| Original Article | ||
Open Vet J. 2022; 12(5): 602-611 Open Veterinary Journal, (2022), Vol. 12(5): 602–611 Original Research The effect of cryopreservation media on the quality of β-thalassemia mouse spermatozoaKakanang Buranaamnuay1*, Suparada Aiemongkot2, Chinarat Changsangfa3 and Saovaros Svasti21Molecular Agricultural Biosciences Cluster, Institute of Molecular Biosciences (MB), Mahidol University, Nakhon Pathom, Thailand 2Thalassemia Research Center (TRC), Institute of Molecular Biosciences (MB), Mahidol University, Nakhon Pathom, Thailand 3Office of Research and Innovation Affair, Institute of Molecular Biosciences (MB), Mahidol University, Nakhon Pathom, Thailand *Corresponding Author: Kakanang Buranaamnuay. Molecular Agricultural Biosciences Cluster, Institute of Molecular Biosciences (MB), Mahidol University, Nakhon Pathom, Thailand. Email: ningkakanang [at] yahoo.com Submitted: 19/05/2022 Accepted: 08/08/2022 Published: 04/09/2022 © 2022 Open Veterinary Journal
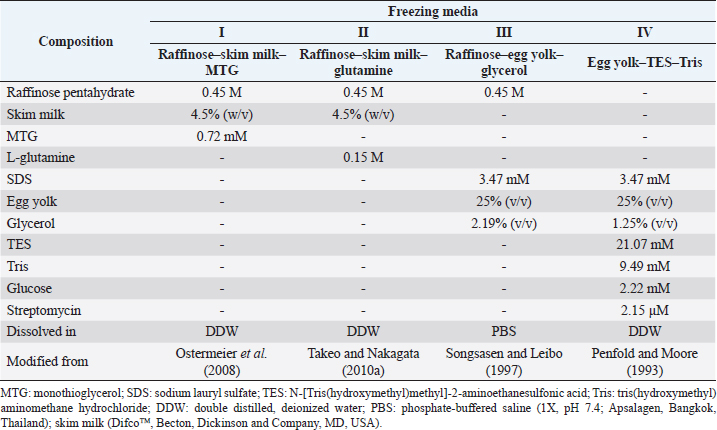
AbstractBackground: The mouse model of human diseases is commonly used for biomedical study, including β-thalassemia (β-thal), an inherited hemoglobin disorder. Maintaining the mice strain by natural mating systems is costly and seems impractical, especially during the COVID-19 pandemic. Sperm-freezing is a cost-effective solution for β-thal mouse colony management. Aim: To determine appropriate cryopreservation media for β-thal mouse spermatozoa to establish a β-thal mouse sperm bank. Methods: The epididymal spermatozoa of C57BL/6 wild-type (WT) and β-globin gene knockout thalassemia (BKO) mice were frozen in four freezing media: I) raffinose–skim milk–monothioglycerol (MTG), II) raffinose–skim milk–glutamine, III) raffinose–egg yolk–glycerol, and IV) egg yolk–TES–Tris. The sperm quality was assessed prior to and following freeze-thawing. Results: Compared with WT counterparts, the viable spermatozoa before freezing exhibiting elevated levels of oxidative stress were significantly greater in BKO (p=0.01). After thawing, the membrane integrity of BKO spermatozoa preserved in I was significantly lower (p=0.001). The sperm viability and membrane integrity of BKO males were also inferior when media III and IV were used (p=0.008–0.027). The amount of oxidative stress in the spermatozoon of BKO mice was significantly greater when preserved in I, III, and IV (p=0.002–0.044). Comparing freezing media, the motility and acrosome integrity of WT and BKO spermatozoa preserved in IV were significantly higher than those in other media (p < 0.001 to p=0.01). Spermatozoa with the highest mitochondrial membrane potential were observed in I in both genotypes (p=0.012 to p > 0.05). The viability, membrane integrity, and oxidative stress of post-thaw BKO spermatozoa did not significantly differ among freezing solutions. Conclusion: Irrespective of freezing media, spermatozoa of BKO males are rather more sensitive to cryopreservation than those of WT. Raffinose–skim milk–MTG/glutamine, raffinose–egg yolk–glycerol, and egg yolk–TES–Tris can all be used to preserve BKO mouse spermatozoa. However, with slightly better sperm characteristics, egg yolk–TES–Tris may be a diluent of choice for BKO mouse sperm cryopreservation. The addition of a reducing agent to thawing media is also strongly recommended to efficiently prevent oxidative stress and therefore improve frozen-thawed sperm survival. Keywords: Anemia, Freezing extender, Mouse, Sperm characteristics. IntroductionMice (Mus musculus) are closely similar to humans in, for example, biological characteristics, genetics, anatomy, and physiology; thus, they are the preferred laboratory animals used for biomedical research (Silver, 2001; Justice and Dhillon, 2016). However, just one mouse strain/stock cannot be a suitable proxy for all the areas studied. Therefore, many mice strains with the induced mutation have been generated worldwide (Bedell et al., 1997; Simpson et al., 1997). Maintaining these strains by natural mating systems controlled by animal facility staff is becoming a significant problem, especially in a COVID-19 outbreak era in which human mobility restriction has been implemented in most countries. Freezing of gametes (oocytes and spermatozoa) and embryos seems a viable solution. Among them, sperm-freezing seems the least complicated and least expensive operation to achieve this goal, since only one sperm donor can provide huge numbers of gametes to be preserved (Nakagata, 2000). Up to now, numerous reports have demonstrated moderately successful mouse sperm cryopreservation by using various procedures. The raffinose–skim milk-based diluent has been refined and applied to epididymal spermatozoa of different mouse strains as a cryopreservation solution (Nakagata and Takeshima, 1993; Nakagata et al., 1995; Nakagata, 1996). Using this diluent, monothioglycerol (MTG) or L-glutamine, an antioxidant, was also included in a certain amount to mitigate sperm cryoinjury and therefore improve sperm quality after cryopreservation. Ostermeier et al. (2008) found that the presence of MTG in the freezing medium increased the fertilizing capacity of frozen-thawed C57BL/6J spermatozoa. Similarly, the in vitro fertilization rates of frozen-thawed C57BL/6J spermatozoa were improved when L-glutamine was added to the raffinose–skim milk-based diluent (Takeo and Nakagata, 2010a). Furthermore, cryopreservation of mouse spermatozoa has been undertaken with other freezing media, such as egg yolk–TES–Tris and raffinose–egg yolk–glycerol-based diluents with promising results (Penfold and Moore, 1993; Songsasen and Leibo, 1997). It has been demonstrated that 25% of the epididymal spermatozoa collected from CBA males regained motility after freezing and thawing in a modified egg yolk–TES–Tris solution. In vitro fertilization with the frozen–thawed sperm population resulted in 50% of oocytes developing to the two-cell stage, and two-cell embryos could develop into normal life offspring after embryo transfer (Penfold and Moore, 1993). Mouse sperm cryopreservation using the raffinose–egg yolk–glycerol-based diluent resulted in varying levels of sperm survival, depending on the mouse strains. Songsasen and Leibo (1997) found that using this diluent, epididymal spermatozoa of the B6D2F1 strain had the highest survival, while those of the C57BL/6J strain were the most sensitive. Such findings imply a genetic basis for the sensitivity of mouse spermatozoa to freezing injury, and there is no one-size-fits-all freezing medium for all mouse strains’ sperm cryopreservation. Therefore, the investigation and development of reliable and effective methods for freezing spermatozoa remain indispensable, primarily in genetically modified mice that have not been examined before. β-thalassemia (β-thal) is an inherited disorder of hemoglobin commonly found in Thais and populations of Mediterranean, African, and South Asian ancestry (Flint et al., 1998). The signs and symptoms of β-thal, such as severe anemia, cardiac failure, and liver cirrhosis, result directly or indirectly from the imbalanced α- or non-α-globin chains in the hemoglobin molecule leading to excess reactive oxygen species (ROS) formation (Higgs et al., 2012). In addition, endocrine dysfunction is one of the significant complications of β-thal that is caused by premature hemolysis and repeated blood transfusions and, therefore, iron deposition in the endocrine glands. Affected individuals with endocrine disorders develop growth retardation, delayed puberty, and diabetes mellitus (Cappellini et al., 2008). The mouse model of human diseases is commonly used for biomedical study, including β-thal. At present, several viable β-thal transgenic mice made on a C57BL/6 (B6) background are maintained in our animal facility. The costs, mouse room space, and related resources are inevitably required to accommodate them. It is intriguing to find efficient and cost-effective alternatives for β-thal mouse colony management. The present study, which aims to establish a β-thal mouse sperm bank, was conducted to determine suitable cryopreservation media for β-thal mouse spermatozoa. Four types of proven freezing solutions, i.e., raffinose–skim milk–MTG, raffinose–skim milk–glutamine, raffinose–egg yolk–glycerol, and egg yolk–TES–Tris-based diluents, were examined. In vitro sperm quality of B6 wild-type (WT) and β-thal mice after freeze-thawing was assessed. Materials and MethodsChemical reagentsUnless otherwise stated, all media components used in the present study were obtained from Sigma Aldrich (St Louis, MO). Animal cohortsWT and β-globin gene knockout thalassemia (BKO) mice on a B6 background used in the present study were maintained in the Laboratory Animal Unit at MB under controlled conditions of light (12L:12D), temperature (22°C ± 2°C), and relative humidity (55% ± 15%). They were fed a regular chow diet and water ad libitum. Sperm collection and processingWT and BKO male mice, aged between 3 and 6 months old, were sacrificed by cervical dislocation. In each replicate, the testes, cauda epididymides, and vas deferens were removed from three males of the same strain using an aseptic technique and then put together in warmed (37°C) standard saline solution (0.9% sodium chloride); this was done to reduce male-to-male variability. At the laboratory, the cauda epididymides and vas deferens were separated from the testes and cut into small pieces using operating curved scissors with sharp blades. Epididymal spermatozoa were released into 800 µl of sperm-Tyrode's albumin lactate pyruvate (sperm-TALP) (Buranaamnuay, 2013) at 37°C. The spermatozoa fluid was filtered through nylon filter mesh to remove unwanted tissues. The obtained fluid, approximately 500 µl, was equally allocated into five aliquots for sperm evaluation (one aliquot) and sperm cryopreservation (four aliquots). Sperm-freezingAt room temperature (26°C–28°C), four aliquots of sperm suspension (100 µl each) were gradually added with a double volume of each of four different freezing media and mixed. According to the previously published methods, these freezing media were prepared beforehand (see the composition and references in Table 1). The sperm-freezing media mixtures were subsequently loaded into 0.25 ml plastic straws (Minitub GmBH, Tiefenbach, Germany), which were prefilled with M2 medium to prevent straw floating during storage in liquid nitrogen (LN2). The order and volume of substances filled from the top to bottom ends of each straw were 100 µl M2 medium, 10 mm air, 20 µl sperm suspension, 5 mm air, 20 µl sperm suspension, 5 mm air, and 10 µl sperm suspension. The loaded straws sealed with polyvinyl powder (IMV Technologies, L Aigle, France) were frozen by being placed horizontally on a 3.5-cm-thick Styrofoam raft floating on the surface 9-cm-deep LN2 for 10 minutes. The straws were maintained in LN2 for long-term storage. Sperm thawingIn the present study, sperm straws were thawed approximately 1 week after freezing. Upon thawing, two straws per group randomly taken from LN2 canisters were plunged in 37°C water for 30 seconds. All contents in the thawed straws, which included sperm suspension and M2 medium, were transferred to a 1.5 ml microtube and diluted with sperm-TALP. Sperm analysisUnless otherwise indicated, sperm parameters were assessed in both fresh and frozen-thawed samples. All flow cytometry experiments were performed on a BD AccuriTM C6 Plus Flow Cytometer (Becton Dickinson, Franklin Lakes, NJ); fluorescent data collected from at least 30,000 gated events per test were acquired with BD Accuri C6 Plus software. Sperm concentrationThe concentration (x106 cells/ml) of epididymal spermatozoa was measured with a counting-chamber device (Hemocytometer; Hausser Scientific, Horsham, PA) after diluting an aliquot of the sample in formal saline [0.9% sodium chloride and 0.1% (v/v) 40% formaldehyde in distilled water] (1:40, v/v). This parameter was measured only in fresh samples. Sperm motilityTen microliters of the sperm sample were placed on a pre-warmed (37°C) glass slide and covered with a pre-warmed coverslip. Total sperm motility was assessed from at least five fields per slide by an experienced technician throughout the study under a phase-contrast microscope (Nikon, Melville, NY) (200×). The percentage of motility was an average of two slides. Sperm viabilityEosin and nigrosin dyes were used to evaluate this sperm parameter (Buranaamnuay et al., 2021). Sperm and dyes, with an equal aliquot, were mixed on a warmed glass slide for 30 seconds. Ten microliters of the mixture were transferred and smeared on another cleaned microscope slide. The dried slide was abruptly checked for the sperm viability using a light microscope (200×) with a white or faint pink sperm head judged as live and a red head deemed dead. The percent sperm viability was calculated from a total of 200 cells counted. Table 1. The composition of sperm-freezing media used in the present study for C57BL/6 WT and BKO mice.
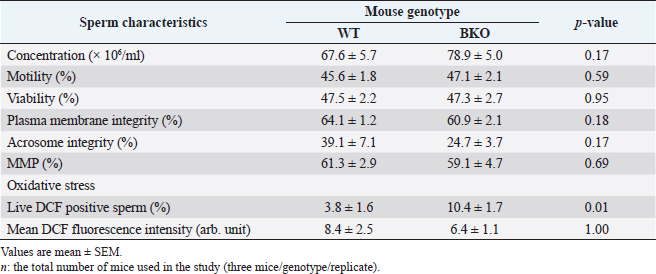
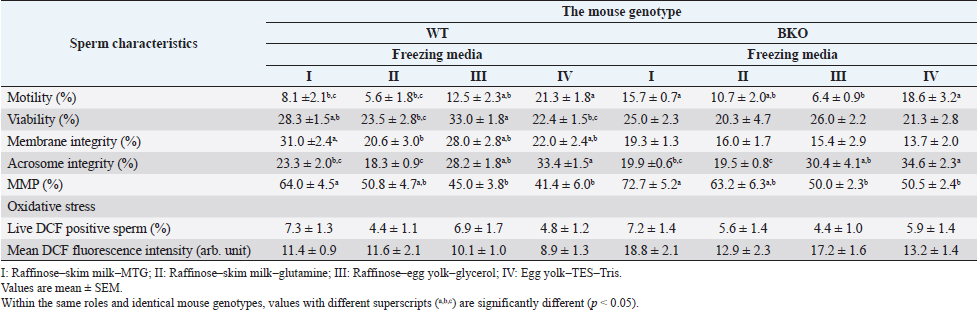
Sperm plasma membrane integrityThe integrity of the sperm plasma membrane in maintaining osmotic balance is one of the essential factors for successful fertilization (Ramu and Jeyendran, 2013). In the hypo-osmotic swelling test (HOST), sperm solution was mixed with 75 mOsm/kg hypo-osmotic solution, prepared with fructose and sodium citrate, at a ratio of 1:10 (v/v), respectively. The mixed solution was left for 20 minutes at 37°C and then aliquoted on a glass slide. The percentage of plasma membrane intact sperm developing tail swellings was given from 200 total spermatozoa per sample observed under a phase-contrast microscope (400×). Acrosome integrityThe acrosome reaction stage of epididymal spermatozoa was determined with a fluorescent dye specifically bound to the outer acrosomal membrane, i.e., fluorescein isothiocyanate-conjugated peanut agglutinin (FITC-PNA). In the staining process modified by Nagy et al. (2003), spermatozoa (2×106/ml, 120 µl) were incubated at 37°C for 10 minutes in phosphate-buffered saline (PBS) (1×, pH 7.4; Apsalagen, Bangkok, Thailand) containing 10 µl of FITC-PNA (25.7 µM). The membrane impermeant vital stain propidium iodide (PI; 74.8 µM) was added to counterstain damaged spermatozoa. The labeled sperm suspensions were added with 300 µl of PBS, remixed, and immediately analyzed. Cell fluorescence was excited in a flow cytometer using a 488-nm argon excitation laser. The red fluorescent signal of PI-positive cells is detected using fluorescence detector 3 (FL-3; 670 nm long-pass filter). FITC-PNA positive cells with a green fluorescent signal are detected using the fluorescence detector 1 (FL-1; 533/30 nm bandpass filters). Two-dimensional plots of forward- and side-scatter properties and FITC-PNA fluorescence versus PI fluorescence were drawn. Quadrants separated subpopulations, and the frequency of each subpopulation was quantified. Only subpopulation negative for PI and FITC-PNA (i.e., sperm cells with intact plasma membrane and acrosome) was reported. Mitochondrial membrane potential (MMP)Tetramethylrhodamine, ethyl ester (TMRE), a cell-permeant, a red-orange fluorescent dye readily sequestered by active mitochondria, is used to measure MMP in various cell types, including spermatozoa (Germain et al., 2021). The sperm MMP was measured with the TMRE solution (25 µM) which was made immediately before use from 2.5 mM stock solution dissolved in dimethyl sulfoxide. Two hundred microliters of samples (2 × 106 sperm/ml) were incubated at 37°C for 30 minutes in 800 µl of PBS with 10 µl of the dye. After that, the spermatozoa were washed by centrifugation (500×g, 5 minutes). The precipitant was resuspended in 500 µl of PBS before flow cytometric analysis. Like FITC-PNA/PI, the fluorochrome TMRE was excited with the 488-nm argon laser. Signals from the TMRE fluorescence were collected through the FL-2 detector (585/40 nm bandpass filter). Control samples with low MMP were performed simultaneously using 20 µM carbonyl cyanide m-chlorophenyl hydrazone, an inducer of mitochondrial damage. A proportion of spermatozoa with high MMP was recorded in each sample. Sperm oxidative stressThe present study used 2,7-dichlorofluorescein diacetate (H2DCFDA) to determine oxidative stress in epididymal spermatozoa. H2DCFDA is a non-fluorescent cell-permeant probe oxidized by intracellular ROS, predominantly H2O2, to form a 2,7-dichlorofluorescein (DCF) that emits green fluorescence at 530 nm in response to 488 nm excitation (Mupfiga et al., 2013). Briefly, 200 µl of the sperm suspension (2 × 106 sperm/ml) was diluted with PBS (800 µl) and added with H2DCFDA (20.5 µM, 20 µl). The mixture was incubated at 37°C for 15 minutes in the dark. Then, the excess unbound dye was removed by centrifugation (500×g, 5 minutes). The DCF-labeled spermatozoa at the bottom of the tubes were resuspended in PBS (1 ml) and added with PI (74.8 µM, 70 µl) to exclude the dead sperm population during flow cytometry analysis. The FL1 (DCF+ cells) and FL3 (PI+ cells) signals were detected through a 533/30 nm bandpass filter and 670 nm long-pass filter. Intracellular ROS levels were determined through mean DCF fluorescence intensity, with a higher fluorescence intensity considered an indicator of higher intracellular ROS. The present study analyzed the percentage of viable spermatozoa with high fluorescence intensity (DCF+ and PI− cells) and the fluorescence intensity per DCF+ and PI− cell. Additionally, as a positive control, freshly prepared H2O2 (10%, v/v) was added to an aliquot of each sample to induce intracellular ROS of spermatozoa. Statistical analysisThe fresh and frozen-thawed epididymal sperm quality of WT and BKO mice was given as mean ± standard error of the mean (SEM). Statistical analyses were performed using SPSS software (IBM SPSS Statistics 26.0). Data were tested for normality using the Shapiro–Wilk test. The Student's t-test and one-way analysis of variances, followed by Tukey’s Multiple Comparison test to figure out which groups differ were used to analyze normally distributed continuous data. Data with non-normal distributions were analyzed using nonparametric statistics, i.e., the Mann–Whitney U test and Kruskal–Wallis test accompanied by the Dunn–Bonferroni post-hoc method. Values of p < 0.05 were considered statistically significant. Ethical approvalAll mice procedures were undertaken following regulations concerning the use and care of experimental animals and were approved by the Animal Care and Use Committee at MB (COA.NO.IMB-ACUC 2021/014). ResultsFresh sperm characteristicsThe characteristics of spermatozoa collected from WT and BKO mice are demonstrated in Table 2. There were no significant differences (p > 0.05) in sperm parameters examined between both genotypes except the percentage of viable spermatozoa exhibiting elevated levels of oxidative stress, in which the value in BKO (10.4% ± 1.7%) was significantly greater than that in WT males (3.8% ± 1.6%, p=0.01). However, the DCF fluorescence intensity was indistinguishable per viable spermatozoon of both genotypes (p > 0.05). Effect of the freezing mediumIrrespective of the mouse genotype, the type of freezing medium (i.e., I–IV) had significant effects on several sperm parameters evaluated except the percentage of viable spermatozoa with high oxidative stress and the level of oxidative stress per viable cell, which did not significantly differ among groups. In WT mice, the motility and acrosome integrity of spermatozoa preserved in egg yolk–TES–Tris (IV) were significantly higher than those in raffinose–skim milk–MTG (I; p=0.001 to p=0.01) and raffinose–skim milk–glutamine (II; p < 0.001 to p=0.001). On the contrary, the sperm characteristics in medium IV became significantly inferior to raffinose–egg yolk–glycerol (III) and medium I in terms of viability (p=0.004) and MMP (p=0.012), respectively. The mean percentages of spermatozoa having intact plasma membrane determined by HOST were comparable across the diluents (p > 0.05) except for the marginal significance found between media I and II (p=0.05). For BKO mice, dilution and cryopreservation of spermatozoa in media I and IV resulted in the post-thaw sperm motility significantly higher than medium III (p=0.007–0.023). The acrosome integrity of spermatozoa in medium IV was also significantly greater than that in media I (p=0.006) and II (p=0.002). Excluding the outstanding MMP observed in the medium I, the percentages of spermatozoa having high MMP in media II, III, and IV did not differ (p > 0.05). The sperm viability and membrane integrity were not different among media I–IV (all p > 0.05). Effect of the mouse genotypeComparing the mouse genotypes, the sperm motility observed in the medium I was significantly lower in WT than BKO mice (p=0.007); however, the membrane integrity of WT spermatozoa in this diluent became more significant (i.e., 31.0% ± 2.4% for WT vs. 19.3% ± 1.3% for BKO, p=0.001). Also, WT sperm viability and membrane integrity were superior to BKO when media III and IV were used (p=0.008–0.027). The amount of oxidative stress in BKO mice’s spermatozoon was more significant than with WT males when preserved in media I, III, and IV (p=0.002–0.044). The acrosome integrity, the MMP, and the number of viable spermatozoa exhibiting high oxidative stress were all commensurate between WT and BKO mice regardless of the type of freezing medium used (all p > 0.05). The sperm characteristics of WT and BKO males examined after cryopreservation are shown in Table 3 and Figure 1. DiscussionThe sperm characteristics of β-thal (BKO) mice following cryopreservation in different freezing media were investigated in the present study. This work is the first report, to the best of our knowledge, especially in the mouse genotype studied. The BKO mice derived from the B6 inbred strain are heterozygous for the β-globin gene deletion. Most heterozygous animals can survive adulthood but generally exhibit pathophysiology comparable to that of patients with β-thal intermedia, including but not limited to abnormal hematological indices, bone deformities, and splenic enlargement (Yang et al., 1995). The sperm quality results of BKO mice revealed in the present study are fundamental and necessary to the successful establishment of the BKO mouse sperm bank. Table 2. The in vitro characteristics of fresh epididymal spermatozoa harvested from WT (n=24) and BKO (n=21) mice.
Table 3. Post-thaw evaluation of the characteristics of WT (n=24) and BKO (n=21) mouse spermatozoa subjected to different freezing media.
Regarding the fresh quality, it was found that most sperm traits evaluated, excluding the sperm cell numbers with high oxidative stress, were very similar between WT and BKO mice. This finding was not beyond expectation and might be explained at least partly by the fact that BKO transgenic mice, on a B6 background, are close to B6 WT mice in the aspect of genetics and that β-globin gene deletion per se, a method of producing BKO mice, does not directly influence gene(s) controlling reproduction (National Center for Biotechnology Information, 2022). In addition, our previous data showed that moderate serum iron levels measured in the non-iron-loaded BKO males did not negatively affect the normal function of the hypothalamic–pituitary–gonadal axis that plays a critical part in the regulation of spermatogenesis and hence sperm quality. Concerning the effect of genetics, earlier studies also found substantial variation in sperm-related parameters between different mouse strains and concluded that the interstrain variation observed was a consequence of genetic differences (Osadchuk and Osadchuk, 2010; Sekine et al., 2021). As stated above, a sole sperm parameter that differed between WT and BKO mice was the percentage of fresh viable spermatozoa having high intracellular ROS, which the levels of ROS were high enough to convert a nonfluorescent probe H2DCFDA used in this study to a green fluorescence DCF. Excessive levels of intracellular and extracellular ROS noticed in BKO mice as well as β-thal patients are almost entirely associated with ineffective erythrocyte generation and oxidative denaturation of unstable hemoglobins (Rifkind et al., 2014; Voskou et al., 2015); and, in the present study, such excess ROS also demonstrated in the male gametes. In contrast to the fresh quality, several sperm characteristics examined after cryopreservation differed slightly between WT and BKO mice depending on freezing diluent types. On the whole, spermatozoa of WT males seemed better able to survive cryopreservation than BKO, indicated by the higher viability (in medium III) and membrane integrity (in media I, III, and IV) of WT spermatozoa. Higher accumulation of intracellular oxidative stress observed in diluents I, III, and IV might be responsible for lower sperm survival in BKO mice. Our finding suggested that quantities of antioxidants and reducing agents in freezing and/or thawing media used in the present study were probably not sufficient to counteract ROS production and the harmful effect of ROS on BKO sperm survival. It has been well documented that oxidative stress is detrimental to the unsaturated fatty acid-enriched membrane, structural components, and nucleus of the spermatozoon (Aitken et al., 1998). This oxidant can self-propagate and lead to membrane injury, loss of functionality, and, eventually, sperm death (Aitken et al., 2014).
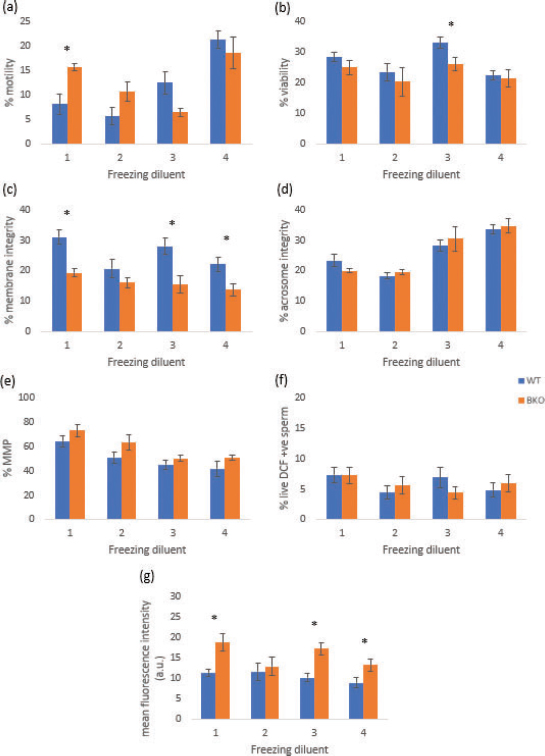
Fig. 1. Comparisons of sperm characteristics between WT and BKO mice after cryopreservation in different freezing media. (a) Sperm motility, (b) sperm viability, (c) plasma membrane integrity, (d) acrosome integrity, (e) MMP, (f) number of viable spermatozoa with high oxidative stress, and (g) amount of oxidative stress in viable spermatozoon. Values are represented as mean ± SEM. *Significant difference between WT and BKO mice (p < 0.05). The composition of freezing media is one of the most important factors influencing the degree of sperm cryodamage and thus the successful sperm cryopreservation (El-Shahat et al., 2020). The present study investigated the cryoprotective efficiency of freezing media for B6-derived transgenic (BKO) and B6 WT mouse spermatozoa. The in vitro sperm characteristics were measured and compared among four freezing diluents. However, regardless of the mouse genotype, the post-thaw sperm characteristics acquired in media I–IV rather varied and, for several parameters, were not significantly different across the diluents. Also, significant linear correlations between sperm variables were hardly found (data not shown). These findings rendered it difficult to promptly reply to the question “What is the most appropriate freezing medium for BKO mouse spermatozoa?” From our viewpoint, a plausible reason for the lack of outstanding advantages of the diluents examined was that they all have been refined and pervasively used for mouse sperm cryopreservation in many laboratories worldwide (for a review, see Takeo and Nakagata, 2010b). All substances included in the freezing media have their functions to protect or mitigate sperm cryodamage. For instance, sugars (i.e., raffinose and glucose) and glycerol acting as non-permeating and permeating agents, respectively, as well as biological materials (i.e., skim milk and egg yolk) play roles in minimizing intracellular ice formation, enhancing the stability of the sperm membranes and reducing the osmotic volume changes while freeze-thawing (Sieme et al., 2016; Chang and Zhao, 2021). Furthermore, the presence of the reducing agent MTG in a medium increased the fertilizing ability of mouse spermatozoa, probably through the modification of ROS generation. Still, it was found that improved fertilization was not associated with an increase in the proportion of motile spermatozoa (Ostermeier et al., 2008). The present results corroborated such finding that the motility of WT mouse spermatozoa frozen in raffinose–skim milk–MTG (medium I) was not superior to the rest three media. There are many reports on the protective effects of the amino acid L-glutamine in the sperm cryopreservation of several mammalian species, including mice (Renard et al., 1996; Amirat-Briand et al., 2009; de Mercado et al., 2009; Takeo and Nakagata, 2010a). L-glutamine can protect the lipid membrane by interacting with the positively charged amino group of amino acids and the negatively charged phospholipids (Anchordoguy et al., 1988) and consequently preserves post-thaw motility and reduces plasma membrane damage to spermatozoa. Nonetheless, the results of the present study did not agree with this statement, as the motility and membrane integrity, measured by vital staining and hypo-osmotic incubation, of spermatozoa preserved in the presence of L-glutamine (freezing medium II), were not different from and even tended to be lower than those of other diluents. Once again, types of freezing media included in our study, which are all specifically developed for mouse spermatozoa, may be used to explain the non-significant outcomes. N-[Tris(hydroxymethyl)methyl]-2-aminoethanesulfonic acid (TES) and tris(hydroxymethyl)aminomethane (Tris) have generally been used as pH regulators for buffering the pH of dilution extenders (Namula et al., 2019). Cold storage of human spermatozoa in an egg yolk extender containing TES and Tris has been demonstrated to maintain sperm motility and enhance in vitro sperm penetration (Chan et al., 1987). In our study, egg yolk–TES–Tris diluent (freezing medium IV) was advantageous to B6 WT and BKO mouse spermatozoa. The sperm motility and acrosome integrity observed in this medium were or tended to be higher than in the others. It was hypothesized that incubation of spermatozoa in egg yolk–TES–Tris buffer would promote capacitation, preserve acrosome reaction, and as a result, increase the percentage of acrosome intact spermatozoa that could successfully penetrate the oocytes (Johnson et al., 1984). The advantages of egg yolk–TES–Tris diluent to the acrosome of mouse spermatozoa found in the present study may partly be supported by this hypothesis. In addition to TES and Tris, the improved sperm motility observed in medium IV could be related to the presence of glucose. Several lines of evidence indicated that, instead of oxidative phosphorylation in the midpiece mitochondria, energy production in B6 spermatozoa is more dependent on the aerobic glycolysis which occurs along the length of the sperm tail. This metabolic pathway requires glucose as a substrate for converting to pyruvate and the high-energy molecule adenosine triphosphate, which is used for sperm movement (Odet et al., 2013; Sansegundo et al., 2022). Moreover, the present study found no significant relationship between the motility and the MMP of post-thaw spermatozoa in both mouse genotypes. Under highly variable, indistinguishable post-thaw sperm characteristics, it was concluded that raffinose–skim milk–MTG, raffinose–skim milk–glutamine, raffinose–egg yolk–glycerol, and egg yolk–TES–Tris can all be used as freezing media for B6 WT and BKO mouse spermatozoa. However, to establish a BKO mouse sperm bank, we prefer egg yolk–TES–Tris diluent with slightly better quality. Also, it seems necessary to supplement thawing media with a reducing agent to efficiently suppress oxidative stress and consequently improve BKO mouse sperm quality after cryopreservation. AcknowledgmentsThe authors thank Assoc. Prof. Dr. Pornthip Chaichompoo for providing TMRE and H2DCFDA. We also thank Ms. Kornkanok Promthep for a phase-contrast microscope and Thalassemia Research Center for a flow cytometer. Mahidol University supported this research project. Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionK.B. designed and performed the experiments, analyzed the data, and wrote, submitted, and revised the manuscript. S.A. and C.C. performed the experiments. S.S. designed the experiments, commented on the manuscript, and supervised the project. ReferencesAitken, R.J., Gordon. E., Harkiss. D., Twigg. J.P., Milne, P., Jennings, Z. and Irvine, D.S. 1998. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol. Reprod. 59, 1037–1046. Aitken, R.J., Smith, T.B., Jobling, M.S., Baker, M.A. and De Iuliis, G. 2014. Oxidative stress and male reproductive health. Asian J. Androl. 16, 31–38. Amirat-Briand, L., Bencharif, D., Vera-Munoz, O., Bel Hadj Ali H., Destrumelle, S., Desherces, S., Schmidt, E., Anton, M. and Tainturier, D. 2009. Effect of glutamine on post-thaw motility of bull spermatozoa after association with LDL (low density lipoproteins) extender: preliminary results. Theriogenology 71, 1209–1214. Anchordoguy, T., Carpenter, J.F., Loomis, S.H. and Crowe, J.H. 1988. Mechanisms of interaction of amino acids with phospholipid bilayers during freezing. Biochim. Biophys. Acta. 946, 299–306. Bedell, M.A., Largaespada, D.A., Jenkins, N.A. and Copeland, N.G. 1997. Mouse models of human disease. Part II: recent progress and future directions. Genes Dev. 11, 11–43. Buranaamnuay, K. 2013. Sperm-TALP: an alternative extender for retrieving and diluting epididymal sperm in the domestic cat. Reprod. Dom. Anim. 48, 912–917. Buranaamnuay, K., Kettawan, A., Changsangfa, C. and Aiemongkot, S. 2021. Effect of chicken bone extract powder on epididymal sperm quality of male Wistar rats. JITV 26(2), 74–81. Cappellini, M.D., Cohen, A., Eleftheriou, A., Piga, A., Porter, J. and Taher, A. 2008. Guidelines for the clinical management of thalassaemia [Internet], 2nd ed. Nicosia, CY: Thalassaemia International Federation. Available via https://www.ncbi.nlm.nih.gov/books/NBK173968/ Chan, S.Y., Li, S.Q. and Wang, C. 1987. TEST-egg yolk buffer storage increases the capacity of human sperm to penetrate hamster eggs in vitro. Int. J. Androl. 10(3), 517–524. Chang, T. and Zhao, G. 2021. Ice inhibition for cryopreservation: materials, strategies, and challenges. Adv. Sci. 8, 2002425. El-Shahat, K., Waheed, M., Ali, A.H., Sallam, A. and El-Saidy, B. 2020. Influence of sugars and osmoregulators on motility and viability of cooled and frozen-thawed ram semen. Animal Sci. Gene. 16(2), 51–57. Flint, J., Harding, R.M., Boyce, A.J. and Clegg, J.B. 1998. The population genetics of the hemoglobinopathies. Bailliere’s Clin. Haematol. 11(1), 1–50. Germain, N., Jouy, N., Marchetti, C. and Marchetti, P. 2021. Determination of mitochondrial membrane potential by flow cytometry in human sperm cells. In Manual of sperm function testing in human assisted reproduction. Eds., Agarwal, A., Henkel, R. and Majzoub A. Cambridge, MA: Cambridge University Press, pp: 58–71. Higgs, D.R., Engel, J.D. and Stamatoyannopoulos, G. 2012. Thalassaemia. Lancet 379(9813), 373–383. Johnson, A.R., Syms, A.J., Lipshultz, L.I. and Smith, R.G. 1984. Conditions influencing human sperm capacitation and penetration of zona-free hamster ova. Fertil. Steril. 41, 603–608. Justice, M.J. and Dhillon, P. 2016. Using the mouse to model human disease: increasing validity and reproducibility. Dis. Model. Mech. 9(2), 101–103. de Mercado, E., Hernandez, M., Sanz, E., Rodriguez, A., Gomez, E., Vazquez, J.M., Martinez, E.A. and Roca, J. 2009. Evaluation of l-glutamine for cryopreservation of boar spermatozoa. Anim. Reprod. Sci. 115, 149–157. Mupfiga, C., Fisher, D., Kruger, T. and Henkel, R. 2013. The relationship between seminal leukocytes, oxidative status in the ejaculate, and apoptotic markers in human spermatozoa. Syst. Biol. Reprod. Med. 59, 304–311. Nagy, S., Jansen, J., Topper, E.K. and Gadella, B.M. 2003. A triple-stain flow cytometric method to assess plasma- and acrosome-membrane integrity of cryopreserved bovine sperm immediately after thawing in presence of egg-yolk particles. Biol. Reprod. 68(5), 1828–1835. Nakagata, N. 1996. Use of cryopreservation techniques of embryos and spermatozoa for production of transgenic (Tg) mice and for maintenance of Tg mouse lines. Lab. Anim. Sci. 46, 236–238. Nakagata, N. 2000. Cryopreservation of mouse spermatozoa. Mamm. Genome. 11(7), 572–576. Nakagata, N. and Takeshima, T. 1993. Cryopreservation of mouse spermatozoa from inbred and F1 hybrid strains. Exp. Anim. 42, 317–320. Nakagata, N., Ueda, S., Yamanouchi, K., Okamoto, M., Matsuda, Y., Tsuchiya, K., Nishimura, M., Oda, S., Koyasu, K., Azuma, S. and Toyoda, Y. 1995. Cryopreservation of wild mouse spermatozoa. Theriogenology 43, 635–643. Namula, Z., Tanihara, F., Wittayarat, M., Hirata, M., Nguyen, N.T., Hirano, T., Le, Q.A., Nii, M. and Otoi, T. 2019. Effects of Tris (hydroxymethyl) aminomethane on the quality of frozen-thawed boar spermatozoa. Acta Vet. Hung. 67(1), 106–114. National Center for Biotechnology Information. 2022. PubChem gene summary for gene 3043, HBB - hemoglobin subunit beta (human). Available via https://pubchem.ncbi.nlm.nih.gov/gene/HBB/human. Odet, F., Gabel, S., London, R.E., Goldberg, E. and Eddy, E.M. 2013. Glycolysis and mitochondrial respiration in mouse LDHC-null sperm. Biol. Reprod. 88(4), 95. Osadchuk, L. and Osadchuk, A. 2010. Genetic variability of spermatozoon production and morphology in laboratory mice. Bull. Exp. Biol. Med. 149, 739–742. Ostermeier, G.C., Wiles, M.V., Farley, J.S. and Taft, R.A. 2008. Conserving, distributing and managing genetically modified mouse lines by sperm cryopreservation. PLoS One 3(7), e2792. Penfold, L.M. and Moore, H.D. 1993. A new method for cryopreservation of mouse spermatozoa. J. Reprod. Fertil. 99(1), 131–134. Ramu, S. and Jeyendran, R.S. 2013. The hypo-osmotic swelling test for evaluation of sperm membrane integrity. Methods Mol. Biol. 927, 21–25. Renard, P., Grizard, G., Griveau, J.F., Sion, B., Boucher, D. and Le Lannou D. 1996. Improvement of motility and fertilization potential of postthaw human sperm using glutamine. Cryobiology 33, 311–319. Rifkind, J.M., Mohanty, J.G. and Nagababu, E. 2014. The pathophysiology of extracellular hemoglobin associated with enhanced oxidative reactions. Front. Physiol. 5, 500. Sansegundo, E., Tourmente, M. and Roldan, E.R.S. 2022. Energy metabolism and hyperactivation of spermatozoa from three mouse species under capacitating conditions. Cells 11, 220. Sekine, N., Yokota, S. and Oshio, S. 2021. Sperm morphology is different in two common mouse strains, BPB Reports, 4(5), 162–165. Sieme, H., Oldenhof, H. and Wolkers, W.F. 2016. Mode of action of cryoprotectants for sperm preservation. Anim. Reprod. Sci. 169, 2–5. Silver, L. 2001. Inbred strain. In Brenner's encyclopedia of genetics, 2nd ed. Eds., Maloy, S. and Hughes, K. Cambridge, MA: Academic Press, 53 p. Simpson, E.M., Linder, C.C., Sargent, E.E., Davisson, M.T., Mobraaten, L.E. and Sharp, J.J. 1997. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat. Genet. 16, 19–27 Songsasen, N. and Leibo, S.P. 1997. Cryopreservation of mouse spermatozoa. II. Relationship between survival after cryopreservation and osmotic tolerance of spermatozoa from three strains of mice. Cryobiology 35(3), 255–269. Takeo, T. and Nakagata, N. 2010a. Combination medium of cryoprotective agents containing L-glutamine and methyl-{beta}-cyclodextrin in a preincubation medium yields a high fertilization rate for cryopreserved C57BL/6J mouse sperm. Lab. Anim. 44(2), 132–137. Takeo, T. and Nakagata, N. 2010b. Mouse sperm cryopreservation and effective embryo production using cryopreserved C57BL/6 mouse sperm. J. Mamm. Ova. Res. 27, 70–78. Voskou, S., Aslan, M., Fanis, P., Phylactides, M. and Kleanthous, M. 2015. Oxidative stress in β-thalassaemia and sickle cell disease. Redox. Biol. 6, 226–239. Yang, B., Kirby, S., Lewis, J., Detloff, P.J., Maeda, N. and Smithies, O. 1995. A mouse model for beta 0-thalassemia. Proc. Natl. Acad. Sci. USA. 92(25), 11608–11612. | ||
| How to Cite this Article |
| Pubmed Style Kakanang Buranaamnuay|. The effect of cryopreservation media on the quality of β-thalassemia mouse spermatozoa. Open Vet J. 2022; 12(5): 602-611. doi:10.5455/OVJ.2022.v12.i5.2 Web Style Kakanang Buranaamnuay|. The effect of cryopreservation media on the quality of β-thalassemia mouse spermatozoa. https://www.openveterinaryjournal.com/?mno=20388 [Access: July 01, 2025]. doi:10.5455/OVJ.2022.v12.i5.2 AMA (American Medical Association) Style Kakanang Buranaamnuay|. The effect of cryopreservation media on the quality of β-thalassemia mouse spermatozoa. Open Vet J. 2022; 12(5): 602-611. doi:10.5455/OVJ.2022.v12.i5.2 Vancouver/ICMJE Style Kakanang Buranaamnuay|. The effect of cryopreservation media on the quality of β-thalassemia mouse spermatozoa. Open Vet J. (2022), [cited July 01, 2025]; 12(5): 602-611. doi:10.5455/OVJ.2022.v12.i5.2 Harvard Style Kakanang Buranaamnuay| (2022) The effect of cryopreservation media on the quality of β-thalassemia mouse spermatozoa. Open Vet J, 12 (5), 602-611. doi:10.5455/OVJ.2022.v12.i5.2 Turabian Style Kakanang Buranaamnuay|. 2022. The effect of cryopreservation media on the quality of β-thalassemia mouse spermatozoa. Open Veterinary Journal, 12 (5), 602-611. doi:10.5455/OVJ.2022.v12.i5.2 Chicago Style Kakanang Buranaamnuay|. "The effect of cryopreservation media on the quality of β-thalassemia mouse spermatozoa." Open Veterinary Journal 12 (2022), 602-611. doi:10.5455/OVJ.2022.v12.i5.2 MLA (The Modern Language Association) Style Kakanang Buranaamnuay|. "The effect of cryopreservation media on the quality of β-thalassemia mouse spermatozoa." Open Veterinary Journal 12.5 (2022), 602-611. Print. doi:10.5455/OVJ.2022.v12.i5.2 APA (American Psychological Association) Style Kakanang Buranaamnuay| (2022) The effect of cryopreservation media on the quality of β-thalassemia mouse spermatozoa. Open Veterinary Journal, 12 (5), 602-611. doi:10.5455/OVJ.2022.v12.i5.2 |