| Research Article | ||
Open Vet J. 2023; 13(12): 1718-1728 Open Veterinary Journal, (2023), Vol. 13(12): 1718–1728 Original Research The role of hesperidin as a cardioprotective strategy against doxorubicin-induced cardiotoxicity: The antioxidant, anti-inflammatory, antiapoptotic, and cytoprotective potentialsFawiziah Khalaf Alharbi1, Zafer S. Alshehri2, Faez F. Alshehri2, Sharif Alhajlah2, Hesham A. Khalifa3, Naief Dahran4 and Wael A. M. Ghonimi5*1Department of Biology, College of Science, Buraydah, Qassim University, Buraydah, Saudi Arabia 2Department of Medical Laboratories, College of Applied Medical Sciences, Shaqra University, Shaqra, Saudi Arabia 3Department of Pharmacology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 4Department of Anatomy, Faculty of Medicine, University of Jeddah, Jeddah, Saudi Arabia 5Department of Histology and Cytology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt *Corresponding Author: Wael A. M. Ghonimi. Department of Histology and Cytology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. Email: ghonimi102030 [at] gmail.com; WAGhonemy [at] vet.zu.edu.eg Submitted: 05/10/2023 Accepted: 12/12/2023 Published: 31/12/2023 © 2023 Open Veterinary Journal
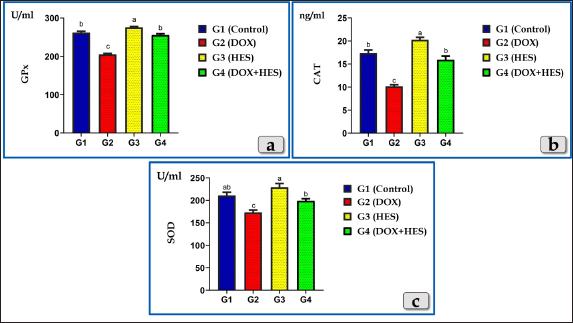
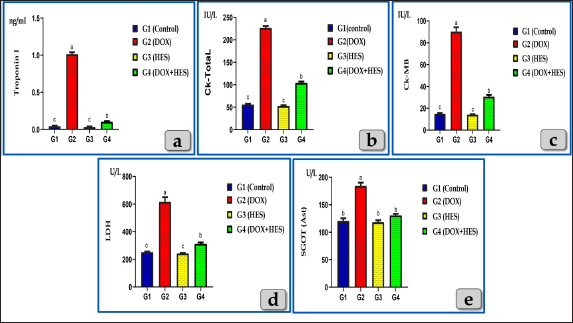
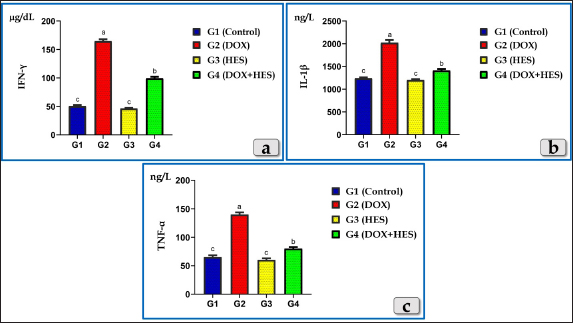
AbstractBackground: Doxorubicin (DOX), an anthracycline antibiotic, is a powerful chemotherapeutic agent effective against multiple types of cancer, particularly lung, breast, bladder and hematologic neoplasia (lymphomas and leukemia). However, its therapeutic usage is restricted by its known cardiotoxicity, which is associated with the production of oxidative stress. Enhancing antioxidant capacity represents a promising approach to mitigate DOX-induced cardiotoxicity. Hesperidin (HES), a citrus bioflavonoid, possesses several pharmacological effects, such as anti-inflammatory and antioxidant characteristics. Aim: This study was designed to evaluate the cardiotoxicity of DOX and assess the possible cardioprotective role of HES. Methods: Groups of Wistar rats were either treated with DOX (4 mg/kg. bw., once a week for five consecutive weeks, intraperitoneally) or received co-treatment with HES (100 mg/kg. bw./day in distilled water, 5 days in a week for five consecutive weeks, administered orally). Heart and blood samples were obtained for histological, immunohistochemical, and biochemical assessments. Results: DOX administration resulted in severe cardiotoxicity, as evidenced by significant elevations in cardiac biomarkers, including Troponin I (CTnI), Creatine kinase (CK-Total), Creatine kinase isoenzyme-MB (CK-MB), lactate dehydrogenase (LDH), and Aspartate aminotransferase (AST). DOX also elevated pro-inflammatory cytokines, such as Interferon γ (IFN-γ), Interleukin 1β (IL-1β), and Tumor necrosis factor α (TNF-α). Furthermore, DOX-induced oxidative stress and substantially reduced the levels of antioxidant enzymes, including Glutathione peroxidase (GPX), Superoxide dismutase (SOD), and Catalase (CAT). Histopathologically, DOX caused severe Zenker's necrosis, cardiomyocyte disarray, sarcoplasmic vacuolizations, cardiomyocyte congestion, and inflammatory cell infiltration. Immunohistochemically, DOX exhibited extensive apoptosis, as indicated by strong positive immuno-localization against anti-caspase-3 antibody. In contrast, co-treatment with HES protected cardiac tissues against cardiotoxicity of DOX, as indicated by the amelioration of histological abnormalities and the normalization of biochemical values. Conclusion: We can conclude that DOX induces severe cardiotoxicity characterized by oxidative stress, inflammation, pathological alterations, and apoptosis. Co-treatment with HES demonstrates significant cardioprotective effects by virtue of its potent anti-inflammatory, antioxidant, cytoprotective, and antiapoptotic characteristics. Keywords: Doxorubicin, Hesperidin, Cardioprotective, Cardiotoxicity, Oxidative damage. IntroductionDoxorubicin (DOX) is considered to be among the most potent broad-spectrum anthracycline antibiotics that play a major role in cancer chemotherapy (Kalyanaraman, 2020). In 1967, DOX was firstly isolated from the Streptomyces peucetius caesius colonies in the labs on Adriatic coast (Bonadonna et al., 1969). Although the developing of further derivatives, DOX maintains to be the most efficient antineoplastic drug against a number of malignancies, such as hematologic neoplasia (lymphomas, leukemias) and several solid malignancies, such as breast, bladder, lung, urogenital, gynecological, brain, endocrine, and stomach cancer (Hortobágyi, 1997; Yu et al., 2018; Wenningmann et al., 2019). DOX suppresses the growth of cancers by the interfering with DNA replication and transcription, (Hortobágyi, 1997). The DOX's major mode of action is its capacity to intercalate among DNA base pairs, which breaks DNA strands and inhibits the synthesis of both RNA and DNA. DOX suppresses topoisomerase II enzyme, leading to the destruction of DNA and activation of apoptosis (Kudo, 2017). The second mode of action of DOX is concerned with the creation of reactive oxygen species (ROS), resulting in cell death for both normal and malignant cells. The generation of ROS in cardiac muscle cells is considered one of the most dangerous adverse effects of DOX medication that ultimately resulting in fatal heart failure (Segredo et al., 2014). DOX is a highly efficient anticancer chemotherapy, however, its therapeutic utilization is restricted by its toxicity, particularly cardiotoxicity. Cardiomyopathy is one of the most hazardous side effects of DOX, resulting in congestive heart failure that is related to morbidity and mortality (Sheibani et al., 2022). Numerous factors and mechanisms were involved and essential in the pathophysiology of cardiotoxicity caused by DOX such as oxidative stress, free radical formation, inflammatory cytokines, mitochondrial damage, intracellular iron accumulation, intracellular calcium overloading, DNA and myocytes membrane injuries, cell necrosis and apoptosis (Alkreathy et al., 2010; Sheibani et al., 2022), as well as myofibrillar disarray (Chen et al., 2012). The cardiotoxicity induced after DOX resulting to the development of cardiomyopathy and then cardiac dysfunction (Fan et al., 2008). For that reason, it makes sense to say that the potentiation of antioxidant, anti-inflammatory, and anti-apoptosis capacities can be an effective approach to attenuate the cardiotoxicity induced after DOX. Natural products have been utilized by humans as a source of nourishment, health maintenance and the treatments of numerous disorders (Dillard and German, 2000). Flavonoid derived-plants were examined and evaluated for their potential to provide cardioprotection against the cardiotoxicity induced after DOX (Sadzuka et al., 1997). Flavonoids have recently received increased attention due to their numerous pharmacological effects, including anti-apoptotic, anti-inflammatory, as well as antioxidant (Feliciano et al., 2015). Hesperidin (HES), a citrus bioflavonoid, is extremely present in citrus fruits (orange and lemon), as well as various vegetables, wine, and green tea (Naewla et al., 2019). The name of HES is derived from hesperidium, a fruit kind that citrus trees produce (Li and Schluesener, 2017). HES has been shown to have numerous pharmacological effects, such as antioxidant (Al-Rikabi et al., 2020), anticarcinogenic, anti-inflammatory effects (Galati et al., 1996; Garg et al., 2001), hepatoprotective (Atta et al., 2023), cardioprotective (Syahputra et al., 2022), immunomodulatory (Sassi et al., 2017), antidiabetic, antihypertensive, and antihyperlipidemic effects (Zanwar et al., 2014). Furthermore, HES mitigates oxidative damage and dysfunction in mitochondria by stimulating antioxidant enzymes (Poetini et al., 2018). Consequently, the purpose of the present investigation is to shed further light on the potential cardiotoxicity, inflammation, oxidative damage and apoptosis associated with DOX intoxication, in addition, to evaluate the possible cardioprotective role of HES against the toxicological effects of DOX. Materials and MethodsChemicals and kitsDOX was obtained as an injectable commercial product (Adriblastina vials, Pharmacia Italia S.P.A., Italy). Each vial has 50 mg DOX hydrochloride (dried powder) and kept at 4°C. Before use, each vial's contents were dissolved in 25 ml of sterile saline solution so each ml contains 2 mg DOX. HES (powder form) was obtained from Sigma Aldrich (St. Louis, MO, USA), kept at 2°C–4°C and freshly dissolved in distilled water just before use. Kits for Superoxide dismutase (SOD), Catalase (CAT), and Glutathione peroxidase (GPX) were purchased from Biodiagnostic Company, Giza, Egypt. In addition, Cardiac markers assessment Kits; Troponin I (CTnI) ELISA Kits were obtained from IMMUNOSPEC Corporation, USA, CAT. No. E29-061. Creatine Kinase Activity (CK) (ab155901), Creatine Kinase MB (CK-MB) (ab285275), and Aspartate Aminotransferase (AST) (ab263883) kits were purchased from Abcam, UK. Lactate dehydrogenase enzyme (LDH) kits were purchased from the Egyptian Company of Biotechnology, EGYPT. ELISA Kits for the exact measurement of Interleukin 1β (IL-1β) Cat. No. E0094Hu, Tumor necrosis factor α (TNF-α) Cat. No. E0082Hu and Interferon γ (IFN-γ) Cat. No. E0105Hu were purchased from Bioassay Technology Laboratory Company, China. Animals and housingForty mature male Wistar albino rats (weighing approximately 180 ± 20 gm and with average three months age) were obtained from animal unite, Veterinary Medicine Faculty, Zagazig University, Egypt. The rats were kept in transparent polypropylene cages under standard laboratory conditions, and provided with balanced diet and water ad libitum. All experimental methods were performed in accordance with guidelines for care and use of laboratory animals and in conformity with the guidelines of the Ethical Committee of the National Research Centre, Egypt. Study design and treatmentsThe animals were randomized into four groups (10 rats for each group): Group I (G1): Rats were served as a control and given tap water and rodent food pellets ad libitum without any treatments; neither DOX nor HES. Group II (G2): Rats were treated intraperitoneally with DOX in a dose of 4 mg/kg. bw once every week for five consecutive weeks. The dose of DOX was performed according to the previous investigation that demonstrated significant cardiotoxicity in rats (Trivedi et al., 2011). Group III (G3): Rats were orally treated with HES at a dose of 100 mg/kg. bw/day in distilled water for 5 days in a week up to five consecutive weeks and the dose was performed according to (Trivedi et al., 2011). Group IV (G4): Rats received DOX 4 mg/kg bw once every week for five consecutive weeks and HES 100 mg/kg bw for 5 days in a week for five consecutive weeks, respectively (co-treatments). Then, a week after the last DOX injection, all animals were euthanized. Samples collection, preparation, and preservationAt the end of the experiment, animals were sacrificed under anesthesia (Ketamin 200 mg/kg bw., intraperitoneally). Animals were dissected to expose the heart. Then, hearts were taken out and then fixed with neutral buffered formalin 10% for histological and immunohistochemical evaluations. In addition, blood samples were withdrawn from the retro-orbital venous plexus into sterilized tubes devoid of anticoagulant in order to separate the serum. Samples were centrifuged for 10 minutes at 3,000 rpm to separate the serum after they had been allowed to clot for 30 minutes at 25°C. The serum was then kept at a temperature of 80°C for various biochemical evaluations (Oxidative stress markers, Cardiac biomarkers, and Pro-inflammatory Cytokines). Oxidative stress and antioxidant enzymes assessmentsFor determination of oxidative stress parameters, the antioxidant enzymes; SOD, CAT, and GPX were performed and measured in serum. Cardiac markers measurementsTroponin I, CK- Total, CK-MB, LDH, and AST were measured in serum. Pro-inflammatory Cytokines assayThree cytokines; IFN-γ, IL-1β, and TNF-α were measured in serum according to the manufacturer’s guidelines. Histopathological evaluationHearts was taken out and directly fixed in 10% neutral buffered formalin for 48 hours. Next, the samples were dehydrated in ascending grades of ethanol, cleared in xylene, and then embedded in paraffin wax to make paraffin blocks. Then, the blocks were sectioned to 5 µm and stained with Harris's Hematoxylin and Eosin (H&E) for routine histopathological evaluations, Periodic acid–Schiff (PAS) for determining the presence of neutral muco-polysaccharides and glycogen and Blue Masson’s Trichrome for determining collagen fibers (Suvarna et al., 2018). Anti-Caspase 3 immunohistochemical reactivityThe immunohistochemical staining was carried out on 5-μm, formalin-fixed, paraffin-embedded sections using anti-caspase-3 (ab184787), rabbit monoclonal primary antibodies at a dilution 1:1,000 as an apoptotic marker, by the streptavidin-biotin complex technique. Deparaffinized sections were stained using an indirect immunoperoxidase technique (Polak and Van Noorden, 2003). Statistical analysisThe results were clarified as mean ± SEM (Standard Error of Mean). To assess how the four treatment groups affected the various biochemical markers, one-way analysis of variance (ANOVA) by Duncan multiple test as post hoc test was utilized. The statistical significance was denoted by the value of P < 0.05. The statistical Package for Social Sciences version 24.0 (SPSS, IBM Corporation, Armonk, NY) and Graph Pad prism 8.0.2 (GraphPad Software, Inc) were utilized for all analyses and charts. Ethical approvalThe Institutional Animal Care and Use Committee at Zagazig University in Egypt has evaluated and approved this research protocol and assigned it with the approval number (ZU—IACUC; No. ZU-IACUC/2/F/249/2023). ResultsEffects on oxidative stress markersAll oxidative stress parameters; GPx, CAT, and SOD were significantly decreased in the DOX-treated group (G2) when compared with the control group. Otherwise, DOX-HES treated group (G4) demonstrated a significant elevation of GPx, CAT, and SOD levels compared with G2 treated with DOX. These impairments were significantly ameliorated by HES and DOX co-administration in G4 (Fig. 1). Effects on Cardiac biomarkersAll tested cardiac biomarkers; Troponin I, CK-Total, CK-MB, LDH, and AST noticed a significant increase in DOX-treated group (G2) confirming cardiotoxicity. While, DOX-HES-treated group (G4) demonstrated a positive effect and noticed a substantial decrease in all cardiac marker measurements when compared to G2, confirming the potent cardioprotective role of HES (Fig. 2). Pro-inflammatory Cytokines assayThe pro-inflammatory cytokines; IFN-γ, IL-1β, and TNF-α were markedly elevated in DOX-treated group (G2) compared to the control group. On the other hand, DOX-HES-treated group (G4) demonstrated a positive effect and noticed a substantial decrease in all tested cytokines values compared to G2 confirming the potent anti-inflammatory effect of HES (Fig. 3). Cardiac histopathological evaluationsThe histopathological evaluation of mature male rats heart myocardium of the G1 (control group) exhibited intact and normal cardiomyocytes without any pathological abnormalities. Where, in the longitudinal sections, cardiomyocytes showed short, branched, and anastomosed forming networks with acidophilic striated sarcoplasm. The majority of the cardiomyocytes have just a single, central, actually large, ovoid or oval pale-stained and more euchromatic nucleus, However, few binucleated cells were infrequently seen with obvious nucleoli (Fig. 4a and b). In addition, the cardiomyocytes were also separated from one another by a significant quantity of highly vascularized connective tissue (Fig. 4b).
Fig. 1. Showing the effect of DOX and HES on all tested antioxidant enzymes; GPX, CAT, and SOD for all experimental groups. According to Duncan multiple test, the different letters (a–c) are significantly different between treatments at the P < 0.05 level.
Fig. 2. Showing the effect of DOX and HES on all tested cardiac biomarkers; Troponin I (CTnI), CK-Total, CK-MB, LDH and AST for all experimental groups. According to Duncan multiple test, the different letters (a–c) are significantly different between treatments at the P < 0.05 level. Meanwhile, the myocardium of the G2 treated with DOX revealed severee Zenker’s degenerations (a form of sever waxy or hyaline degenerations or coagulative necrosis) of the cardiomyocytes characterized by severe muscular damage with loss of their continuity, organization, and cross-striations, fibers swelling, hypereosinophilia of their sarcoplasm, hyaline fragmentation of the sarcoplasm with small dark pyknotic nuclei. In addition, the intercellular spaces were increased with interstitial edema (Fig. 5a and b). Furthermore, disarray of the cardiomyocytes was clearly noticed characterized by the loss of the normal parallel arrangement of the cardiac muscle cells where this change is considered to be a specific histological feature of hypertrophic cardiomyopathy (HCM); abnormal thickened cardiac muscle (Fig. 5c and d).
Fig. 3. Showing the effect of DOX and HES on the tested Pro-inflammatory Cytokines; Interferon γ (IFN-γ), Interleukin 1β (IL-1β) and Tumor necrosis factor α (TNF-α) for all experimental groups. According to Duncan multiple test, the different letters (a–c) are significantly different between treatments at the P < 0.05 level.
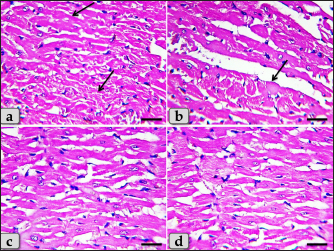
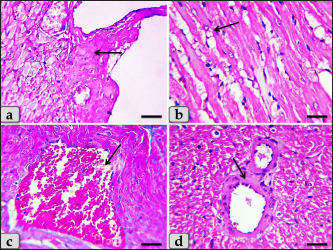
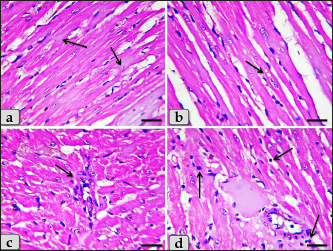
Fig. 4. A photomicrographs of mature male rat’s heart myocardium of G1; control group. (a) Showing normal and intact cardiomyocyes with normal orientations and directions without any pathological lesions. In longitudinal sections, cardiomyocyes are appeared short, branched, anastomosed forming network with single, central, large oval or ovoid, more euchromatic nucleus and acidophilic sarcoplasm (arrow). (b) Showing that the cardiomyocyes are laterally separated from each other by a considerable amount of highly vascularized connective tissue (arrow). Stain: All: Haematoxylin and Eosin (H&E). Scale bars: a,b=40 μm.
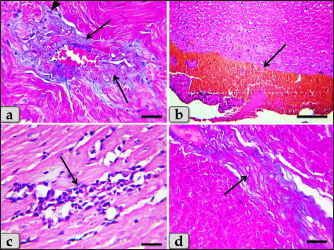
Fig. 5. A photomicrographs of the heart myocardium of the G2 treated with DOX. (a) and (b) Showing sever Zenker’s degenerations of the cardiomyocyes characterized by sever muscular damage with loss of their continuity, loss of cross-striations, fibers swelling, hyper-eosinophilia of their sarcoplasm, hyaline fragmentation of the sarcoplasm with small dark pyknotic nuclei. In addition, the intercellular spaces were increased (arrow). (c) and (d) Showing disarray of the cardiomyocyes characterized with loss of the normal parallel arrangement of the cardiac muscle cells. Stain: All: H&E. Scale bars: All=40 µm. Moreover, with PAS stain, severe Zenker’s necrosis of the cardiomyocytes specially near valves were noticed characterizing by loss of cell architectures, hypereosinophilic with severe glassy or waxy hyaline degenerations of cardiac muscle sarcoplasm with small dark pyknotic nuclei or completely loss of nuclei in some cells (Fig. 6a). Severe vacuolizations were also observed characterized by formation of various scattered vacuoles or vacuole-like structures of variable shape and size within the cardiomyocytes sarcoplasm specially in peri-nuclear area (Fig. 6b). In addition, congested heart muscle with severe prominent blood vessels dilatation engorged with RBCs were clarified (Fig. 6c) with severe thickening of the blood vessels wall (Fig. 6d), accompanied with severe fibroblasts hyperplasia with severe fibrosis (excessive collagen fibers formations) (Fig. 7a). Dense wide prominent area of hemorrhage (excessive extravasated RBCs) was demonstrated in several examined sections (Fig. 7b). Moderate myocarditis were recognized characterizing by chronic inflammatory cells infiltrations specially lymphocytes and plasma cells (Fig. 7c) with fibrous connective tissue proliferations in between cardiomyocytes (Fig. 7d).
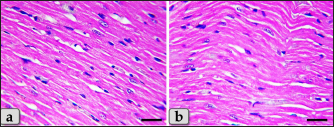
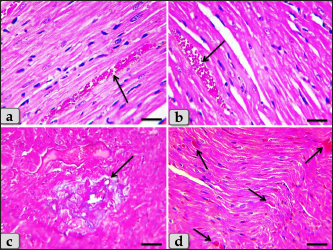
Fig. 6. A photomicrographs of the heart myocardium of the G2 treated with DOX. (a) Showing sever Zenker’s necrosis of the cardiomyocyes specially near valves characterized by loss of cell architectures, hypereosinophilic with severe glassy or waxy hyaline degenerations of cardiac muscle sarcoplasm with small dark pyknotic nuclei or completely loss of nuclei in some cells (arrow). (b) Showing severe vacuolization characterized by formation of various scattered vacuoles or vacuole-like structures with variable shape and size within the cardiomyocytes sarcoplasm specially in peri-nuclear area (arrow). (c) Showing congested heart muscle with severe prominent blood vessels dilatation with severe engorgement with RBCs (arrow). (d) Showing thickening of the blood vessels wall. Stain: (a) PAS, (b) and (d) H&E, (c) Blue Masson’s trichrome. Scale bars: All=40 µm. The cardiomyocytes of the G3 treated with HES demonstrated intact cardiomyocytes with normal organization and no pathological abnormalities, looks like the control group (Fig. 8a and b). Meanwhile, the myocardium of the G4 treated with both DOX and HES revealed normal looking cardiomyocytes with normal muscle directions and fascicles without any pathological effect, except slight blood vessel dilatation (Fig. 9a and b), mild fibrous connective tissue proliferations surrounding blood vessels (Fig. 9c), with individual extravasated RBCs in between the cardiomyocytes indicating slightly hemorrhage when compared with a toxic group; G2 (Fig. 9d). In addition, mild Zenker’s degenerations in very limited areas of the myocardium were also observed (Fig. 10a), with individual small sized vacuoles within the cardiomyocytes sarcoplasm (Fig. 10b). Furthermore, very small focus of inflammatory cells infiltrations between muscle fascicles were recognized (Fig. 10c). And also, individual scattered inflammatory cells between cardiomyocytes especially lymphocytes were clarified (Fig. 10d).
Fig. 7. A photomicrographs of the heart myocardium of the G2 treated with DOX. (a) Showing severe thickening of the blood vessels wall, accompanied with severe fibroblasts hyperplasia (arrowhead) with severe fibrosis (excessive collagen fibers productions) (arrow). (b) Showing wide prominent area of hemorrhage (excessive extravasated RBCs) (arrow). (c) and (d) Showing myocarditis characterized by chronic inflammatory cells infiltrations specially lymphocytes and plasma cells with fibrous connective tissue proliferations in between cardiomyocytes (arrow). Stain: (a), (d) Blue Masson’s trichrome, (b), (c) H&E. Scale bars: All=40 µm, except b=300 µm.
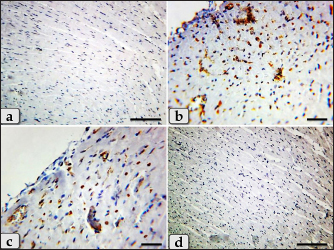
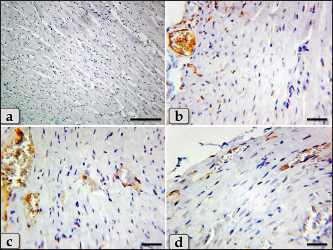
Fig. 8. A photomicrographs of the heart myocardium of the G3 treated with HES. (a) and (b) Showing normal and intact cardiomyocyes with normal organization and directions without any pathological lesions looks like the control group. Stain: All: H&E. Scale bars: a,b=40 µm. Concerning immunohistochemical reactivity of cardiac myocytes against anti-caspase-3 antibody in all tested groups where the positive signal is mostly expressed in the nuclei of morphologically identifiable apoptotic cells. G1 (control group) demonstrated negative expression against anti-caspase-3 antibody in the myocardium (Fig. 11a). Meanwhile, G2 treated with DOX revealed strongly positive immuno-reactivity against anti-caspase-3 antibody that was widely expressed in the nucleus of the cardiac myocytes and also within the interfibrillar connective tissue cells confirming widespread of apoptosis (Fig. 11b and c). Furthermore, G3 treated with HES demonstrated negative expression against anti-caspase-3 antibody in the myocardium resembling the control group (Fig. 11d). Moreover, G4 treated with both DOX and HES clarified negatively immuno-localization against anti-caspase-3 antibody in wide areas of cardiac myocytes in almost of the examined sections (Fig. 12a). But, very few positive immuno-signaling against anti-caspase-3 were expressed in individual cardiomyocytes especially around the blood vessels (Fig. 12b–d).
Fig. 9. A photomicrographs of the heart myocardium of the G4 treated with both DOX and HES. (a) and (b) Showing normal, intact cardiomyocyes with normal directions and orientations looks like normal without any pathological effect, except slightly blood vessels dilatation with congestion (arrow). (c) Showing moderate fibrous connective tissue proliferations surrounding blood vessels (arrow). (d) showing individual extravasated RBCs in between the cardiomyocytes (arrow). Stain: (a) and (b) H&E, (c), (d) Blue Masson’s trichrome. Scale bars: All=40 µm.
Fig. 10. A photomicrographs of the heart myocardium of the G4 treated with both DOX and HES. (a) Showing mild Zenker’s degenerations of the cardiomyocyes. (b) Showing individual small sized vacuoles within the cardiomyocyes sarcoplasm (arrow). (c) Showing very small focus of inflammatory cells infiltrations between muscle fascicles (arrow). (d) showing individual scattered inflammatory cells between cardiomyocyes specially lymphocytes (arrow). Stain: All: H&E. Scale bars: All=40 µm.
Fig. 11. A photomicrographs of the immunohistochemical staining of the cardiac myocytes against anti-caspase-3 antibody, in all experimental groups where, the positive signal is mostly expressed in the nuclei of morphologically identifiable apoptotic cells. (a) G1 (control group) demonstrated completely negative expression against anti-caspase-3 antibody within the cardiac myocytes. (b) and (c) G2 treated with DOX revealed strongly positive immuno-reactivity against anti-caspase-3 antibody that was widely expressed in the nucleus of the cardiac myocytes and also within the interfibrillar connective tissue cells confirming widespread of apoptosis. (d) G3 treated with HES claimed negative expression against anti-caspase-3 antibody in the cardiac myocytes resembling the control group. Stain: (a)–(d) Immunohistochemical stain against anti-caspase-3 antibody. Scale bars: a,d=300 µm, b,c =40 µm. DiscussionDOX, an anthracycline antibiotic and powerful chemotherapeutic agent, is frequently utilized to treat a range of solid cancers, such as malignancies of the breast, ovary, and gastrointestinal tract. However, DOX's considerable cardiotoxicity limits its therapeutic utility (Simoncikova et al., 2008; Sheibani et al., 2022). So, it has recently received increased attention owing to mitigate the cardiotoxicity produced by DOX, particularly when combined with HES, a natural citrus bioflavonoid which is extremely present in citrus fruits and has numerous medical uses.
Fig. 12. A photomicrographs of the immunohistochemical stain of cardiac myocytes against anti-caspase-3 antibody, (a)–(d) G4 treated with DOX+ HES clarified negative expression against anti-caspase-3 antibody in wide areas of cardiac myocytes (a), but few mild positive immuno- localization against anti-caspase-3 antibody in individual cardiomyocytes were also observed specially around the blood vessels (b)–(d). Stain: (a)–(d) Immunohistochemical stain against anti-caspase-3 antibody. Scale bars: a=300 µm, b–d=40 µm. The present investigation clarified a substantial reduction in all tested antioxidant enzymes (SOD, CAT, and GPx,) in the DOX-treated group (G2) confirming the incidence of oxidative stress. The oxidative damage is created because of an imbalance among the production of ROS and activation of the endogenous anti-oxidants as a response when a cell is injured, causing cardiotoxicity. Elevation of oxidative damage with deficiency of anti-oxidant factors plays a crucial function in the cardiotoxicity caused by DOX. Previous investigations clarified decrease in the antioxidant enzymes level as SOD and CAT accompanied byDOX-induced cardiotoxicity (Asensio-López et al., 2017; Xiong et al., 2018). And also, a substantial reduction in tissue CAT, GPX, and SOD was observed with DOX medication (Abdelatty et al., 2021). Moreover, a significant reduction in SOD levels, glutathione (GSH) activity, and increase in lipid peroxide (TBARS) levels were declared in rats treated with DOX (Abdel-Raheem and Abdel-Ghany, 2009). Some investigation suggested that the mitochondria are the desired organelle of DOX generated free radical toxicity in cardiomyocytes consuming the antioxidant enzymes (Danz et al., 2009). These impairments were significantly ameliorated by HES in DOX-HES treated group (G4) that clarified a substantial elevation of GPx, CAT, and SOD levels. Our results were supported by Abdel-Raheem and Abdel-Ghany (2009) who declared that HES ameliorated oxidative stress biomarkers by significant elevating the SOD and GSH activities in cardiac tissues that were decreased after the treatment with DOX. In contrast, HES reduced the increased level of lipid peroxide in rats treated with DOX, indicating that HES may have some cardioprotective effects as a result of its antioxidant and free radical scavenging characteristics. This investigation revealed a substantial elevation in all tested cardiac biomarkers; Troponin I, CK- Total, CK-MB, LDH, and AST in DOX-treated group (G2) confirming cardiotoxicity. Our findings were supported by Abdelatty et al. (2021) who reported a substantial elevation in serum cTnT, CK–MB, and AST activities in rats animal post receiving of 2, 4, or 6 mg of DOX/kg bw and increased to their highest level after being exposed to 8 mg/kg bw. In addition, some investigation was announced that the DOX generated extreme cardiotoxicity as evidenced from the increasing of serum creatine kinase (CK), LDH activities, and nitric oxide (NO) level (Abdel-Raheem and Abdel-Ghany, 2009). While DOX-HES-treated group (G4) demonstrated a positive effect and noticed a substantial reduction in all cardiac marker values compared to G2 confirming the potent cardioprotective role of HES. Previous studies supported our findings by clarifying that the HES treatment of rats protected their cardiac tissues against the cardiotoxicity of DOX by amelioration and normalization of the cardiac biochemical parameters. Where, they announced a substantial reduction in the elevated serum LDH, CK, and NO activities induced after DOX (Abdel-Raheem and Abdel-Ghany, 2009). The pro-inflammatory cytokines; IL-1β, TNF-α, and IFN-γ were markedly elevated in DOX-treated group (G2). So, we observed that the cardiotoxicity induced after DOX is accompanied by inflammation and stimulated cytokine production to enhance immune cell responses. Previously, some investigations declared that DOX stimulated the inflammatory activities by elevation of NF-κB; nuclear factor kappa B and motivated the production of different pro-inflammatory cytokines, as TNF-α (Nagai et al., 2016). On the other hand, DOX-HES-treated group (G4) demonstrated a positive effect and noticed a substantial reduction in all tested cytokines values compared to G2 confirming the potent anti-inflammatory effect of HES. These results were verified by Kuzu et al. (2021) who announced that HES co-treatment has a potent anti-inflammatory effect through the reduction of the elevated IL-1β, TNF-α, and NF-κB levels in the cardiac and brain tissues after the cardiotoxicity and neurotoxicity. Histopathologically, the myocardium of DOX treated group revealed severe Zenker’s degenerations (severe hyaline or coagulative degenerations) of the cardiomyocytes, sever muscular damage with loss of their continuity, organization, and cross-striations, hypereosinophilia of their sarcoplasm, with small dark pyknotic nuclei, interstitial edema, disarray of the cardiomyocytes characterizing with loss of the normal parallel arrangement of the cardiomyocytes, HCM, severe vacuolizations of the cardiomyocytes sarcoplasm, and congested heart muscle with a significant dilated and congested blood vessels accompanied with severe fibroblasts hyperplasia with severe fibrosis, hemorrhage, and moderate myocarditis with inflammatory cells infiltrations and fibrous connective tissue proliferations in between cardiomyocytes. Previous investigations supported our findings and announced that the cardiomyocytes were observed degenerated and disorganized after DOX medication. In addition, wide regions of disorganized cardiomyocytes with loss of their striations, hemorrhage, and sarcoplasmic vacuolations were clarified. They added that thick and irregular collagen bundles among cardiomyocytes were also observed when stained with Masson’s trichrome (Agaba et al., 2021). Other study declared that the cardiomyocytes after DOX medication exhibited acidophilic cytoplasm with loss of striations mostly due to the damage of the contractile banding, coagulative necrosis of cardiomyocytes and sarcoplasmic vacuolations were noticed. Moderate myocarditis with inflammatory cells infiltration was clarified with particularly dilatation of blood capillaries with perivascular edema (Abdelatty et al., 2021). Meanwhile, DOX-HES co-treatment of G4 showed a good impact and ameliorated most of these pathological effects where the myocardium appeared normal, intact cardiomyocytes with normal directions and orientations in most examined sections without any pathological effect, except slight blood vessels dilatation, mild fibrous connective tissue proliferations surrounding blood vessels, mild Zenker’s degenerations, a very small focus of inflammatory cells infiltrations between muscle fascicles were recognized. Our results were supported by Abdel-Raheem and Abdel-Ghany (2009) who demonstrated that HES cotreatment mitigated the majority of histopathological changes caused by DOX. Where HES-DOX-treated group noticed normal myocardial structure with the absence of inflammatory cell infiltration and cardiomyocytes hyalinization. Furthermore, HES minimized the edema and congestion in the blood vessels that were recognized to be significant in the myocardium after DOX medication. Additionally, HES alone was shown to be safe and did not generate any histological abnormalities in the cardiac tissues. Other investigations have confirmed our findings and demonstrated that pretreatment with HES in rats prevented the cardiotoxicity caused after DOX, as evidenced from the improvement of the histological abnormalities and normalization of the cardiac biochemical markers. Moreover, mild hyperemia in interstitial vessels and mild hyaline degeneration were noticed (Kuzu et al., 2021). And added, the co-treatment with HES had anti-inflammatory, antioxidant, antiapoptotic activities on cardiotoxicity and helps in protecting the cardiac tissue by clarifying a regulating impact on all parameters. As a result, the co-treatment with HES clarified a protective impact against cardiotoxicity and neurotoxicity in rats. Regarding the immunohistochemical response of cardiac myocytes against anti-caspase-3 antibody as apoptotic marker, G2 treated with DOX revealed strongly positive immuno-reactivity against anti-caspase-3 antibody which was widely expressed in the nucleus of the cardiac myocytes and also within the interfibrillar connective tissue cells confirming widespread of apoptosis. Meanwhile, DOX-HES treated group clarified negatively immuno-localization against anti-caspase-3 antibody in almost all the examined sections. But, a very few positive immuno-signaling against anti-caspase-3 were expressed in individual cardiomyocytes. Other investigations supported our findings and clarified that HES co-treatment revealed mild Bax expression (a pro-apoptotic marker) in the cardiac tissues confirming antiapoptotic effect of HES against cardiotoxicity (Kuzu et al., 2021). ConclusionWe can conclude that the DOX-induced severe cardiotoxicity through the induction of oxidative stress, inflammation, cytotoxicity, severe pathological changes, and apoptosis. In addition, co-treatment with HES demonstrates significant cardioprotective effects by virtue of its potent anti-inflammatory, antioxidant, cytoprotective, and antiapoptotic properties. AcknowledgmentThe authors extend their appreciation to the deanship of scientific research at Shaqra University, Saudi Arabia for funding this research work through the project number (SU-ANN-202223). We want to express our sincere gratitude to College of Science, Buraydah, Qassim University, Saudi Arabia for its help and support. We would like to extend our sincere appreciation and gratitude to dr. Abdel-rahman A sobeih, Department of Animal Wealth Development, Fac. Vet. Med., Zagazig University, Egypt for his support to finish the statistical analysis. Author contributionsConceptualization: Wael A. M. Ghonimi. Data Curation: Wael A. M. Ghonimi. Methodology: Wael A. M. Ghonimi, Zafer S. Alshehri, Fawiziah Khalaf Alharbi, Faez F. Alshehri, Sharif Alhajlah, Hesham A. Khalifa, Naief Dahran. Histopathological examination—Images analyze: Wael A. M. Ghonimi. Writing-Original draft: Wael A. M. Ghonimi. Review and Editing: Wael A. M. Ghonimi, Zafer S. Alshehri, Fawiziah Khalaf Alharbi, Faez F. Alshehri, Sharif Alhajlah, Hesham A. Khalifa, Naief Dahran. The final version of the manuscript was read and approved by all authors. Conflict of interestAll authors announce that there is no any conflict of interest. FundingThis work received the fund from the deanship of scientific research at Shaqra University, Saudi Arabia through the project number (SU-ANN-202223). Data availabilityAll the data obtained during the present investigation are represented in this manuscript. ReferencesAbdelatty, A., Ahmed, M.S., Abdel-Kareem, M.A., Dmerdash, M., Mady, R., Saad, A.S., Albrakati, A., Elmahallawy, E.K., Elsawak, A. and Abdo, W. 2021. Acute and delayed Doxorubicin-induced myocardiotoxicity associated with elevation of Cardiac biomarkers, depletion of cellular antioxidant enzymes, and several histopathological and ultrastructural Changes. Life (Basel) 11(9), 880. Abdel-Raheem, I.T. and Abdel-Ghany, A.A. 2009. Hesperidin alleviates doxorubicin-induced cardiotoxicity in rats. J. Egypt Natl. Canc. Inst. 21(2), 175–84. Agaba, A.A., Ebada, M.M. and Emara, H.M. 2021. Protective effect of metformin on doxorubicin-induced cardiomyopathy in the adult male Albino rats (light and electron microscopic study). Al-Azhar Int. Med. J. 2(4), 1–8. Alkreathy, H., Damanhouri, Z.A., Ahmed, N., Slevin, M., Ali, S.S. and Osman, A.M. 2010. Aged garlic extract protects against doxorubicin-induced cardiotoxicity in rats. Food Chem. Toxicol. 48, 951–56. Al-Rikabi, R., Al-Shmgani, H., Dewir, Y.H. and El-Hendawy, S. 2020. In vivo and in vitro evaluation of the protective effects of hesperidin in lipopolysaccharide-induced inflammation and cytotoxicity of cell. Molecules 25(3), 478. Asensio-López, M.C., Soler, F., Pascual-Figal, D., Fernández-Belda, F. and Lax, A. 2017. Doxorubicin-induced oxidative stress: the protective efect of nicorandil on HL-1 cardiomyocytes. PLoS ONE 12, e0172803. Atta, I.S., Elnady, M.R., Alghamdi, A.G., Alghamdi, A.H., Aboulata, A.A. and Shatla, I.M. 2023. Assessing the hepatoprotective effects of hesperidin on liver-associated disorders in albino rats with experimentally induced obesity and type II diabetes: a histological and biochemical study. Heliyon 9(5), e16031. Bonadonna, G., Monfardini, S., De Lena, M. and Fossati-Bellani, F. 1969. Clinical evaluation of adriamycin, a new antitumour antibiotic. Br. Med. J. 3, 503–506. Chen, B., Zhong, L., Roush, S.F., Pentassuglia, L., Peng, X., Samaras, S., Davidson, J.M., Sawyer, D.B. and Lim, C.C. 2012. Disruption of a GATA4/Ankrd1 signaling axis in cardiomyocytes leads to sarcomere disarray: Implications for anthracycline cardiomyopathy. PLoS One 7(4), e35743. Danz, E.D., Skramsted, J., Henry, N., Bennett, J.A. and Keller, R.S. 2009. Resveratrol prevents doxorubicin cardiotoxicity through mitochondrial stabilization and the Sirt1 pathway. Free Radic. Biol. Med. 46, 158997. Dillard, C.J. and German, J.B. 2000. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agric. 80, 1744–1756. Fan, G.C., Zhou, X., Wang, X., Song, G., Qian, J. and Nicolaou, P. 2008. Heat shock protein 20 interacting with phosphorylated Akt reduces doxorubicin-triggered oxidative stress and cardiotoxicity. Circ Res. 103, 1270–1279. Feliciano, R.P., Pritzel, S., Heiss, C. and RodriguezMateos, A. 2015. Flavonoid intake and cardiovascular disease risk. Curr. Opin. Food Sci. 2, 92–99. Galati, E.M., Trovato, A., Kirjavainen, S., Forestieri, A.M., Rossitto, A. and Monforte, M.T. 1996. Biological effects of hesperidin, aCitrus flavonoid. (Note III): Antihypertensive and diuretic activity in rat. Farmaco. 51, 219–221. Garg, A., Garg, S., Zaneveld, L.J. and Singla, A.K. 2001. Chemistry and pharmacology of the Citrus bioflavonoid hesperidin. Phytother. Res. 15, 655–669. Hortobágyi, G.N. 1997. Anthracyclines in the treatment of cancer. An overview. Drugs 54 (Suppl. 4), 1–7. Kalyanaraman, B. 2020. Teaching the basics of the mechanism of doxorubicin-induced cardiotoxicity: Have we been barking up the wrong tree? Redox Biol. 29, 101394. Kudo, M. 2017. Systemic therapy for hepatocellular carcinoma: 2017 update. Oncology 93(Suppl1), 135–146. Kuzu, M., Kandemir, F.M., Yıldırım, S., Çağlayan, C. and Küçükler, S. 2021. Attenuation of sodium arsenite-induced cardiotoxicity and neurotoxicity with the antioxidant, anti-inflammatory, and antiapoptotic effects of hesperidin. Environ. Sci. Pollut. Res. Int. 28(9), 10818–10831. Li, C. and Schluesener, H. 2017. Health-promoting effects of the citrus flavanone hesperidin. Crit. Rev. Food Sci. Nutr. 57, 613–631. Naewla, S., Sirichoat, A., Pannangrong, W., Chaisawang, P., Wigmore, P. and Welbat, J.U. 2019. Hesperidin alleviates methotrexate-induced memory deficits via hippocampal neurogenesis in adult rats. Nutrients 11(4), 936. Nagai, K., Fukuno, S., Oda, A. and Konishi, H. 2016. Protective effects of taurine on doxorubicin-induced acute hepatotoxicity through suppression of oxidative stress and apoptotic responses. Anti-cancer Drugs 27, 17–23. Poetini, M.R., Araujo, S.M., Trindade de Paula, M., Bortolotto, V.C., Meichtry, L.B., Polet de Almeida, F., Jesse, C.R., Kunz, S.N. and Prigol, M. 2018. Hesperidin attenuates iron-induced oxidative damage and dopamine depletion in Drosophila melanogaster model of Parkinson's disease. Chem. Biol. Interact. 279, 177–86. Polak, J.M. and Van Noorden, S. 2003. Introduction to immunocytochemistry. 3rd ed. Oxford, UK: Bios Scientific Publishers. Sadzuka, Y., Sugiyama, T., Shimoi, K., Kinae, N. and Hirota, S. 1997. Protective effect of flavonoids on doxorubicin-induced cardiotoxicity. Toxicol. Lett. 92, 1–7. Sassi, A., Mokdad Bzéouich, I., Mustapha, N., Maatouk, M., Ghedira, K. and Chekir-Ghedira, L. 2017. Immunomodulatory potential of hesperetin and chrysin through the cellular and humoral response. Eur. J. Pharmacol. 812, 91–96. Segredo, M.P., Salvadori, D.M., Rocha, N.S., Moretto, F.C., Correa, C.R., Camargo, E.A. and et al. 2014. Oxidative stress on cardiotoxicity after treatment with single and multiple doses of doxorubicin. Hum. Exp. Toxicol. 33, 748–60. Sheibani, M., Azizi, Y., Shayan, M., Nezamoleslami, S., Eslami, F., Farjoo, M.H. and Dehpour, A.R. 2022. Doxorubicin-induced cardiotoxicity: an overview on pre-clinical therapeutic approaches. Cardiovasc. Toxicol. 22(4), 292–310. Simoncikova, P., Ravingerova, T. and Barancik, M. 2008. The effect of chronic doxorubicin treatment on mitogen-activated protein kinases and heat stress proteins in rat hearts. Physiol Res 57(Suppl. 2), S97–S102. Suvarna, S.K., Layton, C. and Bancroft, J.D. 2018. Bancroft's theory and practice of histological techniques, 8th ed. New York, NY and London, UK: Churchill Livingstone, pp: 83–92. Syahputra, R.A., Harahap, U., Dalimunthe, A., Nasution, M.P. and Satria, D. 2022. The role of flavonoids as a cardioprotective strategy against doxorubicin-induced cardiotoxicity: a review. Molecules 27(4), 1320. Trivedi, P.P., Kushwaha, S., Tripathi, D.N. and Jena, G.B. 2011. Cardioprotective effects of hesperetin against doxorubicin-induced oxidative stress and DNA damage in rat. Cardiovasc. Toxicol. 11(3), 215–225. Wenningmann, N., Knapp, M., Ande, A., Vaidya, T.R. and Ait-Oudhia, S. 2019. Insights into doxorubicin-induced cardiotoxicity: molecular mechanisms, preventive strategies, and early monitoring. Molecular Pharmacol 96(2), 219–232. Xiong, C., Wu, Y.Z., Zhang, Y., Wu, Z.X., Chen, X.Y., Jiang, P., Guo, H.C., Xie, K.R., Wang, K.X. and Su, S.W. 2018. Protective effect of berberine on acute cardiomyopathy associated with doxorubicin treatment. Oncol. Lett. 15, 5721–5729. Yu, J., Wang, C., Kong, Q., Wu, X., Lu, J.J. and Chen, X. 2018. Recent progress in doxorubicin-induced cardiotoxicity and protective potential of natural products. Phytomedicine 40, 125–139. Zanwar, A.A., Badole, S.L., Shende, P.S., Hegde, M.V. and Bodhankar, S.L. 2014. Chapter 76—cardiovascular effects of hesperidin: a Flavanone glycoside. In Polyphenols in Human Health and Disease. Eds., Watson, R.R., Preedy, V.R., Zibadi, S. (Eds.), San Diego, CA: Academic Press, pp: 989–992. | ||
| How to Cite this Article |
| Pubmed Style Alharbi FK, Alshehri ZS, Alshehri FF, Alhajlah SA, Khalifa HA, Dahran N, Ghonimi WA. The role of hesperidin as a cardioprotective strategy against doxorubicin-induced cardiotoxicity: The antioxidant, anti-inflammatory, antiapoptotic, and cytoprotective potentials. Open Vet J. 2023; 13(12): 1718-1728. doi:10.5455/OVJ.2023.v13.i12.20 Web Style Alharbi FK, Alshehri ZS, Alshehri FF, Alhajlah SA, Khalifa HA, Dahran N, Ghonimi WA. The role of hesperidin as a cardioprotective strategy against doxorubicin-induced cardiotoxicity: The antioxidant, anti-inflammatory, antiapoptotic, and cytoprotective potentials. https://www.openveterinaryjournal.com/?mno=175261 [Access: June 30, 2025]. doi:10.5455/OVJ.2023.v13.i12.20 AMA (American Medical Association) Style Alharbi FK, Alshehri ZS, Alshehri FF, Alhajlah SA, Khalifa HA, Dahran N, Ghonimi WA. The role of hesperidin as a cardioprotective strategy against doxorubicin-induced cardiotoxicity: The antioxidant, anti-inflammatory, antiapoptotic, and cytoprotective potentials. Open Vet J. 2023; 13(12): 1718-1728. doi:10.5455/OVJ.2023.v13.i12.20 Vancouver/ICMJE Style Alharbi FK, Alshehri ZS, Alshehri FF, Alhajlah SA, Khalifa HA, Dahran N, Ghonimi WA. The role of hesperidin as a cardioprotective strategy against doxorubicin-induced cardiotoxicity: The antioxidant, anti-inflammatory, antiapoptotic, and cytoprotective potentials. Open Vet J. (2023), [cited June 30, 2025]; 13(12): 1718-1728. doi:10.5455/OVJ.2023.v13.i12.20 Harvard Style Alharbi, F. K., Alshehri, . Z. S., Alshehri, . F. F., Alhajlah, . S. A., Khalifa, . H. A., Dahran, . N. & Ghonimi, . W. A. (2023) The role of hesperidin as a cardioprotective strategy against doxorubicin-induced cardiotoxicity: The antioxidant, anti-inflammatory, antiapoptotic, and cytoprotective potentials. Open Vet J, 13 (12), 1718-1728. doi:10.5455/OVJ.2023.v13.i12.20 Turabian Style Alharbi, Fawiziah K., Zafer S. Alshehri, Faez F. Alshehri, Sharif A. Alhajlah, Hesham A. Khalifa, Naief Dahran, and Wael A.m. Ghonimi. 2023. The role of hesperidin as a cardioprotective strategy against doxorubicin-induced cardiotoxicity: The antioxidant, anti-inflammatory, antiapoptotic, and cytoprotective potentials. Open Veterinary Journal, 13 (12), 1718-1728. doi:10.5455/OVJ.2023.v13.i12.20 Chicago Style Alharbi, Fawiziah K., Zafer S. Alshehri, Faez F. Alshehri, Sharif A. Alhajlah, Hesham A. Khalifa, Naief Dahran, and Wael A.m. Ghonimi. "The role of hesperidin as a cardioprotective strategy against doxorubicin-induced cardiotoxicity: The antioxidant, anti-inflammatory, antiapoptotic, and cytoprotective potentials." Open Veterinary Journal 13 (2023), 1718-1728. doi:10.5455/OVJ.2023.v13.i12.20 MLA (The Modern Language Association) Style Alharbi, Fawiziah K., Zafer S. Alshehri, Faez F. Alshehri, Sharif A. Alhajlah, Hesham A. Khalifa, Naief Dahran, and Wael A.m. Ghonimi. "The role of hesperidin as a cardioprotective strategy against doxorubicin-induced cardiotoxicity: The antioxidant, anti-inflammatory, antiapoptotic, and cytoprotective potentials." Open Veterinary Journal 13.12 (2023), 1718-1728. Print. doi:10.5455/OVJ.2023.v13.i12.20 APA (American Psychological Association) Style Alharbi, F. K., Alshehri, . Z. S., Alshehri, . F. F., Alhajlah, . S. A., Khalifa, . H. A., Dahran, . N. & Ghonimi, . W. A. (2023) The role of hesperidin as a cardioprotective strategy against doxorubicin-induced cardiotoxicity: The antioxidant, anti-inflammatory, antiapoptotic, and cytoprotective potentials. Open Veterinary Journal, 13 (12), 1718-1728. doi:10.5455/OVJ.2023.v13.i12.20 |