| Research Article | ||
Open Vet J. 2024; 14(11): 3004-3016 Open Veterinary Journal, (2024), Vol. 14(11): 3004-3016 Research Article Histological evaluation of the regenerative potential of injectable hyaluronic acid hydrogel or collagen with blood clot as scaffolds during revascularization of immature necrotic dog’s teethMohamed S. Abdelsalam1, Abeer A. Elgendy1, Ashraf M. Abu-Seida2,3*, Tarek M. Abdelaziz1, Mohamed H. Issa4 and Khaled E. El-Haddad5,61Department of Endodontics, Faculty of Dentistry, Ain Shams University, Cairo, Egypt 2Department of Surgery, Anesthesiology & Radiology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt 3Faculty of Dentistry, Galala University, New Galala City, Egypt 4Department of Conservative Dentistry and Endodontics, Faculty of Dentistry, University of Tripoli, Tripoli, Libya 5Department of Basic Oral Medical Sciences, College of Dentistry, Qassim University, Buraydah, Saudi Arabia 6Department of Oral Biology, Faculty of Dentistry, Ain Shams University, Cairo, Egypt *Corresponding Author: Ashraf M. Abu-Seida. Department of Surgery, Anesthesiology & Radiology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt. Email: ashrafseida [at] cu.edu.eg Submitted: 27/08/2024 Accepted: 15/10/2024 Published: 30/11/2024 © 2024 Open Veterinary Journal
AbstractBackground: Regenerative endodontics’ primary objective is to establish a favorable environment in the root canal by removing infection, providing a sturdy scaffold, and sealing the apical end of the tooth tightly. These actions should promote pulp regeneration and root development. Aim: This study evaluated histologically the regenerative potential of injectable hyaluronic acid (HA) hydrogel or collagen with blood clot as scaffolds during revascularization of immature necrotic dog’s teeth. Methods: Sixteen permanent immature necrotic premolars with 32 roots were chosen from two 4–5-month-old mongrel dogs. Out of the 32 roots, 24 roots were sealed for 2 weeks after being cleaned with CaOH2. According to the treatment protocol, the 24 cleaned roots were randomly assigned to one of three treatment groups (each with 8 roots): blood clot + HA group, blood clot + collagen group, and blood clot group. The control group consisted of the eight infected roots that remained untreated. Three months after the surgery, the assessment of tissue ingrowth in the pulp cavity took into account the kind of cellular components, the intercellular matrix, angiogenesis, and the occurrence of any hard tissue formation. The pulp’s capacity for regeneration was described descriptively, taking into account the type of regenerated tissue, the root’s apical closure, and any potential periodontal and periapical histological alterations. Semi-quantitative analysis was used to assess the degree of pulp tissue regeneration. Tukey’s post hoc test was used after a two-way ANOVA for statistical analysis of all the data. Results: When comparing the treated groups to the control group, a significant increase in tissue ingrowth and a significant decrease in the periapical inflammatory reaction were noted (p < 0.05). It’s interesting to note that blood clot + HA group’s inflammatory cell count was significantly greater (p < 0.05) than that of the other treatment groups. Furthermore, blood clot + collagen group and blood clot group did not differ significantly in inflammatory cell count from one another. Tissue ingrowth did not differ significantly (p > 0.05) amongst treated groups. Conclusion: The tissue regeneration of the immature necrotic teeth is improved by revascularization, whether or not it is combined with HA or collagen scaffolds. After revascularization, using HA as a scaffold is less efficient in reducing the inflammation than collagen. Keywords: Dental pulps, Periapical pathosis, Regenerative endodontic, Revascularization, Scaffolds. IntroductionAn immature permanent tooth with pulp necrosis has thin, fragile walls that are prone to breaking and stop the tooth’s continued development. Such a tooth presents a significant difficulty for endodontists, as it is hard to seal using traditional methods or novel techniques (Murray et al., 2007). Regenerative endodontics (RE) has been called a “paradigm shift” in the treatment of immature teeth with infected root canals. RE is an interesting and emerging area that can lead to sustained root development and apical closure. For endodontic disease, this method offers a cutting-edge and unique selection of clinical treatments based on biology (Banchs and Trope, 2004; El-Tayeb et al., 2019; Abdelsalam et al., 2020; Eldessoky et al., 2023). When applied to permanent immature teeth that had pulp necrosis and apical periodontitis, the findings of RE showed that pulp functions could be successfully restored and that normal physiological root development could be stimulated (Alobaid et al., 2014; Saoud et al., 2014; El Halaby et al., 2020). In contrast to apexification and artificial apical barrier procedures, revascularization is a regenerative procedure and a scientifically grounded alternative method for treating necrotic immature teeth that permits root development to continue (Alobaid et al., 2014; Saoud et al., 2014; Abbas et al., 2020; El Halaby et al., 2020). The apical papilla stem cells play an important role in physiologic root development and may also contribute to further root development during regenerative endodontic procedures (REPs). Many case reports supported survival and continued potential differentiation of the apical papilla after endodontic infection (Palma et al., 2019). On the other hand, it was found that there is insufficient data to support the effectiveness of revitalization in treating apical periodontitis in (im)mature permanent teeth. There was no discernible difference in the success and survival rates of totally pulpectomized (im)mature permanent teeth and revitalized teeth (Meschi et al., 2023). Research on tissue engineering has advanced by concentrating on three main areas. First, stem cells, which are capable of both differentiation and multiplication. The second is the scaffold, which is a three-dimensional framework that promotes vascularization and cell arrangement. Third, the growth factors which are secreted extracellularly and control morphogenesis (Tawfik et al., 2013; Chrepa et al., 2017). The pulpal tissues of immature teeth have a plentiful blood supply and include stem cells that are the potential for tissue regeneration (Lovelace et al., 2011). For a more useful endodontic tissue engineering therapy, pulp stem cells need to be arranged into a three-dimensional framework that can support cell organization and vascularization. For cell growth and differentiation, the scaffold offers a three-dimensional physicochemical and biological milieu that promotes cell adhesion and migration (Nagy et al., 2014; El Halaby et al., 2020; Abada et al., 2022). Within RE, choosing a scaffold is a difficulty. A scaffold needs to be immune-genicity-low, safe, biodegradable, biocompatible, and able to promote cell proliferation. Good biological properties for cell regeneration are demonstrated by the use of natural or synthetic polymer scaffolds (El Halaby et al., 2020; Sugiaman et al., 2023). In addition to generating a fibrin clot that can serve as a scaffold to support cellular proliferation and can supply growth factors, blood clots transport stem cells from the periradicular tissues to the canal system (Iwaya et al., 2001; Thibodeau and Trope, 2007). The host compatibility, autologous growth factors, cost-effectiveness, and clinical simplicity of blood clot scaffolds are just a few of their many benefits (Iwaya et al., 2001; Nagy et al., 2014; El Ashry et al., 2016; El Halaby et al., 2020). Nevertheless, the blood clot has many drawbacks, such as instability, uneven results, difficulties with bleeding, and inadequate mechanical strength (Raddall et al., 2019). Collagen is a scaffold material with viscoelasticity closest to that of natural pulp tissue (Erisken et al., 2015). Low antigenicity, high biocompatibility, biodegradability, bioactivity, appropriate cell adhesion, inadequate mechanical strength, and cross-linking capabilities are just a few of its many encouraging qualities (Erisken et al., 2015). Additionally, a collagen sponge can provide the ideal environment for stem cell growth and proliferation and is frequently utilized as a scaffold in regeneration operations. However, collagen has drawbacks as well, such as a high rate of degradation, challenges with processing and sterilization, pathogen dissemination, inadequate mechanical strength, uneven biodegradation, immunogenicity risks, and the development of tissues that in vivo resemble connective tissue rather than dentin (Kim et al., 2009; Sugiaman et al., 2023). Hyaluronic acid (HA), a biopolymer that can be altered and processed for use in biomedical applications, is one of the often-used natural scaffold materials in REPs (Inuyama et al., 2010; Ayala-Ham et al., 2021). Because of its qualities of biocompatibility and biodegradation, it has been suggested as a viable scaffold for dental pulp regeneration (Inuyama et al., 2010). HA can promote the formation of reparative dentin when administered to the exposed pulp. This is because it provides an environment that is conducive to the growth of blood vessels and the differentiation of stem cells (Nowicka et al., 2021). HA does, however, have a few disadvantages, including poor mechanical qualities, and the potential for hypersensitivity reactions – a serious side effect – and the likelihood that bacterial contaminants are secondary (Friedman et al., 2002; Raddall et al., 2019). It is crucial to compare the two types of scaffold materials in the RE because collagen and HA scaffolds have been found to have benefits and drawbacks. Consequently, the effectiveness of collagen and HA hydrogel with revascularization as scaffolds in pulp regeneration of immature permanent necrotic dog teeth was studied histologically in this study. Materials and MethodsAnimal modelBased on the findings of a prior study’s 3-month root length increase, the sample size was determined (Tawfik et al., 2013). Size 3.738, alpha error 0.05, and 99% power necessitated a minimum of 12 total sample sizes. In this investigation, two male mongrel puppies, around 4–5 months old, were used. For every dog, eight immature necrotic premolars were utilized. Each quadrant’s second and third premolars were chosen, for a total of 16 premolars and 32 roots. Every group was represented by a single dog. The dogs were randomly assigned to separate kennels with good ventilation, cleanliness, feeding, and a 12-hour light-dark cycle for a period of 4 months (1 month for infection and disinfection and 3 months for follow-up). The dogs were fed on a soft diet, soup, and bread twice daily. Fresh water was available ad libitum. Surgical proceduresPremedication for the dogs involved intramuscular injection of Xylazine HCl (1 mg/kg) and subcutaneous Atropine sulphate (0.05 mg/kg body weight). Ketamine HCl was given intravenously at a dose of 5 mg/kg body weight through an intravenous cannula inserted into the cephalic vein to induce anesthesia. Thiopental sodium was administered intravenously at a dose of 25 mg/kg body weight 2.5% solution to sustain the anesthesia (dose to effect). The previously outlined approach was followed in order to infect the pulps of 16 experimental teeth (Tawfik et al., 2013). In short, the root canals were left open to the oral cavity for 15 days to allow for microbial contamination after the pulps were mechanically exposed to an abundance of saline solution and extracted. Five days following surgery, the animals received 1.1 mg kg of intramuscular Diclofenac sodium as a pain reliever once daily for 5 days (Abu-Seida, 2012). The previously described anesthetic regimen was used to re-anesthetize the dogs following the infection phase. Ten milliliters of 1.25% NaOCl were used to lightly irrigate the root canals of 12 infected premolars. Next, CaOH2 was injected into the coronal two thirds of the canal length and up to the cement-enamel junction. The temporary restoration, Coltosol F (Ultimate Dental, Switzerland), was then placed on top of the canals and left for 15 days. After 15 days, the temporary restoration and CaOH2 in the disinfected teeth were removed with sterile saline. The root canals were irrigated with 17% EDTA solution to induce the release of growth factors from dentin (El Ashry et al., 2016). Then, the canals were dried with sterile paper points. The total 24 roots were assigned randomly into three groups (8 roots each) according to the treatment protocol as follows: Blood clot and HA hydrogel group: A sterile #30 K-file was inserted 2 mm beyond the canal terminus to induce bleeding and blood clots inside the canal. Then, HA gel (Gengigel: gel form in a syringe) was injected on the top of the blood clot with the delivery tip till the middle of the canal. The canal orifice located in the cervical part of the root canal, measuring 2–3 mm, was sealed using a white mineral trioxide aggregate (MTA) orifice plug. Then, for maximum adaptation and to reduce shrinkage-causing polymerization, the access cavity was sealed using smart dentin replacement (SDR) bulk-fill flow composite (SDR®, Dentsply Sirona, Charlotte, NC). For improved mechanical properties, a nano-composite (Neo Spectra ST®, Dentsply Sirona, Charlotte, NC) was used. Collagen and blood clot group: as previously indicated; bleeding was generated. After using tweezers to insert collagen into the canal, an endodontic plugger was used to pack it in. The access cavity was sealed and the MTA orifice stopper was installed as indicated. Blood clot group: the previously described procedures for inducing bleeding and forming a blood clot inside the canal were followed. The MTA orifice stopper was placed, and the access cavity was sealed, as shown. Control group (no treatment): This was a control group (n=8 roots) consisting of two infected premolar teeth per dog that were not given any therapy. As previously stated, coronal access has been restored. Histological evaluationThree months after the surgery, the dogs were euthanized with 20 ml of general anesthetic Thiopental sodium 5% solution (Thiopental sodium vial®, EPICO, Egypt) given by rapid intravenous injection. Segments of the jaws, including the tested teeth, were dissected and fixed in 10% neutral buffer formalin. They were then trimmed, decalcified in 10% formic acid, and dehydrated in increasing grades of ethyl alcohol before being cleaned in xylene and embedded in paraffin. For histopathology examination, six-micron serial sections were cut longitudinally through the roots and stained with hematoxylin and eosin (Bancroft, 2008). To assess the histological alterations, the histological study was carried out under a light microscope at magnifications of 40×, 100×, and 200×. The assessment of tissue invasion into the pulp cavity took into account the kind of cellular components, the intercellular matrix, angiogenesis, and the occurrence of any hard tissue formation. Two sections were obtained from each sample. All the sections were evaluated for the descriptive analysis. However, regarding the numerical analysis, the non-representative samples with artifacts that would affect the data collection were excluded. The pulp’s capacity for regeneration was described descriptively, taking into account the type of regenerated tissue, the root’s apical closure, and any potential periodontal and periapical histological alterations. Semi-quantitative analysis was used to assess the extent of pulp tissue regeneration was performed by a single specialist observer using the following scores (Tawfik et al., 2013): Score 0: The canal space shows no discernible in-growth of tissue. Score 1: One-third of the canal shows evidence of tissue in-growth. Score 2: More than one third but less than two thirds of the canal show signs of tissue in-growth. Score 3: More than two thirds of the canal shows signs of tissue ingrowth. Any symptoms of degeneration or distortion pertaining to the periodontal tissues were documented and examined. High power fields (×400) were used to count the number of inflammatory cells in a specific area of the periapical tissue. Using an image analyzer computer system, the number of inflammatory cells was counted in five distinct fields per slide (QuPath-0.3.2 software, UK). The experimental procedures are summarized in Figure 1. Statistical analysisThe mean and standard deviation (SD) of numerical data were displayed. Shapiro-Wilk’s test and a visual inspection of the distribution were used to determine their normality. All data were found to be parametric and were analyzed using a one-way ANOVA test followed by Tukey’s post hoc test. The significance level was set at p < 0.05 within all tests. Statistical analysis was performed with R statistical analysis software version 4.3.1 for Windows (R Core Team (2023). R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria). Ethical approvalThe Ethical Committee of Ain Shams University’s Faculty of Dentistry approved the research protocol (Approval number: FDASU-Rec; ID062018). Every procedure was carried out in compliance with the applicable rules and regulations. Every attempt was made to reduce the suffering experienced by the studied dogs. Moreover, all dogs were monitored for any sign of pain by an expert veterinarian (Ashraf M. Abu-Seida, PhD.V.Sc.) and any dog showed signs of severe pain was discarded from the study and treated with the recommended medications. Furthermore, all the requirements outlined in Animal Research: Reporting of in vivo Experiments were adhered to.
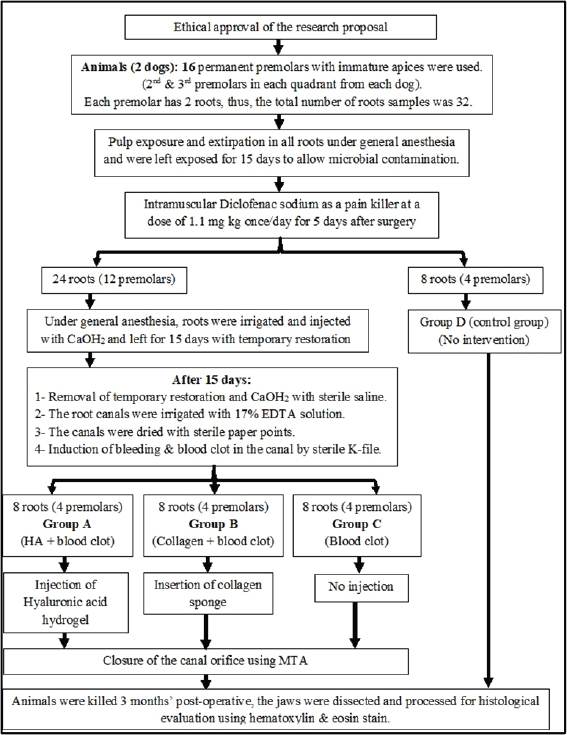
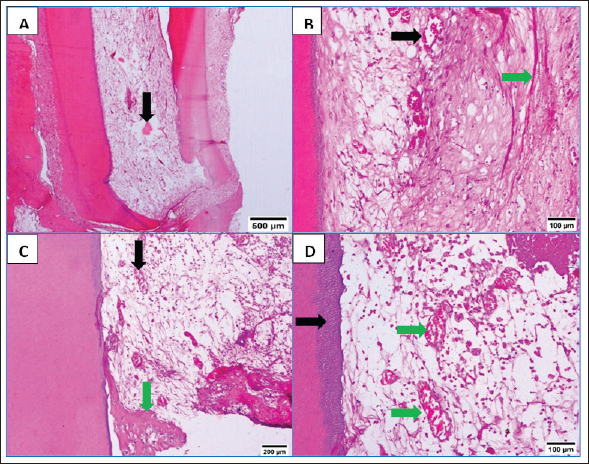
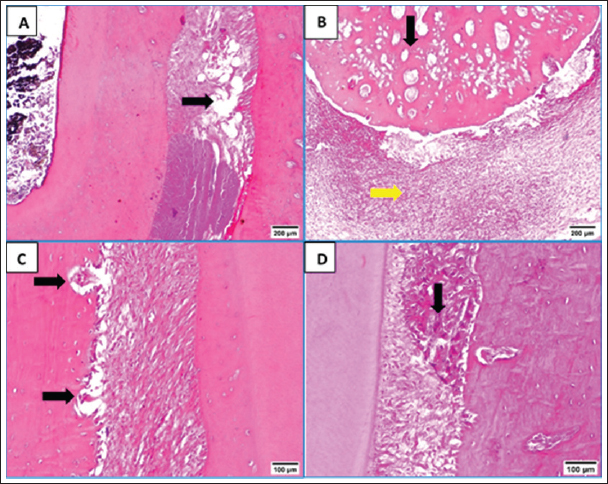
Fig. 1. Flowchart showing the main experimental procedures in the present study. ResultsThe intra-canal pulp tissue regenerative potentialThe pulpal tissue regeneration in Group A (blood clot + HA) was more extensive and occasionally covered nearly the whole root canal. Regular connective tissue with visible blood vessels and sporadic calcified lumps made up the developed tissue. In the pulp core, there were patches of dense collagenous fibrous tissues (Fig. 2A and B). Three of the samples in group B (blood clot + collagen) had empty root canals, while the other five samples had clearly defined connective tissue with established blood vessels, unique cellular components, and newly formed dentin at the pulpal side of the root dentin. Additionally, calcified formations containing entrapped cells were seen (Fig. 2C and D). Only the blood clot samples from group C’s samples displayed empty canals with soft tissue strands and no bone or root resorption (Fig. 3A). On the other hand, five samples from group D (control group) displayed an empty intracanal area (Fig. 3B). There was very little tissue formation next to the canal’s dentin wall in two of the samples. One specimen occasionally showed heterogeneous connective tissue development, with calcified masses distributed across hyalinized regions and fibrous areas (Fig. 3C). While aggregations of red blood corpuscles were found in one specimen (Fig. 3D), no distinguishable cellular features were observed in the intracanal cavities.
Fig. 2. A&B: Representative photomicrographs of blood clot + HA group showing more extended pulpal tissue regeneration with calcified mass (black arrow, A), blood vessels (black arrow, B) and dense collagenous fibrous tissues (green arrow, B). C: Representative photomicrograph of blood clot + collagen group showing well-defined connective tissue with developed blood vessels (black arrow) and calcified structures with entrapped cells (green arrow). D: Representative photomicrograph of blood clot + collagen group showing distinct cellular elements and new dentin formation at the pulpal side of root dentin (black arrow) and scattered blood vessels (green arrows) (H&E. A: ×40, B: 200×, C: ×100, D: ×200).
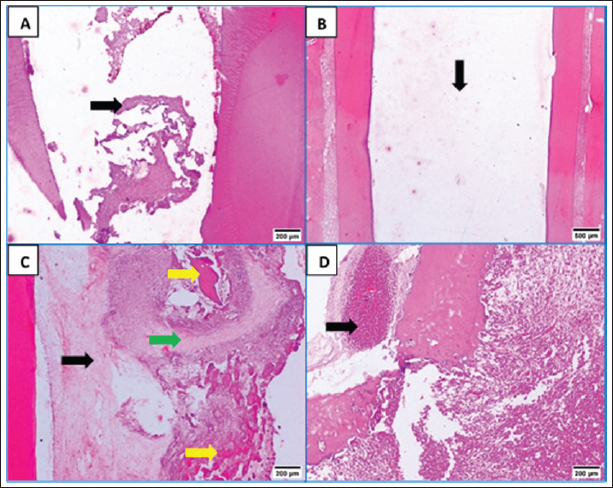
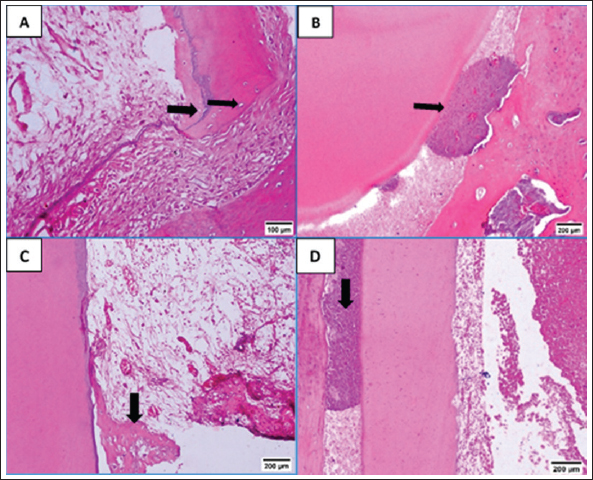
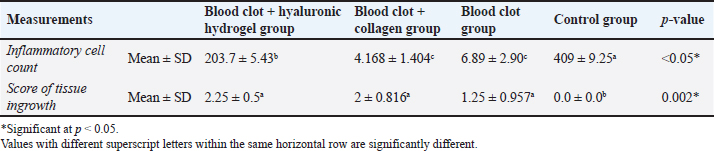
Fig. 3. A: Representative photomicrograph of blood clot group showing strands of soft tissue (black arrow). B: Representative photomicrograph of the control group (no treatment) showing empty intracanal space (black arrow). C: Representative photomicrograph of the control group showing heterogeneous connective tissue with fibrous areas (black arrow) and hyalinized regions (green arrow) with scattered calcified masses (yellow arrows). D: Representative photomicrograph of the control group showing aggregations of congested red blood corpuscles (black arrow). (H&E. A: ×100, B: ×40, C and D: ×100). Fibroblasts and inflammatory cells, among other cell types, were among the cellular components that were distributed throughout the regenerated tissues in groups A and B. In relation to groups C and D, a number of regions displayed amorphous areas devoid of distinct cell types. Changes to the periapical and periodontal tissuesBoth groups A and B had normal periodontal ligaments with an active osteoblastic layer around the alveolar bone. In both groups, there was evidence of cementocytes inside the apical apertures and apical closure with cementum-like tissue (Fig. 4A and B). Both groups also showed characteristic thick basophilic condensations of fibrous tissues (Fig. 4C and D). Group C showed sporadic degenerative alterations along with heavily pigmented basophilic condensation of fibrous tissues. Seldom did the roots exhibit cementum-like tissue at the apical closure, an interlacing pattern containing cavities of varying sizes, and lacunae devoid of cementocytes (Fig. 5A). This group did not exhibit any signs of bone or root resorption. The control group, group D, displayed osteoclasts and Howships lacunae at the sites of bone resorption. An extensive collection of inflammatory cells was seen in the periapical tissues. Additionally, there were sporadic degenerative alterations and densely stained basophilic condensation of fibrous structures seen. Seldom did the roots exhibit cementum-like tissue around voids of varying sizes near the apical closure of the root canal, nor did they exhibit cementocytes in their lacunae (Fig. 5B–D). Statistical results for the histological findingsAll data were found to be parametric and were analyzed using one-way ANOVA test followed by Tukey’s post hoc test. In several of the fields under investigation, the inflammatory cells were primarily acute inflammatory cells. In addition to neutrophils, one sample also occasionally showed plasma cells and eosinophils. The control group, group D, had a significantly greater mean count of inflammatory cells (p < 0.05) than the treatment groups, groups A, B, and C. Interestingly, compared to the two treatment groups (B and C), group A (blood clot + HA) had a significantly higher (p < 0.05) inflammatory cell count. Furthermore, no discernible change was seen between group C (blood clot only) and group B (blood clot + collagen). According to Table 1, group B (blood clot + collagen) had the lowest number of inflammatory cells.
Fig. 4. A: Representative photomicrographs of blood clot + HA group showing apical closure with cementum like tissue, evidence of cementocytes inside the apical openings (arrows) and dense basophilic condensations (blue arrow, B). C: Representative photomicrograph of blood clot + collagen group showing cemntum like tissue inside the root canal (arrow). D: Photomicrograph of blood clot + collagen group dense basophilic condensations (arrow). (H&E. A: 200, B, C and D: ×100). As indicated in Table 1, the treatment groups (A, B, and C) exhibited significantly higher values (p < 0.05) for tissue ingrowth than the control group (D). There were no noteworthy distinctions (p > 0.05) observed among the A, B, and C treatment groups. Group D displayed the lowest tissue ingrowth value (0.0 ± 0.0). DiscussionDue to the lack of an apical stop and the fragile dentin walls, teeth with immature apices pose challenges for standard root canal therapy (Tawfik et al., 2013). Various approaches have been proposed to address these issues, such as calcium hydroxide apexification (Andreasen et al., 2002) and MTA (Holden et al., 2008; Nabeel et al., 2024). As a biologically based substitute that enables more root maturation in length and thickness through the regeneration of essential tissue, RE has attracted a lot of interest lately (Hargreaves and Law, 2011; Talaat et al., 2024). The current study compared the histological efficacy of collagen and HA hydrogel as scaffolds for pulp regeneration in immature, necrotic dog teeth following revascularization. The current study is based on experimental animals. Animal studies have a crucial role in healthcare investigations particularly when meta-analyses of animal studies are conducted (Hooijmans et al., 2014). Additionally, animal models are commonly used to test the efficacy of drugs or techniques before proceeding to clinical trials (Roberts et al., 2002). However, animal studies possess some limitations, including the diversity of the experimental design and study characteristics (Hooijmans et al., 2014) and the poor methodological quality which increases the risk of bias (Kilkenny et al., 2009). One of the most important limitations of animal studies is that there are critically important physiological and genetic differences between humans and animals, which can invalidate the use of animals to study human diseases, and treatments. Animal experimentation generally, are inadequate bases for predicting clinical outcomes in human beings in the great bulk of biomedical science (Akhtar, 2015).
Fig. 5. A: Representative photomicrograph of blood clot group showing regions of minor degenerative changes (arrow). B: Representative photomicrograph of the control group (no treatment) showing periapical tissues with dense aggregation of inflammatory cells (yellow arrow), apical closure with cementum like tissue without cementocytes in their lacunae (black arrow). C: Representative photomicrograph of the control group showing bone resorption sites indicated by Howships lacunae and osteoclasts (arrows). D: Representative photomicrograph of the control group showing regions of densely stained basophilic condensation (arrow) (H&E. A and B: ×100, C and D: ×200). In the current study, we used the dog which is one of the most frequently used large-animal models because its dental tissues resemble human tissues in structure and size in addition to the dog’s compliant nature (Wang et al., 2020). Particularly in dentin, there is a great resemblance between dog and human (Dehghani et al., 2019) The rationale behind using dogs in biological endodontics investigations was that their teeth are a lot like human teeth, which makes it possible to conduct tests that mimic real-world clinical settings (El-Tayeb et al., 2019; Abdelsalam et al., 2020; El Halaby et al., 2020; Eldessoky et al., 2022; Talaat et al., 2024). Moreover, information gathered from dogs’ research can be utilized for upcoming human investigations and full orthotopic pulp regeneration (Tawfik et al., 2013; Nakashima et al., 2019; Al-Anesi et al., 2024). Premolars have average-sized canals that allow for endodontic manipulation and are accessible for endodontic operations (Tawfik et al., 2013; Eldessoky et al., 2022). The purpose of inducing periapical infection was to mimic clinical settings, as noted in numerous prior investigations (Tawfik et al., 2013; El-Tayeb et al., 2019; El Halaby et al., 2020; Eldessoky et al., 2022; Talaat et al., 2024). As previously noted, access cavies were left open for a period of 15 days to allow enough time for the development of periapical pathosis (Tawfik et al., 2013; Abdelsalam et al., 2020; El Halaby et al., 2020). After the disinfection process, samples were kept for 15 days, which was enough time to thoroughly disinfect the canals (Sato et al., 1996). For intracanal disinfection during RE operations, Calcium hydroxide is advised (Diogenes and Ruparel, 2017). Consequently, in the current study, it was applied to disinfect the necrotic premolars. Table 1. Mean ± SD values of inflammatory cell count and score of tissue ingrowth in all tested groups.
The fact that undifferentiated dental pulp stem cells can survive exposure to MTA has made MTA the material of choice for crucial pulp capping and RE treatments up to this point. Because of its biocompatibility, bioactivity, and biomineralization characteristics, MTA has the ability to generate new hard tissues (Hassanien et al., 2015; Ulusoy et al., 2019). As a result, in the treatment groups in the current investigation, MTA orifice plug was employed. Additionally, a blood clot was used to provide uniformity among the groups that received treatment. The RE was employed in several researches (Tawfik et al., 2013; El Halaby et al., 2020; Eldessoky et al., 2022; Talaat et al., 2024) to treat necrotic pulps. As in many other investigations, histological evaluation was used to evaluate the outcomes in order to measure tissue in-growth inside the pulp space and periapical reaction (Tawfik et al., 2013; Abdelsalam et al., 2020). The current histology findings demonstrated that, in comparison to revascularization alone, pulp tissue regeneration was not significantly stimulated by either HA hydrogel or collagen used as scaffolds following revascularization. Similar results were reported by Palma et al. (2017) who recorded that the development of new mineralized tissues along the root canal walls and the histologic evidence of the regeneration of a pulp-dentin complex in dogs undergoing regenerative treatments were not enhanced by the addition of chitosan scaffolds to a blood clot. According to a previous report, injectable HA hydrogels have supporting qualities and encourage cell migration toward dentin surfaces (Astudillo-Ortiz et al., 2021). We found blood vessels in the HA-injected samples, which is consistent with another work that found HA 3D-sponge can create an environment that is conducive to blood vessel growth and stem cell differentiation (Nowicka et al., 2021). The biocompatibility and biodegradation characteristics of HA may account for the broad distribution of the regenerated pulp tissue in the HA group (Inuyama et al., 2010). The type of regenerated tissue is relatively enhanced by this scaffold, leading to tissue regeneration. Our findings showed that the collagen scaffold promotes angiogenesis and pulp tissue regeneration. This could be explained by the collage scaffold’s strong biocompatibility, low antigenicity, and biodegradability (Sugiaman et al., 2023), as well as its viscoelasticity, which is similar to that of genuine pulp tissue (Erisken et al., 2015). Remaining portions of a few essential pulp cells at the apical canal end may be the cause of the occasional presence of soft tissue in the root canal in certain control group samples. This is consistent with the work of previous authors Kenny and Hitzig, 1979). It is possible that these cells can divide and multiply. The control group’s inflammatory cell count showed much higher levels. This illustrates the body’s normal reaction to the infection in teeth that have not been cleaned or treated. In contrast, the three treatment groups’ inflammatory cell counts were lower than those of the control group. Because MTA is biocompatible, the reduced inflammatory response in the treatment groups may be related to the periapical lesion’s ongoing healing (Shabahang et al., 1999; Gomes-Filho et al., 2013). Moreover, Calcium hydroxide paste works well to remove endodontic infections and increases the likelihood that the periapical space will recover. The three treated groups revealed a decrease in inflammatory cell count in comparison to the control group, indicating the healing potential. However, in some samples of HA group, the inflammatory cell count was high. Taking into consideration that the teeth were left 15 days for infection and 15 days after disinfection procedure then the HA was injected. Thus, any detected inflammatory cells could be interpreted as the results of the experimental procedure which was more accentuated in the control group. This could be attributed to the hypersensitivity reactions secondary to bacterial fermentation contaminants which might be induced by HA (Friedman et al., 2002; Raddall et al., 2019). The role of HA acid in inflammatory regulation is extensively discussed in many studies. HA is considered one of the key players in the tissue regeneration process. It modulates the inflammation via specific HA receptors. Studies have revealed that most HA properties depend on its molecular size. High molecular weight HA (HMWHA) displays anti-inflammatory and immunosuppressive properties, whereas low molecular weight HA (LMWHA) is a potent proinflammatory molecule (Rayahin et al., 2015; Litwiniuk et al., 2016; Galvez-Martin et al., 2023; Zhang et al., 2023). LMWHA binds to either TLR2 or TLR4 to elicit pro-inflammatory action, while HMWHA dampens inflammation by inhibiting TLR2 or TLR4 signaling (Scheibner et al., 2006; Campo et al., 2010; Maharjan et al., 2011; Galvez-Martin et al., 2023; Zhang et al., 2023). On the other hand, other researchers reported that there are other studies do not support this conception and emphasize the importance of the form of HA, particularly regarding its purity and origin (Schmidt et al., 2020). Šafránková et al. (2018) reported that the purity of HA is important because it may contain endotoxins and other impurities that provoke an inflammation that may be incorrectly attributed to HA. The authors concluded via a comparative study between several HMWHA and LMWHA that LMWHA did not reveal itself to have significantly higher immunostimulatory activity compared to HMWHA. This could explain why the used HMWHA in the current study could not reduce the inflammatory process in some samples. Additionally, the timing of the HA application could affect the inflammation modulatory effects of HA. HMWHA was able to significantly diminish TLR4 and TLR2 expression in the murine model of osteoarthritis. Interestingly, this effect was evident only when HMWHA was administered in an early inflammatory phase of osteoarthritis. The authors proposed that HMWHA may mask TLR2 and TLR4 to prevent their stimulation. However, the exact mechanism in which HMWHA interacts with TLR receptors is not known (Campo et al., 2016; Litwiniuk et al., 2016). Additionally, the circumstances of the periapical tissue could affect the inflammation modulatory effects of HA. For example, HA is pH-sensitive hydrogel, the action of which is affected by the pH levels (Li et al., 2022). In the current study, the injection of HMWHA in the already infected tissues which could have occasional unfavorable pH for the optimum modulatory effects of HA. Moreover, it has been reported that the HMWHA can be broken down into smaller molecular weight fragments in response to many environmental cues such as the pH (Stern et al., 2007; Rayahin et al., 2015). Consistent with the current findings, a recent radiography investigation determined that revascularization allowed the developing immature permanent teeth with necrotic pulps to proceed, independent of the use of collagen or HA as a scaffold (Abdelsalam et al., 2024). The small sample size resulting from bioethical concerns for animal research was one of the limitations of the current study. However, the current histology findings provide insight into the scaffold materials’ potential for regeneration. The absence of immunohistochemistry analysis in this investigation was another limitation. It is advised that future research be done on the application of scaffolds and growth factors that control tissue regeneration, as well as on extended follow-up periods for tracking the healing process and immunohistochemistry examination of the regenerated tissues. Within the limitations of the current study, our results emphasize the efficiency of the revascularization in the RE even when applied without concomitant scaffolds. This outcome necessitates more clarification via further investigations regarding the effects of different parameters of the revascularization procedures, such as the used disinfection protocol, the time frame of the process, and the instrumentation variables. Moreover, the current study outcomes revealed promising effects of using scaffolds for augmentation of the regeneration. However, their promoting effects were limited and the specific mechanisms of their action are still not fully elucidated. This potentiates further investigations using immunohistochemical analysis of the possible factors affecting the regeneration. Moreover, future research is recommended regarding the use of scaffolds and growth factors governing tissue regeneration. ConclusionThe tissue regeneration of the immature necrotic teeth is improved by revascularization, whether or not it is combined with HA or collagen scaffolds. After revascularization, using HA as a scaffold is less efficient in reducing inflammation than collagen. AcknowledgmentsNone. Conflict of interestThe authors declare no conflicts of interest to report. FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for profit sectors. Authors’ contributionsMSA, AMA, and TMA carried out all surgeries. AMA followed up on the experimental animals. AAE evaluated the radiographs and supervised the study. KEE examined the histopathology specimens and wrote the draft manuscript. AAE, AMA, and MHI wrote the main manuscript text. MSA and MHI prepared figures. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript. Data availabilityThe article includes all data supporting the study’s findings. In case additional data are needed, the corresponding author can provide it upon reasonable request. ReferencesAbada, H.M., Hashem, A.A.R., Abu-Seida, A.M. and Nagy, M.M. 2022. The effect of changing apical foramen diameter on regenerative potential of mature teeth with necrotic pulp and apical periodontitis. Clin. Oral Investig. 26(2), 1843–1853. Abbas, K.F., Tawfik, H., Hashem, A.A., Ahmed, H.M.A., Abu-Seida, A.M. and Refai, H.M. 2020. Histopathological evaluation of different regenerative protocols using Chitosan-based formulations for management of immature non-vital teeth with apical periodontitis: in vivo study. Aust. Endod. J. 46, 405–414. Abdelsalam, N., Abu Seida, A.M., Fayyad, D. and Tawfik, H. 2020. Radiographic and histopathologic outcomes of immature dog teeth with apical periodontitis after revascularization using propolis. Saudi Endod. J. 10(3), 199–207. Abdelsalam, M.S., Elgendy, A.A., Abu-Seida, A.M., Abdelaziz, T.M. and El-Haddad, K.E. 2024. Radiography evaluation of the regenerative potential of injectable hyaluronic acid hydrogel or collagen with blood clot as scaffolds during revascularization of immature necrotic dog’s teeth. Ain Shams Dent. J. 2024 [In Press]. Abu-Seida, A.M.A. 2012. Efficacy of diclofenac sodium, either alone or together with cefotaxime sodium, for control of postoperative pain, in dogs undergoing ovariohysterectomy. Asian J. Anim. Vet. Adv. 7, 180–186 Akhtar, A. 2015. The flaws and human harms of animal experimentation. Camb. Q. Health Ethics. 24(4), 407–419. Al-Anesi, M.A., Abu-Seida A.M., Abd-Elhamid, E.S., Mahran, A.H., El Ashry, S.H., Issa, M.H. and Nagy, M.M. 2024. Influence of azathioprine on healing of exposed dogs’ dental pulp capped with three different materials. Open Vet. J. 14(7), 1614–1624. Alobaid, A.S., Cortes, L.M., Lo, J., Nguyen, T.T., Albert, J., Abu-Melha, A.S., Lin, L.M. and Gibbs, J.L. 2014. Radiographic and clinical outcomes of the treatment of immature permanent teeth by revascularization or apexification: a pilot retrospective cohort study. J. Endod. 40(8), 1063–1070. Andreasen, J., Farik, B. and Munksgaard, E. 2002. Long term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent. Traumatol. 18, 134–137. Astudillo-Ortiz, E., Babo, P.S., Reis, R.L. and Gomes, M.E. 2021. Evaluation of injectable hyaluronic acid-based hydrogels for endodontic tissue regeneration. Materials (Basel) 14(23), 7325. Ayala-Ham, A., López-Gutierrez, J., Bermúdez, M., Aguilar-Medina, M., Sarmiento-Sánchez, J.I., López-Camarillo, C., Sanchez-Schmitz, G. and Ramos-Payan, R. 2021. Hydrogel-based scaffolds in oral tissue engineering. Front. Mater. 8, 708945. Banchs, F. and Trope, M. 2004. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J. Endod. 30(4), 196–200. Bancroft, J.D. 2008. Theory and practice of histological techniques, 6th ed. Oxford, UK: Elsevier Health Sciences. Campo, G.M., Avenoso, A., Campo, S., D’Ascola, A., Nastasi, G. and Calatroni, A. 2010. Molecular size hyaluronan differently modulates toll-like receptor-4 in LPS-induced inflammation in mouse chondrocytes. Biochimie. 92(2), 204–215. Campo, G.M., Avenoso, A., Nastasi, G., Micali, A., Prestipino, V., Vaccaro, M., D’Ascola, A., Calatroni, A. and Campo, S. 2011. Hyaluronan reduces inflammation in experimental arthritis by modulating TLR-2 and TLR-4 cartilage expression. Biochem. Biophys. Acta. 1812(9), 1170–1181. Chrepa, V., Austah, O. and Diogenes, A. 2017. Evaluation of a commercially available hyaluronic acid hydrogel (Restylane) as injectable scaffold for dental rulp regeneration: an in vitro evaluation. J. Endod. 43(2), 257–262. Dehghani, N.A., Dehghanpour, F.H., Haddadi, P. and Dehghani N.F. 2019. Ultrastructural and chemical composition of dentin and enamel in lab animals. J. Dent. (Shiraz, Iran), 20(3), 178–183. Diogenes, A. and Ruparel, N.B. 2017. Regenerative endodontic procedures: clinical outcomes. Dent. Clin. 61, 111–125. El Ashry, S.H., Abu-Seida, A.M., Bayoumi, A.A. and Hashem, A.A. 2016. Regenerative potential of immature permanent non-vital teeth following different dentin surface treatments. Exp. Toxicol. Pathol. 68, 181–190. Eldessoky, A.E., Khalefa, M.M. and Abu-Seida, A.M. 2022. Comparison of antibacterial activity of diode laser 980 nm and double antibiotic paste during regenerative endodontic therapy of mature necrotic teeth. G. Ital. Endod. 36(2), 10–18. Eldessoky, A.E., Khalefa, M.M. and Abu-Seida, A.M. 2023. Regenerative endodontic therapy in mature teeth with necrotic pulp and apical periodontitis using two disinfection protocols. BMC Oral Health. 23(1), 163. El Halaby, H.M., Abu-Seida, A.M., Fawzy, M.I., Farid, M.H. and Bastawy, H.A. 2020. Evaluation of regenerative potential of dentin conditioning and naturally derived scaffold for necrotic immature permanent teeth in a dog model. Int. J. Exp. Pathol. 101(6), 264–276. El-Tayeb, M.M., Abu-Seida, A.M., El Ashry, S.H. and El-Hady, S.A. 2019 Evaluation of antibacterial activity of propolis on regenerative potential of necrotic immature permanent teeth in dogs. BMC Oral Health. 19, 174. Erisken, C., Kalyon, D.M., Zhou, J., Kim, S.G. and Mao, J.J. 2015. Viscoelastic properties of dental pulp tissue and ramifications on biomaterial development for pulp regeneration. J. Endod. 41, 1711–1717. Friedman, P.M., Mafong, E.A., Kauvar, A.N. and Geronemus, R.G. 2002. Safety data of injectable nonanimal stabilized hyaluronic acid gel for soft tissue augmentation. Dermatol. Surg. 8(6), 491–494. Galvez-Martin, P., Soto-Fernandez, C., Romero-Rueda, J., Cabañas, J., Torrent, A., Castells, G. and Martinez-Puig, D. 2023. A novel hyaluronic acid matrix ingredient with regenerative, anti-aging and cntioxidant capacity. Int. J. Mol. Sci. 24, 4774. Gomes-Filho, J.E., Duarte, P.C., Ervolino, E., Mogami Bomfim, S.R., Xavier Abimussi, C.J., Mota da Silva Santos, L., Lodi, C.S., Penha De Oliveira, S.H., Dezan, E. Jr. and Cintra, L.T. 2013. Histologic characterization of engineered tissues in the canal space of closed-apex teeth with apical periodontitis. J. Endod. 39(12), 1549–1556. Hargreaves, K. and Law, A. 2011. Regenerative endodontics. In Pathways of the pulp, 10th ed. Eds., Hargreaves, K. and Cohen, S. St. Louis, MO: Mosby Elsevier, pp: 602–619. Hassanien, E.E., Abu-Seida, A.M., Hashem, A.A. and Khanbash, S.S. 2015. Histologic evaluation of furcation perforation treated with mineral trioxide aggregate and bioaggregate. Asian J. Anim. Sci. 9, 148–156. Hooijmans, C.R., IntHout, J., Ritskes-Hoitinga, M. and Rovers, M.M. 2014. Meta-analyses of animal studies: an introduction of a valuable instrument to further improve healthcare. ILAR J. 55(3), 418–426. Holden, D.T., Schwartz, S.A., Kirkpatrick, T.C. and Schindler, W.G. 2008. Clinical outcomes of artificial root end barriers with mineral trioxide aggregate in teeth with immature apices. J. Endod. 34, 812–817. Inuyama, Y., Kitamura, C., Nishihara, T., Morotomi, T., Nagayoshi, M., Tabata, Y., Matsuo, K., Chen, K. and Terashita, M. 2010. Effects of hyaluronic acid sponge as a scaffold on odontoblastic cell line and amputated dental pulp. J. Biomed. Mater. Res. B Appl. Biomater. 92, 120–128. Iwaya, S.I., Ikawa, M. and Kubota, M. 2001. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent. Traumatol. 17 (4), 185–187. Kenny, A.B. and Hitzig, W.H. 1979. Bone marrow transplantation for severe combined immunodeficiency disease. Reported from 1968 to 1977. Eur. J. Pediatr. 131, 155–177. Kilkenny, C., Parsons, N., Kadyszewski, E., Festing, M.F., Cuthill, I.C., Fry, D., Hutton, J. and Altman, D.G. (2009). Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PLoS One 4(11), e7824. Kim, N.R., Lee, D.H., Chung, P.H. and Yang, H.C. 2009. Distinct differentiation properties of human dental pulp cells on collagen, gelatin, and chitosan scaffolds. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 108 (5), e94–e100. Li, M., Lv, J., Yang, Y., Cheng, G., Guo, S., Liu, C. and Ding, Y. 2022. Advances of hydrogel therapy in periodontal regeneration-A materials perspective review. Gels 8(10), 624. Litwiniuk, M., Krejner, A., Speyrer, M.S., Gauto, A.R. and Grzela, T. 2016. Hyaluronic acid in inflammation and tissue regeneration. Wounds Compendium Clin. Res. Pract. 28(3), 78–88. Lovelace, T., Henry, M., Hargreaves, K. and Diogenes, A. 2011. Evaluation of the delivery of mesenchymal stem cells into the root canal space of necrotic immature teeth after clinical regenerative endodontic procedure. J. Endod. 37, 133–138. Maharjan, A.S., Pilling, D. and Gomer, R.H. 2011. High and low molecular weight hyaluronic acid differentially regulate human fibrocyte differentiation. PLoS One 6(10), e26078. Meschi, N., Palma, P.J. and Cabanillas-Balsera, D. 2023. Effectiveness of revitalization in treating apical periodontitis: a systematic review and meta-analysis. Int. Endod. J. 56 (S3), 510–523. Murray, P.E., Garcia-Godoy, F. and Hargreaves, K.M. 2007. Regenerative endodontics: a review of current status and a call for action. J. Endod. 33(4), 377–390. Nabeel, M., Abu-Seida, A.M., Elgendy, A.A. and Tawfik, H.M. 2024. Biocompatibility of mineral trioxide aggregate and biodentine as root-end filling materials: an in vivo study. Sci. Rep. 14(1), 3568. Nagy, M.M., Tawfik, H.E., Hashem, A.A. and Abu-Seida, A.M. 2014. Regenerative potential of immature permanent teeth with necrotic pulps after different regenerative protocols. J. Endod. 40(2), 192–198. Nakashima, M., Iohara, K., Bottino, M.C., Fouad, A.F., Nör, J.E. and Huang, G.T. 2019. Animal models for stem cell-based pulp regeneration: Foundation for human clinical applications. Tissue Eng. Part B Rev. 25(2), 100–113. Nowicka, A., Miller-Burchacka, M., Lichota, D., Metlerska, J. and Gońda-Domin, M. 2021. Tissue engineering application in regenerative endodontics. Pomeranian J. Life Sci. 67, 10–17. Palma, P.J., Martins, J., Diogo, P., Sequeira, D., Ramos, J.C., Diogenes, A. and Santos, J.M. 2019. Does apical papilla survive and develop in apical periodontitis presence after regenerative endodontic procedures? Appl. Sci. 9, 3942. Palma, P.J., Ramos, J.C., Martins, J.B., Diogenes, A., Figueiredo, M.H., Ferreira, P., Viegas, C. and Santos, J.M. 2017. Histologic evaluation of regenerative endodontic procedures with the use of chitosan scaffolds in immature dog teeth with apical periodontitis. J. Endod. 43(8), 1279–1287. Raddall, G., Mello, I. and Leung, B.M. 2019. Biomaterials and scaffold design strategies for regenerative endodontic therapy. Front. Bioeng. Biotechnol. 7, 317. Rayahin, J.E., Buhrman, J.S., Zhang, Y., Koh, T.J. and Gemeinhart, R.A. 2015. High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomat. Sci. Engin. 1(7), 481–493. Roberts, I., Kwan, I., Evans, P. and Haig, S. 2002. Does animal experimentation inform human healthcare? Observations from a systematic review of international animal experiments on fluid resuscitation. BMJ (Clinical research ed.) 324(7335), 474–476. Šafránková, B., Hermannová, M., Nešporová, K., Velebný, V. and Kubala, L. 2018. Absence of differences among low, middle, and high molecular weight hyaluronan in activating murine immune cells in vitro. Int. J. Biol. Macromol. 107(Pt A), 1–8. Saoud, T.M., Zaazou, A., Nabil, A., Moussa, S., Lin, L.M. and Gibbs, J.L. 2014 Clinical and radiographic outcomes of traumatized immature permanent necrotic teeth after revascularization/ revitalization therapy. J. Endod. 40(12), 1946–1952. Sato, I., Kurihara-Ando, N., Kota, K., Iwaku, M. and Hoshino, E. 1996. Sterilization of infected root canal dentine by topical application of a mixture ciprofloxacin, metronidazole and minocycline in situ. Int. Endod. J. 29, 118–124. Scheibner, K.A., Lutz, M.A., Boodoo, S., Fenton, M.J., Powell, J.D. and Horton, M.R. 2006. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J. Immunol. (Baltimore, Md.: 1950), 177(2), 1272–1281. Schmidt, J., Pilbauerova, N., Soukup, T., Suchankova-Kleplova, T. and Suchanek, J. 2020. Low molecular weight hyaluronic acid effect on dental pulp stem cells in vitro. Biomolecules 11(1), 22. Shabahang, S., Torabinejad, M., Boyne, P.P., Abedi, H. and McMillan, P. 1999. A comparative study of root end induction using osteogenic protein-1, calcium hydroxide and mineral trioxide aggregate in dogs. J. Endod. 25(1), 1–5. Stern, R., Kogan, G., Jedrzejas, M.J. and Soltés, L. 2007. The many ways to cleave hyaluronan. Biotechnol. Adv. 25(6), 537–557. Sugiaman, V.K., Jeffrey, Naliani, S., Pranata, N., Djuanda, R. and Saputri, R.I. 2023. Polymeric scaffolds used in dental rulp regeneration by tissue engineering approach. Polymers (Basel) 15(5), 1082. Talaat, S., Hashem, A.A., Abu-Seida, A., Abdel Wahed, A. and Abdel Aziz, T.M. 2024. Regenerative potential of mesoporous silica nanoparticles scaffold on dental pulp and root maturation in immature dog’s teeth: a histologic and radiographic study. BMC Oral Health. 24, 817. Tawfik, H., Abu-Seida, A.M., Hashem, A.A. and Nagy, M.M. 2013. Regenerative potential following revascularization of immature permanent teeth with necrotic pulps. Int. Endod. J. 46, 910–922. Thibodeau, B. and Trope, M. 2007. Pulp revascularization of a necrotic infected immature permanent tooth: case report and review of the literature. Pediatr. Dent. 29, 47–50. Ulusoy, A.T., Turedi, I., Cimen, M. and Cehreli, Z.C. 2019. Evaluation of blood clot, platelet-rich plasma, platelet-rich fibrin, and platelet pellet as scaffolds in regenerative endodontic treatment: a prospective randomized trial. J. Endod. 45(5), 560–566. Wang, W., Yuan, C., Liu, Z., Geng, T., Li, X., Wei, L., Niu, W. and Wang, P. 2020. Characteristic comparison between canine and human dental mesenchymal stem cells for periodontal regeneration research in preclinical animal studies. Tiss. Cell. 67, 101405. Zhang, G., Gao, Y., Zhao, Z., Pyykko, I. and Zou, J. 2023. Low-molecular-weight hyaluronic acid contributes to noise-induced cochlear inflammation. Audiol. Neurootol. 28(5), 380–393. | ||
| How to Cite this Article |
| Pubmed Style Abdelsalam MS, Elgendy AA, Abu-seida AM, Abdelaziz TM, Issa MH, El-haddad KE. Histological evaluation of the regenerative potential of injectable hyaluronic acid hydrogel or collagen with blood clot as scaffolds during revascularization of immature necrotic dog’s teeth. Open Vet J. 2024; 14(11): 3004-3016. doi:10.5455/OVJ.2024.v14.i11.29 Web Style Abdelsalam MS, Elgendy AA, Abu-seida AM, Abdelaziz TM, Issa MH, El-haddad KE. Histological evaluation of the regenerative potential of injectable hyaluronic acid hydrogel or collagen with blood clot as scaffolds during revascularization of immature necrotic dog’s teeth. https://www.openveterinaryjournal.com/?mno=217532 [Access: July 01, 2025]. doi:10.5455/OVJ.2024.v14.i11.29 AMA (American Medical Association) Style Abdelsalam MS, Elgendy AA, Abu-seida AM, Abdelaziz TM, Issa MH, El-haddad KE. Histological evaluation of the regenerative potential of injectable hyaluronic acid hydrogel or collagen with blood clot as scaffolds during revascularization of immature necrotic dog’s teeth. Open Vet J. 2024; 14(11): 3004-3016. doi:10.5455/OVJ.2024.v14.i11.29 Vancouver/ICMJE Style Abdelsalam MS, Elgendy AA, Abu-seida AM, Abdelaziz TM, Issa MH, El-haddad KE. Histological evaluation of the regenerative potential of injectable hyaluronic acid hydrogel or collagen with blood clot as scaffolds during revascularization of immature necrotic dog’s teeth. Open Vet J. (2024), [cited July 01, 2025]; 14(11): 3004-3016. doi:10.5455/OVJ.2024.v14.i11.29 Harvard Style Abdelsalam, M. S., Elgendy, . A. A., Abu-seida, . A. M., Abdelaziz, . T. M., Issa, . M. H. & El-haddad, . K. E. (2024) Histological evaluation of the regenerative potential of injectable hyaluronic acid hydrogel or collagen with blood clot as scaffolds during revascularization of immature necrotic dog’s teeth. Open Vet J, 14 (11), 3004-3016. doi:10.5455/OVJ.2024.v14.i11.29 Turabian Style Abdelsalam, Mohamed S., Abeer A. Elgendy, Ashraf M. Abu-seida, Tarek M. Abdelaziz, Mohamed H. Issa, and Khaled E. El-haddad. 2024. Histological evaluation of the regenerative potential of injectable hyaluronic acid hydrogel or collagen with blood clot as scaffolds during revascularization of immature necrotic dog’s teeth. Open Veterinary Journal, 14 (11), 3004-3016. doi:10.5455/OVJ.2024.v14.i11.29 Chicago Style Abdelsalam, Mohamed S., Abeer A. Elgendy, Ashraf M. Abu-seida, Tarek M. Abdelaziz, Mohamed H. Issa, and Khaled E. El-haddad. "Histological evaluation of the regenerative potential of injectable hyaluronic acid hydrogel or collagen with blood clot as scaffolds during revascularization of immature necrotic dog’s teeth." Open Veterinary Journal 14 (2024), 3004-3016. doi:10.5455/OVJ.2024.v14.i11.29 MLA (The Modern Language Association) Style Abdelsalam, Mohamed S., Abeer A. Elgendy, Ashraf M. Abu-seida, Tarek M. Abdelaziz, Mohamed H. Issa, and Khaled E. El-haddad. "Histological evaluation of the regenerative potential of injectable hyaluronic acid hydrogel or collagen with blood clot as scaffolds during revascularization of immature necrotic dog’s teeth." Open Veterinary Journal 14.11 (2024), 3004-3016. Print. doi:10.5455/OVJ.2024.v14.i11.29 APA (American Psychological Association) Style Abdelsalam, M. S., Elgendy, . A. A., Abu-seida, . A. M., Abdelaziz, . T. M., Issa, . M. H. & El-haddad, . K. E. (2024) Histological evaluation of the regenerative potential of injectable hyaluronic acid hydrogel or collagen with blood clot as scaffolds during revascularization of immature necrotic dog’s teeth. Open Veterinary Journal, 14 (11), 3004-3016. doi:10.5455/OVJ.2024.v14.i11.29 |