| Research Article | ||
Open Vet J. 2024; 14(5): 1098-1102 Open Veterinary Journal, (2024), Vol. 14(5): 1098–1102 Research Article Study of the therapeutic efficacy of DOKSI AVZ 500 in bacterial respiratory diseases in young pigsSergey Vladimirovich Engashev1, Aleksey Vladimirovich Savinkov2, Ekaterina Sergeevna Engasheva3, Artyom Viktorovich Lyamin4, Danir Damirovich Ismatullin4, Aleksandr Viktorovich Zhestkov4, Pavel Vladimirovich Iliasov4*, Konstantin Mikhailovich Sadov2 and Aleksandr Anatolievich Komarov51K.I. Skryabin Moscow State Academy of Veterinary Medicine and Biotechnology, Moscow, Russian Federation 2Samara State Agrarian University, Kinel, Russian Federation 3All-Russian Research Institute for Veterinary Sanitation, Hygiene and Ecology, Moscow, Russian Federation 4Samara State Medical University, Ministry of Health of the Russian Federation, Samara, Russian Federation 5Russian Biotechnological University, Moscow, Russian Federation *Corresponding Author: Pavel V. Iliasov. Samara State Medical University, Ministry of Health of the Russian Federation, Samara, Russian Federation. Email: p.v.ilyasov [at] samsmu.ru Submitted: 14/12/2023 Accepted: 04/04/2024 Published: 31/05/2024 © 2024 Open Veterinary Journal
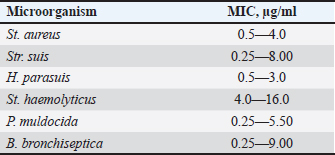
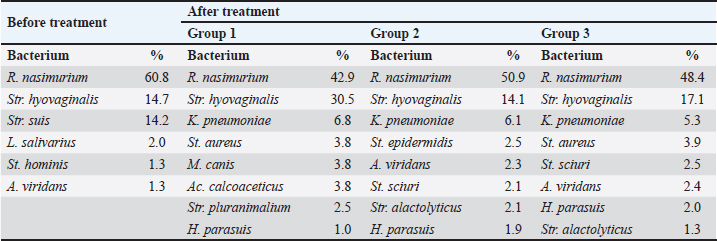
AbstractBackground: Young farm animals are susceptible to opportunistic infections which may cause economic losses due to mortality and poor weight gain. The development of antimicrobial resistance and the desire to improve therapy efficacy and safety are the reasons to seek for new antibacterial drugs ensuring rapid recovery with minimum adverse events. Aim: To estimate the efficacy of DOKSI AVZ 500 in respiratory pathologies in young pigs. Methods: The study was conducted in 65–70-day-old Yorkshire piglets with signs of bacterial respiratory pathologies. The animals were treated with the test drug for 3 or 5 days. The reference group received TETRAMAX 500 which is similar to the test drug in terms of chemical structure, mechanism of action, and activity spectrum. The animal's status was assessed using clinical examination, clinical blood count, and bacteriological tests. Results: Both test and reference drugs were well tolerated and ensured the animal recovery within about 4 days. The recovery was accompanied by normalization of hematological parameters and flora composition. The bacterium associated with the disease development, Streptococcus suis, was virtually completely eliminated in all groups. No adverse events were noted. After the treatment, all the animals readily gained weight and live market quality. Conclusion: DOKSI AVZ 500 was a highly efficient therapy for respiratory pathologies caused by the resident opportunistic flora in piglets. It has also shown noninferiority vs. TETRAMAX 500 in terms of all the health-related parameters and thus can be recommended for introduction in veterinary practice in pig farms. Keywords: Piglets, Opportunistic infection, Respiratory pathology, Tetracyclines, Drug testing. IntroductionBacterial respiratory diseases of young farm animals are common in agricultural holdings and inflict high economic losses due to animal mortality and poor weight gain (te Beest et al., 2011; van Duijkeren et al., 2011; Wilkinson et al., 2011; Crisci et al., 2013; Zhang et al., 2013; Reynaga et al., 2017). Their treatment is compounded by the adaptation of the animal’s microbiota to the drugs via development of the antimicrobial resistance or microbial succession. These factors, together with a need to improve the therapies, make the search for new drugs an ongoing hot topic. In this regard, AVZ LTD proposes the possibility of group therapy of piglets with a novel DOKSI AVZ 500 drug for oral administration. The study is aimed to estimate the efficacy of DOKSI AVZ 500 in respiratory pathologies in young pigs. The study involved an evaluation of the recovery dynamics using clinical blood count (CBC) and bacteriological analysis of nasal washings of test animals who received the drug orally for three and five days. Materials and MethodsThe study was conducted in Myasoagroprom LLC (Samara region, Russian Federation) in 65–70-day-old Yorkshire piglets of both sexes with an average weight of 17.1 kg which displayed clinical signs of a bronchopulmonary inflammatory pathology. At the beginning of the study, 3 groups with 12 animals each were formed. Baseline examination has shown no significant differences between the groups in terms of body temperature, weight, hematological parameters, and disease severity. The test drug was administered for the animals in a group orally in a dosage of 10 mg of the active substance per 1 kg of body weight, with drinking water, for 3 days in Group 1 and for 5 days in Group 2. Group 3 received a reference drug in a dose of 60 mg/kg of body weight two times a day, 12 hours apart. The animals of each group were housed in separate pens. All groups received the same diet matching the species- and age-specific feed standards. The animals were handled and treated in accordance with bioetics principles and applicable local and international animal testing regulations. The test drug was DOKSI AVZ 500, a powder for oral administration. It was developed and manufactured by AVZ S-P LLC (Russian Federation), and the pilot batch of the drug was provided for this study. 1 g of the drug contains 500 mg of doxycycline hyclate. It looks like a pale-yellow powder. Doxycycline which is comprised in the drug is a semi-synthetic tetracycline antibiotic active against a broad spectrum of both Gram-positive and Gram-negative bacteria. A bacteriostatic effect of doxycycline is based on the inhibition of the enzymes that catalyze the binding of aminoacyl tRNAs with ribosomal acceptors, followed by protein synthesis disturbance and bacterial cell death. The reference drug was TETRAMAX 500 (FISH Corp. 2,000 d.o.o., Serbia). It contains, as an active substance, another tetracycline antibiotic, oxytetracycline, which has a mechanism of action and spectrum similar to those of the doxycycline. The reference drug’s indications include but are not limited to bronchial pneumonia in piglets. The test drug efficacy was estimated based on the findings of clinical monitoring, CBC, and bacteriological analysis. At the baseline, nasal washings and blood for CBC were drawn. Seven days after the last drug administration, nasal washings and blood were drawn again. To assess the completeness of the recovery, the animals were followed up until Day 12 when they were weighed to determine the average daily gain during this period. The blood samples were drawn from the vena cava cranialis with a disposable sterile vacuum system into EDTA tubes. CBC was performed on the Mindray Вс-5,300 blood analyzer (Mindray Medical International Limited, China) and included the determination of RBC, WBC, platelets, hemoglobin level, and hematocrit. WBC differential was determined by microscopy of Pappenheim-stained blood smears. The nasal samples for bacteriology were taken using sterile swabs. Bacteria from the nasal mucosa were inoculated on 5% blood agar and universal chromogenic media (Bio-Rad Laboratories, Inc., USA). The cultures were incubated for 2 days at 37°С. All growing bacteria were identified using Microflex LT MALDI-ToF mass spectrometer (Bruker®, USA) by direct application. The drug sensitivity was determined using 2–fold serial dilution method. At the end of the study, the data obtained were subjected to statistical processing using MedCalc v. 20 (Medcalc Software Ltd., USA). The data between and within groups were compared using the Wilcoxon signed-rank test for independent or paired samples, respectively. р ≤ 0.05 was considered a significance threshold. Logistic regression was estimated using the forward method, with p < 0.05 being used as an inclusion criterion (Culliford, 2022). Numeric data were provided in terms of WHO-recommended SI units. Ethical approvalThe present study was approved by the Institutional Review Board and Ethical Committee of the Samara State Agrarian University. The entire study and all procedures were designed so as to minimize the damage to animals and avoid their mortality. ResultsAt the baseline, the test animals displayed shortness of breath and disruptive cough. During the cough, the piglets stood with their fore limbs astride, craned neck, and gathered-in stomach. Appetite and motor activities were decreased. The appearance was unsatisfactory, with constrained posture, weak body build, poor live market quality, sallow skin, pale dry conjunctiva and oral mucosa, sunken eyes, and catarrhal purulent nasal discharge; some piglets had also copious conjunctival discharge. These signs were characteristic of infectious inflammatory airway or lung pathologies. Necropsy of animals that died due to such diseases typically reveals cases of catarrhal and purulent rhinitis, laryngitis, tracheitis, and bronchitis. Some animals had lobular pneumonia of varying severity. Clinical dynamicsBefore the treatment, the body temperature in all groups was slightly above 40°С. Within the first 4 days of treatment, the temperature dynamically reduced by 3.71% (р ≤ 0.01), 2.97% (р ≤ 0.01), and 2.94% (р ≤ 0.01) in Groups 1, 2, and 3, respectively. Furthermore, the temperature varied within the reference range (39.0°С–40.0°С). In all groups, the changes vs. baseline were significant. The animal’s clinical status improved with the decrease in its body temperature. As indicated above, on Day 1 100% of animals had cough and unsatisfactory appearance. One day after the start of treatment, 33.3%, 25.0%, and 33.0% of animals in Groups 1, 2, and 3, respectively, improved their appetites and motor activities. Two days after the start of treatment, 75%, 66.7%, and 66.7% of piglets in Groups 1, 2, and 3, respectively, had no cough. Appetites and motor activities improved in all groups (91.7%, 83.3%, and 83.3% animals). 50.0%, 58.3%, and 50.0% piglets improved their appearances in Groups 1, 2, and 3, respectively. Three days after the start of treatment all animals displayed improved appetites, motor activities, and appearances. Cough was only recorded in 8.3%, 16.7%, and 8.3% of piglets in Groups 1, 2, and 3, respectively. On Day 4, all animals in all groups had reached a complete recovery in terms of the above parameters, with no significant differences found between groups and uniform clinical dynamics. None of the animals used in the study died during the experiments and follow-up period. On Day 12, median body weight was 24.0, 24.5, and 23.8 kg in Groups 1, 2, and 3, respectively, with average daily gain of 0.58, 0.62, and 0.55 kg. Considering that the average daily gain in stall-fed pigs is 0.54 kg, DOKSI AVZ 500 was allowed to surpass this technological standard value by 0.04 and 0.08 kg in Groups 1 and 2, although these values were not significant. Thus, DOKSI-AVZ 500, when used to treat respiratory pathologies in piglets in accordance with the instructions, allowed to reach clinical recovery on Day 4. Moreover, we observed the achievement of the holding-specific technological body weight standards. No adverse events were recorded after the drug administration. Hematological parametersAt the baseline, WBC in animals (21.3; 20.7–21.8) reflected inflammatory processes in their bodies and was above the ULN (reference range is 8.0—16.0×109/l). WBC differential parameters showed no apparent differences vs. reference ranges. After the treatment, the animals that were treated with the test drug for 3 days had significant changes in bands (−59.3%) and segmented neutrophils (25.7%), eosinophils (−49.4%), lymphocytes (−13.1%), and monocytes (−55.6%). WBC showed no significant changes in this group (−8.3%, p > 0.05), while reaching the reference values characteristic for healthy pigs. The animals treated with the test drug for 5 days had significant changes in WBC (−24.9%), bands (−71.8%) and segmented neutrophils (23.4%), lymphocytes (−13.4%), and monocytes (−47.3%). The animals treated with TETRAMAX 500 also had significant changes in WBC (−17.5%), bands (−64.3%), segmented neutrophils (24.7%), lymphocytes (−12.8%), and monocytes (−51.5%). All animals had RBC within the reference range at any time. Baseline hemoglobin was low and did not reach LLN in all groups. After the treatment, the changes in RBC (9.2%), hemoglobin (10.4%), and hematocrit (13.8%) in Group 1, as well as in hemoglobin level (8.0% and 9.1%) were noted in Groups 2 and 3. At the end of the experiment, hemoglobin level was within the reference range (90–130 g/l) in all animals. Therefore, the use of DOKSI AVZ 500 in respiratory pathologies in young pigs was accompanied by the restoration of normal WBC levels indicating inhibition of the inflammatory processes. An increase in the segmented neutrophils suggested maintenance of the active cellular immunity. DOKSI AVZ 500 also promoted the restoration of hemoglobin levels which might be associated with pathogen elimination, reduction of inflammatory intoxication, and restoration of body functions. Considering the above, one can conclude that the use of DOKSI AVZ 500 in respiratory pathologies in pigs results in a decrease in WBC, restructuring of WBC differential, and a significant increase in hemoglobin levels. Bacteriological testsThe antimicrobial activity of DOKSI AVZ 500 was determined in the microorganisms isolated from the sick animals (Table 1). The assessment of the sensitivity of the bacteria isolated from the nasal washings of sick piglets to DOKSI AVZ 500 has shown high drug sensitivity of Streptococcus suis (MIC=0.25—8.00 µg/ml), Pasteurella muldocida (MIC=0.25—5.50 µg/ml), and Bordetella bronchiseptica (MIC=0.25–9.00 µg/ml). High sensitivity values in terms of MIC were also obtained for Staphylococcus aureus (0.5–4.0 µg/ml), Haemophilus parasuis (MIC=0.5–3.0 µg/ml), and Staphylococcus haemolyticus (4–16 µg/ml). Therefore, DOKSI AVZ 500 would be quite efficient against both Gram-positive and Gram-negative flora. All the bacteria tested were sensitive to the drug. The percent composition of the piglet respiratory tract microbiota is provided in Table 2 (which only includes bacteria accounting for at least 1% of total bacterial content). As a result of treatment, all groups showed a decrease in the content of S. suis and, to a lesser extent, of Rothia nasimurium. Instead, there was an increase in the percent contents of Klebsiella pneumoniae, H. parasuis, Moraxella canis, Acinetobacter calcoaceticus, as well as a number of staphylocicci (including St. aureus), and streptococci which apparently were relatively resistant to tetracyclines. Surprisingly, the increase in the percentage of K. pneumoniae and St. aureus had no adverse effect on animal health. Most of the recorded changes in the flora were insignificant. The exceptions were the decrease in contents of R. nasimurium (−17.9%), Str. suis (−13.4%), and an increase in Streptococcus hyovaginalis (15.8%) in Group 1, as well as the decrease in R. nasimurium (−9.9%), S. suis (−13.7%), and total bacterial content (−55.8%) in Group 2; marginal changes (p=0.05—0.07) also took place in Group 2 for Str. hyovaginalis (−0.6%) and K. pneumoniae (5.9%). Group 3 which received the reference drug also showed a significant decrease in the contents of R. nasimurium (−12.4%) and S. suis (−13.6%), as well as marginal changes in Str. hyovaginalis (−2.4%), K. pneumoniae (4.5%), and St. aureus (3.0%). Table 1. Sensitivity of the microorganisms isolated from the nasal washings of sick animals to DOKSI AVZ 500, μg/ml.
Table 2. Percent composition of the test animal respiratory tract microbiota.
Table 3. Logistic regression parameters when assessing effect of bacteria on animal health.
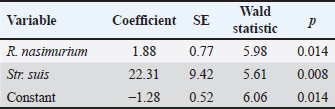
Between groups 1 and 2 which received the test drug for 3 and 5 days, significant differences for Str. hyovaginalis (−16.4%) and marginal differences for Staphylococcus epidermidis (0.49%) and Aeromonas viridans (0.23%) were recorded after the treatment. These bacteria, except for Str. hyovaginalis, did not play a prominent part in the total flora of the piglet respiratory tract. Group 2 also showed a 2-fold decrease in the total microbial content vs. baseline (with the virtual absence of changes in this parameter in Group 1). The joined Groups 1 and 2 had significant changes in the contents of R. nasimurium (−15.9%), Str. suis (−13.6%), Enterococcus hirae (−0.64%), and K. pneumoniae (6.3%) vs. baseline. Marginal changes (p=0.05—0.07) were also recorded for H. parasuis (1.3%). After the treatment, no significant changes were revealed in the percent content of the flora in joined Groups 1 and 2 vs. Group 3, except for the marginal increase in A. viridans (0.7%), Staphylococcus sciuri (0.6%), and Escherichia coli (0.6%), as well as the decrease in Staphylococcus chromogenes (−0.3%) in Group 3 which received TETRAMAX 500. Percentages of these bacteria in the piglet respiratory tract did not exceed 1% both before and after treatment; hence, they would not significantly affect the animal's health. Logistic regression which was calculated to assess the effect of the flora on the animal health, had revealed a significant association of Str. suis and far weaker but also significant association of R. nasimurium with the pathology treated. The total model significance level was 0.001, with the area under the curve of 0.786 (95% CI 0.643–0.891), which suggests a sufficiently high model reliability. The regression coefficients are provided in Table 3. DiscussionAs shown above, both the test or reference drugs virtually eliminated Str. suis and significantly reduced percentage of R. nasimurium in the flora of the respiratory tract in the test animals which apparently was a key to their recovery. It should be noted that Rothia genus includes common opportunistic bacteria that are prevalent in the healthy mammalian saliva and can only cause diseases in immunocompromised animals. Therefore, the contribution of R. nasimurium in the pathology treated was likely secondary to the effect of Str. suis which apparently resulted in decay in the piglets’ immune protection. When Str. suis was eliminated, R. nasimurium no longer posed injury to the animals. K. pneumoniae and St. aureus strains whose percentages increased in the treated animals were unlikely to be highly pathogenic. Hence, after the treatment, the respiratory tract microbiota comprised no bacteria which could have an adverse effect on the animal health in the concentrations presented. These findings allow us to conclude that DOKSI AVZ 500 was a highly efficient therapy for respiratory pathologies caused by the resident opportunistic flora in piglets. This is supported by improvements in the animal's clinical status, changes in the composition of nasal washing flora, and hematological parameters. Administration of the drug to sick piglets resulted in a decrease in WBC, restructuring of WBC differential, and increase in hemoglobin levels. It also ensured complete therapeutic efficacy (in terms of reaching recovery) in pigs with respiratory pathologies on Day 4 after the start of treatment. The recovered animals readily achieved holding-specific technological body weight standards. DOKSI AVZ 500 virtually eliminated pathogenic flora represented by Str. suis and significantly reduced the percent content of R. nasimurium which were significantly associated with the bronchopulmonary pathologies in the test animals. Animals well tolerated the drug, with no adverse events noted. Moreover, DOKSI AVZ 500 has shown noninferiority vs. TETRAMAX 500 which is approved in this pathology, in terms of all the health-related parameters studied. ConclusionConsidering the above, we recommend the use of DOKSI AVZ 500 in acute infectious inflammatory respiratory pathologies caused by resident opportunistic flora in young pigs, in a dosage of 10 mg of the active substance/kg of body weight with drinking water for 3 days, as this time period ensures the therapeutic effect similar to the results of 5-day therapy. AcknowledgmentsThe authors would like to thank Grigory G. Pratasov, the General Manager of Myasoagroprom LLC, and Pyotr N. Zhirnov, the Chief Veterinarian of Myasoagroprom LLC, for their contribution to the study. Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionsAll authors directly contributed to the manuscript’s composing, revising, and approving its final version. S.V. Engashev, K.M. Sadov, and A.A. Komarov developed the study design and controlled its progress. A.V. Savinkov and E.S. Engasheva performed the animal treatment, examination, and follow-up. A.V. Lyamin, D.D. Ismatullin, and A.V. Zhestkov analyzed the blood samples and nasal washings obtained throughout the study. P.V. Iliasov performed the statistical processing of the experimental data. FundingThe study was funded by AVZ LTD (Russian Federation) within the program of development and testing of new drugs for veterinary use. Data availabilityThe data that support the findings of this study are available within the manuscript. ReferencesCrisci, E., Mussa, T., Fraile, L. and Montoya, M. 2013. Influenza virus in pigs. Mol. Immunol. 55(3-4), 200–211. Culliford, D. 2022. Applied Statistical Considerations for Clinical Researchers. Berlin, Germany: Springer. Reynaga, E., Torres, C., Garcia-Nunez, M., Navarro, M., Vilamala, A., Puigoriol, E., Lucchetti, G.E. and Sabria, M. 2017. Clinical impact and prevalence of MRSA CC398 and differences between MRSA-TetR and MRSA-TetS in an area of Spain with a high density of pig farming: a prospective cohort study. Clin. Microbiol. Infect. 23(9), 678.e1–678.e4. te Beest, D.E., Hagenaars, T.J., Stegeman, J.A., Koopmans, M.P. and van Boven, M. 2011. Risk based culling for highly infectious diseases of livestock. Vet. Res. 42(1), 81. van Duijkeren, E., Catry, B., Greko, C., Moreno, M.A., Pomba, M.C., Pyorala, S., Ruzauskas, M., Sanders, P., Threlfall, E.J., Torren-Edo, J. and Torneke, K. 2011. Review on methicillin-resistant Staphylococcus pseudintermedius. J. Antimicrob. Chemother. 66(12), 2705–2714. Wilkinson, K., Grant, W.P., Green, L.E., Hunter, S., Jeger, M.J., Lowe, P., Medley, G.F., Mills, P., Phillipson, J., Poppy, G.M. and Waage, J. 2011. Infectious diseases of animals and plants: an interdisciplinary approach. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 366(1573), 1933–1942. Zhang, Q., Hu, R., Tang, X., Wu, C., He, Q., Zhao, Z., Chen, H. and Wu, B. 2013. Occurrence and investigation of enteric viral infections in pigs with diarrhea in China. Arch. Virol. 158, 1631–1636. | ||
| How to Cite this Article |
| Pubmed Style Engashev SV, Savinkov AV, Engasheva ES, Lyamin AV, Ismatullin DD, Zhestkov AV, Iliasov PV, Sadov KM, Komarov AA. Study of the therapeutic efficacy of DOKSI AVZ 500 in bacterial respiratory diseases in young pigs. Open Vet J. 2024; 14(5): 1098-1102. doi:10.5455/OVJ.2024.v14.i5.2 Web Style Engashev SV, Savinkov AV, Engasheva ES, Lyamin AV, Ismatullin DD, Zhestkov AV, Iliasov PV, Sadov KM, Komarov AA. Study of the therapeutic efficacy of DOKSI AVZ 500 in bacterial respiratory diseases in young pigs. https://www.openveterinaryjournal.com/?mno=180107 [Access: June 30, 2025]. doi:10.5455/OVJ.2024.v14.i5.2 AMA (American Medical Association) Style Engashev SV, Savinkov AV, Engasheva ES, Lyamin AV, Ismatullin DD, Zhestkov AV, Iliasov PV, Sadov KM, Komarov AA. Study of the therapeutic efficacy of DOKSI AVZ 500 in bacterial respiratory diseases in young pigs. Open Vet J. 2024; 14(5): 1098-1102. doi:10.5455/OVJ.2024.v14.i5.2 Vancouver/ICMJE Style Engashev SV, Savinkov AV, Engasheva ES, Lyamin AV, Ismatullin DD, Zhestkov AV, Iliasov PV, Sadov KM, Komarov AA. Study of the therapeutic efficacy of DOKSI AVZ 500 in bacterial respiratory diseases in young pigs. Open Vet J. (2024), [cited June 30, 2025]; 14(5): 1098-1102. doi:10.5455/OVJ.2024.v14.i5.2 Harvard Style Engashev, S. V., Savinkov, . A. V., Engasheva, . E. S., Lyamin, . A. V., Ismatullin, . D. D., Zhestkov, . A. V., Iliasov, . P. V., Sadov, . K. M. & Komarov, . A. A. (2024) Study of the therapeutic efficacy of DOKSI AVZ 500 in bacterial respiratory diseases in young pigs. Open Vet J, 14 (5), 1098-1102. doi:10.5455/OVJ.2024.v14.i5.2 Turabian Style Engashev, Sergey Vladimirovich, Aleksey Vladimirovich Savinkov, Ekaterina Sergeevna Engasheva, Artyom Viktorovich Lyamin, Danir Damirovich Ismatullin, Aleksandr Viktorovich Zhestkov, Pavel Vladimirovich Iliasov, Konstantin Mikhailovich Sadov, and Aleksandr Anatolievich Komarov. 2024. Study of the therapeutic efficacy of DOKSI AVZ 500 in bacterial respiratory diseases in young pigs. Open Veterinary Journal, 14 (5), 1098-1102. doi:10.5455/OVJ.2024.v14.i5.2 Chicago Style Engashev, Sergey Vladimirovich, Aleksey Vladimirovich Savinkov, Ekaterina Sergeevna Engasheva, Artyom Viktorovich Lyamin, Danir Damirovich Ismatullin, Aleksandr Viktorovich Zhestkov, Pavel Vladimirovich Iliasov, Konstantin Mikhailovich Sadov, and Aleksandr Anatolievich Komarov. "Study of the therapeutic efficacy of DOKSI AVZ 500 in bacterial respiratory diseases in young pigs." Open Veterinary Journal 14 (2024), 1098-1102. doi:10.5455/OVJ.2024.v14.i5.2 MLA (The Modern Language Association) Style Engashev, Sergey Vladimirovich, Aleksey Vladimirovich Savinkov, Ekaterina Sergeevna Engasheva, Artyom Viktorovich Lyamin, Danir Damirovich Ismatullin, Aleksandr Viktorovich Zhestkov, Pavel Vladimirovich Iliasov, Konstantin Mikhailovich Sadov, and Aleksandr Anatolievich Komarov. "Study of the therapeutic efficacy of DOKSI AVZ 500 in bacterial respiratory diseases in young pigs." Open Veterinary Journal 14.5 (2024), 1098-1102. Print. doi:10.5455/OVJ.2024.v14.i5.2 APA (American Psychological Association) Style Engashev, S. V., Savinkov, . A. V., Engasheva, . E. S., Lyamin, . A. V., Ismatullin, . D. D., Zhestkov, . A. V., Iliasov, . P. V., Sadov, . K. M. & Komarov, . A. A. (2024) Study of the therapeutic efficacy of DOKSI AVZ 500 in bacterial respiratory diseases in young pigs. Open Veterinary Journal, 14 (5), 1098-1102. doi:10.5455/OVJ.2024.v14.i5.2 |