| Review Article | ||
Open Vet J. 2021; 11(2): 188-202 Open Veterinary Journal, (2021), Vol. 11(2): 188–202 Review Article Clinical application of mesenchymal stem cells therapy in musculoskeletal injuries in dogs—a review of the scientific literatureInês E. Dias1,2, Diogo F. Cardoso1, Carla S. Soares3,4, Luís C. Barros2,3, Carlos A. Viegas1,6,7,8, Pedro P. Carvalho2,5* and Isabel R. Dias1,6,7,81Department of Veterinary Sciences, ECAV, UTAD, Vila Real, Portugal 2CIVG—Vasco da Gama Research Center, Vasco da Gama University School, Av. José R. Sousa Fernandes, Campus Universitário, Coimbra, Portugal 3VetLamaçães Small Animal Clinic, Braga, Portugal 4CECAV – Animal and Veterinary Research Centre (CECAV), University of Trás-os-Montes and Alto Douro (UTAD), Vila Real, Portugal 5Vetherapy, 479. St, San Francisco, CA 94103, USA 63B’s Research Group, I3Bs – Research Institute on Biomaterials, Biodegradables and Biomimetics, University of Minho, Headquarters of the European Institute of Excellence on Tissue Engineering and Regenerative Medicine, AvePark, Parque de Ciência e Tecnologia, Zona Industrial da Gandra, Guimarães, Portugal 7ICVS/3B’s - Government Associate Laboratory, CITAB – Center for the Research and Technology of Agro-Environmental and Biological Sciences, University of Minho, 4805-017 Braga/Guimarães, Portugal 8CITAB – Center for the Research and Technology of Agro-Environmental and Biological Sciences, UTAD, Vila Real, Portugal *Corresponding Author: Pedro P. Carvalho. CIVG—Vasco da Gama Research Center, Vasco da Gama University School, Campus Universitário, Lordemão, Portugal. Email: pedro.carvalho [at] euvg.pt Submitted: 10/02/2021 Accepted: 25/03/2021 Published: 12/04/2021 © 2021 Open Veterinary Journal
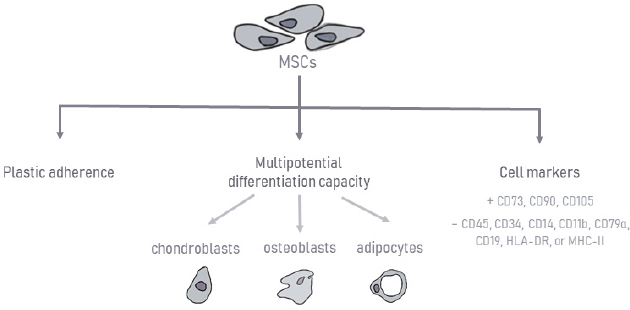
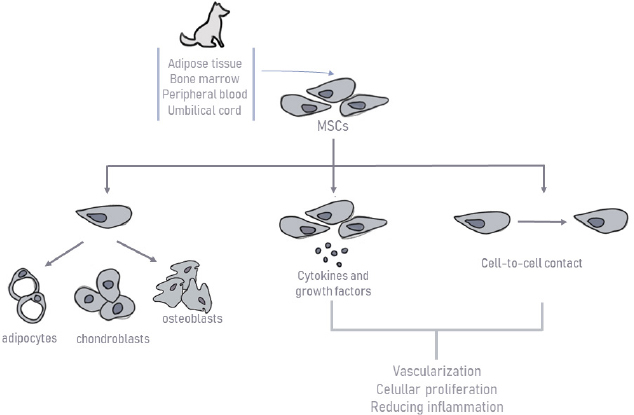
AbstractMesenchymal stem cells (MSCs) are multipotent, which is defined by their ability to self-renew while maintaining the capacity to differentiate into a certain number of cells, presumably from their own germinal layer. MSCs therapy is based on their anti-inflammatory, immunomodulatory (immunosuppressive), and regenerative potential. This review aims to provide a clinical overview of the MSCs potential as a therapeutic option for orthopedic diseases in dogs. A total of 25 clinical studies published in the scientific literature in the last 15 years on various diseases will be presented: semitendinosus myopathy, supraspinatus tendinopathy, cruciate ligament rupture, bone fractures and defects, and also osteoarthritis (OA). All articles involved in this study include only diseases that have naturally occurred in canine patients. MSCs therapy in the veterinary orthopedic field has great potential, especially for OA. All studies presented promising results. However, MSCs bone healing capacity did not reveal such favorable outcomes in the long term. Besides, most of these clinical studies did not include immunohistochemistry, immunofluorescence, and histopathology to confirm that MSCs have differentiated and incorporated into the injured tissues. This review summarizes the current knowledge of canine MSCs biology, immunology, and clinical application in canine orthopedic diseases. Despite the positive results in its use, there is still a lack of defined protocols, heterogeneous samples, and concomitant medications used with MSCs therapy compromising therapeutic effects. Further studies are needed in the hope of overcoming its limitation in upcoming trials. Keywords: Bone fractures, Cruciate ligament rupture, Osteoarthritis, Semitendinosus myopathy, Supraspinatus tendinopathy. IntroductionRegenerative medicine aims to restore the organism’s function and structure using its own biological mechanisms (Bogers, 2018; Dias et al., 2019). Over the last few years, new and promising biological therapies have emerged, contributing to the therapeutic management of orthopedic, inflammatory, and immune pathologies (Ogliari et al., 2014; Jiménez and Guerrero, 2017; Dias et al., 2019). The main focus of these cellular therapies is mesenchymal stem cells (MSCs), defined as multipotent cells of non-hematopoietic origin, with self-renewal capacity and present in connective tissues throughout the body (De Witte et al., 2016; Zhao et al., 2016; Dias et al., 2019). These cells were first reported in the literature by Friedenstein et al. (1968). Since then, the interest in their therapeutic potential has grown, leading to the emergence of innovative approaches (Friedenstein et al., 1968). One alternative source of cells for cartilage, muscle, tendon, or bone regeneration is MSCs, which are readily available and harvestable from different tissues, hold excellent proliferation ability, and can differentiate into various cell types in the body, such as osteoblast and chondroblasts (Freitag et al., 2016; Reissis et al., 2016). Whereas some tissues can recover their original or almost original strength and stiffness, other tissues, such as cartilage, have a poor healing capacity, leading to the necessity of searching for new alternative therapies (Carr and Canapp, 2016). Regenerative medicine has been used to stimulate healing in areas that have not responded to more conventional treatments, promoting injured tissue heal to their original or near-original condition (Carr and Canapp, 2016). Several authors have studied MSCs therapy alone or in combination with platelet-rich plasma (PRP), plasma-rich in growth factors (PRGF) or hyaluronic acid, and have reported of very satisfactory results in human and veterinary orthopedic diseases. Moreover, dogs can serve as experimental or translational clinical study models for tendons, muscle, cartilage, and bone repair in humans (Hoffman and Dow, 2016). They naturally develop diseases that share a close analogy with human conditions, such as osteoarthritis (OA), making them good preclinical models and providing valuable information for human therapies (Sasaki et al., 2019). This review aims to provide a clinical overview of the MSCs potential as a therapeutic option for orthopedic diseases in dogs, particularly in supraspinatus tendinopathy (ST), semitendinosus myopathy cruciate ligaments rupture, bone fractures and defects, and also OA. Canine MSCsMSCs biology and immunologyMSCs have a great self-renewal capacity while maintaining their multipotency (Zhao et al., 2016; Arnhold et al., 2019; Dias et al., 2019). Their high proliferative and self-renewal capacity allows obtaining the number of cells necessary for clinical application (Paterson et al., 2018; Arnhold et al., 2019). These cells can be isolated from the embryonic attachments, and in the adult individual, it can be isolated from a large variety of tissues, such as bone marrow (BM), umbilical cord, adipose tissue (AT), placenta, amnion, dental pulp, periosteum, among others (Kern et al., 2006; Klingemann et al., 2008; Webster et al., 2012). MSCs present remarkable pleiotropic properties, such as anti-apoptosis, angiogenesis, growth factor production, antifibrosis, and the ability to migrate toward injury sites through chemotaxis (Arnhold et al., 2019; Ayala-cuellar et al., 2019; Dias et al., 2019). In veterinary medicine, they are frequently obtained from AT and BM (Gonçalves et al., 2014; Bogers, 2018; Arnhold et al., 2019). This procedure usually requires surgical intervention, using general anesthesia and appropriate aseptic care. Although many of the alternatives are quite invasive, BM samples’ harvesting presents a higher technical difficulty (Carvalho et al., 2012; Sullivan et al., 2016). Some studies have even demonstrated a higher proliferation ability for MSCs derived from AT than BM (Kang et al., 2012; Russell et al., 2016; Sasaki et al., 2019). Other authors suggest that BM has a more significant osteogenic potential, making it a better choice (Alves et al., 2014). Further differences between cells’ sources are described depending on the donor’s age or the passage number of MSCs in terms of proliferation capacity (Volk et al., 2012; Sasaki et al., 2019). To date, there is no evidence to support the superiority of one source over another in terms of viability or efficiency of the derived stem cells. There is still no consensus between authors (Carr and Canapp, 2016). Stem cells’ therapy can be autologous or allogeneic, depending on whether the source of stem cells comes from the same patient or from a different patient of the same species. Recent researches with the application of xenogeneic stem cells from different species have revealed good and safe results (Tsai et al., 2014; Requicha et al., 2016; Daems et al., 2019). In the culture, MSCs have a predominantly fusiform shape and have plastic adhesion ability (Kolf et al., 2007). The expression of cell surface markers is one of the identification criteria for MSCs. To create a broader consensus on the universal characterization of MSCs, the International Society for Cellular Therapy formulated the minimal criteria for defining human MSCs (Dominici et al., 2006), as shown in Figure 1. These criteria include the ability to adhere to plastic under standardized culture conditions; cell surface expression (>95%) of CD73, CD90, and CD105, as well as the absence of hematopoietic stem cell markers: CD45, CD34, CD14 or CD11b, CD79α (<2% positive) or CD19, and human leukocyte antigen – DR isotype (HLA-DR); and the ability to differentiate, under standardized in vitro conditions, into osteoblasts, adipocytes, and chondroblasts. It is known that human MSC markers may not be fully compatible with those required for canine MSCs due to interspecies differences. The definitive expression of surface antigens by canine MSCs has not yet been recognized (Sasaki et al., 2019). MSCs mechanism of actionMSCs therapy is based on their anti-inflammatory, immunomodulatory, and immunosuppressive potential (Peroni and Borjesson, 2011; Dias et al., 2019), as shown in Figure 2. These cells can differentiate into the targeted cell type allowing the repair of the damaged area. Also, they have a tremendous immunomodulatory capacity through paracrine effects by secreting various molecules to adjacent cells (cytokines, growth factors, and microvesicles capable of carrying a cargo of proteins and other bioactive molecules) and by cell-to-cell contact, leading to vascularization, cellular proliferation in damaged tissues, and reducing inflammation (Dias et al., 2019; Torres-Torrillas et al., 2019). Although MSCs mechanism of action is not yet fully understood, it is known that they are capable of interacting with various types of immune cells, including T and B lymphocytes, natural killer (NK) cells, and macrophages, neutrophils, and monocytes (Peroni and Borjesson, 2011). MSCs act on the innate immune system through three different mechanisms. In the first one, with the presence of MSCs and their secretome [interleukin-6, prostaglandin E2 (PGE2), transforming growth factor β (TGF-β), and hepatocyte growth factor (HGF)], the M1 pro-inflammatory macrophages are converted into M2 anti-inflammatory macrophages. In the second one, MSCs inhibit monocytes’ development into dendritic cells, decreasing their immune response even more (Maggini et al., 2010; Quimby, 2019). Finally, through the production of immunomodulatory mediators [indoleamine 2,3-dioxygenase (IDO), TGF-β, PGE2] and by cell-to-cell contact, MSCs also inhibit the proliferation and cytotoxicity of NK cells (Maggini et al., 2010; Kim and Cho, 2015; Quimby, 2019).
Fig. 1. Characterization of mesenchymal stem cells.
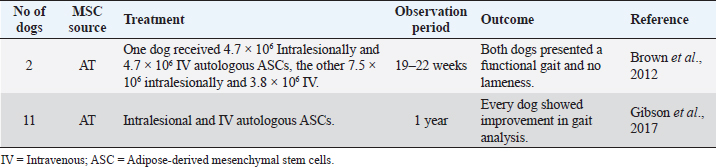
Fig. 2. Schematic representation of canine mesenchymal stem cells mechanism of action. The acquired immune system’s interaction occurs through the secretion of PGE2, HGF, hemoxygenase, nitric oxide (NO), IDO, cyclooxygenase, and by cell-to-cell contact. MSCs suppress the proliferation of T cells and modulate their response. They also inhibit B-cell proliferation through cell-to-cell contact and through an arrest in the G0/G1 phase of the cell cycle (Kim and Cho, 2015; Chow et al., 2017). Furthermore, MSCs show significant immunomodulatory capacity (Arnhold et al., 2019), making them especially useful for clinical application since their transplantation does not require the use of additional immunosuppressive therapy. This happens due to the lack of major histocompatibility complex class II (MHC-II) expression and costimulatory molecules, such as CD40, CD80, and CD86, since they escape T cells and NK receptors (Peroni and Borjesson, 2011; Arnhold et al., 2019; Quimby, 2019). A recent study suggests that canine MSCs are distinct from human MSCs in their immune suppressive pathways. For canine MSCs, T-cell suppression relies primarily on cyclooxygenase and TGF-β pathways, instead of NO or IDO-mediated pathways, which occur in human MSCs (Chow et al., 2017). The mechanisms of T-cell suppression were also investigated for BM and AT sources of MSCs in dogs. However, they realized that both sources were equivalent in suppressing T-cell activation by using different biochemical pathways. Adipose-derived mesenchymal stem cells (ASC) utilize TGF-β signaling pathways and adenosine signaling to suppress T-cell activation. On the contrary, BM-derived mesenchymal stem cells (BMSCs) use cyclooxygenase, TGF-β, and adenosine signaling pathways (Chow et al., 2017). Clinical application of canine MSCsSemitendinosus myopathySemitendinosus myopathy is an uncommon disease in canine patients, documented primarily on German Shepherd and Belgian Shepherd breeds. Although the muscle has some intrinsic regeneration capacity to repair damaged tissue, complete functional recovery of the muscle after a severe trauma remains a challenge (Milner et al., 2018; Torres-Torrillas et al., 2019). After a blunt muscle trauma, secondary fibrosis and muscle atrophy develop, affecting muscle fiber number, cross-sectional muscle area, and eventually, muscle force and function (Torres-Torrillas et al., 2019). Its exact etiology is unknown. However, it occurs with a higher incidence in working dogs (competition or work-protecting functions). Regular practice of intense and excessive exercise in the hamstring muscle group may culminate in secondary fibrosis and muscle contracture. Animals present limb lameness with shortened stride, external rotation of the hock, and internal rotation of the stifle. Semitendinosus myopathy has been treated with several surgical techniques (myotenectomy, myectomy, etc.) and non-surgical therapies (rest with a combination of anti-inflammatory medications), resulting in the recurrence of lameness in 2–9 months. Conventional therapy has been ineffective at preventing fibrous tissue formation, leading to the need to search for alternative therapies. MSCs are a promising option as recent studies suggest that ASCs promote myoblasts’ proliferation, explaining the regenerative capacity in vivo (Schaakxs et al., 2013; Forcales, 2015; Lee et al., 2015). Brown et al. (2012) reported two cases of working German Shepherds with acute semitendinosus myopathy of one or both hind limbs (Table 1), confirmed by surgeons’ evaluation and ultrasound. Both dogs were injected with autologous ASCs, one with a total of 4.7 × 106 cells intralesionally and 4.7 × 106 intravenously (IV); the other received 7.5 × 106 cells intralesionally (for both affected muscles) and 3.8 × 106 IV. Outcome measures for both dogs included owners and surgeons’ assessments as well as ultrasound control. The results were very encouraging. Both dogs returned to their previous training and occupations with a functional gait and no lameness (Brown et al., 2012). Gibson et al. (2017) conducted a study with 11 dogs with semitendinosus myopathy (Table 1), and eight of them were police dogs. All dogs were treated with ASCs and retrospectively evaluated. Autologous ASCs were obtained from falciform ligament. Some of them were injected intralesionally under ultrasound guidance and some IV through a previously placed IV catheter in each patient. One-year post-treatment, every dog showed improvement in gait analysis, and eight of them were already classified with a normal gait. Every police dog used in the study returned to their normal professional life (Gibson et al., 2017). Table 1. Preclinical trials carried out with MSCs in canine semitendinosus myopathy.
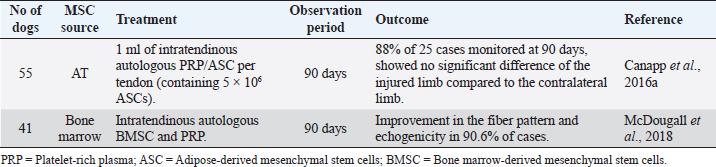
Intralesional injection of MSCs for the treatment of semitendinosus myopathy in dogs has proved to be effective in preventing the formation of fibrous tissue through the regenerative capacity they possess, demonstrated by clinical improvement and ultrasound control. Supraspinatus tendinopathy (ST)Tendon injuries represent a clinical challenge, as their natural healing process is slow, inefficient, and poorly responsive to treatments. The tendon has an inherently limited healing capacity, as it is a slightly cellular and poorly vascularized tissue (Schneider et al., 2018; Torres-Torrillas et al., 2019; Steinmann et al., 2020). A fibrous scar is formed after a traumatic event, producing significant dysfunction and joint movement inability, making it more susceptible to re-rupture (Schneider et al., 2018; Torres-Torrillas et al., 2019). ST is a common cause of forelimb lameness in dogs, especially those with sports functions. This condition is usually associated with excessive effort and repetitive movements that do not allow adequate tissue remodeling (Canapp et al., 2016a). Inflammation may play a vital role in triggering ST. However, it does not appear to be involved in the disease’s progression (Lafuente et al., 2009; Canapp et al., 2016a). Histologically speaking, ST shows minimal inflammation, hypocellularity, loss of collagen, increase of proteoglycan content, and lack of neovascularization. The damaged tendon shows discontinuous and disorganized tendon fibers, and occasional mineralization. In chronic cases, calcification may also occur at the site of insertion (Lafuente et al., 2009; Canapp et al., 2016a). Conservative therapy includes the use of non-steroidal anti-inflammatory drugs (NSAIDs), rest, rehabilitation therapy program with massages and hot therapy, acupuncture, ultrasound or laser therapy, and also focal shockwave therapy (Lafuente et al., 2009; Canapp et al., 2016a). Additionally, surgical management involves extracting the calcified biological material or even releasing the tendon at its origin (Lafuente et al., 2009; Canapp et al., 2016a). Despite these options, the percentage of therapeutic success, according to previous studies, is relatively low (Laitinen and Flo, 2000; Canapp et al., 2016a). Therapy with MSCs has been showing great results for ST in canine patients due to the secretion of cytokines and growth factors that reduce inflammation, inhibit programmed cell death in the cells inside the tissue, and recruit circulating stem cells to the affected area. Moreover, it increases the density of collagen fibers, improves tissue architecture, restores an almost standard tendon–bone interface, and improves biochemical strength (Canapp et al., 2016a). Two different studies, one with BMSC and the other with ASC, have been published to treat canine ST. The first study (Table 2) involved 55 dogs with ST that did not respond to conservative management with NSAIDs (61.8% with no response) and a rehabilitation program (45.5% refractory to rehabilitation). ASCs were obtained from the falciform ligament of each patient and blood samples for autologous PRP therapy. Every patient received 1 ml of PRP/ASC per tendon (containing 5 × 106 ASCs). After ultrasound-guided injection of PRP/ASC, objective gait analysis was available in 25 out of the 55 dogs at 90 days after treatment. On the 90th day, 88% of these 25 available cases showed no significant difference in the total pressure index percentage of the injured limb compared to the contralateral limb (Canapp et al., 2016a). The second study (Table 2) involved 41 dogs with ST treated with a combination of autologous BMSC and PRP, evaluating the ultrasound evolution with treatment. This study has proved a significant reduction in the cross-sectional area of the affected tendon. On the 45th day post-treatment, the comparison of the treated limb with the contralateral limb showed substantial improvements. On the 90th day post-treatment, in 90.6% of cases, the fiber pattern and echogenicity improved considerably. Furthermore, in 13.8% of the patients, the fiber pattern and echogenicity abnormalities were considered resolved (Mcdougall et al., 2018). Intratendinous injection of MSCs in addition to PRP therapy for ST treatment in canine patients has demonstrated auspicious clinical benefits (Tornero-Esteban et al., 2015; Canapp et al., 2016a; Mcdougall et al., 2018). This approach represents a minimally invasive treatment option, revealing positive sonographic results. This treatment combination appears to provide an adequate biologic effect, leading to tendon healing and improved function (Mcdougall et al., 2018). Table 2. Preclinical trials carried out with MSCs in canine ST.
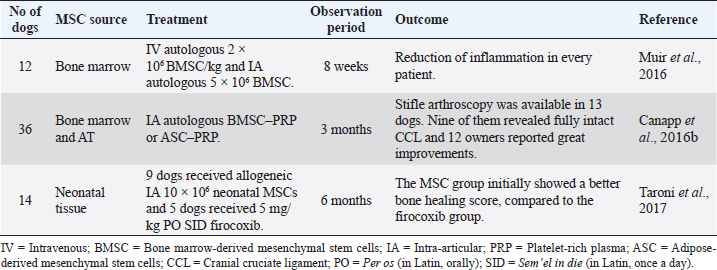
Cruciate ligament ruptureCranial cruciate ligament (CCL) rupture is one of the main causes of pelvic limb lameness in dogs. The etiology occurs primarily due to the progressive degeneration of unknown cause, leading to a partial or complete rupture (Ferreira et al., 2019). The insufficient ligament incites an internal rotation of the tibia and instability of the knee articulation, leading to lameness, pain, inflammation, and, in the long term, OA (Fitzpatrick et al., 2010; Ferreira et al., 2019). Conservative treatment, including rest, weight management, and anti-inflammatory drugs, is often used in small dogs (<15 kg body weight) and presents acceptable results (Ferreira et al., 2019). However, this approach is not consensual between authors. Some defend the surgical treatment as a first choice in all patients (Ferreira et al., 2019). There are many surgical techniques for CCL rupture, whereas nowadays extracapsular and osteotomy techniques are often used (Canapp et al., 2016b; Pinna et al., 2019). However, no specific surgical procedure has been established as the gold standard in veterinary medicine. Muir et al. (2016) conducted a study to investigate whether the use of MSCs could reduce systemic and stifle joint inflammatory response in 12 dogs affected by CCL rupture (Table 3). Admission criteria included the presence of a total unilateral rupture and a stable partial rupture in the contralateral knee. BM aspirates were obtained during surgical resolution of complete CCL rupture tibial plateau leveling osteotomy (TPLO). BMSC was cultured and expanded from this material. Subsequently, stem cells were injected IV (2 × 106 BMSC/kg) and intra-articularly (5 × 106 BMSC) into the contralateral stifle joint. The authors reported no adverse reactions. The results revealed a substantial improvement in the reduction of inflammation by assessing circulating T lymphocyte by flow cytometry, C-reactive protein (CPR), cytokine concentrations in serum, and synovial fluid by ELISA. Circulating CD8+ T-lymphocytes and CPR in serum and synovial fluid were lower after BMSC injection, proving the anti-inflammatory potential of these cells. They also stated that BMSC may have preventive effects, reducing the contralateral tendon’s risk of rupture (Muir et al., 2016). Canapp et al. (2016b) investigated the use of autologous BMSC–PRP or ASC–PRP combination for the treatment of early partial CCL rupture (≤50%) in 36 dogs (Table 3). Intra-articular (IA) injections of BMSC-PRP or ASC–PRP were carried out in the affected knee. Anti-inflammatory treatments (NSAIDs, corticosteroids, etc.) were discontinued 2 weeks before the injections. A rehabilitation program was recommended to all owners. Some therapies were banned due to the unknown effect on MSC/PRP. Objective gait analysis, diagnostic arthroscopy, and validated functional questionnaire were carried out on patients. At 90 days after treatment, stifle arthroscopy findings were available in 13 of the 36 dogs. In nine of the 13 dogs, arthroscopy revealed a fully intact CCL with marked neovascularization and a normal fiber pattern. One of the 13 dogs showed significant improvement and received an additional injection. In the remaining three dogs, TPLO was carried out due to a >50% CCL rupture. The questionnaires were completed by 12 owners, who reported a very good or excellent increase in their dogs’ quality of life. Eight among these 12 dogs were sport dogs. Seven of them have ultimately returned to their normal activity life (Canapp et al., 2016b). Taroni et al. (2017) compared post-TPLO treatment with NSAIDs and neonatal canine MSCs therapy (Table 3). Allogeneic neonatal canine MSCs were obtained from fetal attachments of healthy pregnant bitches. This pilot study involved 14 dogs with unilateral ruptures divided into two groups. The first group involved nine dogs administered intra-articularly with 10 × 106 MSCs and received an oral placebo for 30 consecutive days. The second group involved five dogs that received an IA culture medium and were prescribed 5 mg/kg per os (PO) sem’el in die (SID) firocoxib. One month after surgery, the dogs of the MSC group showed a better bone-healing score. At 1, 3, and 6 months after surgery, no significant differences were detected between the two groups for clinical signs and gait evaluation (Taroni et al., 2017). Table 3. Preclinical trials carried out with MSCs in canine cruciate ligament rupture.
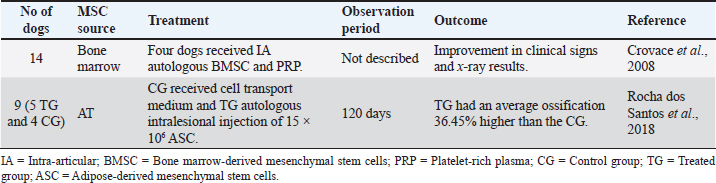
IA injection of MSCs for the treatment of CCL in dogs has demonstrated great clinical benefits and systemic and anti-inflammatory potential by decreasing CD8 lymphocytes c-reactive protein (CRP) and inflammatory cytokines in serum and synovial fluid in canine patients. Bone fractures and defectsBone fractures and segmental bone defects are important causes of patient morbidity and imply a sizeable economic burden. They are frequently secondary to trauma, post-tumor resection, or post-debridement infection (Morcos et al., 2015; Oryan et al., 2017; Torres-Torrillas et al., 2019). Conventional therapy includes autologous bone grafts and distraction osteogenesis. However, these approaches have some limitations, such as long periods of immobilization, muscular atrophy, and surgical complications such as infection, pain, or hemorrhage (Morcos et al., 2015; Oryan et al., 2017; Mousaei et al., 2019; Torres-Torrillas et al., 2019). MSCs have been adopted as an alternative therapy to promote accelerated bone repair with successful results. Crovace et al. (2008) evaluated the use of BM mononuclear cells and cultured BMSC to treat some bone defects in 14 dogs (Table 4). In four cases (one case of non-union of the tibia and three cases of Legg–Calvé–Perthés disease), a 3D scaffold was used in combination with BMSCs to treat these bone defects. Although the treated dogs showed outstanding clinical and X-ray results, this study compares different dogs in age, size, and breed, as well as various lesions or diseases (Crovace et al., 2008). Another study, by Rocha dos Santos et al. (2018), applied ASCs in osteotomy repair after tibial tuberosity advancement (TTA) surgical technique in dogs with CCL rupture (Table 4). Animals were divided into two groups: the treated group (TG) and the control group (CG). All of them were subjected to TTA. In the TG (five dogs), 15 × 106 of ASCs were implanted at the osteotomy site after TTA. The CG (four dogs) received only a cell transport medium at the osteotomy site. One month after treatment, dogs that received ASCs had an average ossification of 36.45% higher than the CG. However, over time, there were no statistical differences between groups (Rocha dos Santos et al., 2018). These studies have demonstrated that the use of MSCs can be a potential therapy to conventional treatment for fractures and bone defects. They speed up ossification allowing faster recovery and avoiding the consequences of having a patient being immobilized for an extended period. Osteoarthritis (OA)Canine OA is a degenerative disease of all joint tissues, resulting in the loss of articular cartilage, the release of inflammatory and regulatory cytokines, causing pain and lameness (Kalamegam et al., 2018). The cartilage has inadequate self-healing capacity due to its avascular nature. After an injury, fibrous tissue is formed with different functional properties of native hyaline cartilage, promoting joint degeneration (Bogers, 2018; Kalamegam et al., 2018; Torres-Torrillas et al., 2019). The OA pathophysiology is multifactorial, with a robust inflammatory component (Harman et al., 2016; Kalamegam et al., 2018). It is often secondary to anatomical abnormalities or injuries, causing joint instability. Although it can affect dogs of all breeds and sizes, it is more prevalent in large animals (Zeira et al., 2018). Currently, there is no cure for OA, and most treatment regimens focus on symptoms management and pain reduction. Conventional therapy is based on the long-term usage of NSAIDS drugs, physical therapy, diet, weight management, and dietary supplements (Sanderson et al., 2009). Alternatives such as acupuncture or shockwave therapy have also been studied (Sanderson et al., 2009). Nevertheless, it is still necessary to find treatments that relieve pain more effectively. Surgical therapy such as joint replacement is available for the hip and elbow joints; however, surgery is often expensive. Recently, MSCs have received increasing attention as promising tools for OA treatment. In vitro studies have demonstrated that ASCs can differentiate toward chondrocytes when they are cultured alone or in combination with growth factors (such as insulin-like growth factor (IGF-1 or TGF-β), making them interesting for OA therapy (Longobardi et al., 2006; Sun et al., 2018; Torres-Torrillas et al., 2019). Table 4. Preclinical trials carried out with MSCs in canine bone fractures and defects.
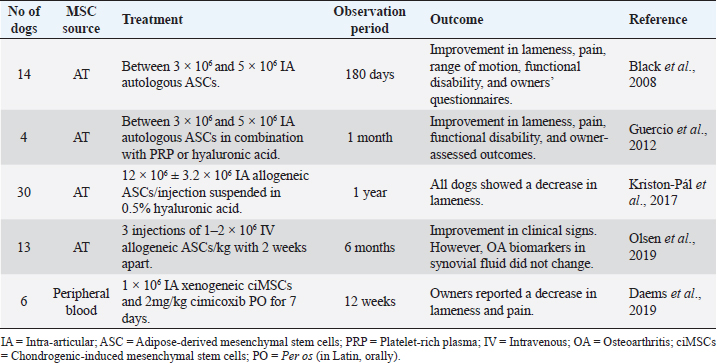
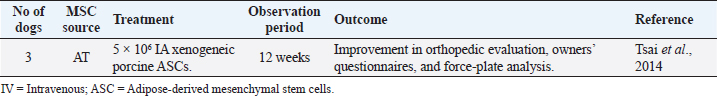
Elbow joint OASeveral studies have demonstrated remarkable results with intra-articular administration of ASCs for the treatment of canine elbow OA, with improvements in lameness, pain, range of motion, functional disability, and owners’ questionnaires (Black et al., 2008; Guercio et al., 2012; Kriston-pál et al., 2017), as described in Table 5. Some studies have associated stem cells treatment with PRP or hyaluronic acid, reaching similar good results. Kriston-Pál et al. (2017) have even demonstrated that in one of the dogs that underwent control arthroscopy, the cartilage had fully regenerated. Another study by Olsen et al. (2019) uses a different approach, choosing the IV route instead of the local administration (Table 5). This study included 13 dogs with elbow OA that received three IV injections (1–2 × 106 cells/kg body weight) of allogeneic ASCs, 2 weeks apart. No acute adverse effects were observed, and a significant improvement in clinical signs and owner’s perception was noted. However, synovial fluid OA biomarkers did not change after MSCs administration. Despite subjective outcomes showing good improvements, such as the dog’s clinical signs, objective outcome measures did not confirm similar results, such as reducing the OA biomarkers measurement in synovial fluid. Larger sample sizes and CGs are needed to interpret these findings (Olsen et al., 2019). Daems et al. (2019), unlike the previous studies, used xenogeneic peripheral blood-derived equine chondrogenically induced MSCs (ciMSCs) for the treatment of six dogs with elbow OA (Table 5). After orthopedic examination, pressure plate analysis, radiographs, and general clinical examinations, a placebo control (0.9% saline solution) was intra-articularly administered to all dogs. After 6 weeks, all tests were repeated, and equine ciMSCs were administered to the same joints. The six dogs received cimicoxib (2 mg/kg PO once daily for 7 days) in both intra-articular administrations. After another 6 weeks, dogs returned for a final follow-up, with no severe adverse reactions during the study. Two adverse events were observed (vomiting and diarrhea), both in the same dog, one after placebo treatment and the other after ciMSCs treatment, probably due to the concomitant NSAID administration. Although there were no significant differences in the orthopedic examination, radiographs, synovial fluid sampling, and pressure plate analysis between MSCs treatment compared to placebo treatment, all dogs showed a reduction in lameness and pain after MSCs therapy, according to the owner’s evaluation (Daems et al., 2019). Stifle joint OASimilar to Daems et al.’s (2019) study, another study conducted by Tsai et al. (2014) reported the use of xenogeneic MSCs to treat canine OA with success (Table 6). They used porcine ASCs to treat stifle joints OA in three dogs. A total of 5 × 106 ASCs were injected into each dog’s diseased joint and orthopedic controls, owners’ questionnaires, radiographs, and force-plate gait analysis were carried out. The authors reported no adverse effects of ASCs therapy. Two of the three dogs improved in the orthopedic evaluation. All dogs showed decreased pain and improvement in force-plate analysis. However, they found no radiographic changes before and after treatment (Tsai et al., 2014). Table 5. Preclinical trials carried out with MSCs in canine elbow OA.
Table 6. Preclinical trials carried out with MSCs in canine stifle OA.
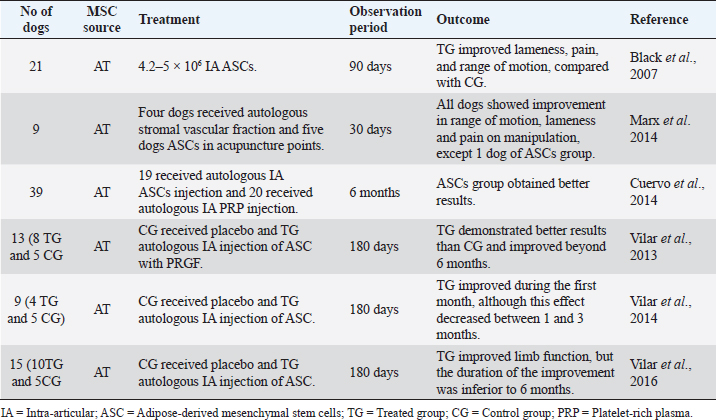
Table 7. Preclinical trials carried out with MSCs in canine hip OA.
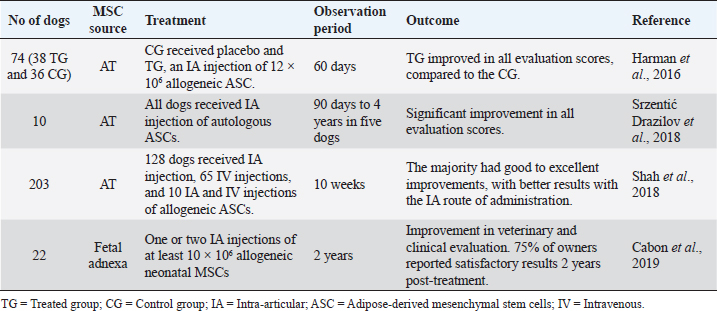
Hip joint OARegarding the OA of the hip joint, Black et al. (2007) conducted a study on 21 dogs with chronic hip OA (for at least more than 6 months), as described in Table 7. Dogs treated with intra-articular autologous ASCs had significantly improved scores for lameness, pain, and range of motion when compared with the CG (Black et al., 2007). Marx et al. (2014) used a totally different administration route than the previous studies, combining acupuncture with stem cells therapy (Table 7). This study evaluated the effect of autologous stromal vascular fraction, and allogeneic ASCs injected into acupuncture points in dogs with hip dysplasia. After 30 days of treatment, all dogs showed improvement in range of motion, lameness, and pain on manipulation, except one dog of the ASC group (Marx et al., 2014). Another study by Cuervo et al. (2014) compared the efficacy of autologous ASCs versus PRGF as a treatment for hip OA (Table 7). This study included 39 dogs with hip OA, divided into two groups: 19 dogs received an intra-articular injection of ASCs and 12 dogs received an intra-articular injection of PRGF. The results showed that ASCs and PRGF are a safe and effective therapeutic option, as they significantly reduced the dogs’ pain and improved physical function at 1, 3, and 6 months post-treatment. The ASCs group obtained better results at 6 months post-treatment. In all cases, owners reported positive results to therapy (Cuervo et al., 2014). The majority of the studies used subjective methods to evaluate therapy improvement, such as lameness, pain, and range of motion scores, and owners’ questionnaires. Three studies have used a much more objective method with gait analysis using a force platform to demonstrate the efficacy of a single IA injection of ASCs (Table 7). In the first study, Vilar et al. (2013) showed how the effect of the association of ASCs with PRGF was prolonged beyond 6 months. On the contrary, in the second study, Vilar et al. (2014) showed that dogs treated with ASCs alone seemed to improve during the first month after treatment with reductions in pain and lameness. However, this effect decreased progressively between 1 and 3 months (Vilar et al., 2014). In 2016, these authors compared pain scales with the use of a force platform with the same animals during 6 months after ASCs therapy. They realized that using pain assessment scales to measure lameness associated with OA did not show great accuracy and agreement compared to the quantitative force platform gait analysis (Vilar et al., 2016). Multiple joint OASome studies have been published regarding the treatment with ASCs in canine OA of multiple joints: elbow, stifle, hip, or shoulder (Table 8). These studies have proved the effectiveness and safety of ASCs intra-articular injection in canine OA (Harman et al., 2016; Srzentić Dražilov et al., 2018). Shah et al. (2018) used allogeneic ASCs in 203 dogs diagnosed with OA and other joint defects (Table 8). These dogs were divided into three groups: 128 dogs received IA injections of allogeneic ASCs, 65 dogs received IV injections of allogeneic ASCs, and ten dogs received intra-articular and IV injections of allogeneic ASCs. In the first group (128 dogs), 114 dogs were reported to have good to excellent improvement. In the second group (65 dogs), 25 dogs showed remarkable improvement and 25 of the animals good improvement. In the third group (ten dogs), due to the small sample size, statistical analysis could not be carried out. However, nine of the ten dogs were reported to have either good or excellent improvement. Of the 203 dogs, only one showed worsening of the symptoms at the end of the 10-week study. This study supports the safety and efficacy of allogeneic ASCs, with promising results using the intra-articular route of administration (Shah et al., 2018). Cabon et al. (2019) studied the use of allogeneic neonatal MSCs for the treatment of 22 dogs with OA in one or more joints (Table 8). All dogs discontinued anti-inflammatory therapy at least 1 week before treatment. Every dog received an intra-articular injection of at least 10 × 106 viable neonatal MSCs (obtained from fetal attachments). Clinical evaluation showed significant improvements up to 6 months after MSCs administration. Eight dogs were re-injected 6 months after the first injection, offering clinical benefits for up to 1 year. The owners’ evaluation was obtained up to 2 years after treatment, with 75% reporting satisfactory results. This study also measured the humoral response against cellular therapy by flow cytometric cross-match analysis. The absence of alloantibodies after one or two IA injections of neonatal allogeneic MSC suggests that this therapeutic approach is well tolerated and can be repeated if necessary (Cabon et al., 2019). DiscussionThe aim of this review was to provide a clinical overview of the therapeutic potential of MSCs for orthopedic diseases in dogs. A total of 25 studies published in the last 15 years were reviewed, with applications for musculoskeletal systems: muscle, ligament, tendon, and bone, to evaluate these therapies in the veterinary orthopedic field. All studies have shown positive results when using MSCs therapy. However, MSCs bone-healing capacity did not reveal such promising outcomes in the long term (Rocha dos Santos et al., 2018). MSCs immunomodulatory properties, anti-inflammatory potential, and the ability to differentiate toward adipocytes, osteocytes, and chondrocytes make them a unique cell type capable of repairing injured tissues. These cells are a safer alternative to embryonic or induced pluripotent stem cells, which can present a risk of tumorigenesis if complete reprogramming is not achieved (Gibson et al., 2017). Nevertheless, there are still concerns about the source of cells, administration route, and additional therapy, such as PRP or PRGF. Table 8. Preclinical trials carried out with MSCs in canine multiple joints OA.
Regarding MSCs source, the most commonly used in veterinary medicine are AT and BM. None of these studies has demonstrated the superiority of one cell source over another. However, stem cells derived from AT were the most used by the authors, probably due to their easier extraction, namely in elective ovariohysterectomy. MSCs therapy can be autologous/allogeneic or xenogeneic depending on whether stem cells are obtained, respectively, from the same animal, same species donor or from different species. Two studies regarding canine OA treatment have used xenogeneic MSCs from porcine or horses, reporting excellent results with no severe adverse effects in none of the dogs (Tsai et al., 2014; Daems et al., 2019). Regarding the routes of administration, the one that is preferentially selected is the injection of MSCs directly at the injury site. Experimental studies support the greater effectiveness of the intralesional route of administration when comparing to others. Intra-articular injection of ASCs is the most commonly reported route to deliver cells into the damaged cartilage; however, in most cases, it requires anesthesia or sedation, and synovial fluid from arthritic joints is reported to be cytotoxic to cultured MSCs (Kiefer et al., 2015). Therefore, new protocols with the intravenous route have been developed. The intravenous administration of MSCs promotes a better interaction with the immune system, which may lead to systemic anti-inflammatory effects and reduce pain at multiple sites. However, despite the attraction of MSCs to the injured areas by the intravenous route, its effectiveness was reported to be low in some studies (Eggenhofer et al., 2014; De Becker and Van Riet, 2016). Shah et al. (2018) compared ASC therapy by different administration routes in dogs with OA. They realized that the intra-articular way had better improvements than the intravenous one. Olsen et al. (2019) studied the effect of ASCs by intravenous route in dogs with OA. Despite the improvement in clinical outcome, subjective outcome measures (such as OA biomarkers in synovial fluid) did not confirm similar progress. These results reveal the need for larger samples sizes and CGs to interpret the outcomes and more research before intravenous MSCs becomes a therapeutic option for dogs with OA. It is necessary to increase our knowledge of the MSC migration process to improve it. According to previous studies, it has been reported that treatment with ASC or BMSC in combination with PRP or PRGF improves clinical signs, reducing lameness and providing notable recovery of previous limited sports activities (Cuervo et al., 2014). The effect of PRP is due to the behavior of the platelet concentrate, acting as a scaffold which, through the release of growth factors (such as TGF-β, fibroblast growth factor and IGF-1), promotes the stimulation of chondrogenesis, increases hyaluronic acid production, promotes angiogenesis, and differentiation of the existing cells in the treated area (Cuervo et al., 2014; Upchurch et al., 2016). The PRGF is considered a better choice by some authors due to the optimal concentration of platelets and absence of white blood cells (Nishiyama et al., 2016). Cuervo et al. (2014) demonstrated that a single IA injection of ASCs is significantly more effective than one IA injection of PRGF. Therapy with PRGF alone showed similar improvements as ASC in the first month post-treatment. However, better results were observed in the long term in patients with ASCs. Other studies had great results in the combination therapy: MSCs with PRP or PRGF (Cuervo et al., 2014; Canapp et al., 2016c; Mcdougall et al., 2018). MSCs therapy with specific concomitant medication proved detrimental to treatment, particularly with NSAIDs, generally used for these conditions. Shah et al. (2018) studied allogeneic ASC therapy in dogs with OA, reporting only one case that did not respond to the treatment and had shown worsening of the condition. This dog was the only one receiving NSAIDs therapy at the same time as MSCs therapy. Most studies did not include immunohistochemistry, immunofluorescence, and histopathology to confirm that MSCs have differentiated and/or incorporated into the injured tissues. Further studies will be needed to definitely prove stem cell differentiation and engraftment. However, their initial cell-to-cell contact and cytokine release effect seems to be the most significant therapeutic impact of these cells. ConclusionMSCs have been shown to have an excellent potential for orthopedic therapy in dogs, especially for OA, where all studies presented promising results. This therapy has shown no evident adverse effects, even with its allogeneic administration, making them a safe and promising therapeutic option. The main obstacles to this therapy are the inconsistency of protocols applied to date, the small samples used for clinical trials, patient variability, and the follow-up time of cases after therapy. Besides, it should be noted that cell therapies in veterinary patients are not strictly supervised by regulatory agencies in most countries. There is an urgent need for health agencies, national and across borders, such as European Medicines Agency (EMA), to define and regulate the standardization and criteria for the safe and efficient application of these therapeutic approaches. Future trials should have more homogeneous and larger samples, predefined standardized stem cell protocols for treatment groups, presence of CGs, and continue data collection for more extended periods (more than 6 months) for appropriate long-term conclusions. Currently, therapy based on MSCs requires, more than ever before, a thorough analysis and consideration in the hope of overcoming its limitation in upcoming trials. AcknowledgmentsThis research was funded by National Funds by FCT (Portuguese Foundation for Science and Technology) under the project UIDB/AGR/04033/2020. Conflict of interestP.P.C. declares to be the founder and CEO of Vetherapy, a biotech company focused on regenerative products. P.P.C. also declares that his company had no influence or involvement with any of the results in analysis in this study. The remaining authors declare that they have no competing interests. Authors’ contributionIED, DFC, and PPC were responsible for the bibliographic research and redaction of the manuscript; CSS, LCB, CAV, and IRD were responsible for the tables, clinical data analysis, and critical review of the manuscript; IED was responsible for the adaption of the tables and text; PPC and IRD were responsible for the overall supervision and critical review of the manuscript. ReferencesAlves, E.G.L., Serakides, R., Boeloni, J.N., Rosado, I.R., Ocarino, N.M., Oliveira, H.P., Goés, A. and Rezende, C. 2014. Comparison of the osteogenic potential of mesenchymal stem cells from the bone marrow and adipose tissue of young dogs. BMC Vet. Res. 10(1), 1–9. Arnhold, S., Elashry, M.I., Klymiuk, M.C. and Wenisch, S. 2019. Biological macromolecules and mesenchymal stem cells: basic research for regenerative therapies in veterinary medicine. Int. J. Biol. Macromol. 123, 889–899. Ayala-cuellar, A.P., Kang, J., Jeung, E. and Choi, K. 2019. Roles of mesenchymal stem cells in tissue regeneration and immunomodulation. Biomol. Ther. 27(1), 25–33. Black, L.L., Gaynor, J., Adams, C., Dhupa, S., Sams, A., Taylor, R., Harman, S., Gingerich, D.A. and Harman, R. 2008. Effect of intraarticular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. Vet. Ther. 9(3), 192–200. Black, L.L., Gaynor, J., Gahring, D., Adams, C., Aron, D., Harman, S., Gingerich, D.A. and Harman, R. 2007. Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: a randomized, double-blinded, multicenter, controlled trial. Vet. Ther. 8(4), 272–284. Bogers, S.H. 2018. Cell-based therapies for joint disease in veterinary medicine: what we have learned and what we need to know. Front. Vet. Sci. 5, 1–17. Brown, S.G., Harman, R.J. and Black, L.L. 2012. Adipose-derived stem cell therapy for severe muscle tears in working German shepherds: two case reports. Stem Cell Discov. 2(2), 41–44. Cabon, Q., Febre, M., Gomez, N., Cachon, T., Pillard, P., Carozzo, C., Saulnier, N., Robert, C., Livet, V., Rakic, R., Plantier, N., Saas, P., Maddens, S. and Viguier, E. 2019. Long-term safety and efficacy of single or repeated intra-articular injection of allogeneic neonatal mesenchymal stromal cells for managing pain and lameness in moderate to severe canine osteoarthritis without anti-inflammatory pharmacological support: Pi. Front. Vet. Sci. 6, 1–14. Canapp, S.O., Canapp, D.A., Carr, B.J., Cox, C. and Barrett, J.G. 2016b. Supraspinatus tendinopathy in 327 dogs : a retrospective study. Vet. Evid. 1(3), 1–14. Canapp, S.O., Canapp, D.A., Ibrahim, V., Carr, B.J., Cox, C. and Barrett, J.G. 2016a. The use of adipose-derived progenitor cells and platelet-rich plasma combination for the treatment of supraspinatus tendinopathy in 55 dogs: a retrospective study. Front. Vet. Sci. 3, 1–11. Canapp, S.O., Leasure, C.S., Cox, C., Ibrahim, V. and Carr, B.J. 2016c. Partial cranial cruciate ligament tears treated with stem cell and platelet-rich plasma combination therapy in 36 dogs: a retrospective study. Front. Vet. Sci. 3, 1–9. Carr, B.J. and Canapp, S.O. 2016. Regenerative medicine for soft tissue injury and osteoarthritis. Today’s Vet. Paract. 53, 53–60. Carvalho, P.P., Hamel, K.M., Duarte, R., King, A.G., Haque, M., Dietrich, M.A., Wu, X., Shah, F., Burk, D., Reis, R.L., Rood, J., Zhang, P., Lopez, M., Gimble, J.M. and Dasa, V. 2012. Comparison of infrapatellar and subcutaneous adipose tissue stromal vascular fraction and stromal/stem cells in osteoarthritic subjects. J. Tissue Eng. Regen. Med. 1, 1–6. Chow, L., Johnson, V., Coy, J., Regan, D. and Dow, S. 2017. Mechanisms of immune suppression utilized by canine adipose and bone marrow-derived mesenchymal stem cells. Stem Cells Dev. 26(5), 374–389. Crovace, A., Favia, A., Lacitignola, L., Di Comite, M.S., Staffieri, F. and Francioso, E. 2008. Use of autologous bone marrow mononuclear cells and cultured bone marrow stromal cells in dogs with orthopaedic lesions. Vet. Res. Commun. 32(1), 39–44. Cuervo, B., Rubio, M., Sopena, J., Carrillo, J.M., Cugat, R., Dominguez, J.M., Vilar, J., Morales, M., Cugat, R. and Carrillo, J.M. 2014. Hip osteoarthritis in dogs: a randomized study using mesenchymal stem cells from adipose tissue and plasma rich in growth factors. Int. J. Mol. Sci. 15(8), 13437–13460. Daems, R., Hecke, L., Van Schwarzkopf, I, Depuydt, E., Broeckx, S.Y., David, M., Beerts, C., Vandekerckhove, P. and Spaas, J.H. 2019. A feasibility study on the use of equine chondrogenic induced mesenchymal stem cells as a treatment for natural occurring osteoarthritis in dogs. Stem Cells Int. 6, 1–11. De Becker, A. and Van Riet, I. 2016. Homing and migration of mesenchymal stromal cells: how to improve the efficacy of cell therapy? World J. Stem Cells 8(3), 73–87. De Witte, S.F.H., Franquesa, M., Baan, C.C. and Hoogduijn, M.J. 2016. Toward development of imesenchymal stem cells for immunomodulatory therapy. Front. Immunol. 6, 1–9. Dias, I.E., Pinto, P.O., Barros, L.C., Viegas, C.A., Dias, I.R. and Carvalho, P.P. 2019. Mesenchymal stem cells therapy in companion animals: useful for immune-mediated diseases? BMC Vet. Res. 15, 1–14. Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., Krause, D., Deans, R., Keating, A., Prockop, D. and Horwitz, E. 2006. Minimal criteria for defining multipotent mesenchymal stromal cells. International Society for Cellular Therapy position statement. Cytotherapy 8(4), 315–317. Eggenhofer, E., Luk, F., Dahlke, M.H. and Hoogduijn, M.J. 2014. The life and fate of mesenchymal stem cells. Front. Immunol. 5, 1–6. Ferreira, A.J.A., Bom, R.M. and Tavares, S.O. 2019. Tibial tuberosity advancement technique in small breed dogs: study of 30 consecutive dogs (35 stifles). J. Small Anim. Pract. 60(5), 305–312. Fitzpatrick, N., Johnson, J., Hayashi, K., Girling, S. and Yeadon, R. 2010. Tibial plateau leveling and medial opening crescentic osteotomy for treatment of cranial cruciate ligament rupture in dogs with tibia vara. Vet. Surg. 39(4), 444–453. Forcales, S.V. 2015. Potential of adipose-derived stem cells in muscular regenerative therapies. Front. Aging Neurosci. 7, 1–12. Freitag, J., Bates, D., Boyd, R., Shah, K., Barnard, A., Huguenin, L. and Tenen, A. 2016 Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy - a review. BMC Musculoskelet. Disord. 17(1), 1–13. Friedenstein, A., Petrakova, K., Kurolesova, A. and Frovola, G. 1968. Heterotopic transplants of bone marrow. Transplantation 6(2), 230–247. Gibson, M.A., Brown, S.G. and Brown, N.O. 2017. Semitendinosus myopathy and treatment with adipose-derived stem cells in working German shepherd police dogs. Can. Vet. J. 58(4), 241–246. Gonçalves, N.N., Ambrósio, C.E. and Piedrahita, J.A. 2014. Stem cells and regenerative medicine in domestic and companion animals: a multispecies perspective. Reprod. Domest. Anim. 49(4), 2–10. Guercio, A., Di Marco, P., Casella, S., Cannella, V., Russotto, L., Purpari, G., Bella, S. and Piccione, G. 2012. Production of canine mesenchymal stem cells from adipose tissue and their application in dogs with chronic osteoarthritis of the humeroradial joints. Cell Biol. Int. 36(2), 189–194. Harman, R., Carlson, K., Gaynor, J., Gustafson, S., Dhupa, S., Clement, K., Hoelzler, M., McCarthy, T., Schwartz, P. and Adams, C. 2016. A prospective, randomized, masked, and placebo-controlled efficacy study of intraarticular allogeneic adipose stem cells for the treatment of osteoarthritis in dogs. Front. Vet. Sci. 3, 1–10. Hoffman, A. and Dow, S. 2016. Concise review: stem cell trials using companion animal disease models. Stem Cells 34, 1709–1729. Jiménez, A. and Guerrero, F. 2017. Células madre mesenquimales como nueva terapia en dermatología: conceptos básicos. Rev. Clín. Dermatol. Vet. 9, 8–18. Kalamegam, G., Memic, A., Budd, E., Abbas, M. and Mobasheri, A. 2018. A comprehensive review of stem cells for cartilage regeneration in osteoarthritis. Adv. Exp. Med. Biol. 1089, 23–36. Kang, B.J., Ryu, H.H., Park, S.S., Koyama, Y., Kikuchi, M., Woo, H.M., Kim, W.H. and Kweon, O.K. 2012. Comparing the osteogenic potential of canine mesenchymal stem cells derived from adipose tissues, bone marrow, umbilical cord blood, and Wharton’s jelly for treating bone defects. J. Vet. Sci. 13(3), 299–310. Kern, S., Eichler, H., Stoeve, J., Klüter, H. and Bieback, K. 2006. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24(5), 1294–1301. Kiefer, K.M., O’Brien, T.D., Pluhar, E.G. and Conzemius, M. 2015. Canine adipose-derived stromal cell viability following exposure to synovial fluid from osteoarthritic joints. Vet. Rec. Open 2(1), 1–7. Kim, N. and Cho, S.G. 2015. New strategies for overcoming limitations of mesenchymal stem cell-based immune modulation. Int. J. Stem Cells 8(1), 54–68. Klingemann, H., Matzilevich, D. and Marchand, J. 2008. Mesenchymal stem cells - sources and clinical applications. Transfus. Med. Hemotherapy 35(4), 272–277. Kolf, C.M., Cho, E. and Tuan, R.S. 2007. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res. Ther. 9(1), 1–10. Kriston-pál, É., Czibula, Á., Gyuris, Z., Balka, G., Seregi, A. and Sükösd, F. 2017. Characterization and therapeutic application of canine adipose mesenchymal stem cells to treat elbow osteoarthritis. Can. J. Vet. Res. 81, 73–78. Lafuente, P.M., Fransson, B.A., Lincoln, J.D., Martinez, S.A., Gavin, P.R., Lahmers, K.K. and Gay, J.M. 2009. Surgical treatment of mineralized and nonmineralized supraspinatus tendinopathy in twenty-four dogs. Vet. Surg. 38, 380–387. Laitinen, O.M. and Flo, G.L. 2000. Mineralization of the supraspinatus tendon in dogs: a long-term follow-up. J. Am. Anim. Hosp. Assoc. 36(3), 262–267. Lee, E., Kim, A., Lee, E., Park, J., Lee, M., Hwang, M., Kim, C., Kim, S. and Jeong, K. 2015. Therapeutic effects of mouse adipose-derived stem cells and losartan in the skeletal muscle of injured Mdx mice. Cell Transplant. 24, 939–953. Longobardi, L., O’Rear, L., Aakula, S., Johnstone, B., Shimer, K., Chytil, A., Horton, W.A., Moses, H.L. and Spagnoli, A. 2006. Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-β signaling. J. Bone Miner. Res. 21(4), 626–636. Maggini, J., Mirkin, G., Bognanni, I., Holmberg, J., Piazzón, I.M., Nepomnaschy, I., Costa, H., Cañones, C., Raiden, S., Vermeulen, M. and Geffner, J.R. 2010. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 5(2), 1–13. Marx, C., Silveira, M.D., Selbach, I., Silva, A.S., Braga, L.M., Camassola, M. and Nardi, N.B. 2014. Acupoint injection of autologous stromal vascular fraction and allogeneic adipose-derived stem cells to treat hip dysplasia in dogs. Stem Cells Int. 1, 1–6. Mcdougall, R.A., Canapp, S.O. and Canapp, D.A. 2018. Ultrasonographic findings in 41 dogs Treated with bone marrow aspirate concentrate and platelet- rich plasma for a supraspinatus tendinopathy: a retrospective study. Front. Vet. Sci. 5, 1–10. Milner, D.J., Bionaz, M., Cameron, J.A., Wheeler, M.B. and Wheeler, M.B. 2018. Myogenic potential of mesenchymal stem cells isolated from porcine adipose tissue. Cell Tissue Res. 372(3), 507–522. Morcos, M.W., Al-Jallad, H. and Hamdy, R. 2015. Comprehensive review of adipose stem cells and their implication in distraction osteogenesis and bone regeneration. Biomed. Res. Int. 1, 1–20. Mousaei, G.M., Matin, M.M., Kazemi, M.H., Naderi-Meshkin, H., Moradi, A., Rajabioun, M., Alipour, F., Ghasemi, S., Zare, M., Mirahmadi, M., Bidkhori, H.R. and Bahrami, A.R. 2019. Application of mesenchymal stem cells to enhance non-union bone fracture healing. J. Biomed. Mater. Res. A. 107(2), 301–311. Muir, P., Hans, E.C., Racette, M., Volstad, N., Sample, S.J., Heaton, C., Holzman, G., Schaefer, S.L., Bloom, D.D., Bleedom, J.A., Hao, Z., Amene, E., Suresh, M. and Hematti, P. 2016. Autologous bone marrow-derived mesenchymal stem cells modulate molecular markers of inflammation in dogs with cruciate ligament rupture. PLoS One. 11(8), 1–21. Nishiyama, K., Okudera, T., Watanabe, T., Isobe, K., Suzuki, M., Masuki, H., Okudera, H., Uematsu, K., Nakata, K. and Kawase, T. 2016. Basic characteristics of plasma rich in growth factors (PRGF): blood cell components and biological effects. Clin. Exp. Dent. Res. 2(2), 96–103. Ogliari, K.S., Marinowic, D., Brum, D.E. and Loth, F. 2014. Stem cells in dermatology. An. Bras. Dermatol. 89(2), 286–291. Olsen, A., Johnson, V., Webb, T., Santangelo, K.S., Dow, S. and Duerr, F.M. 2019. Evaluation of intravenously delivered allogeneic mesenchymal stem cells for treatment of elbow osteoarthritis in dogs: a pilot study. Vet. Comp. Orthop. Traumatol. 32(3), 173–181. Oryan, A., Kamali, A., Moshirib, A. and Eslaminejad, M.B. 2017. Role of mesenchymal stem cells in bone regenerative medicine: what is the evidence? Cells Tissues Organs 204(2), 59–83. Paterson, Y.Z., Kafarnik, C. and Guest, D.J. 2018. Characterization of companion animal pluripotent stem cells. Cytom. Part A. 93(1), 137–148. Peroni, J.F. and Borjesson, D.L. 2011. Anti-inflammatory and immunomodulatory activities of stem cells. Vet. Clin. North Am. Equine Pract. 27(2), 351–362. Pinna, S., Lambertini, C., Grassato, L. and Romagnoli, N. 2019. Evidence-based veterinary medicine: a tool for evaluating the healing process after surgical treatment for cranial cruciate ligament rupture in dogs. Front. Vet. Sci. 6, 1–8. Quimby, J.M. 2019. Stem cell therapy. Vet. Clin. North Am. Small Anim. Pract. 49(2), 223–231. Reissis, D., Tang, Q.O., Cooper, N.C., Carasco, C.F., Gamie, Z., Mantalaris, A. and Tsiridis, E. 2016. Current clinical evidence for the use of mesenchymal stem cells in articular cartilage repair. Expert. Opin. Biol. Ther. 16(4), 535–557. Requicha, J.F., Carvalho, P.P., Pires, M.A. and Dias, M.I. 2016. Evaluation of canine adipose-derived stem cells in a healthy mice subcutaneous model. J. Stem Cell Res. Ther. 6(9), 1–5. Rocha dos Santos, C., Rocha Filgueiras, R., Furtado, M.P., Rodrigues da Cunha Barreto-Vianna, A., Nogueira, K., Silva Leite, C. and Mendes de Lima, E.M. 2018. Mesenchymal stem cells in osteotomy repair after tibial tuberosity advancement in dogs with cranial cruciate ligament injury. J. Exp. Orthop. 5(1), 17. Russell, K.A., Chow, N.H.C., Dukoff, D., Gibson, T.W.G., La Marre, J., Betts, D.H. and Koch, T.G. 2016. Characterization and immunomodulatory effects of canine adipose tissue- and bone marrow-derived mesenchymal stromal cells. PLoS One. 11(12), e0167442. Sanderson, R.O., Beata, C., Flipo, R.M., Genevois, J.P., Macias, C., Tacke, S., Vezzoni, A. and Innes, J.F. 2009. Systematic review of the management of canine osteoarthritis. Vet. Rec. 164(14), 418–424. Sasaki, A., Mizuno, M., Mochizuki, M. and Sekiya, I. 2019. Mesenchymal stem cells for cartilage regeneration in dogs. World J. Stem Cells. 11(5), 254–269. Schaakxs, D., Kalbermatten, D., Raffoul, W., Wiberg, M. and Paul, K. 2013. Regenerative cell injection in denervated muscle reduces atrophy and enhances recovery following nerve repair. Muscle Nerve. 1, 691–701. Schneider, M., Angele, P., Järvinen, T.A.H. and Docheva, D. 2018. Rescue plan for achilles: therapeutics steering the fate and functions of stem cells in tendon wound healing. Adv. Drug Deliv. Rev. 129, 352–375. Shah, K., Drury, T., Roic, I., Hansen, P., Malin, M., Boyd, R., Sumer, H. and Ferguson, R. 2018. Outcome of allogeneic adult stem cell therapy in dogs suffering from osteoarthritis and other joint defects. Stem Cells Int. 2018, 1–7. Srzentić Dražilov, S., Mrkovački, J., Spasovski, V., Fazlagić, A., Pavlović, S. and Nikčević, G. 2018. The use of canine mesenchymal stem cells for the autologous treatment of osteoarthritis. Acta Vet. Hung. 66(3), 376–389. Steinmann, S., Pfeifer, C.G., Brochhausen, C. and Docheva, D. 2020. Spectrum of tendon pathologies: triggers, trails and end-state. Int. J. Mol. Sci. 21(3), 1–20. Sullivan, M.O., Gordon-Evans, W.J., Fredericks, L.P., Kiefer, K., Conzemius, M.G. and Griffon, D.J. 2016. Comparison of mesenchymal stem cell surface markers from bone marrow aspirates and adipose stromal vascular fraction sites. Front. Vet. Sci. 2, 1–9. Sun, Q., Zhang, L., Xu, T., Ying, J., Xia, B., Jing, H. and Tong, P. 2018. Combined use of adipose derived stem cells and TGF-β3 microspheres promotes articular cartilage regeneration in vivo. Biotech. Histochem. 93(3), 168–176. Taroni, M., Cabon, Q., Fèbre, M., Cachon, T., Saulnier, N., Carozzo, C., Maddens, S., Labadie, F., Robert, C. and Viguier, E. 2017. Evaluation of the effect of a single intra-articular injection of allogeneic neonatal mesenchymal stromal cells compared to oral non-steroidal anti-inflammatory treatment on the postoperative musculoskeletal status and gait of dogs over a 6-month period. Front. Vet. Sci. 4, 1–11. Tornero-Esteban, P., Hoyas, J.A., Villafuertes, E., Rodríguez-Bobada, C., López-Gordillo, Y., Rojo, F.J., Guinea, G.V., Paleczny, A., Lópiz-Morales, Y., Rodriguez-Rodriguez, L., Marco, F. and Fernández-Gutiérrez, B. 2015. Efficacy of supraspinatus tendon repair using mesenchymal stem cells along with a collagen I scaffold. J. Orthop. Surg. Res. 10(1), 1–7. Torres-Torrillas, M., Rubio, M., Damia, E., Cuervo, B., Romero, A., Peláez, P., Chicharro, D., Miguel, L. and Sopena, J.J. 2019. Adipose-derived mesenchymal stem cells: a promising tool in the treatment of musculoskeletal diseases. Int. J. Mol. Sci. 20(12), 3105. Tsai, S., Huang, Y., Chueh, L., Yeh, L. and Lin, C. 2014. Intra-articular transplantation of porcine adipose-derived stem cells for the treatment of canine osteoarthritis: a pilot study. World J. Transplant. 4(3), 196. Upchurch, D.A., Renberg, W.C., Roush, J.K., Milliken, G.A. and Weiss, M.L. 2016. Effects of administration of adipose-derived stromal vascular fraction and platelet-rich plasma to dogs with osteoarthritis of the hip joints. Am. J. Vet. Res. 77(9), 940–951. Vilar, J.M., Batista, M., Morales, M., Santana, A., Cuervo, B., Rubio, M., Cugat, R., Sopena, J. and Carrillo, J.M. 2014. Assessment of the effect of intraarticular injection of autologous adipose-derived mesenchymal stem cells in osteoarthritic dogs using a double blinded force platform analysis. BMC Vet. Res. 10(1), 1–7. Vilar, J.M., Cuervo, B., Rubio, M., Sopena, J., Domínguez, J.M., Santana, A. and Carrillo, J.M. 2016. Effect of intraarticular inoculation of mesenchymal stem cells in dogs with hip osteoarthritis by means of objective force platform gait analysis: concordance with numeric subjective scoring scales. BMC Vet. Res. 12(1), 1–10. Vilar, J.M., Morales, M., Santana, A., Spinella, G., Rubio, M., Cuervo, B., Cugat, R. and Carrillo, J.M. 2013. Controlled, blinded force platform analysis of the effect of intraarticular injection of autologous adipose-derived mesenchymal stem cells associated to PRGF-Endoret in osteoarthritic dogs. BMC Vet. Res. 9, 1–6. Volk, S.W., Wang, Y. and Hankenson, K.D. 2012. Effects of donor characteristics and ex vivo expansion on canine mesenchymal stem cell properties: implications for MSC-based therapies. Cell Transplant. 23(1), 1–7. Webster, R.A., Blaber, S.P., Herbert, B.R., Wilkins, M.R. and Vesey, G. 2012. The role of mesenchymal stem cells in veterinary therapeutics - a review. N. Z. Vet. J. 60(5), 265–272. Zeira, O., Scaccia, S., Pettinari, L., Ghezzi, E., Asiag, N., Martinelli, L., Zahirpour, D., Dumas, M.P., Konar, M., Lupi, D.M., Fiette, L., Pascucci, L., Leonardi, L., Cliff, A., Alessandri, G., Pessina, A., Spaziante, D. and Aralla, M. 2018. Intra-articular administration of autologous micro-fragmented adipose tissue in dogs with spontaneous osteoarthritis: safety, feasibility, and clinical outcomes. Stem Cells Transl. Med. 7(11), 819–828. Zhao, Q., Ren, H. and Han, Z. 2016. Mesenchymal stem cells: immunomodulatory capability and clinical potential in immune diseases. J. Cell Immunother. 2(1), 3–20. | ||
| How to Cite this Article |
| Pubmed Style IED, Cardoso DF, CSS, LCB, CAV, PPC, Dias IR. Clinical application of mesenchymal stem cells therapy in musculoskeletal injuries in dogs: A review of the scientific literature. Open Vet J. 2021; 11(2): 188-202. doi:10.5455/OVJ.2021.v11.i2.2 Web Style IED, Cardoso DF, CSS, LCB, CAV, PPC, Dias IR. Clinical application of mesenchymal stem cells therapy in musculoskeletal injuries in dogs: A review of the scientific literature. https://www.openveterinaryjournal.com/?mno=50829 [Access: October 06, 2024]. doi:10.5455/OVJ.2021.v11.i2.2 AMA (American Medical Association) Style IED, Cardoso DF, CSS, LCB, CAV, PPC, Dias IR. Clinical application of mesenchymal stem cells therapy in musculoskeletal injuries in dogs: A review of the scientific literature. Open Vet J. 2021; 11(2): 188-202. doi:10.5455/OVJ.2021.v11.i2.2 Vancouver/ICMJE Style IED, Cardoso DF, CSS, LCB, CAV, PPC, Dias IR. Clinical application of mesenchymal stem cells therapy in musculoskeletal injuries in dogs: A review of the scientific literature. Open Vet J. (2021), [cited October 06, 2024]; 11(2): 188-202. doi:10.5455/OVJ.2021.v11.i2.2 Harvard Style , I. E. D., Cardoso, . D. F., , . C. S. S., , . L. C. B., , . C. A. V., , . P. P. C. & Dias, . I. R. (2021) Clinical application of mesenchymal stem cells therapy in musculoskeletal injuries in dogs: A review of the scientific literature. Open Vet J, 11 (2), 188-202. doi:10.5455/OVJ.2021.v11.i2.2 Turabian Style , Ines Esteves Dias, Diogo Filipe Cardoso, Carla Sofia Soares, Luis Carlos Barros, Carlos Antunes Viegas, Pedro Pires Carvalho, and Isabel Ribeiro Dias. 2021. Clinical application of mesenchymal stem cells therapy in musculoskeletal injuries in dogs: A review of the scientific literature. Open Veterinary Journal, 11 (2), 188-202. doi:10.5455/OVJ.2021.v11.i2.2 Chicago Style , Ines Esteves Dias, Diogo Filipe Cardoso, Carla Sofia Soares, Luis Carlos Barros, Carlos Antunes Viegas, Pedro Pires Carvalho, and Isabel Ribeiro Dias. "Clinical application of mesenchymal stem cells therapy in musculoskeletal injuries in dogs: A review of the scientific literature." Open Veterinary Journal 11 (2021), 188-202. doi:10.5455/OVJ.2021.v11.i2.2 MLA (The Modern Language Association) Style , Ines Esteves Dias, Diogo Filipe Cardoso, Carla Sofia Soares, Luis Carlos Barros, Carlos Antunes Viegas, Pedro Pires Carvalho, and Isabel Ribeiro Dias. "Clinical application of mesenchymal stem cells therapy in musculoskeletal injuries in dogs: A review of the scientific literature." Open Veterinary Journal 11.2 (2021), 188-202. Print. doi:10.5455/OVJ.2021.v11.i2.2 APA (American Psychological Association) Style , I. E. D., Cardoso, . D. F., , . C. S. S., , . L. C. B., , . C. A. V., , . P. P. C. & Dias, . I. R. (2021) Clinical application of mesenchymal stem cells therapy in musculoskeletal injuries in dogs: A review of the scientific literature. Open Veterinary Journal, 11 (2), 188-202. doi:10.5455/OVJ.2021.v11.i2.2 |