lineola), and green-cheeked parakeet (Pyrrhura molinae). Excreted feces were collected and stored in microtubes under refrigeration, and 30 samples were collected from each group. Group (A) samples were collected from the cage when the birds came to the hospital, and the amount of stool collected was small when the birds were symptomatic. Group (B) samples were collected 2–4 weeks after the end of treatment, and 1–4 feces were collected per day for 1 week. Group (B) underwent fecal examination at least 5 times during the treatment to confirm the disappearance of M. ornithogaster. Group (C) samples were collected from the cage when the patients came to the hospital. Groups (B) and (C) were promptly collected and mailed, but for Group (A), which was requested as a research sample, it took 1 month for the sample to be stored in the hospital refrigerator for an extended period. PCR testing of the 18S ribosomal RNA gene of M. ornithogaster was performed at a commercial laboratory; Canine Lab. Inc. (Tokyo, Japan). Feces samples were 10-fold diluted with physiologic saline solution. 400 μl of feces suspension was used for genomic DNA extraction. Extracted DNA was eluted with 50 μl TE buffer. Real-time PCR was conducted using TB Green® Premix Ex Taq™ II (TaKaRa, Shiga, Japan). The reaction solution contained 10 μl TB Green® Premix Ex Taq™ II, 0.4 μl of each primer (10 μM), 2 μl of genomic DNA, and nuclease-free water (total volume 20 μl). The primer sequences of the 18S ribosomal RNA gene of M. ornithogaster were the following: forward: 5′-ggacttatattactagtcagatgg-3′ and reverse: 5′-caatacgcctgctttgaacactc-3 (Tomaszewski et al., 2003). The PCR program was as follows: initial denaturation at 95°C for 10 seconds, followed by 40 cycles of denaturation at 95°C for 5 seconds, annealing, and extension at 58°C for 30 seconds. Finally, a melting curve analysis was performed to determine the melting temperature of the amplification products. Statistical analysis of the collected data was performed using Fisher’s exact test (p < 0.01), and 95% confidence intervals (CIs) were calculated for the sensitivity, specificity, prevalence, positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio for the PCR test. Detection of M. ornithogaster in PCRThe PCR tests indicated that group (A) had 25 positive and 5 negative cases, with a positive rate of 83%; group (B) had 2 positive, 27 negative, and 1 indeterminate case, with a positive rate of 6%; and Group (C) had 30 negative cases, with a positive rate of 0% (Table 1). Among the 25 positive cases in group (A), the direct fecal examination also detected the presence of M. ornithogaster, thereby confirming them as true positives. The 5 negative cases (#26–30) were deemed false negatives because M. ornithogaster was detected by direct fecal examination and the patients had clinical signs of Macrorhabdosis. These false-negative cases presented with notably severe clinical signs of black stools, dehydration, vomiting, and nausea, which made it difficult to collect sufficient fecal samples. Out of these cases, # 26, 27, 29, and 30 responded well to Macrorhabdus treatment, which included administration of amphotericin B and micafungin sodium, resulting in the resolution of clinical signs, and the disappearance of Macrorhabdus were confirmed by fecal examination, while # 28 died in the middle of treatment. Due to clinical signs of black stools, dehydration, and other clinical manifestations, the amount of fecal samples collected from group (A) was lower than that from groups (B) and (C). Table 1. Group (A): bird species, FT results, PCR results, judgment results, age, symptoms, and keel score (KS).
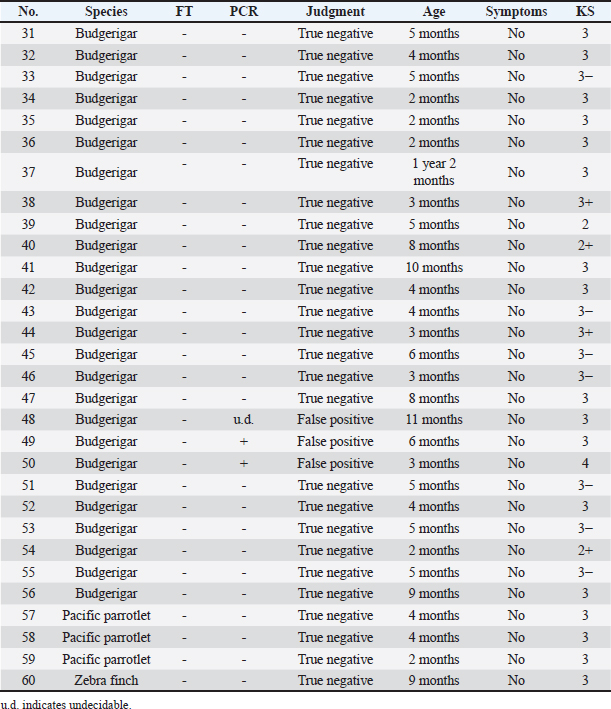
In group (B), the 27 negative cases were considered true negatives because M. ornithogaster was not detected by direct fecal examination on more than one occasion following the PCR test, and there were no clinical signs present (Table 2). For the 2 positive cases (#49 and 50), sequencing tests were conducted by the canine lab, Inc. The #49 case was identified as a false positive, as the sequencing test could not be performed, and the sample was re-collected for PCR testing, which yielded a negative result with no clinical signs present. In the case #50, the M. ornithogaster was not detected by direct fecal examination after multiple tests. In this case, symptoms that were absent at the time of the sample collection were confirmed over time. Although the gene sequence matched that of M. ornithogaster, case #50 was determined to be a false positive because the positive PCR result may have been a residual fragment in the specimen rather than live M. ornithogaster. The #48 case had a pending decision because the PCR test had been performed multiple times with mixed positive and negative results. Upon re-collection of the sample and PCR testing, the result was negative with no symptoms present, leading to the conclusion that the initial PCR test decision was a false positive. Table 2. Group (B): bird species, FT results, PCR results, judgment results, age, symptoms, and KS.
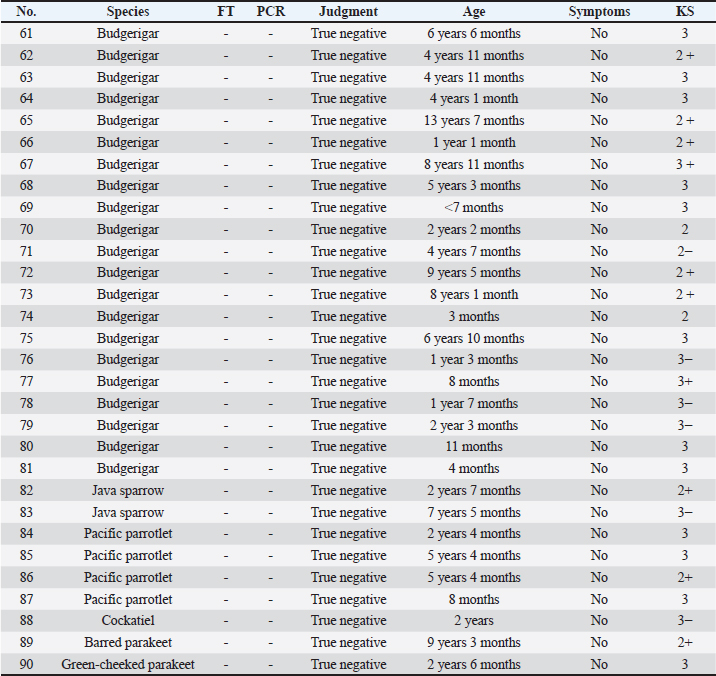
All 30 negative cases in group (C) were confirmed negative by multiple direct FTs and had no clinical signs, and were therefore classified as true negatives (Table 3). All specimens were assessed based on the original criteria, resulting in 25 true positives, 3 false positives, 5 false negatives, and 57 true negatives. Calculation of index valuesThe sensitivity was 83.33% (95% CI, 65.28%–94.36%), specificity was 95.00% (95% CI, 86.08%–98.96%), the false negative rate was 16.67%, the false positive rate was 5.00%, positive predictive value was 89.29% (95% CI, 73.22%–96.21%), negative predictive value was 91.94% (95% CI, 83.64%–96.22%), prevalence was 33.33% (95% CI, 23.74%–44.05%), positive likelihood ratio was 16.67 (95% CI, 5.47–50.80), negative likelihood ratio was 0.18 (95% CI, 0.08–0.39), and the diagnostic odds ratio was 95.00 (95% CI, 21.06–428.58) for the PCR test. DiscussionThe PCR test for M. ornithogaster has a specificity of 95.00%, resulting in a high diagnostic accuracy for correctly determining that an uninfected subject is negative. However, with a sensitivity of 83.33%, the probability of correctly identifying an infected subject is relatively low. Nonetheless, the positive predictive value is as high as 89.29%, indicating that a positive test result has a high probability of indicating an actual case. The test’s positive likelihood ratio is greater than 10, indicating good true positive extraction and a high discernment, as demonstrated by a large diagnostic odds ratio value of 95.00. Based on these findings, although the direct FT is more appropriate for use as a primary screening test in routine practice than the PCR test, the PCR test is more likely to lead to a more accurate diagnosis, closer to a definitive diagnosis, when M. ornithogaster is not detected by the FT, owing to its high specificity. Table 3. Group (C): bird species, FT results, PCR results, judgment results, age, symptoms, and KS.
When conducting diagnostic accuracy analyses, typically only groups (A) and (C) are included due to their clear diagnosis (White et al., 2011). Group (A) is diagnosed via fecal examination and PCR testing is generally not performed at the owner’s expense. Group (C) may also not undergo PCR testing as it is cost-prohibitive for some owners. Group (B) is often excluded from diagnostic accuracy analyses as it is uncertain if the animal is fully cured or not. However, if M. ornithogaster is confirmed via fecal examination in group (B), owners may want to verify the disease has been fully cured, leading to post-treatment PCR testing even if direct fecal examination is negative. Therefore, it is important to include group (B) data in current diagnostic accuracy analyses, even if additional PCR testing and product sequencing are required. Since this study was performed using clinical specimens from birds visiting the veterinary hospital, individual sample volumes were not measured and standardized. Group (A) had samples with extremely low defecation volume compared to groups (B) and (C). In addition, group (A) lacked sample freshness compared to groups (B) and (C) due to a longer storage period in the in-hospital refrigerator, which may have affected the detection rate of PCR test results for group (A). Fungi are eukaryotes like animals, and their cell structures are very similar. Thus, fungi have tough cell walls, making nucleotide extraction more difficult than with bacteria and viruses (Fredricks et al., 2005). The PCR detection rate decreases with increasing contamination of the specimen. A gross excretory cavity swab is less contaminated than a fecal sample from the outside environment (Cressman et al., 2010). However, taking gross excretory cavity swabs can be burdensome to birds and may lead to sample contamination due to bleeding caused by the technique or underlying disease. Primers designed for the 18S rRNA gene of M. ornithogaster may amplify similar sequences in host animal genes, resulting in false positives. Although sequencing tests can confirm the gene sequence and clarify this point, it is impractical to perform sequencing tests every time in a clinical setting due to cost. The sequencing test is an option when results are in doubt. The diagnostic accuracy for direct fecal examination may vary depending on the veterinarian who performs the examination. A small amount of M. ornithogaster infection may be below the detection limit of a fecal examination, resulting in a false-negative. In addition, there are multiple forms of M. ornithogaster, which can result in false negatives if they cannot be found by an inexperienced observer. Moreover, different structures of fungi may be erroneously recognized as M. ornithogaster, resulting in false positives. Therefore, a combination with the PCR test should be used to reduce the overall number of missed tests, rather than relying on one method. Macrorhabdosis is a disease commonly encountered by avian veterinarians, but still difficult to diagnose and treat reliably (Speer et al., 2004; Poleschinski et al., 2019). Early detection and thorough treatment using the properties of the PCR test can prevent the disease from silently developing in seemingly healthy birds, leading to gastritis that persists even after the Macrorhabdus has been eliminated. AcknowledgmentNone. Conflict of interestThe authors declare no conflicts of interest. FundingThis study has not received specific funding from any funding agency. Author contributionsSK: conceptualization, data curation, investigation, methodology, writing manuscript; YM: data curation, investigation, writing manuscript. All authors reviewed the results and approved the final version of the manuscript. Data availabilityAll data are available within the article. ReferenceArabkhazaeli, F., Madani, S.A. and Ghavami, S. 2016. Outbreak of an unusual tracheal mite, Ptilonyssus morofskyi (Acarina: Rhinonyssidae), in canaries (Serinus canaria) with concurrent infection with Staphylococcus aureus and Macrorhabdus ornithogaster. J. Avian. Med. Surg. 30, 269–273. Baron, H.R., Stevenson, B.C. and Phalen, D.N. 2021. Comparison of in-clinic diagnostic testing methods for Macrorhabdus ornithogaster. J. Avian. Med. Surg. 35, 37–44. Behnke, E.L. and Fletcher, O.J. 2011. Macrorhabdus ornithogaster (Megabacterium) infection in adult hobby chickens in North America. Avian. Dis. 55, 331–334. Cressman, M.D., Yu, Z., Nelson, M.C., Moeller, S.J., Lilburn, M.S. and Zerby, H.N. 2010. Interrelations between the microbiotas in the litter and in the intestines of commercial broiler chickens. Appl. Environ. Microbiol. 76, 6572–6582. Fredricks, D.N., Smith, C. and Meier, A. 2005. Comparison of six DNA extraction methods for recovery of fungal DNA as assessed by quantitative PCR. J. Clin. Microbiol. 43, 5122–5128. Hanafusa, Y., Costa, E. and Phalen, D.N. 2013. Infection trials in mice suggest that Macrorhabdus ornithogaster is not capable of growth in mammals. Med. Mycol. 51, 669–672. Hannafusa, Y., Bradley, A., Tomaszewski, E.E., Libal, M.C. and Phalen, D.N. 2007. Growth and metabolic characterization of Macrorhabdus ornithogaster. J. Vet. Diagn. Invest. 19, 256–265. Kojima, A., Osawa, N., Oba, M., Katayama, Y., Omatsu, T. and Mizutani, T. 2022. Validation of the usefulness of 26S rDNA D1/D2, internal transcribed spacer, and intergenic spacer 1 for molecular epidemiological analysis of Macrorhabdus ornithogaster. J. Vet. Med. Sci. 84, 244–250. Lanzarot, P., Blanco, J.L., Alvarez-Perez, S., Abad, C., Cutuli, M.T. and Garcia, M.E. 2013. Prolonged fecal shedding of ‘megabacteria’ (Macrorhabdus ornithogaster) by clinically healthy canaries (Serinus canaria). Med. Mycol. 51, 888–891. Martins, N.R.S., Horta, A.C., Siquerira, A.M., Lopes, S.Q., Resende, J.S., Jorge, M.A., Assis, R.A., Martins, N.E., Fernandes, A.A., Barrios, P.R., Costa, T.J.R. and Guimarães, L.M.C. 2006. Macrorhabdus ornithogaster in ostrich, rhea, canary, zebra finch, free range chicken, turkey, guinea-fowl, columbina pigeon, toucan, chuckar partridge and experimental infection in chicken, Japanese quail and mice. Arq. Bras. Med. Vet. Zootec. 58, 291–298. Poleschinski, J.M., Straub, J.U. and Schmidt, V. 2019. Comparison of two treatment modalities and PCR to assess treatment effectiveness in macrorhabdosis. J. Avian Med. Surg. 33, 245–250. Powers, L.V., Mitchell, M.A. and Garner, M.M. 2019. Macrorhabdus ornithogaster infection and spontaneous proventricular adenocarcinoma in budgerigars (Melopsittacus undulatus). Vet. Pathol. 56, 486–493. Püstow, R. and Krautwald-Junghanns, M.E. 2017. The incidence and treatment outcomes of Macrorhabdus ornithogaster infection in budgerigars (Melopsittacus undulatus) in a veterinary clinic. J. Avian. Med. Surg. 31, 334–350. Rinder, M., Schmitz, A., Peschel, A., Wörle, B., Gerlach, H. and Korbel, R. 2017. Molecular characterization of a recently identified circovirus in zebra finches (Taeniopygia guttata) associated with immunosuppression and opportunistic infections. Avian. Pathol. 46, 106–116. Speer, B., Phalen, D.N., Powers, L.V., Filippich, L.J. and Antinoff, N. 2004. Diagnosis and treatment options for megabacteria (Macrorhabdus ornithogaster). J. Avian. Med. Surg. 18, 189–195. Sullivan, P.J., Ramsay, E.C., Greenacre, C.B., Cushing, A.C., Zhu, X. and Jones, M.P. 2017. Comparison of two methods for determining prevalence of Macrorhabdus ornithogaster in a flock of captive budgerigars (Melopsittacus undulatus). J. Avian. Med. Surg. 31, 128–131. Tomaszewski, E.K., Logan, K.S., Snowden, K.F., Kurtzman, C.P. and Phalen, D.N. 2003. Phylogenetic analysis identifies the megabacterium of birds as a novel anamorphic ascomycetous yeast, Macrorhabdus ornithogaster gen. nov., sp. nov. Int. J. Syst. Evol. Microbiol. 53, 1201–1205. Van Herck, H., Duijser, T., Zwart, P., Dorrestein, G.M., Buitelaar, M. and Van Der Hage, M.H. 1984. A bacterial proventriculitis in canaries (Serinus canaria). Avian. Pathol. 13, 561–572. White, S., Schultz, T. and Enuameh, Y.A.K. 2011. The nature of diagnostic test accuracy. In Synthesizing evidence of diagnostic accuracy. Philadelphia, PA: Lippincott Wiliams & Wilkins, pp:11–15. | ||
| How to Cite this Article |
| Pubmed Style Kanno S, Matsumoto Y. Analysis for the diagnostic accuracy of PCR detection of Macrorhabdus in companion birds. Open Vet J. 2023; 13(12): 1769-1775. doi:10.5455/OVJ.2023.v13.i12.26 Web Style Kanno S, Matsumoto Y. Analysis for the diagnostic accuracy of PCR detection of Macrorhabdus in companion birds. https://www.openveterinaryjournal.com/?mno=173202 [Access: May 13, 2024]. doi:10.5455/OVJ.2023.v13.i12.26 AMA (American Medical Association) Style Kanno S, Matsumoto Y. Analysis for the diagnostic accuracy of PCR detection of Macrorhabdus in companion birds. Open Vet J. 2023; 13(12): 1769-1775. doi:10.5455/OVJ.2023.v13.i12.26 Vancouver/ICMJE Style Kanno S, Matsumoto Y. Analysis for the diagnostic accuracy of PCR detection of Macrorhabdus in companion birds. Open Vet J. (2023), [cited May 13, 2024]; 13(12): 1769-1775. doi:10.5455/OVJ.2023.v13.i12.26 Harvard Style Kanno, S. & Matsumoto, . Y. (2023) Analysis for the diagnostic accuracy of PCR detection of Macrorhabdus in companion birds. Open Vet J, 13 (12), 1769-1775. doi:10.5455/OVJ.2023.v13.i12.26 Turabian Style Kanno, Shoko, and Yusuke Matsumoto. 2023. Analysis for the diagnostic accuracy of PCR detection of Macrorhabdus in companion birds. Open Veterinary Journal, 13 (12), 1769-1775. doi:10.5455/OVJ.2023.v13.i12.26 Chicago Style Kanno, Shoko, and Yusuke Matsumoto. "Analysis for the diagnostic accuracy of PCR detection of Macrorhabdus in companion birds." Open Veterinary Journal 13 (2023), 1769-1775. doi:10.5455/OVJ.2023.v13.i12.26 MLA (The Modern Language Association) Style Kanno, Shoko, and Yusuke Matsumoto. "Analysis for the diagnostic accuracy of PCR detection of Macrorhabdus in companion birds." Open Veterinary Journal 13.12 (2023), 1769-1775. Print. doi:10.5455/OVJ.2023.v13.i12.26 APA (American Psychological Association) Style Kanno, S. & Matsumoto, . Y. (2023) Analysis for the diagnostic accuracy of PCR detection of Macrorhabdus in companion birds. Open Veterinary Journal, 13 (12), 1769-1775. doi:10.5455/OVJ.2023.v13.i12.26 |