| Case Report | ||
Open Vet J. 2022; 12(6): 910-918 Open Veterinary Journal, (2022), Vol. 12(6): 910–918 Case Report Partial lung lobectomy with the Caiman® Seal & Cut device in a dog with spontaneous pneumothorax: Case reportAnne Zobel*, Thomas Rohwedder and Peter BöttcherSmall Animal Clinic, Freie Universität Berlin, Berlin, Germany Submitted: 26/04/2022 Accepted: 01/11/2022 Published: 01/12/2022 *Corresponding Author: Anne Zobel. Small Animal Clinic, Freie Universität Berlin, Berlin, Germany. Email: anne.zobel [at] fu-berlin.de © 2022 Open Veterinary Journal
AbstractBackground: Spontaneous pneumothorax in dogs is characterized by an accumulation of air in the interpleural space without underlying trauma and consecutive acute onset of respiratory distress. Underlying causes for spontaneous pneumothorax vary with ruptured bullae being one of the main causes. Treatment after initial stabilization often requires partial or complete surgical resection of affected lung lobes. Partial lung lobectomy can be performed with stapling devices or sealing devices for example, by different surgical approaches including video-assisted thoracoscopic surgery. However, inter-thoracic surgery in small-sized dogs using either of the techniques is challenging. Case Description: A 12-year-old Shih Tzu was presented with spontaneous pneumothorax. Further diagnostics with computed tomography and intercostal thoracoscopy revealed a bulla in the right middle lung lobe. Partial lung lobectomy (2.5 × 2.5 × 2 cm) of the respective lung lobe was performed by an intercostal approach using the Caiman® 5 Seal & Cut sealing device. The Caiman® 5 Seal & Cut device allowed quick and safe partial lung lobectomy in the treatment of spontaneous pneumothorax without intra- or post-operative complications. The dog was discharged 2 days after surgery in good clinical condition. This report demonstrates the in-vivo efficacy and safety of the Caiman® 5 Seal & Cut sealing device for partial lung lobectomy in a small breed dog. Conclusion: Using the Caiman® 5 Seal & Cut device lung tissue could be resected without intra- or post-operative complications in a small breed dog. This case may emphasize the use of the device in fully video-assisted thoracoscopic surgery also in small-sized patients. Keywords: Dog, Lobectomy, Pneumothorax, Sealing device, Lung. IntroductionPneumothorax is a potentially life-threatening condition. Leakage of air from the pulmonary tissue, without any trauma, is defined as spontaneous pneumothorax (Johnston and Tobias, 2018). A considerable proportion of patients with spontaneous pneumothorax suffer from bilateral pneumothorax and consecutive tension pneumothorax (Holtsinger et al., 1993). In contrast to spontaneous pneumothorax in humans, a causative pathology is commonly found in dogs with spontaneous pneumothorax (Holtsinger and Ellison, 1995). Bullae or blebs, as well as pulmonary abscesses, plant migration, bacterial or fungal pneumonia, angiostrongylus vasorum infection, heart worm infection, or neoplasia have been reported in this context (Busch and Noxon, 1992; Puerto et al., 2002; Lipscomb et al., 2003; Au et al., 2006; Oliveira et al., 2010; Gallagher et al., 2012; Boudreau et al., 2013; Spodsberg et al., 2013; Trempala and Herold, 2013). Depending on the underlying disease, complete or partial lung lobectomy is performed and early surgical treatment has been reported to improve survival rate (Puerto et al., 2002). Recurrence rates following surgical intervention vary between 0% and 3% and up to 14% to 25% (Holtsinger and Ellison, 1995; Puerto et al., 2002; Lipscomb et al., 2003; Case et al., 2015; Howes et al., 2020; Dickson et al., 2021). In contrast, non-surgical treatment bears a risk of recurrence of up to 50% with associated increased mortality in dogs (Puerto et al., 2002). Affected lung lobes can be resected through different surgical approaches including median sternotomy, intercostal thoracotomy, thoracoscopic-assisted intercostal thoracotomy, or thoracoscopy alone (Brissot et al., 2003; Laksito et al., 2010; Wormser et al., 2014; Case et al., 2015; Johnston and Tobias, 2018). Partial lung lobectomy can be achieved by different techniques such as suture ligation or the use of stapling or sealing devices (LaRue et al., 1987; Walshaw, 1994; Faunt et al., 1998; Brissot et al., 2003; Mayhew et al., 2012; Johnston and Tobias, 2018; Oberhaus and Mcfadden, 2020). Several factors including underlying disease, location, and distribution of lung pathologies as well as patient size may influence the choice of the surgical approach and resection technique (Lipscomb et al., 2003). If feasible, minimally invasive techniques are preferred over open surgery, since post-operative pain and morbidity are minimized (Landreneau et al., 1993; Walsh et al., 1999; Bleakley et al., 2018). Especially in small-sized patients, intra-thoracic surgery can be challenging. The visualization may be limited, maneuvering of instruments can be difficult, and quite extensive tissue retraction is needed for the use of an endoscopic stapling device, because the smallest device is 12 mm in diameter which limits their use to extra-corporal application in these patients (Brissot et al., 2003; Mayhew et al., 2012; Case et al., 2015; Park et al., 2017; Johnston and Tobias, 2018). Various studies describe the use of bipolar sealing devices for thoracic surgery including pericardectomy (Mayhew et al., 2009; Michelotti et al., 2019), treatment of persistent right aortic arch (Townsend et al., 2016), lymph node extirpation (Steffey et al., 2015), lung biopsies (Mayhew et al., 2012), partial lung lobectomy (Oberhaus and Mcfadden, 2020), and complete lung lobectomy in combination with standard suture ligation of the vessels (Kanai et al., 2019). The Caiman® Seal & Cut sealing device (Aesculap AG, Tuttlingen, Germany) is available with two different jaw sizes [5 mm (Fig. 1) and 12 mm]. Both safely seal vessels up to a diameter of 7 mm (Eick et al., 2013). For partial lung lobectomy in dogs, the Caiman® 12 has been evaluated in a cadaveric model with excellent results regarding efficacy and safety (Brückner et al., 2019). So far, no in vivo report exists for either the Caiman® 5 or 12 Seal & Cut sealing device for partial lung lobectomy in small animals. This case report describes the successful clinical application of the Caiman® 5 Seal & Cut sealing device for partial lung lobectomy in a dog with spontaneous pneumothorax.
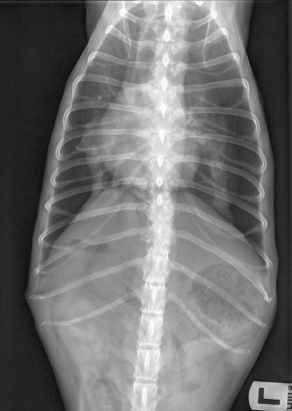
Fig. 1. Caiman® 5 Seal & Cut vessel sealing device and lektrafuse generator. Picture kindly provided by Aesculap AG, Tuttlingen, Germany. Case DetailsA 12-year-old male neutered Shih Tzu with a body weight of 6 kg was presented to the emergency service of the Small Animal Clinic of the Freie Universität of Berlin due to acute onset of respiratory distress without any history of trauma or preceding respiratory symptoms. At presentation, the dog showed mild dyspnea with a respiratory rate of 40 breaths per minute with increased abdominal effort but reduced thoracic motion. Auscultation of the thorax revealed damped respiratory sounds on both sides without alteration in heart sounds indicating a pneumothorax. All other parameters of the general examination were within normal range. Bilateral thoracocentesis was performed until dyspnea had significantly improved, evacuating 250 ml air in total dyspnea resolved after initial thoracocentesis, and a dorso-ventral thoracic radiograph was taken. Subsequent radiography of the chest in the dorso-ventral projection showed marked retraction and increased radiopacity of the right and left lung lobes, confirming severe bilateral pneumothorax and the tentative diagnosis of spontaneous bilateral pneumothorax was made (Fig. 2). No radiographic signs of bullae or other pathologic conditions such as lung tissue masses could be detected on the dorso-ventral view. Further radiographic imaging was suspended to reduce the patient’s stress level and to prevent possible recurrence of dyspnea. Besides mild thrombocytosis (742 103/µl; range 165–400), no abnormalities were found on complete blood count and blood chemistry analysis.
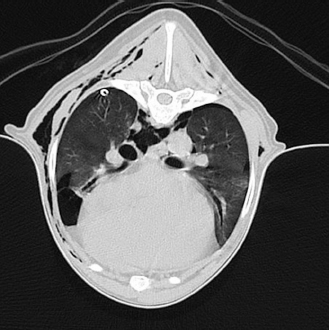
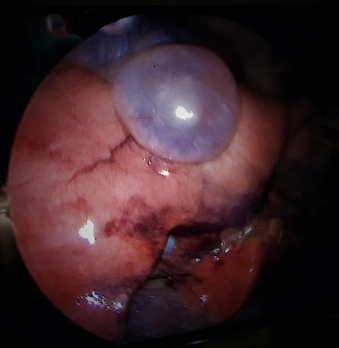
Fig. 2. Radiographic image of the thorax in dorso-ventral view with the dog in sternal recumbency after thoracocentesis for initial patient stabilization. Severely collapsed lung lobes with retracted lung lobe margins are visible on both sides. Since dyspnea relapsed within 4 hours following initial thoracocentesis, the patient was anesthetized for bilateral chest tube placement, to allow for effective repetitive or if needed continuous drainage. The patient was pre-oxygenated for approximately 5 minutes. After pre-medication with midazolam (0.5 mg/kg i.v., Midazolam 5 mg/ml, B.Braun Melsungen AG, Melsungen, Germany) and methadone (0.2 mg/kg i.v., Comfortan 10 mg/ml, Dechra Veterinary Products Deutschland GmbH, Aulendorf, Germany) anesthesia was induced with propofol intravenously (Narcofol 10 mg/ml, cp-pharma, Burgdorf, Germany) titrated to effect. An endotracheal tube was placed and anesthesia was maintained with isoflurane in oxygen. Bilateral chest tubes were placed at the level of the 8th intercostal space with an over the needle technique (Pleuracan®, B.Braun Melsungen AG, Melsungen, Germany) and 400 ml of air were evacuated. Continuous drainage of air was provided by attaching modified Heimlich valves (LEO – Ventil Berlin, Covetrus DE GmbH, Hamburg, Germany) to the drains, allowing for passive evacuation of air but without measuring its volume. Orthogonal radiographs confirmed proper tube placement as well as the absence of etiological findings. Recovery from anesthesia was uneventful. The patient remained in the intensive care unit under strict surveillance for recurrent dyspnea and further medical treatment, including continuous intravenous fluids using a balanced crystalloid solution (40 ml/kg/24 hours, Sterofundin ISO®, B.Braun Melsungen AG, Melsungen, Germany), intravenous buprenorphine (0.02 mg/kg q8h, Buprenovet 0.3 mg/ml, Elanco Germany, Bad Homburg, Germany) and metamizole (40 mg/kg q8h, Novacen 500 mg/ml, cp-pharma, Burgdorf, Germany). After 2 days of conservative treatment and absence of clinical signs, indicative for pneumothorax, the modified Heimlich valves were closed as a trial, and the patient was closely monitored for recurrent pneumothorax. Within 2 hours dyspnea relapsed and approximately 200 ml of air were evacuated from the chest tubes indicating persistent spontaneous pneumothorax. Because pneumothorax had not resolved by continuous chest drainage within 2 days computed tomography (CT) was performed with the goal to diagnose the causative factor(s) of persistent spontaneous pneumothorax as well as to propose a definitive treatment plan to the owners. General anesthesia was performed using the same protocol outlined previously. Using a 16 slice helical CT scanner (Philips Brilliance; Philips Medical Systems, Best, Netherlands) the thorax was scanned with and without intravenous contrast media (600 mg/kg i.v.; Unilux® 300 mg/ml, Sanochemia Pharmazeutika GmbH, Neufeld An Der Leitha, Germany) at a slice thickness of 2 mm and an increment of 1 mm using a high-resolution soft tissue kernel with the dog positioned in sternal recumbency Images were assessed in the transverse and reconstructed sagittal and coronal planes using a lung window (window width: 1,400 HU, window level: −400 HU). CT imaging revealed a mild bilateral pneumothorax and a roundish radiolucent, bullous, subpleural air-filled structure at the caudo-ventral aspect of the right middle lung lobe at the level of the 6th to 7th intercostal space with a size of 1.8 × 1.8 × 1.4 cm (Fig. 3). Additionally, a mild to moderate bilateral subcutaneous emphysema and pneumomediastinum were detected bilaterally at that level (Fig. 4). Mild to moderate atelectasis of the right middle, right caudal, and accessory lung lobe was evident in the ventral aspect of the respective lung lobes. CT-imaging post-contrast media application did not reveal any further findings. Therefore, the tentative diagnosis of spontaneous pneumothorax associated with a bulla located in the right middle lung lobe was made and surgical treatment was discussed with the owner. Immediately following CT imaging, the patient underwent thoracoscopy of the right hemithorax, to confirm bullous emphysema and confirmation of computer tomograhical results, as well as for further diagnostics. The surgical field was clipped and aseptically prepared. The dog was positioned in left lateral recumbency with the affected side uppermost (Johnston and Tobias, 2018). Perioperative medical treatment consisted of cefazoline, administered 30 minutes before the skin incision (22 mg/kg, Cephazolin Fresenius 1 g, Fresenius Kabi GmbH, Bad Homburg, Germany), fentanyl, ketamine, and lidocaine as a constant rate infusion (2.5 µg/kg/hour; Fentadon 50 mcg/ml, Dechra Veterinary Products Deutschland GmbH, Aulendorf, Germany; 0.2 mcg/kg/hour, Anesketin, Albrecht GmbH, Aulendorf, Germany; LidoCARD 2%, B.Braun Melsungen AG, Melsungen, Germany) and a constant rate infusion of a balanced crystalloid solution (Sterofundin ISO®, B. Braun Melsungen AG, Melsungen, Germany). Anesthesia was maintained with isoflurane in oxygen at an end-tidal volume percentage of 0.8–1.0. The dog was mechanically ventilated with volume-controlled intermittent positive pressure ventilation with a maximum pressure of 15 cmH2O with the addition of a positive end expiratory pressure of 2–5 cmH2O. Ventilatory parameters were adjusted to maintain normocapnia. The right-sided chest tube was removed to allow for unrestricted inspection of the right thoracic cavity and to prevent potential interference with instrumentation during thoracoscopy in the small-sized patient. A skin incision was made at the level of the 4th intercostal space at the junction between the ventral and middle third of the thorax. Blunt dissection was performed until the pleura was perforated. A 5 mm endoscopic threaded shaft cannula with an open valve (Ternamian Endotip; Carl Storz Endoskope, Tuttlingen, Germany) was placed at the junction between the middle and the dorsal third of the 6th intercostal space. A 5 mm 30° fore oblique Hopkins endoscope (Carl Storz Endoskope, Tuttlingen, Germany) was used for exploration of the right thoracic cavity. Thoracoscopy confirmed the bulla within the right middle lung lobe (Fig. 5). No further pathological findings were detected on arthroscopic exploration.
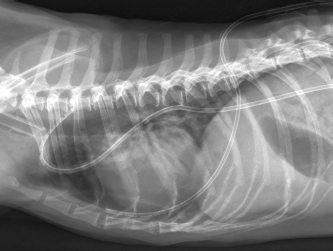
Fig. 3. Radiographic image of the thorax in latero-lateral view with the dog laying on the left side. Bilateral chest tubes are placed at the level of the 8th intercostal space. No etiological findings for pneumothorax are visible and the thorax is completely evacuated from air. The endotracheal tube is visible at the level of the thoracic aperture. The procedure was converted to a “mini” intercostal thoracotomy by extension of the initial thoracoscopic portal to an incision of about 3–4 cm with manual retraction of the ribs to allow for extracorporeal partial lung lobe resection. The right middle lung lobe was gently mobilized under thoracoscopic control and exteriorized from the thoracic cavity. Resection of the distal aspect of the right middle lung lobe, approximately 1 cm proximal to the bulla was performed by using the 5 mm Caiman® sealing device. The sample was fixated in formalin and sent for histopathological examination. After repositioning the partially amputated lung lobe, a leakage test was performed, by filling the right thoracic cavity with sterile warmed lactated Ringer’s solution (Ringer B.Braun Ecotainer, B. Braun Melsungen AG, Melsungen, Germany) followed by careful manual lung insufflation with a maximum pressure of 35 mmH2O for several intervals of approximately 10 seconds. No air bubbles, as an indicator for air leakage, were detected and the resection side was considered safely sealed. A new chest tube was placed in the 7th intercostal space of the right hemithorax under arthroscopic control. The thorax was closed routinely in four layers, including circumcostal sutures. The pleural cavity was evacuated via the chest tube to re-establish negative pressure. Post-operative orthogonal radiographs of the thorax confirmed proper chest tube placement, as well as absence of pneumothorax.
Fig. 4. Transverse CT-image of the thorax at the level of the 5th rip (lung window). A 1.8 × 1.8 × 1.4 cm radiolucent, bullous, air-filled structure within the caudo-ventral aspect of the right medial lung lobe is visible; the air-filled cavity is located at the periphery of the respective lung lobe and seems surrounded by a thin soft tissue dense margin. Additionally, mild bilateral pneumothorax, as well as mild to moderate pneumomediastinum and bilateral subcutaneous emphysema are visible. The patient recovered uneventfully from anesthesia and remained in the intensive care unit for another day post-operatively. Fluid therapy was continued using Sterofundin ISO® and analgesia was maintained by a fentanyl-ketamine-lidocaine constant rate infusion (2–5 mcg/kg/hour, Fentadon, Dechra Veterinary Products Deutschland GmbH, Aulendorf, Germany; 0.2 mcg/kg/hour, Anesketin, Albrecht GmbH, Aulendorf, Germany; LidoCARD 2%, B.Braun Melsungen AG, Melsungen, Germany). Additional analgesia was provided by intravenous administration of metamizole 40 mg/kg three times daily. During the first 2 days following surgery, neither fluid nor gas could be drained from the chest tube, which prompted drain removal and discharge of the patient. Oral metamizole (Novaminsulfon 500 mg, ratiopharm GmbH, Ulm, Germany) was prescribed in a total dosage of 200 mg three times daily for another 5–7 consecutive days in combination with omeprazole 10 mg (Antra Mups 10mg, AstraZeneca GmbH, Wedel, Germany) two times daily for 5–7 consecutive days as a gastroprotective.
Fig. 5. Thoracoscopic image of the right lung lobe. An air filled bullous structure with a thin membrane can be seen on the caudo-ventral aspect of the right medial lung lobe indicating a bulla. Thoracoscopic images corresponded with CT findings. Histopathology of the resected lung tissue diagnosed pulmonary adenoma with bullous cyst formation. The tumor was resected completely with clean surgical margins. The dog was presented back 1 week after discharge due to anorexia and reduced general condition, but without any signs of dyspnea, as reported by the owners. Gastro-intestinal ulceration was suspected due to the presence of melena and pale mucous membranes as well as a decreased hematocrite. The dog was hospitalized again. The condition resolved under medical treatment and the dog was ultimately lost to follow up after discharge. No complications regarding the pneumothorax were detected until then. DiscussionThe presented case strengthens the safety and efficacy of the Caiman® 5 Seal & Cut sealing device for partial lung lobectomy in dogs, which has only been reported in an ex-vivo experiment, so far (Brückner et al., 2019). Even though, partial lung lobectomy had been performed for the treatment of spontaneous pneumothorax caused by a bulla, the authors would not limit the use of the Caiman® 5 Seal & Cut device to only that indication. In the hereby presented case, a radiolucent, bullous, subpleural air-filled structure with a size of 1.8 × 1.8 × 1.4 cm was present at the caudo-ventral aspect of the right middle lung lobe indicating a bulla within that lung lobe. Several studies report poor sensitivity for identification of bullae using CT (Au et al., 2006; Reetz et al., 2013; Case et al., 2015; Dickson et al., 2021). Therefore, thoracoscopy was performed in the present case to confirm the tentative CT diagnosis. The success of identifying bullae by thoracoscopy varies among the studies. Several studies report that conversion to open surgery/median sternotomy was necessary in up to 87.5% of cases, because no lesion could be identified (Case et al., 2015; Howes et al., 2020; Dickson et al., 2021). One study, however, demonstrates better results and corresponding lesions could safely be identified by thoracoscopy (Brissot et al., 2003). In the present case, no other lesion was found during CT or thoracoscopy. But it should be acknowledged that by limiting thoracoscopy to the right hemithorax, lesions affecting the left lung lobes might have been missed. Since pneumothorax resolved after resection of the detected bulla and did not re-occur, the authors assume not having missed other lesions and the resected bulla being solely responsible for the pneumothorax. Anesthetic protocols for thoracoscopy usually include the use of one-lung ventilation. In the case presented, endotracheal intubation and volume-controlled ventilation were used. This type of ventilation was chosen to ensure that all lung areas were ventilated with an appropriate tidal volume. However, to ease navigation and visualization a maximum pressure of ≤15 cmH2O and a more conservative tidal volume were set. Since we did not have a pressure-controlled volume guaranteed option in the available anesthetic machine, we worried the pressure-controlled mode might lead to insufficient ventilation and potentially atelectasis, especially in the lungs of the other hemithorax. Overall, of the resulting inflation of the lung lobes using the described ventilation protocol did neither hinder thoracoscopic exploration, nor subsequent thoracoscopic assisted lung lob resection. Our positive experience is also supported by Brissot et al. (2003) and Wormser et al. (2014), who showed that one lung ventilation does not seem to be mandatory for successful thoracoscopy in the context of pulmonary bullae. Several surgical techniques for partial lung lobectomy have been reported, including suture ligation, stapling, and sealing devices (LaRue et al., 1987; Walshaw, 1994; Faunt et al., 1998; Brissot et al., 2003; Johnston and Tobias, 2018; Oberhaus and Mcfadden, 2020). In small animals no in vivo data exist whether the use of a sealing device for partial lung lobectomy is beneficial compared to stapling or other techniques in terms of intra- or post- operative complications. However, multiple studies have shown, that safe vessel closure is achieved in a significantly shorter time using a sealing device compared to suture ligation in ovariectomy (Mayhew and Brown, 2007; Schwarzkopf et al., 2015) or stapling in splenectomy (Monarski et al., 2014) without negatively altering the post-operative outcome (Sirochman et al., 2020). However, no time-sparing effect could be shown in patients undergoing liver lobe biopsies (Risselada et al., 2010). Regarding thoracic surgery data from human medicine indicate that there is no significant difference in duration of pulmonary resection between sealing devices compared to endoscopic stapling devices (Kovács et al., 2009; Toishi et al., 2014). However, in people, sealing devices are mainly used for isolated vessel and bronchi sealing when performing total lung lobectomy. Reported data indicate that the use of sealing devices decreases intra-operative blood loss significantly and reduces thoracic drainage exudation following surgery, thus allowing earlier drain removal compared to endostaplers (Toishi et al., 2014). The use of sealing devices for partial or wedge resection or treatment of pulmonary fissures is less common (Santini et al., 2006; Kovács et al., 2009). Since the intracorporal use of stapling devices for thoracic surgery seems to be limited especially in small-sized patients, it is crucial to have an easy to use and safe instrument of appropriate size for thoracoscopic lung tissue resection. Furthermore, in human medicine, it has been stated that the use of a sealing device compared to stapling devices could save functional lung tissue in non-anatomical resection because of more accurate resection margins (Santini et al., 2006). Furthermore, we speculate that lung tissue resection is possible in a shorter time using sealing devices compared to staplers in small animals. For partial lung lobe resection two or three rows of staple lines have to be set for adequate tissue sealing which makes cartridge reload necessary, which adds to the high costs for stapling procedures (Santini et al., 2006; Johnston and Tobias, 2018). In comparison, using a sealing device like the Caiman® 5, allows consecutive tissue sealing without interruption as well as without additional costs. Although designed for single use in human medicine, sealing devices are often re-sterilized and used multiple times in veterinary medicine. To do so, the devices must provide safe tissue sealing after re-sterilization, which has been confirmed in an ex-vivo study, observing no seal failure as well as supra-physiologic burst pressures when using devices being re-sterilized multiple times (Gardeweg et al., 2019). Whether multiple sterilization cycles have an impact on the sealing efficacity of bronchi has not been investigated so farand warrants further investigation. Literature on vessel burst pressure is controversial. The evidence available is predominately based on studies in people or experimental data in animals. One major aspect regarding sealing failure is vessel diameter (Toishi et al., 2014; Wyatt et al., 2016). Ex vivo studies on porcine carotid arteries showed that the seal strength highly correlates with the outer diameter of the vessel – a diameter reduction results in a significantly increased seal strength along the same artery (Wyatt et al., 2016). Compared to other sealing devices, the Caiman® 5 has been shown to be superior with regard to effective vessel sealing and burst pressure for different vessel diameters (Okhunov et al., 2018). The Caiman® 5 offers a larger sealing surface compared to other sealing devices and provides homogenous pressure distribution along the sealing electrodes, distributed equally to the upper and lower jaw (Eick et al., 2013). Furthermore, due to the larger sealing surface, fewer sealing cycles are needed to achieve safe tissue sealing (Heblinski and Brückner, 2018). However, the overall surgical time does not seem to be affected by jaw configuration (Heblinski and Brückner, 2018; Okhunov et al., 2018). In case of thoracic surgery, not only the safety and efficacy of vessel sealing is of important but also possible air leakage from the resection side. Permanent leakage of air could result in permanent pneumothorax and ultimately tension pneumothorax. In people, complication rates of 5% to 10 % are reported in association with air leakage after using a sealing device for lung lobe wedge resection (Santini et al., 2006; Kovács et al., 2009). Sealing devices have to seal lung tissue that withstands at least a physiological airways pressure of 20 mmHg in the bronchi to be considered safe (Santini et al., 2006). Similar to vessel diameter, airway diameter is associated with sealing failure, too. Sealing safety decreases with increasing diameter of the bronchus. In an experimental ex vivo study (Santini et al., 2006) on porcine bronchi, tissues with a small diameter (1–3 mm) could be safely sealed and withstood supra- physiological airway pressures of more than 20 mmHg, while larger diameters of 4–7 and 6–7 mm showed partial or complete sealing failure, respectively, and could not withstand the defined “critical pressure” which was defined as three times the maximal physiological pressure (Santini et al., 2006). This statement is supported by an ex vivo study on canine lung tissue where tissue samples with larger diameters were used and many of the sealed resection planes showed leakage of air in the central region of the resection site. Interestingly, in this study, stapling devices showed similar failure patterns and only suture or loop ligation was significantly safer regarding bronchial closure and prevention of air leakage (Marvel and Monnet, 2013). While it is known that the type of sealing device impacts sealing safety and air burst pressure measured by the existence and duration of post-operative pneumothorax (Koga et al., 2014), studies comparing the different devices are lacking. For the Caiman Seal & Cut device only experimental ex vivo data on canine lung tissue is available (Brückner et al., 2019). In that study, lung tissue was safely sealed and no air leakage was evident at supra-physiological pressures. Moreover, one tissue sample exceeded bronchial diameter of 7 mm, the threshold for vessel diameter that can safely be sealed according to the manufacture’s recommendations and could also safely be sealed without air leakage at supra-physiological pressures (Brückner et al., 2019). However, these results were achieved using the 12 mm device, while in the present case the 5 mm device was used. To the knowledge of the authors, no other report of the in vivo application of the Caiman® 5 Seal & Cut device for partial lung lobectomy exists. However, in other studies promising results have been reported using different sealing devices (Mayhew et al., 2012; Oberhaus and Mcfadden, 2020). These results are not limited to open surgical procedures, but also report on the safe use in minimal invasive lung lobectomy via thoracoscopy, which is superior to open surgery in terms of post-operative pain and morbidity, such as wound infections or post-operative pleural effusion, which are predominantly associated with the surgical approach used in small animals (Landreneau et al., 1993; Walsh et al., 1999; Bleakley et al., 2018). Although the device used in this report is designed for intra-corporal use, a “mini”-thoracotomy and extra-corporal partial resection of the affected lung tissue were performed after thoracoscopic evaluation of the right thoracic cavity and identification of the bulla. This was done due to safety concerns since the device has never been used in vivo for this procedure before. In case of sealing failure or hemorrhage from the resection plane, the authors wanted to be able to intervene immediately. Beside different sizes, the Caiman is also available with a 80 degree articulating jaw tip, which was developed especially for the use in challenging anatomic conditions, which certainly includes the thoracic cavity in small-sized patients. No in vivo data exist whether intra-corporal use of the angulated device is superior to the straight tip device in terms of maneuvering the instrument within the thorax. With the positive experience in the present case in mind, the authors will consider future application of the sealing devices in a fully thoracoscopic setting. We did not encounter any intra-operative complications and the dog could be discharged 2 days after the operation. Whether the uneventful course of the surgery, short surgical as well as hospitalization time were significantly associated with the use of the Caiman® warrants further investigation. ConclusionThe Caiman® 5 Seal & Cut device allowed quick and safe partial lung lobectomy in the treatment of spontaneous pneumothorax in a Shih Tzu with pulmonary adenoma and bullous cyst. AcknowledgmentsWe acknowledge support by the Open Access Publication Initiative of Freie Universität Berlin. Conflict of interestThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Author contributionsA.Z. contributed to writing the manuscript, interpreting and describing the imaging findings and surgical treatment, and literature review and has first authorship. T.R. contributed to writing and editing the manuscript and interpreting and describing the imaging findings and surgical treatments. P.B. was the main surgeon and contributed to interpreting and describing the imaging findings and surgical treatments. All authors contributed to the article and approved the submission. ReferencesAu, J.J., Weisman, D.L., Stefanacci, J.D. and Palmisano, M.P. 2006. Use of computed tomography for evaluation of lung lesions associated with spontaneous pneumothorax in dogs: 12 cases (1999-2002). J. Am. Vet. Med. Assoc. 228(5), 733–737. Bleakley, S., Phipps, K., Petrovsky, B. and Monnet, E. 2018. Median sternotomy versus intercostal thoracotomy for lung lobectomy: a comparison of short-term outcome in 134 dogs. Vet. Surg. 47(1), 104–113. Boudreau, B., Nelson, L.L., Carey, S.A. and Williams, K.J. 2013. Spontaneous pneumothorax secondary to reactive bronchopneumopathy in a dog. J. Am. Vet. Med. Assoc. 242(5), 658–662. Brissot, H.N., Dupre, G.P., Bouvy, B.M. and Paquet, L. 2003. Thoracoscopic treatment of bullous emphysema in 3 dogs. Vet. Surg. 32(6), 524–529. Brückner, M., Heblinski, N. and Henrich, M. 2019. Use of a novel vessel-sealing device for peripheral lung biopsy and lung lobectomy in a cadaveric model. J. Small. Anim. Pract. 60(7), 411–416. Busch, D.S. and Noxon, J.O. 1992. Pneumothorax in a dog infected with Dirofilaria immitis. J. Am. Vet. Med. Assoc. 201(12), 1893. Case, J.B., Mayhew, P.D. and Singh, A. 2015. Evaluation of video-assisted thoracic surgery for treatment of spontaneous pneumothorax and pulmonary bullae in dogs. Vet. Surg. 44(Suppl 1), 31–38. Dickson, R., Scharf, V.F., Michael, A.E., Walker, M., Thomson, C., Grimes, J., Singh, A., Oblak, M., Brisson, B. and Case, J.B. 2021. Surgical management and outcome of dogs with primary spontaneous pneumothorax: 110 cases (2009–2019). J. Am. Vet. Med. Assoc. 258(11), 1229–1235. Eick, S., Loudermilk, B., Walberg, E. and Wente, M.N. 2013. Rationale, bench testing and in vivo evaluation of a novel 5 mm laparoscopic vessel sealing device with homogeneous pressure distribution in long instrument jaws. Ann. Surg. Innov. Res. 7(1), 15. Faunt, K.K., Jones, B.D., Turk, J.R., Cohn, L.A. and Dodam, J.R. 1998. Evaluation of biopsy specimens obtained during thoracoscopy from lungs of clinically normal dogs. Am. J. Vet. Res. 59(11), 1499–1502. Gallagher, B., Brennan, S.F., Zarelli, M. and Mooney, C.T. 2012. Geographical, clinical, clinicopathological and radiographic features of canine angiostrongylosis in Irish dogs: a retrospective study. Ir. Vet. J. 65(1), 5. Gardeweg, S., Bockstahler, B. and Duprè, G. 2019. Effect of multiple use and sterilization on sealing performance of bipolar vessel sealing devices. PLoS One 14(8), e0221488. Heblinski, N. and Brückner, M. 2018. Comparison of two vessel-sealing devices for laparoscopic-assisted ovariohysterectomy in dogs. Tierarztl. Prax. Ausg. K. Kleintiere. Heimtiere. 46(6), 363–369. Holtsinger, R.H., Beale, B., Bellah, J. and King, R.R. 1993. Spontaneous pneumothorax in the dog: a retrospective analysis of 21 cases. J. Am. Anim. Hosp. Assoc. 29, 195–210. Holtsinger, R.L. and Ellison, G. 1995. Spontaneous pneumothorax. Compendium on continuing education for the practising veterinarian North American edition, pp 197–209, vol. 17. Howes, C.L., Sumner, J.P., Ahlstrand, K., Hardie, R.J., Anderson, D., Woods, S., Goh, D., de La Puerta, B., Brissot, H.N., Das, S., Nolff, M., Liehmann, L. and Chanoit, G. 2020. Long-term clinical outcomes following surgery for spontaneous pneumothorax caused by pulmonary blebs and bullae in dogs - a multicentre (AVSTS Research Cooperative) retrospective study. J. Small. Anim. Pract. 61(7), 436–441. Johnston, S.A. and Tobias, K.M. 2018. Veterinary surgery: small animal. St. Louis, MO: Elsevier, pp: 82. Kanai, E., Matsutani, N., Hanawa, R. and Takagi, S. 2019. Video-assisted thoracic surgery anatomical lobectomy for a primary lung tumor in a dog. J. Vet. Med. Sci. 81(11), 1624–1627. Koga, H., Suzuki, K., Nishimura, K., Okawada, M., Doi, T., Lane, G.J., Inada, E., Okazaki, T. and Yamataka, A. 2014. Comparison of the value of tissue-sealing devices for thoracoscopic pulmonary lobectomy in small children: a first report. Pediatr. Surg. Int. 30(9), 937–940. Kovács, O., Szántó, Z., Krasznai, G. and Herr, G. 2009. Comparing bipolar electrothermal device and endostapler in endoscopic lung wedge resection. Interact. Cardiovasc. Thorac. Surg. 9(1), 11–14. Laksito, M.A., Chambers, B.A. and Yates, G.D. 2010. Thoracoscopic-assisted lung lobectomy in the dog: report of two cases. Aust. Vet. J. 88(7), 263–267. Landreneau, R.J., Hazelrigg, S.R., Mack, M.J., Dowling, R.D., Burke, D., Gavlick, J., Perrino, M.K., Ritter, P.S., Bowers, C.M. and DeFino, J. 1993. Postoperative pain-related morbidity: video-assisted thoracic surgery versus thoracotomy. Ann. Thorac. Surg. 56(6), 1285–1289. LaRue, S.M., Withrow, S.J. and Wykes, P.M. 1987. Lung resection using surgical staples in dogs and cats. Vet. Surg. 16(3), 238–240. Lipscomb, V.J., Hardie, R.J. and Dubielzig, R.R. 2003. Spontaneous pneumothorax caused by pulmonary blebs and bullae in 12 dogs. J. Am. Anim. Hosp. Assoc. 39(5), 435–445. Marvel, S. and Monnet, E. 2013. Ex vivo evaluation of canine lung biopsy techniques. Vet. Surg. 42(4), 473–477. Mayhew, P.D. and Brown, D.C. 2007. Comparison of three techniques for ovarian pedicle hemostasis during laparoscopic-assisted ovariohysterectomy. Vet. Surg. 36(6), 541–547. Mayhew, P.D., Culp, W.T.N., Pascoe, P.J. and Arzi, N.V. 2012. Use of the ligasure vessel-sealing device for thoracoscopic peripheral lung biopsy in healthy dogs. Vet. Surg. 41(4), 523–528. Mayhew, K.N., Mayhew, P.D., Sorrell-Raschi, L. and Brown, D.C. 2009. Thoracoscopic subphrenic pericardectomy using double-lumen endobronchial intubation for alternating one-lung ventilation. Vet. Surg. 38(8), 961–966. Michelotti, K.P., Youk, A., Payne, J.T. and Anderson, J. 2019. Outcomes of dogs with recurrent idiopathic pericardial effusion treated with a 3-port right-sided thoracoscopic subtotal pericardiectomy. Vet. Surg. 48(6), 1032–1041. Monarski, C.J., Jaffe, M.H. and Kass, P.H. 2014. Decreased surgical time with a vessel sealing device versus a surgical stapler in performance of canine splenectomy. J. Am. Anim. Hosp. Assoc. 50(1), 42–45. Oberhaus, A. and Mcfadden, M. 2020. Use of vessel sealing system for multiple partial lung lobectomies for spontaneous pneumothorax. Can. Vet. J. 61(8), 875–879. Okhunov, Z., Yoon, R., Lusch, A., Spradling, K., Suarez, M., Kaler, K.S., Patel, R., Hwang, C., Osann, K., Huang, J., Lee, T. and Landman, J. 2018. Evaluation and comparison of contemporary energy-based surgical vessel sealing devices. J. Endourol. 32(4), 329–337. Oliveira, C., Rademacher, N., David, A., Vasanjee, S. and Gaschen, L. 2010. Spontaneous pneumothorax in a dog secondary to Dirofilaria immitis infection. J. Vet. Diagn. Invest. 22(6), 991–994. Park, J., Lee, H.B. and Jeong, S.M. 2017. Treatment of a giant pulmonary emphysematous cyst with primary bronchoalveolar papillary carcinoma in a Shih Tzu dog. Vet. Surg. 46(1), 158–164. Puerto, D.A., Brockman, D.J., Lindquist, C. and Drobatz, K. 2002. Surgical and nonsurgical management of and selected risk factors for spontaneous pneumothorax in dogs: 64 cases (1986-1999). J. Am. Vet. Med. Assoc. 220(11), 1670–1674. Reetz, J.A., Caceres, A.V., Suran, J.N., Oura, T.J., Zwingenberger, A.L. and Mai, W. 2013. Sensitivity, positive predictive value, and interobserver variability of computed tomography in the diagnosis of bullae associated with spontaneous pneumothorax in dogs: 19 cases (2003–2012). J. Am. Vet. Med. Assoc. 243(2), 244–251. Risselada, M., Ellison, G.W., Bacon, N.J., Polyak, M.M.R., van Gilder, J., Kirkby, K. and Kim, S.E., 2010. Comparison of 5 surgical techniques for partial liver lobectomy in the dog for intraoperative blood loss and surgical time. Vet. Surg. 39(7), 856–862. Santini, M., Vicidomini, G., Baldi, A., Gallo, G., Laperuta, P., Busiello, L., Di Marino, M.P. and Pastore, V. 2006. Use of an electrothermal bipolar tissue sealing system in lung surgery. Eur. J. Cardiothorac. Surg. 29(2), 226–230. Schwarzkopf, I., van Goethem, B., Vandekerckhove, P.M. and Rooster, H. 2015. Vessel sealing versus suture ligation for canine ovarian pedicle haemostasis: a randomised clinical trial. Vet. Rec. 176(5), 125. Sirochman, A.L., Milovancev, M., Townsend, K. and Grimes, J.A. 2020. Influence of use of a bipolar vessel sealing device on short-term postoperative mortality after splenectomy: 203 dogs (2005-2018). Vet. Surg. 49(2), 291–303. Spodsberg, E.H., Miles, J.E., McEvoy, F.J. and Willesen, J.L. 2013. Spontaneous pneumothorax secondary to granulomatous pneumonia caused by Angiostrongylus vasorum in a dog in Denmark. J. Small. Anim. Pract. 54(2), 114. Steffey, M.A., Daniel, L., Mayhew, P.D., Affolter, V.K., Soares, J.H.N. and Smith, A. 2015. Video-assisted thoracoscopic extirpation of the tracheobronchial lymph nodes in dogs. Vet. Surg. 44(Suppl 1), 50–58. Toishi, M., Yoshida, K., Agatsuma, H., Sakaizawa, T., Eguchi, T., Saito, G., Hashizume, M., Hamanaka, K. and Shiina, T. 2014. Usefulness of vessel-sealing devices for ≤7 mm diameter vessels: a randomized controlled trial for human thoracoscopic lobectomy in primary lung cancer. Interact. Cardiovasc. Thorac. Surg. 19(3), 448–455. Townsend, S., Oblak, M.L., Singh, A., Steffey, M.A. and Runge, J.J. 2016. Thoracoscopy with concurrent esophagoscopy for persistent right aortic arch in 9 dogs. Vet. Surg. 45(S1), O111–O118. Trempala, C.L. and Herold, L.V. 2013. Spontaneous pneumothorax associated with Aspergillus bronchopneumonia in a dog. J. Vet. Emerg. Crit. Care. 23(6), 624–630. Walsh, P.J., Remedios, A.M., Ferguson, J.F., Walker, D.D., Cantwell, S. and Duke, T. 1999. Thoracoscopic versus open partial pericardectomy in dogs: comparison of postoperative pain and morbidity. Vet. Surg. 28(6), 472–479. Walshaw, R. 1994. Stapling techniques in pulmonary surgery. Vet. Clin. North. Am. Small. Anim. Pract. 24(2), 335–366. Wormser, C., Singhal, S., Holt, D.E. and Runge, J.J. 2014. Thoracoscopic-assisted pulmonary surgery for partial and complete lung lobectomy in dogs and cats: 11 cases (2008-2013). J. Am. Vet. Med. Assoc. 245(9), 1036–1041. Wyatt, H.L., Richards, R., Pullin, R., Yang, T.J., Blain, E.J. and Evans, S.L. 2016. Variation in electrosurgical vessel seal quality along the length of a porcine carotid artery. Proc. Inst. Mech. Eng. H. 230(3), 169–174. | ||
| How to Cite this Article |
| Pubmed Style Zobel A, Rohwedder T, Bottcher P, . Partial lung lobectomy with the Caiman® Seal & Cut device in a dog with spontaneous pneumothorax: case report. Open Vet J. 2022; 12(6): 910-918. doi:10.5455/OVJ.2022.v12.i6.17 Web Style Zobel A, Rohwedder T, Bottcher P, . Partial lung lobectomy with the Caiman® Seal & Cut device in a dog with spontaneous pneumothorax: case report. https://www.openveterinaryjournal.com/?mno=27186 [Access: October 06, 2024]. doi:10.5455/OVJ.2022.v12.i6.17 AMA (American Medical Association) Style Zobel A, Rohwedder T, Bottcher P, . Partial lung lobectomy with the Caiman® Seal & Cut device in a dog with spontaneous pneumothorax: case report. Open Vet J. 2022; 12(6): 910-918. doi:10.5455/OVJ.2022.v12.i6.17 Vancouver/ICMJE Style Zobel A, Rohwedder T, Bottcher P, . Partial lung lobectomy with the Caiman® Seal & Cut device in a dog with spontaneous pneumothorax: case report. Open Vet J. (2022), [cited October 06, 2024]; 12(6): 910-918. doi:10.5455/OVJ.2022.v12.i6.17 Harvard Style Zobel, A., Rohwedder, T., Bottcher, P. & (2022) Partial lung lobectomy with the Caiman® Seal & Cut device in a dog with spontaneous pneumothorax: case report. Open Vet J, 12 (6), 910-918. doi:10.5455/OVJ.2022.v12.i6.17 Turabian Style Zobel, Anne, Thomas Rohwedder, Peter Bottcher, and . 2022. Partial lung lobectomy with the Caiman® Seal & Cut device in a dog with spontaneous pneumothorax: case report. Open Veterinary Journal, 12 (6), 910-918. doi:10.5455/OVJ.2022.v12.i6.17 Chicago Style Zobel, Anne, Thomas Rohwedder, Peter Bottcher, and . "Partial lung lobectomy with the Caiman® Seal & Cut device in a dog with spontaneous pneumothorax: case report." Open Veterinary Journal 12 (2022), 910-918. doi:10.5455/OVJ.2022.v12.i6.17 MLA (The Modern Language Association) Style Zobel, Anne, Thomas Rohwedder, Peter Bottcher, and . "Partial lung lobectomy with the Caiman® Seal & Cut device in a dog with spontaneous pneumothorax: case report." Open Veterinary Journal 12.6 (2022), 910-918. Print. doi:10.5455/OVJ.2022.v12.i6.17 APA (American Psychological Association) Style Zobel, A., Rohwedder, T., Bottcher, P. & (2022) Partial lung lobectomy with the Caiman® Seal & Cut device in a dog with spontaneous pneumothorax: case report. Open Veterinary Journal, 12 (6), 910-918. doi:10.5455/OVJ.2022.v12.i6.17 |