| Original Article | ||
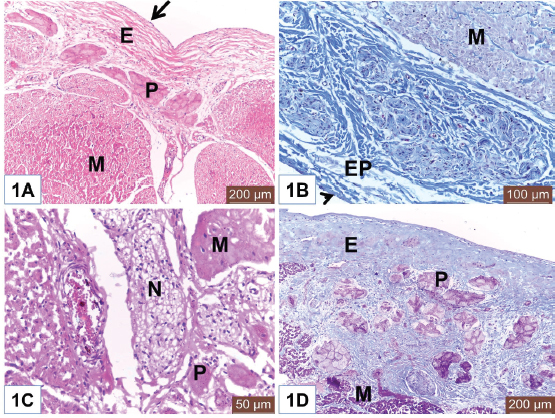
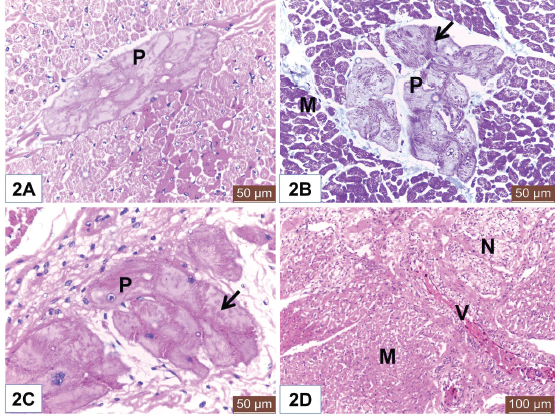
Open Vet J. 2019; 9(4): 281-286 doi: 10.4314/ovj.v9i4.1 Open Veterinary Journal, (2019), Vol. 9(4): 281–286 Original Research DOI: http://dx.doi.org/10.4314/ovj.v9i4.1 Histological study on the heart ventricle of Egyptian bovines (Bos aegyptiacus)Mahmoud Abdelghaffar Emam1* and Badia Abugherin21Histology Department, Faculty of Veterinary Medicine, Benha University, Moshtohor, Egypt 2Department of Anatomy, Histology and Embryology, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya *Corresponding Author: Mahmoud Abdelghaffar Emam. Histology Department, Faculty of Veterinary Medicine, Benha University, Moshtohor 13736, Egypt. Email: mahmoud.hussein [at] fvtm.bu.edu.eg Submitted: 18/05/2019 Accepted: 27/09/2019 Published: 20/10/2019 © 2019 Open Veterinary Journal AbstractBackground: The heart ventricles have thicker walls than atrium as they pump blood through blood vessels into all body organs. Aim: This study aimed to describe the histological changes of the heart ventricles in Egyptian bovine (Bos aegyptiacus) with special reference to Purkinje fibers. Methods: A total of 10 male Egyptian bovines of 1–10 years old were divided into three groups according to age; immature, mature, and adult animals. Results: The histological sections from all examined animals’ groups revealed three different layers of the wall of both right and left ventricles; endocardium, myocardium, and epicardium. The endocardium was lined with endothelium and filled with fibrous connective tissue. The endocardium of adult bovine was the thickest. Purkinje fibers appeared of pale cytoplasm with few myofibrils. They were present in the deep layer of the endocardium and in the myocardium. The size of Purkinje fibers and the amount of their myofibrils appeared to be increased with advanced age. Bundles of cardiac muscles were the main constituent of the myocardium. The myocardial bundles were separated by fine connective tissue in immature animals that showed an increased amount in the adult animals. The hypereosinophilic cardiac muscle cells were observed in the ventricles of both mature and adult animals suggesting hypercontraction during rigor mortis. An external layer of the ventricles was the epicardium which consisted of connective tissue and covered with mesothelium. Conclusion: Overall, this study revealed histological changes in the wall of the ventricle and Purkinje fibers of Egyptian bovines (B. aegyptiacus) in relation to age. Additionally, the hypereosinophilia of the cardiac muscle cells was recorded in the ventricles of mature and adult bovines. Keywords: Bovine, Heart, Histology, Purkinje fiber, Ventricle. IntroductionThe heart is a hollow organ that pumps blood through blood vessels into all body organs (Pathak and Kumar, 2010). In mammals, it consists of two atria and two ventricles. The ventricles have thicker walls than atria. The required physiological load on the ventricles to pump blood throughout the body is much greater than the load exerted by the atria to fill the ventricles. The histological architecture of ventricular myocardial fibers is critical for cardiac functions (Gouda et al., 2015). The anatomical features of the ventricles in most animal species were reported (Crick et al., 1998; Erdoǧan et al., 2014; Getty et al., 1975; Halıgür and Dursun, 2009; Panhwar et al., 2007). Also, the histological studies on the ventricles of various animals and human were done (Ates et al., 2017; Babiker, 2010; El-Khouly et al., 2001; Motabagani, 2006; Nabipur, 2002). The reports about the histological changes of the ventricles in Egyptian bovine (Bos aegyptiacus) were little especially those discussing the effect of age on the ventricular structure and the Purkinje fibers. However, Ryu et al. (2009) referred to Purkinje fibers in the sheep ventricles. Therefore, this study aimed to deal with the histological changes of the ventricles of Egyptian bovines (B. aegyptiacus) with the advancement of age. Also, a special reference to Purkinje fibers was studied. Materials and MethodsAnimalsThis study was conducted on the hearts of 10 male apparently healthy Egyptian bovines (B. aegyptiacus) collected from a local abattoir in Kalubyia Governorate, Egypt. The animals were 1–10 years old. The animals were divided according to their ages into three groups: immature (n=3), mature (n=3), and adult animals (n=4). Specimen collectionThe heart of each animal was washed with physiological saline and then small specimens from both right and left ventricles were fixed in 10% neutral buffered formalin for 72 hours. Tissue specimens were dehydrated in ascending grades of alcohol, cleared in xylene, and embedded in paraffin. Histological examinationParaffin blocks were cut into sections of 5 μm thick. The sections were stained with hematoxylin and eosin for general structure and Masson’s trichrome for the identification of collagen fibers as outlined by Bancroft and Gamble (2007). ResultsThe wall of the ventricles of all examined Egyptian bovines consisted of endocardium, myocardium, and epicardium (Fig. 1A and 1B). The endocardium is lined with endothelium (Fig. 1A) and filled with fibrous connective tissue. Also, it contains Purkinje fibers, blood vessels, and nerve fibers (Fig. 1C). The endocardium appeared the thickest in adult animals (Fig. 1D). Purkinje fibers are present in the deep part of the endocardium (Fig. 1A and 1D), between the myocardial bundles (Fig. 2A) as well as intramural fibers among the myocardial fibers (Fig. 2B). The cytoplasm of Purkinje fibers appears pale and contains few myofibrils that are thin fibers confined to the periphery of the cells. Additionally, the amount of myofibrils increased in the ventricles in adult animals (Fig. 2C).
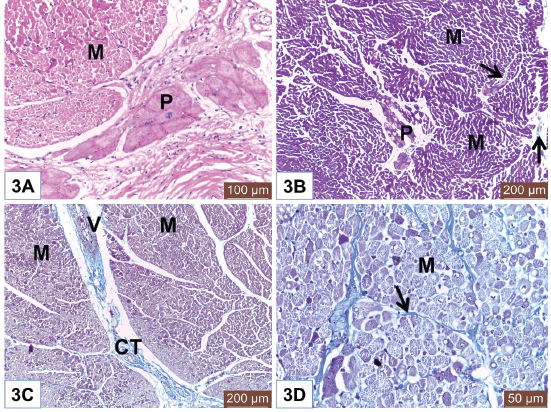
Fig. 1. (A) Photomicrograph of the ventricle from mature Egyptian bovine showed endocardium (E) and myocardium (M). Notice endothelial lining (Arrow) of the endocardium. Also, Purkinje fiber (P) in the endocardial layer. H&E. Scale bars=200 μm. (B) Photomicrograph of the ventricle from mature Egyptian bovine showed epicardium (EP) and myocardial bundles (M). Notice outer mesothelium (Arrowhead). Masson’s trichrome method. Scale bars=100 μm. (C) Photomicrograph of the ventricle from immature Egyptian bovine showed myocardium (M), Purkinje fiber (P), and nerve fibers (N). H&E. Scale bars=50 μm. (D) Photomicrograph of the ventricle from adult Egyptian bovine showed wide endocardium (E). Notice Purkinje fibers (P) and cardiac muscles of the myocardium (M). Masson’s trichrome method. Scale bars=200 μm. Myocardium consisted of bundles of cardiac muscles (Fig. 2D). In both mature and adult animals, some cardiac muscles show dark cytoplasm (hypereosinophilia) and others had lighter cytoplasm (Fig. 3A and 3D). Fine connective tissue (CT) among myocardial bundles is observed in immature animal (Fig. 3B); however, the amount of CT is increased and become the widest in adult animal (Fig. 3C). Fine collagen fibers are surrounding myocardial cells (Fig. 3D). Epicardium consists of CT and is covered externally with mesothelium. Nerve fibers can be seen within this layer (Fig. 2D). DiscussionThe heart is a muscular pump that contracts rhythmically to propel blood around the body via the blood vessels. It is well known that the wall of the heart consists of three layers; endocardium, myocardium, and epicardium. The ventricles of Egyptian bovine, like other animals, consisted of those three layers that were similar to reported by Ghazy et al. (2018). The endocardium is the innermost that was covered with a thin layer of endothelium underlying the subendothelial connective tissue layer. This finding was similar to Gálfiová et al. (2017). Deep to the endocardium, clusters of Purkinje fibers were distributed, as a part of the conducting system of the heart. This supports the finding of Ansari et al. (1999). Like finding of Koprla and Nemeséri (1984), our histological results showed few amount of myofibrils in a pale cytoplasm of the Purkinje fibers. This histological feature varied depending on ages (Forsgren, 1982); therefore, our results revealed a higher amount of myofibrils in the Purkinje fibers of older animals. The present study observed the close localization of the nerve fibers to Purkinje fibers that point to the nerve-Purkinje fiber relationship as reported by Jensen et al. (1978) and Crick et al. (1999) as they referred to the importance of the nervous control for cardiac performance in rhythm. Also, Kawano et al. (2003) demonstrated the presence of more autonomic nerves in the subendocardial area than in the subepicardial area of the human ventricle.
Fig. 2. (A) Photomicrograph of the ventricle from mature Egyptian bovine showed intramural Purkinje fiber (P) in between myocardial bundles. Notice hypereosinophilia of some cardiac muscle cells. H&E. Scale bars=50 μm. (B) Photomicrograph of the ventricle from adult Egyptian bovine showed intramural Purkinje fibers (P). Notice myofibrils (Arrow) and cardiac muscles of the myocardium (M). Masson’s trichrome method. Scale bars=200 μm. (C) Photomicrograph of the ventricle from mature Egyptian bovine showed Purkinje fiber (P) with pale cytoplasm and few myofibrils (Arrow). H&E. Scale bars=50 μm. (D) Photomicrograph of the ventricle from immature Egyptian bovine showed myocardium (M), blood vessels (V), and nerve fibers (N). H&E. Scale bars=100 μm. The intramural Purkinje fibers, in this study, were seen among the myocardium that confirms the ramification of Purkinje fibers within endocardium and myocardium as recorded by Ryu et al. (2009) in sheep and Ghonimi et al. (2014) in camel but, these fibers were not identified in the heart of human (Durrer et al., 1970; Oosthoek et al., 1993). The presence of intramural Purkinje fibers in the myocardium results in a more efficient excitation of the ventricles compared with the absence of intramural Purkinje fibers (Oosthoek et al., 1993; Tranum-Jensen et al., 1991) as they prevent intercyclic pre-excitation of the ventricular myocardium. Our observation revealed a relative increase in the thickness of the endocardium with the advanced age that in accordance with Centurion et al. (2003).
Fig. 3. (A) Photomicrograph of the ventricle from mature Egyptian bovine showed different forms of muscles in the myocardium (M). Notice Purkinje fiber (P) in the endocardial layer. H&E. Scale bars=100 μm. (B) Photomicrograph of the ventricle from immature Egyptian bovine showed fine connective tissue (Arrows) in between myocardial fibers (M). Notice Purkinje fibers (P) among myocardial bundles. Masson’s trichrome method. Scale bars=200 μm. (C) Photomicrograph of the ventricle from adult Egyptian bovine showed wide connective tissue (CT) in between myocardial bundles (M). Notice blood vessel (V). Masson’s trichrome method. Scale bars=200 μm. (D) Photomicrograph of the ventricle from adult Egyptian bovine showed fine collagen fibers (Arrow) surrounding myocardial cells (M). Notice hypereosinophilia of the cardiac muscle cells. Masson’s trichrome method. Scale bars=50 μm. For the myocardial layer of Egyptian bovine, the most predominant constituent was the cardiac muscles which arranged in bundles that were inconsistent with that reported by Ghonimi et al. (2104) in camel. It was noteworthy to mention that some cardiac muscle cells of the ventricles of both mature and adult Egyptian bovine showed hypereosinophilic cytoplasm while the others had light ones. This feature was recorded by Dettmeyer (2011) and Turillazzi et al. (2014) and owed this to the hypercontraction of the myocardial cells during rigor mortis. Also, Faa et al. (2012) observed hypereosinophilia of the cardiomyocytes due to cardiac hypoxia in newborn piglets. Another important notice was the increase in the amount of connective tissue among myocardial bundles in relation to the age. This was similar to the finding of Emam and Abo-Ahmed (2019) in the moderator bands of cattle. The external layer of the ventricle was the epicardium which consisted of connective tissue and covered externally with mesothelium. The identification of nerve fibers within the epicardium was similar to the finding of Crick et al. (1999). In conclusion, the histological structure of the ventricle of Egyptian bovine (B. aegyptiacus) was similar to other animals and human. However, the intramural Purkinje fiber was characteristic of bovine and absent in human. Both the thickness of endocardium and the amount of connective tissue among the myocardial bundles were relatively increased with the advanced age. Additionally, the hypereosinophilia of the cardiac muscle cells was characteristic for the ventricles of mature and adult bovines. Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionMAE collected and prepared the specimens for histological examination. Also, MAE captured the histological sections by Leica DM3000 microscope at the Histology Department, Faculty of Veterinary Medicine, Benha University, Egypt. Both MAE and BA interpreted the photomicrographs and shared the editing of the manuscript. MAE responded to the comments of reviewers. Ethical ApprovalSlaughtering of the animals was carried out in accordance with the Guidelines Animal Ethics Committee at the Faculty of Veterinary Medicine, Benha University. ReferencesAnsari, A., Ho, S.Y. and Anderso, R.H. 1999. Distribution of the Purkinje fibres in the sheep heart. Anat. Rec. 254, 92–97. Ates, S., Karakurum, E., Takci, L., Başak, F. and Kürtül, I. 2017. Morphology of the atrioventricular valves and related Intraventricular structures in the wild pig (Sus scrofa). Folia Morphol. 7(4), 650–679. Babiker, M.A.M. 2010. Morphological, Histochemical and Biochemical studies on the Heart of the Dromedary Camel (Camelus dromedarius). Master thesis, University of Bahr El-Ghazal, Sudan. Bancroft, J.D. and Gamble, M. 2007. Theory and Practice of Histological Techniques. 6th ed. London, UK: Churchill Livingstone. Centurion, O.A., Isomoto, S., Shimizu, A., Konoe, A., Kaibara, M., Hirata, T., Hano, O., Sakamoto, R., Hayano, M. and Yano, K. 2003. The effects of aging on atrial endocardial electrograms in patients with paroxysmal atrial fibrillation. Clin. Cardiol. 26, 435–438. Crick, S.J., Anderson, R.H., Ho, S.Y. and Sheppard, M.N. 1999. Localisation and quantitation of autonomic innervation in the porcine heart II: endocardium, myocardium and epicardium. J. Anat. 195(3), 359–373. Crick, S.J., Sheppard, M.N., Ho, S.Y., Gebstein, L. and Anderson, R.H. 1998. Anatomy of the pig heart: comparisons with normal human cardiac structure. J. Anat. 193(1), 105–119. Dettmeyer, R.B. 2011. Forensic histopathology. Berlin Heidelberg: Springer-Verlag, Germany. Durrer, D., Van Dam, R.T., Freud, G.E., Janse, M.J., Meijler, F.L. and Arzbaecher, R.C. 1970. Total excitation of the isolated human heart. Circulation 41, 899–912. El-Khouly, A.A., Abbas, T.A. and Moustafa, T. 2001. Myocardial dystrophy in camel calves in the United Arab Emirates. Emir. J. Agri. Sci. 13, 11–17. Emam, M.A. and Abo-Ahmed, A.I. 2019. Age-related histomorphometric and immunohistochemical changes of the moderator band in Egyptian Baladi cattle. Anat. Histol. Embryol. 48(2), 149–156. Erdoǧan, S., Lima, M. and Pérez, W. 2014. Inner ventricular structures and valves of the heart in white rhinoceros (Ceratotherium simum). Anat. Sci. Int. 89(1), 46–52. Faa, A., Iacovidou, N., Xanthos, T., Locci, A., Pampaloni, P., Aroni, F., Papalois, A., Faa, G. and Fanos,V. 2012. Hypoxia/reoxygenation-induced myocardial lesions in newborn piglets are related to interindividual variability and not to oxygen concentration. Clinics (Sao Paulo). 67(5), 503–508. Forsgren, S. 1982. Differentiation of Heart Purkinje Fibres An immuno- and enzyme histochemical and ultrastructural study. Umeâ University Medical Dissertations, Sweden, New Series No. 83. ISSN 0346-6612. Gálfiová, P., Polák, S., Mikušová, R., Andrea Gažová, A., Kosnáč, D., Barczi, T., Kyselovič, J. and Varga, I. 2017. The three-dimensional fine structure of the human heart: a scanning electron microscopic atlas for research and education. Biologia 72(12), 1521–1528. Getty, R., Sisson, S. and Grossman, J.D. 1975. The Anatomy of the Domestic Animals, Vol 2. 5th ed. New York: WB. Saunders Company. Ghazy, T., Dittfeld, C., Jannasch, A., Haase, M., Galli, R., Plötze, K., Waldow, T. and Matschke, K. 2018. Subclinical endocarditis might be a hidden trigger of early prosthetic valve calcification: a histological study. Heart Surg. Forum 21(4), E300–E304. Ghonimi, W., Abuel-Atta, A.A., Bareedy, M.H. and Balah, A. 2014. Left ventricles of the mature camel heart (Camelus dromedaries) with special references to the structure and distribution of the Purkinje cardiomyocytes: microanatomy. J. Cytol. Histol. 5, 247; doi:10.4172/2157-7099.1000247. Gouda, Z.A., Elewa, Y.H.A. and Selim, A.O. 2015. Histological architecture of cardiac myofibers composing the left ventricle of murine heart. J. Histol. Histopathol. 2(2); doi:10.7243/2055-091X-2-2. Halıgür, A. and Dursun, N. 2009. Morphological and morphometric investigation of the musculus papillaris and chordae tendineae of the donkey (Equus asinus L). J. Animal Vet. Adv. 8(4), 726–733. Jensen, H., Holtet, L. and Hoen, R. 1978. Nerve-Purkinje fiber relationship in the moderator band of bovine and caprine heart. Cell Tiss. Res. 188, 11–18. Kawano, H., Okada, R., Yano, K. 2003. Histological study on the distribution of autonomic nerves in the human heart. Heart Vessels. 18(1), 32–39. Koprla, E.C. and Nemeséri, L. 1984. Essential features of endocardial and myocardial morphology: SEM and TEM studies. Acta Physiol. Hung, 64(1), 65–79. Motabagani, M.A.B. 2006. Comparative anatomical, morphometric and histological studies of the tricuspid valve-complex in human and some mammalian hearts. J. Anat. Soc. 55(1), 1–23. Oosthoek, P.W., Viragh, S., Lamers, W.H. and Moorman, A.F.M. 1993. Immuno-histochemical delineation of the conduction system II: the atrioventricular node and purkinje fibres. Circ. Res. 73, 482–491. Nabipur, A. 2002. Anatomy and histology of the atrio-ventricular node of goats (Capra hircus). J. Appl. Anim. Res. 2, 255–260. Panhwar, S.P., Rind, M.M., Khan, H., Tufail, M., Rind, B. and Rind, R. 2007. Gross Anatomical Studies on Normal Heart of Buffalo (Bubalus bubalis). Int. J. Agri. Biol. 9(1), 162–166. Pathak, A. and Kumar, P. 2010. Anatomy and Biometry of Heart of Sheep (Ovis aries) of Jammu Region. Indian J. Vet. Anat. 22(1), 12–14. Ryu, S., Yamamoto, S., Andersen, C.R., Nakazawa, K., Miyake, F. and James, T.N. 2009. Intramural purkinje cell network of sheep ventricles as the terminal pathway of conduction system. Anat. Rec. 292, 12–22. Tranum-Jensen, J., Wilde, A.A.M., Vermeulen, J.T. and Janse, M.J. 1991. Morphology of electrophysiologically identified junctions between Purkinje fibers and ventricular muscle in rabbit and pig hearts. Circ. Res. 69, 429–437. Turillazzi, E., Di Paolo, M., Neri, M., Riezzo, I. and Fineschi, V. 2014. A theoretical timeline for myocardial infarction: immunohistochemical evaluation and western blot quantification for Interleukin-15 and Monocyte chemotactic protein-1 as very early markers. J. Transl. Med. 12, 188. | ||
| How to Cite this Article |
| Pubmed Style Emam MA, Abugherin B, . Histological Study on the Heart Ventricle in Egyptian bovine (Bos aegyptiacus) . Open Vet J. 2019; 9(4): 281-286. doi:10.4314/ovj.v9i4.1 Web Style Emam MA, Abugherin B, . Histological Study on the Heart Ventricle in Egyptian bovine (Bos aegyptiacus) . https://www.openveterinaryjournal.com/?mno=49420 [Access: July 27, 2024]. doi:10.4314/ovj.v9i4.1 AMA (American Medical Association) Style Emam MA, Abugherin B, . Histological Study on the Heart Ventricle in Egyptian bovine (Bos aegyptiacus) . Open Vet J. 2019; 9(4): 281-286. doi:10.4314/ovj.v9i4.1 Vancouver/ICMJE Style Emam MA, Abugherin B, . Histological Study on the Heart Ventricle in Egyptian bovine (Bos aegyptiacus) . Open Vet J. (2019), [cited July 27, 2024]; 9(4): 281-286. doi:10.4314/ovj.v9i4.1 Harvard Style Emam, M. A., Abugherin, B. & (2019) Histological Study on the Heart Ventricle in Egyptian bovine (Bos aegyptiacus) . Open Vet J, 9 (4), 281-286. doi:10.4314/ovj.v9i4.1 Turabian Style Emam, Mahmoud Abdelghaffar, Badia Abugherin, and . 2019. Histological Study on the Heart Ventricle in Egyptian bovine (Bos aegyptiacus) . Open Veterinary Journal, 9 (4), 281-286. doi:10.4314/ovj.v9i4.1 Chicago Style Emam, Mahmoud Abdelghaffar, Badia Abugherin, and . "Histological Study on the Heart Ventricle in Egyptian bovine (Bos aegyptiacus) ." Open Veterinary Journal 9 (2019), 281-286. doi:10.4314/ovj.v9i4.1 MLA (The Modern Language Association) Style Emam, Mahmoud Abdelghaffar, Badia Abugherin, and . "Histological Study on the Heart Ventricle in Egyptian bovine (Bos aegyptiacus) ." Open Veterinary Journal 9.4 (2019), 281-286. Print. doi:10.4314/ovj.v9i4.1 APA (American Psychological Association) Style Emam, M. A., Abugherin, B. & (2019) Histological Study on the Heart Ventricle in Egyptian bovine (Bos aegyptiacus) . Open Veterinary Journal, 9 (4), 281-286. doi:10.4314/ovj.v9i4.1 |