| Original Article | ||
Open Vet J. 2022; 12(6): 839-850 Open Veterinary Journal, (2022), Vol. 12(6): 839–850 Original Research Pathogenicity of Salmonella enterica subspecies enterica serovar Enteritidis phage type 1 in one-day-old specific pathogen-free chicksSadik Ahmad1, Mohamed Hair-Bejo1,2*, Elawad A. Hussein1, Elmutaz Atta Awad3, Mohamed Ibrahim Saeed4, Pit Sze Liew1 and Zunita Zakaria1,21Department of Veterinary Pathology and Microbiology, Faculty of Veterinary Medicine, Universiti Putra Malaysia, Serdang, Malaysia 2Institute of Bioscience, Universiti Putra Malaysia, Serdang, Malaysia 3Institute of Tropical Agriculture, Universiti Putra Malaysia, Serdang, Malaysia 4Department of Microbiology and Parasitology, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, Serdang, Malaysia Submitted: 26/07/2022 Accepted: 20/10/2022 Published: 14/11/2022 *Corresponding Author: Mohamed Hair-Bejo. Department of Veterinary Pathology and Microbiology, Faculty of Veterinary Medicine, Universiti Putra Malaysia, Serdang, Malaysia. Email: mdhair [at] upm.edu.my © 2022 Open Veterinary Journal
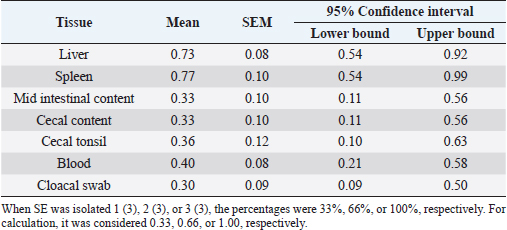
AbstractBackground: The studies about Salmonella infection in newly hatched chicks were not extensive. Aim: The objective of this study was to determine the pathogenicity of Salmonella enterica subspecies enterica serovar Enteritidis (SE) phage type (PT) 1 in one-day-old specific pathogen-free (SPF) chicks. Methods: Seventy, one-day-old SPF chicks, were divided into SE group (30 chicks), mortality group (10 chicks), both orally inoculated (1.0 ml) with SE PT1 (1 × 108 colony-forming unit per 1.0 ml), and one control group (30 chicks). The chicks were sacrificed at 6 and 12 hours, and days 1, 2, 3, 5, 7, 10, 14, and 21 post-inoculation (pi). Samples were collected for bacterial isolation, histological examination, and ultrastructural examination. Results: Starting from day 2 pi, the body weight in the SE group significantly (p < 0.05) decreased. The SE isolation percentages from the liver, spleen, mid-intestinal content, cecal content, cecal tonsil, blood, and cloacal swab were 0.73, 0.77, 0.33, 0.33, 0.36, 0.40, and 0.30, respectively. The isolation percentage in the liver was significantly (p < 0.05) higher than the blood and cloacal swab. The villi heights and crypt depths in the SE group were significantly (p < 0.05) greater and smaller, respectively. Ultrastructurally, erosion and necrosis were observed in the microvilli of the cecal tonsil. The bacteria were engulfed by macrophages at the interepithelial clefts of the M-like M cells. Conclusion: It was concluded that the inoculation of SE PT 1 in one-day-old chicks caused a systemic infection with diarrhea, a decrease in the body weight and villi height in the duodenum, jejunum, and ileum, and high bacterial loading in the liver with mild gross and histological lesions of organs, erosion, and necrosis of microvilli and low mortality. The bacteria entered the body system from the intestinal tract through the interepithelial clefts of the M-like M cells of the cecal tonsil. Keywords: Salmonella enterica, Phage type 1, SPF chicks, Villi and crypt heights, Lesions. IntroductionSalmonella is a causative agent of cross-infection between humans and animals (Ajmera and Shabbir, 2020). It is characterized as a Gram-negative, motile, hydrogen sulfide-producing, an acid-labile facultative intracellular bacterium (Ajmera and Shabbir, 2020). Globally, Salmonella enterica and Salmonella typhimurium represent the major causative agents of human Salmonellosis (Havelaar et al., 2015). The typing of Salmonella is important for the identification of the bacteria, tracing, epidemiological investigation, monitoring antibody susceptibility, food safety, and public health measures. Despite the low resolution, serotyping and multilocus sequence typing (MLST) analyses are considered the standard methods for typing Salmonella (Yan et al., 2021). Based on the phage typing method, Salmonella enterica subspecies enterica serovar Enteritidis (SE) phage type (PT) 1 is a causative agent of human Salmonella infection in Sweden (Nygård et al., 2004), Canada (Khakhria et al., 1991), Italy (Vencia et al., 2015), and Finland (Lukinmaa et al., 1999). However, recently, a high-resolution molecular typing method was established. It was core genome MLST. Such a method underwent whole genomic sequencing for accurate bacterial tracing (Yan et al., 2021). Enteric pathogens, in general, and Salmonella in particular, attach and invade the intestinal epithelium. The infection brings on some adverse consequences to the intestinal function and structure comprising a reduction in the length of the epithelial villi and damage in the intestinal crypts (Bellido-Carreras et al., 2019). This infection initiates activation of the natural killer cells accompanied by intraepithelial T cells. Then, it leads to macrophage infiltration and activation of dendritic cells. Also, there is a proliferation of T cells in the spleen and antibody release in the serum (Meijerink et al., 2021). The studies, about this infection, in newly hatched chicks, were not extensive (McWhorter and Chousalkar, 2019). Thus, the investigation of the infection, with light microscopy and scanning electron microscopy (SEM), would be expected to reveal some consequent influences on the function and architectural physique of the intestine. Moreover, it might uncover some details about the entry mechanism of the microbe and the defensive reaction of the host. The objective of this study was to determine the pathogenicity of SE PT 1 in one-day-old specific pathogen-free (SPF) chicks. Materials and MethodsSalmonella enterica isolateSalmonella enterica was isolated from the liver samples of commercial broiler chickens in Melaka, Malaysia. The method of isolation and identification was described by Kyung-Min et al. (2015). In brief, the samples were collected in Rappaport–Vassiliadis (RV) broth incubated at 37°C for 24 hours and then cultured on both xylose lysin deoxycholate (XLD) and brilliant green (BG) agar at 37°C for 24 hours. The suspected colonies of Salmonella were cultured on urease and triple sugar iron (TSI) biochemical test. Positive cultures from the biochemical test were then identified serologically by Salmonella poly-O antisera using slide agglutination test (SAT). Phage typingPositive SE colonies were characterized by phage typing at the Laboratory of Enteric Pathogens Center for Infections Institute, 61 Colindale Avenue, London, UK by the method described by Demczuk et al. (2003). A loopful of frozen nutrient broth was cultured on nutrient agar at 37°C for 24 hours. Positive colonies were incubated in blood agar (BA) culture at 37°C for 24 hours. The colonies from BA were inoculated into 3 ml of phage broth for 2 hours with vigorous shaking. The phage broth was then poured on a phage agar plate after the removal of excess broth. Next, bacteriophages were left overnight to dry at room temperature. The phage lysis pattern of cultures was evaluated according to Demczuk et al. (2003) scheme. It was identified that the three samples were SE PT 1. SerotypingSalmonella polyvalent O (A–I, Vi) antiserum was used at room temperature. Some bacterial growth was emulsified and examined under light microscopy. Clumping, of Salmonella polyvalent O antiserum represented a positive result. Positive colonies were tested with monovalent group D antiserum. Positive growth of group D antisera was tested with H antisera by adding 1 ml of solid nutrient agar. The agar was melted by boiling water, and incubated in a water bath for 30 minutes at 45°C. 10 μl of heterologous H antiserum was added, shaken, and left to solidify. Heavy suspensions of the bacterial growth and normal saline were streaked and incubated overnight at 37°C. Using a loop, a speck of the bacterial growth was emulsified with normal saline. Then, 10 μl of H antiserum was mixed. Clumping was considered a positive result for SE (Vandepitte et al., 2003). Inoculum preparationSE was cultured with nutrient broth on nutrient agar and incubated for 24 hours at 37°C. The cultures were transferred to BA, incubated for 24 hours at 37°C, harvested, and suspended in normal saline, and eventually an inoculum of 1 × 108 colony-forming unit (cfu) per 1.0 ml was prepared. SPF chicksSPF embryonated chick eggs were incubated in Siam Incubator System, Thailand, and allowed to hatch. One-day-old chicks were provided with water and an antibiotic-free ration at all times. Experimental designSeventy, one-day-old SPF chicks were divided into three groups, namely SE group (30 chicks), control group (30 chicks), and mortality group (10 chicks). Chicks in SE and mortality groups were infected orally with 1.0 ml of 1 × 108 cfu per 1.0 ml of SE while the chicks of the control group were not infected. Sampling was carried out on the SE group at hours 6 and 12, and on days 1, 2, 3, 5, 7, 10, 14, and 21 post-inoculation (pi). Samples of liver, spleen, mid-intestinal contents, cecum, cecal tonsils, and cloacal swabs were collected and incubated in RV broth for 24 hours at 37°C for SE isolation. Mortality group chicks were not sacrificed but left either to record the pathogenicity of the microbe or to cause death throughout the trial when the chicks did not undergo any disturbance. Clinical signs, body weights, and gross lesions were recorded. Samples of liver, spleen, duodenum, jejunum, ileum, and cecum were fixed in 10% buffered formalin, embedded in paraffin, and processed for histopathological examination. Cecal tonsils were fixed in 2.5% glutaraldehyde cacodylate buffer, pH 7.2 at 4°C for ultrastructural examination by SEM. Bacterial isolationA loopful of RV broth was cultured onto XLD and BG agar and incubated for 24 hours at 37°C. The positive black colonies (due to the production of hydrogen sulfide) and rounded pink colonies from BG agar were then cultured onto TSI and urease for 24 hours at 37°C. The positive results from TSI were then transferred to BA and incubated for 24 hours at 37°C. The positive colonies were then tested by SAT using poly-O antisera (Kyung-Min et al., 2015). Morphometric analysis by measurement of villi height and crypts depthThe intestinal segments from the duodenum, jejunum, and ileum samples were processed and stained with hematoxylin and eosin (HE). The tissues were examined under light microscopy (Motic BA410) connected with a camera (Motic Moticam pro 285A) for determination of the villi height and crypt depth using the software of motic images plus 2.0 ML. The villi heights and the crypt depths were measured from the tip to the crypt junction and from the base to the villi junction, respectively (Gava et al., 2015) (Fig. 1). Three readings were recorded for every collected sample. Scanning electron microscopyThe cecal tonsil tissues were fixed in 2.5% glutaraldehyde cacodylate buffer for 12 hours. Then, the tissues were washed with 0.1 M sodium cacodylate buffer, fixed with osmium tetroxide for 2 hours at 4°C, and washed with 0.1 M sodium cacodylate buffer. The next step was dehydration with acetone and run for critical point drying. After that, the cecal tonsil tissues were mounted with double-sided tape or colloidal silver. Eventually, they were coated with gold sputter or coater samples and viewed under SEM (Mogana and Patchamuthu, 2016). Statistical analysisUsing IBM Statistical Package for the Social Sciences Statistics 25 software, the technique of T-test (independent sample) was applied for the result of body weight. While for the result of SE isolation, when SE was isolated 1(3) (one positive sample out of three examined samples), 2(3), or 3(3), the percentages were considered 33%, 66%, or 100%, respectively. Hence, for the calculation, the values, of SE isolation, were considered 0.33, 0.66, or 1.00, respectively. Then, one-way analysis of variance (ANOVA) with repeated measures was applied. The level of significance was considered when p < 0.05.
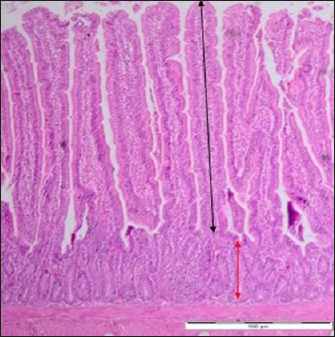
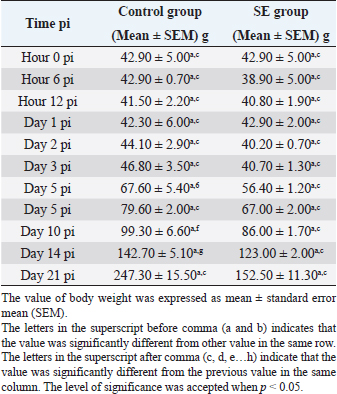
Fig. 1. Villi height and crypt depth of the intestine of SPF chick. Black arrow (villi height) extending along the villi of the duodenum. Red arrow (crypt depth) extending from the base to the villi junction. Control group. Day 7 pi. HE staining. Bar=4 μm (40×). Ethical approvalAll experimental procedures were undertaken in accordance with the Research Policy on Animal Ethics of Universiti Putra Malaysia. ResultsBody weightThe body weight of the chicks in the SE group was fluctuating at the beginning of the experiment. Starting from day 2 pi till the end, every time there was an increase. The body weight in the SE group was lower (p > 0.05) in comparison with the control group before day 2 pi. However, it was also significantly lower (p < 0.05) in comparison with the control group starting from day 2 pi till the end of the experiment (Table 1). The body weight of the chicks in the control group increased continuously starting from hour 12 pi till the end of the experiment. Starting from day 5 pi till the end of the experiment, it was significantly greater (p < 0.05) in comparison with the value in the time before (Table 1). Table 1. Body weight of chick infected with S. enterica (SE PT 1) throughout the 21 days experiment.
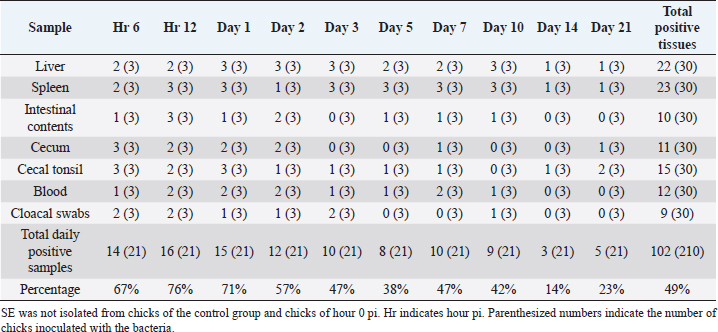
Clinical signsIn the SE group, at hour 6 pi, one chick was affected with diarrhea, then diarrhea was observed in all chicks from hour 12 pi till the end of the experiment. From day 2 pi till the end of the experiment, the chicks were affected by inappetence. From day 3 pi till the end of the experiment, there was a sign of ruffled feathers. From day 5 pi till the end of the experiment, there was stunting. In the mortality group, all the chicks were affected with diarrhea from hour12 till the end of the experiment. From day 3 pi till the end of the experiment, there were inappetence, ruffled feathers, and stunting. No abnormal clinical signs were observed in the control group. Gross lesionsIn the SE group, at hours 6, 12 pi, and day 1 pi, gross lesions were not observed. From day 2 pi till the end of the experiment, mild airsacculitis and mild peritonitis were recorded in all chicks. Also, from day 2 pi until day 21 pi, congestion, in the liver and kidney, was observed in some chicks. One chick died on day 3 pi and another chick died on day 5 pi. Dead chicks were affected by dehydration. In the control group, no gross lesions were recorded. Bacterial isolationLiverIn the SE group, SE was isolated at hours 6, 12 pi from the liver tissues (33%). On days 1, 2, and 3 pi, it was isolated 100%. On days 5 and 7 pi, it was isolated at 33%. On day 10 pi, it was isolated 100%. On days 14 and 21 pi, it was isolated at 33% (Table 2). SpleenIn the SE group, SE was isolated at hour 6 pi (66%). At hour 12, days 1, 3, 5, 7, and 10 pi, it was isolated 100%. On days 2, 14, and 21 pi, it was isolated at 33% (Table 2). BloodIn the SE group, SE was isolated at hour 6 pi (33%). At hour 12 pi, it was isolated 66%. On days 1, 2, and 7 pi, it was isolated at 66%. On days 3, 5, and 10 pi, it was isolated at 33%. On days 14 and 21 pi, it was not isolated (Table 2). Mid-intestinal contentIn the SE group, SE was isolated at hour 6 pi (33%). At hour 12 pi, it was isolated 100%. On day 1 pi, it was isolated at 33%. On day 2 pi, it was isolated at 66%. On days 5, 7, and 10 pi, it was isolated at 33%. On day 3 pi, it was not isolated (Table 2). Cecal contentIn the SE group, SE was isolated at hour 6 pi (100%). At hour 12 pi, days 1 and 2 pi, it was isolated at 66%. On days 7 and 21 pi, it was isolated at 33%. It was not isolated on days 3, 5, 10, and 14 pi (Table 2). Cecal tonsilIn the SE group, SE was isolated at hour 6 pi (100%). At hour 12 pi, it was isolated 66%. On day 1 pi, it was isolated 100%. On days 2, 3, 5, 7, and 14 pi, it was isolated at 33%. On day 21 pi, it was isolated at 66% (Table 2). Cloacal swabIn the SE group, SE was isolated at hours 6 and 12 pi (66%). On days 1 and 2 pi, it was isolated at 33%. On day 3 pi, it was isolated at 66%. On day 10 pi, it was isolated at 33%. It was not isolated on days 14 and 21 pi (Table 2). Table 2. Isolation of SE from different tissues of chicks from hour 6 till day 21 pi.
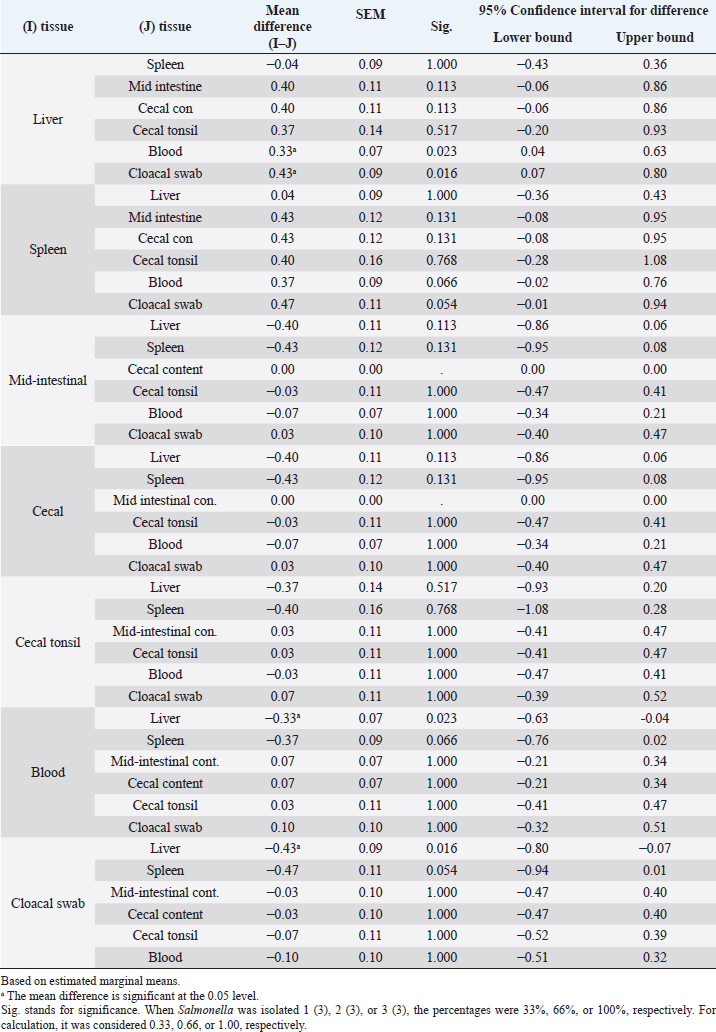
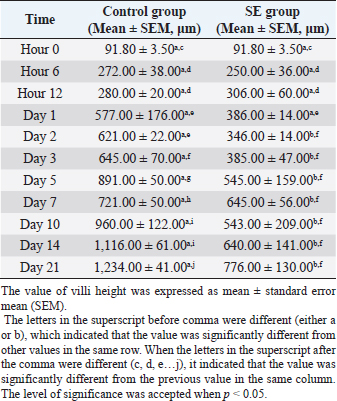
Statistical analysis for the result of isolation of SE from different tissues of chicks from hour 6 till day 21 piOne-way ANOVA with repeated measures was applied on the result of the isolation of SE from different tissues of the chicks from hour 6 till day 21 pi to determine the overall mean and standard error of the mean for the isolation of SE 10 times in the liver, the overall mean for the isolation of SE 10 times in spleen, the overall mean for the isolation of SE 10 times in mid-intestinal content, and so on. There were significances (p < 0.05) for the differences obtained when the mean of the liver was compared with the mean of blood and also when the mean of the liver was compared with the mean of the cloacal swab (Tables 3 and 4). Villi heightDuodenumThe villi height in the duodenum of the SE group was rising throughout the experiment. But there was a drop on day 2 pi. The height was significantly (p < 0.05) greater than the height in the previous time of tissue collection of the same group at hour 6, days 1 and 2 pi. The villi height in the SE group was significantly (p < 0.05) smaller than the height in the control group from day 2 pi till the end with the exception of day 7 pi (Table 5). Table 3. Overall means and SEM for isolation of S. enterica (SE PT 1) 10 times from different tissues of chicks from hour 6 till day 21 pi.
Table 4. Comparison of overall means for isolation of S. enterica (SE PT 1) 10 times from different tissues with each other for chicks from hour 6 till day 21 pi.
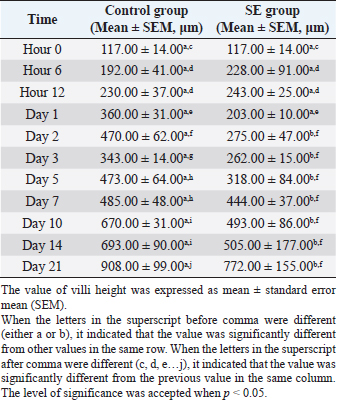
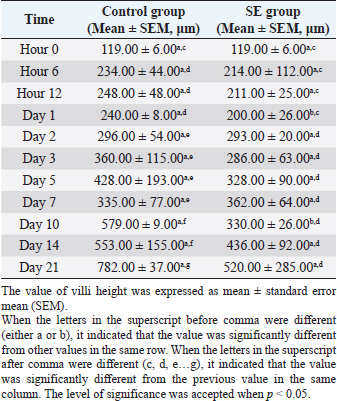
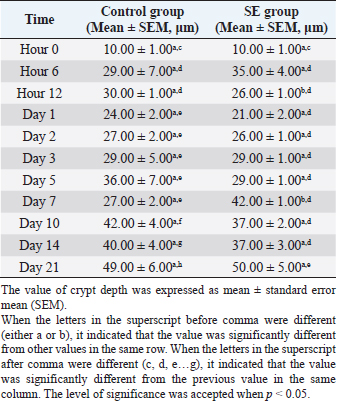
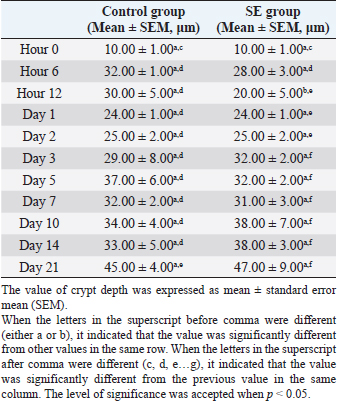
The villi height in the control group was significantly (p < 0.05) greater than the height in the previous time at hour 6, days 1,3, 5, 7, 10, and 21 pi (Table 5). JejunumThe villi height in the jejunum of the SE group was rising every time apart from days 1 and 3 pi. The height was significantly (p < 0.05) greater than the height in the same group (SE) at hour 6 days 1 and 2 pi. The villi height in the control group increased every time apart from day 3 pi. The height was significantly (p < 0.05) greater than the height in the same group (control) at hour 6, days 1, 2, 3, 5, 10, and 21 pi. When the height value in the SE group was compared with the height in the control group, it was significantly (p < 0.05) smaller than the height in the control group on days 1, 2, 3, 5, and 10 pi (Table 6). IleumThe villi height in the ileum of the SE group was fluctuating, in other words, sometimes, it increased and sometimes it decreased in comparison with the measurement of the time before. However, only on day 2 pi, there was a significant (p < 0.05) increase. When the height value in the SE group was compared with the height in the control group, it was significantly (p < 0.05) greater in the control group only on days 1 and 10 pi. The villi height in the control group increased significantly (p < 0.05) more than the height in the previous time in the control group only at hour 6, days 2, 10, and 21 pi (Table 7). Table 5. Villi height in the duodenum of chicks infected with S. enterica (SE PT 1) from hour 0 till day 21 pi.
Table 6. Villi height in jejunum of chicks infected with S. enterica (SE PT 1) from hour 0 till day 21 pi.
Table 7. Villi height in ileum of chicks infected with S. enterica (SE PT 1) from hour 0 till day 21 pi.
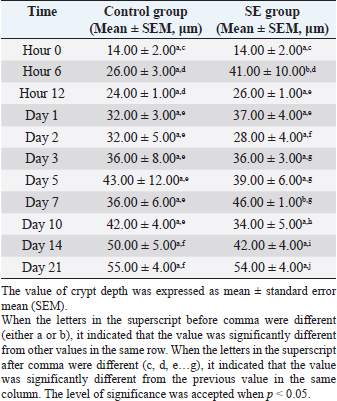
Table 8. Crypts depths in duodenum of chicks infected with S. enterica (SE PT 1) from hour 0 till day 21 pi.
Crypt depthDuodenumThe crypt depth in the duodenum of the SE group was significantly (p < 0.05) greater than the depth in the duodenum of the control group at hour 6 and day 7 pi. The crypt depth in the SE group was significantly greater than the previous value in the same group (SE) at hours 6, 12, days 2, 3, 10, 14, and 21 pi. The crypt depth in the control group was significantly (p < 0.05) greater than the previous value in the control group at hour 6, days 1 and 14 pi (Table 8). Table 9. Crypts depths in jejunum of chicks infected with S. enterica (SE PT 1) from hour 0 till day 21 pi.
JejunumThe value of the crypt depths in the jejunum of the SE group was significantly (p < 0.05) greater than the value in the control group on day 7 pi. The crypt depth value in the SE group was significantly (p < 0.05) greater than the previous value in the SE group at hour 6 and day 21 pi. The crypt depth value in the control group was significantly (p < 0.05) greater than the previous value in the control group at hour 6, days 1, 10, 14, and 21 pi (Table 9). IleumThe value of the crypt depths in the ileum of the SE group was significantly (p < 0.05) smaller than the value in the control group at hour 12 pi. The crypt depth value in the SE group was significantly (p < 0.05) greater than the previous value in the SE group at hours 6, 12, and day 3 pi. The crypt depth in the control group was significantly (p < 0.05) greater than the previous value in the control group at hour 6, and day 21 pi (Table 10). Microscopic lesionsIn the liver, there was mild heterophilic infiltration and fatty degeneration at hour 6 pi. Then at hour 12 pi, there was congestion. On days 1, 2, and 3 pi, there was mild to moderate congestion, fatty degeneration, and heterophilic infiltration. On days 5, 7, 10, 14, and 21 pi, there were necrosis, inflammatory cell infiltration, and congestion. In the spleen, there was congestion at hour 6 and 12 pi. Then on days 1 and 2 pi, there were heterophilic infiltration and congestion. On days 3, 5, and 7 pi, there were heterophilic infiltration, edema, and congestion. On days 10, 14, and 21 pi, there were some hyperplasia and edema. Table 10. Crypts depths in ileum of chicks infected with S. enterica (SE PT 1) from hour 0 till day 21 pi.
In the bursa of Fabricius, at hours 6, 12, days 1, 2, and 3 pi, there were degeneration and congestion. From day 5 pi until the end of the experiment, there were congestion, necrosis, and thickening in the interfollicular area. In the duodenum, at hours 6 pi, there were mild heterophilic infiltration and congestion. At hour 12 pi, there were congestion and necrosis. On days 1, 2, 3, and 5 pi, there were more heterophilic infiltration, congestion, and necrosis. On days 7, 10, 14, and 21 pi, there were inflammatory cell infiltration and necrosis. In the jejunum, at hours 6 and 12 pi, there were heterophilic infiltration and congestion. On days 1, 2, and 3 pi, necrosis was observed. On days 5, 7, 10, and 21 pi, the same previous lesions persisted. In the ileum, at hours 6 and 12 pi, there was mild heterophilic infiltration. From day 1 pi till the end of the experiment, there were heterophilic infiltration, congestion, and necrosis. In the cecum, at hours 6 and 12 pi, there was mild heterophilic infiltration in the lamina propria. On days 1, 2, and 3 pi, some mild congestion and moderate heterophilic infiltration in the lamina propria were recorded. On days 5, 7, 10, 14, and 21 pi, necrosis was detected. On day 5 pi, moderated heterophilic infiltration, congestion, and necrosis in the lamina propria were detected. On days 7, 10, 14, and 21 pi, moderated heterophilic infiltration in the lamina propria and muscular layer beside necrosis of the epithelial cells. Dead chicksOn days 3 and 5 post-challenged, severe infiltration of inflammatory cells, congestion, and multiple areas of focal coagulative necrosis were observed in the hepatic tissue of the dead chicks. Also, increased lymphocytic activity was recorded in the spleen tissue. The intestine was severely autolyzed. The bursa of Fabricius tissue was congested and infiltrated by heterophils. The interfollicular tissue was thickened, whereas, the lymphoid follicles were invaded by focal areas of necrosis. Scanning electron microscopyHours 6 and 12 piThere was some roughening in the microvilli of the cecal tonsils and macrophage engulfment of some individual or bacterial clusters near the interepithelial clefts.
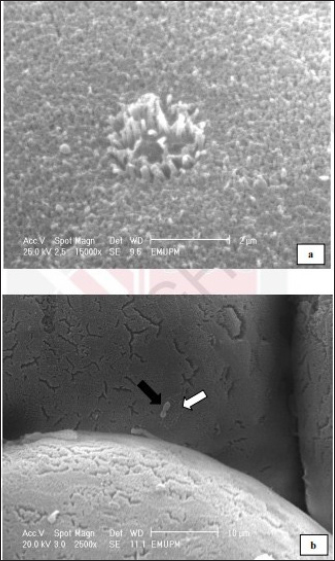
Fig. 2. Cecal tonsil of SPF chicks. Control group, cecal tonsil microvilli. Day 2 pi. SEM. Bar=2 μm.
Fig. 3. Cecal tonsil of SPF chicks. SE group. Hour 12 pi. Cecal tonsil. Salmonella enterica (SE PT 1) (white arrow) attacked by macrophage (black arrow). SEM. Bar=5 μm.
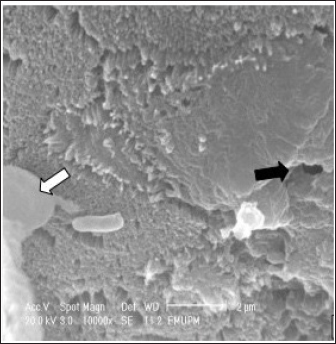
Fig. 4. Cecal tonsil of SPF chicks. SE group. Microvilli necrosis (black arrow). Macrophage approaching the S. enterica (SE PT 1) (white arrow). SEM. Bar=2 μm. Day 2 piThe microvilli surface was affected by erosion and necrosis. Bacterial infiltration through the erosions and bacterial engulfment by the microphage were observed (Figs. 2–4). Days 5, 7, and 21 piThere were necrosis and sloughing of the microvilli of the cecal tonsil’s surface. Some individual bacteria were detected approaching the M-like M cells of the microvilli of the cecal tonsils (Fig. 5). DiscussionThe study demonstrated that the oral inoculation of 1.0 ml of SE PT 1 with a dose of 1 × 108 cfu/1.0 ml in one-day-old SPF chicks resulted in a moderate infection. In addition, some morphometric alterations were detected in the villi height and crypt depth of the intestine. The technique for typing Salmonella in this study, phage typing, is a phenotypical technique that has been applied for a long time. It is a rapid and low-cost technique (Mohammed, 2017). Whereas the technique itself necessitates specialized phage collections and bacterial strains for propagation. Thus, it is solely carried out in a limited number of laboratories (Bertrand et al., 2012). It was reported that the use of temperate phage 1, from the phage typing conducted by Ward et al. (1987), resulted in the conversion of PTs 1–20 (Rankin and Platt, 1995). In general, such a technique is unstable due to its limited reproducibility (Bertrand et al., 2012). There were 2 (6%) dead chicks. However, in a study conducted by Lima et al. (2016), it was found that the mortality rate, in one-day-old chicks experimentally infected with different strains of SE, was 69.5%. Also, in an outbreak of Salmonella in many poultry farms in four states in Nigeria, the mortality rate was ranging from 40% to 80% (Mshelbwala et al., 2017). While, in a study, conducted by Fernández et al. (2001), one-day-old chicks were inoculated with SE PT 4, and the mortality rate was 23%.
Fig. 5. Cecal tonsil of SPF chicks. (a) Control group on day 5 pi. (b) SE group. Bacterial rod (black arrow) multiplying on microvilli surface of the Cecal tonsil upon M-like M cell (white arrow). Day 5 pi. SEM. Bar=10 μm. The huge difference in the mortality rate between the chicks in this study and the chicks in other cited studies could be attributed to the differences in the virulence of different SE PTs (Dhillon et al., 2001), and different serovars (Cheng et al., 2019). The body weight value, of the chicks in this study, was significantly (p < 0.05) smaller in the SE group than the body weight value in the control group from day 2 pi till the end of the experiment. On day 21 pi, the body weight in the control group reached a peak, which was greater by 39% than the body weight in the SE group. Such reduction in the body weight of the infected chicks might be due to profuse diarrhea. The significantly lower values of body weight in the SE group in comparison with the control group reflected the economic importance of SE infection with respect to commercial loss which could affect the farms’ broilers. In a research conducted by Alisantosa et al. (2000), the body weights in 15 groups of one-day-old SPF chicks were recorded on 14, 21, and 28 days pi of SE PTs 4, 8, and 23. It was found that in the majority of the groups, the average body weights were significantly lower than the uninoculated controls. Also, Dhillon et al. (1999) and Fernández et al. (2001) found a decrease in the body weights with percentages ranging from 10% to 24% in Salmonella inoculated newly hatched chicks compared with uninoculated chicks. Since the surface area of the small intestine is significantly enhanced by the presence of villi and microvilli (Kiela and Ghishan, 2016). So, the intestinal function can be evaluated with the villi height, cell area, and cell mitosis (Yamauchi et al., 1996). However, the villi are covered with columnar epithelial cells or enterocytes at the tip. These are mostly absorptive cells, whereas crypt cells are generally regarded as secretory. Furthermore, most of the nutrient transport occurs in the small intestine (Kiela and Ghishan, 2016). In this study, the villi height in the duodenum of the control group started to increase and became greater significantly (p < 0.05) than the height of the villi in the SE group from day 2 pi till the end of the experiment apart from day 7 pi. The villi height in the jejunum of the control group was greater significantly (p < 0.05) than the height of the villi in the SE group on days 1, 2, 3, 5, and 10 pi. In the ileum, the villi heights were greater significantly (p < 0.05) on days 1 and 10 pi than the height of the villi in the SE group. The depth, of the crypt in the duodenum of the control group, was smaller significantly (p < 0.05) than the depth of the crypts in the duodenum of the SE group at hour 6 and day 7 pi. In the jejunum, the crypt depth, in the control group, was smaller significantly (p < 0.05) than the crypt depth of the SE group at hour 12 and day 7 pi. However, in the ileum, the crypt depth in the control group was also smaller significantly (p < 0.05) than the crypt depth in the ileum of the SE group at hours 6 and 12 pi. The results of villi height and crypt depth in this study indicated that the intestinal absorption in the SE group decreased. In contrast, the secretion of crypts in the SE group increased. However, in one previous study conducted by Rebollada-Merin et al. (2020), in which 7-day-old chicks were challenged with 3.3 × 105 cfu/ml of the monophasic variant of colistin-resistant S. typhimurium, the duodenum villi heights were found slightly lower (539.81 μm) on day 7 of life, higher (896.95 and 1,208.45 μm) on days 14 and 21 of life, respectively. The values obtained in the study of Rebollada-Merin et al. (2020), were also smaller than the values in the control of the present study. In addition, in the study of Rebollada-Merin et al. (2020), the duodenum crypt depths were found greater (78.14, 93.91, and 128.09 μm) on days 7, 14, and 21 of life, respectively. However, greater values in the study of Rebollada-Merin et al. (2020), in comparison with this study, were expected since the chicks in their study were challenged too late in comparison with the chicks in this study. In this study, SE was not isolated from the blood on days 14 and 21 pi. It might be an indication of some clearance of the microbe from the blood. However, Buck et al. (2004) found that fimD mutant of SE PT 4 was present in the blood for 3 weeks after infection of laying hens (ISA Warren Brown) at the age of 19 weeks. Whereas, the wild-type parent strain of the microbe was cleared from the blood within the first 3 days. Using SEM, SE PT 1 was detected in the tissue of the cecal tonsil at hour 6 pi. The bacteria were engulfed by the macrophages at the interepithelial clefts of the M-like M cells of the cecal tonsil which might provide a suggestion about the route of entry of the bacteria from the intestine to the body system. The bacteria also caused some erosion and necrosis of the microvilli which definitely lead to the disturbance and decreased absorption of the feed materials and some reduction in the body weight of the chicks. The significant reduction in the body weight of the SE group in comparison with the control group might also be due to diarrhea and a decrease in villi heights of the duodenum, jejunum, and ileum. The result of SEM supported some previous studies that M cells were the target cells invaded by Salmonella typhi and S. typhimurium (Jepson and Clark, 2001). Another action, induced by Salmonella, was programmed cell death in the macrophage (Bruno et al., 2009). Furthermore, Huang et al. (2020) reported that Salmonella crossed the intestinal epithelium. Then it was engulfed by some phagocytic cells such as dendritic cells and macrophages. It was recommended in future studies to subtype the causative agent, SE PT 1, with a more sophisticated, stable, and accurate technique. ConclusionIt was concluded that the inoculation of SE PT 1 in 1-day-old chicks caused a systemic infection with diarrhea and a decrease in body weight and villi height in the duodenum, jejunum, and ileum, and high bacterial loading in the liver with mild gross and histological lesions of organs, erosion, and necrosis of microvilli and low mortality. The bacteria were engulfed by macrophages at the interepithelial clefts of the M-like M cells of the cecal tonsil and entered the body system from the intestinal tract. Data availabilityThe data used to support the findings of this study are included in the article. Conflict of interestNo potential conflict of interest was reported by the authors. FundingThis research was funded by the Ministry of Science, Technology and Innovations, Malaysia (MOSTI), under TechnoFund grants no. 6363001 and 6364002. Authors’ contributionsMHB and ZZ: Conceived and designed the study. AS: Performed the study. MHB and EHA: Analyzed the data, Drafted, updated the references, and revised the manuscript. All authors have read and approved the manuscript. ReferencesAjmera, A. and Shabbir, N. 2020. Salmonella. Treasure Island, FL: StatPearls Publishing. Available via https://www.ncbi.nlm.nih.gov/books/NBK555892/ Alisantosa, B., Shivaprasad, H.L., Dhillon, A.S., Jack, O., Schaberg, D. and Bandli, D. 2000. Pathogenicity of Salmonella enteritidis phage types 4, 8 and 23 in specific pathogen free chicks. Avian Pathol. 29(6), 583–592. Bellido-Carreras, N., Argüello, H., Zaldívar-López, S., Jiménez-Marín, Á., Martins, R.P., Arce, C., Morera, L., Carvajal, A. and Garrido, J.J. 2019. Salmonella typhimurium infection along the porcine gastrointestinal tract and associated lymphoid tissues. Vet. Pathol. 56(5), 681–690. Bertrand, T.S., Hailu, K. and Isha, W. 2012. Development of a sequence typing scheme for differentiation of Salmonella Enteritidis strains. FEMS Microbiol. Lett. 331(2), 165–175. Bruno, V.M., Hannemann, S., Lara-Tejero, M., Flavell, R.A., Kleinstein, S.H. and Galán, J.E. 2009. Salmonella typhimurium type III secretion effectors stimulate innate immune responses in cultured epithelial cells. PLoS Pathog. 5(8), e1000538. Buck, J. D., Immerseel, F. V., Haesebrouck, F. and Ducatelle, R. 2004. Effect of type 1 fimbriae of Salmonella enterica serotype Enteritidis on bacteraemia and reproductive tract infection in laying hens. Avian Pathol. 33(3), 314–320. Cheng, R.A., Eade, C.R. and Wiedmann, M. 2019. Embracing diversity: differences in virulence mechanisms, disease severity, and host adaptations contribute to the success of nontyphoidal Salmonella as a foodborne pathogen. Front. Microbiol. 10, 1368. Demczuk, W., Soule, G., Clark, C., Ackermann, H.W., Easy, R., Khakhria, R., Rodgers, F. and Ahmed, R. 2003. Phage-based typing scheme for Salmonella enterica serovar Heidelberg, a causative agent of food poisonings in Canada. J. Clin. Microbiol. 41(9), 4279–4284. Dhillon, A.S., Shivaprasad, H.L., Roy, P., Alisantosa, B., Schaberg, D., Bandli, D. and Johnson, S. 2001. Pathogenicity of environmental origin salmonellas in specific pathogen-free chicks. Poult. Sci. 80(9), 1323–1328. Dhillon, A.S., Alisantosa, B., Shivaprasad, H.L., Jack, O., Schaberg, D. and Bandli, D. 1999. Pathogenicity of Salmonella enteritidis phage types 4, 8 and 23 in broiler chicks. Avian Dis. 73, 506–515. Fernández, A., Lara, C., Loste, A., Calvo, S. and Marca, M.C. 2001. Control of Salmonella enteritidis phage type 4 experimental infection by fosfomycin in newly hatched chicks. Comp. Immunol. Microbiol. Infect. Dis. 24(4), 207–216. Gava, M.S., Moraes, L.B., Carvalho, D., Chitolina, G.Z., Fallavena, L.C.B., Moraes, H.L., Herpich, J. and Salle, C.T.P. 2015. Determining the best sectioning method and intestinal segment for morphometric analysis in broilers. Brazil. J. Poult. Sci. 17(2), 145–149. Havelaar, A.H., Kirk, M.D., Torgerson, P.R., Gibb, H.J., Hald, T., Lake, R.J., Praet, N., Bellinger, D.C., De Silva, N.R. and Gargouri, N. 2015. World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 12, e1001923. Huang, K., Fresno, A.H., Skov, S. and Olsen, J.E. 2020. Dynamics and outcome of macrophage interaction between Salmonella gallinarum, Salmonella typhimurium, and Salmonella Dublin and macrophages from chicken and cattle. Front. Cell. Infect. Microbiol. 9, 420. Jepson, M.A., Ann, M. and Clark, M. 2001. The role of M cells in Salmonella infection. Microbes Infect. 3(14–15), 1183–1190. Khakhria, R., Duck, D. and Lior, H. 1991. Distribution of Salmonella enteritidis phage types in Canada. Epidemiol. Infect. 106(1), 25–32. Kiela, P.R. and Ghishan, F.K. 2016. Physiology of intestinal absorption and secretion. Best Pract. Res. Clin. Gastroenterol. 30(2), 145–159. Kyung-Min, L., Mick, R., Timothy, J., Herrman, R.P. and Hsieh, J. 2015. Review of Salmonella detection and identification methods: aspects of rapid emergency response and food safety. Food Control 47, 264–276. Lima, D.A., Furian, T.Q., Pillati, R.M., Silva, G.L., Morgam, R.B., Borges, K.A., Fortes, F.B.B., Moraes, H.L.S., Brito, B.G., Brito, K.C.T. and Salle, C.T.P. 2016. Establishment of a pathogenicity index in Salmonella Enteritidis and Salmonella typhimurium strains inoculated in one-day-old broiler chicks. Arq. Bras. Med. Vet. Zoo. 68(2), 257–264. Lukinmaa, S., Schildt, R., Rinttilä, T. and Siitonen, A. 1999. Salmonella enteritidis phage types 1 and 4: pheno- and genotypic epidemiology of recent outbreaks in Finland. J. Clin. Microbiol. 37(7), 2176–2182. McWhorter, A.R. and Chousalkar, K.K. 2019. From hatch to egg grading: monitoring of Salmonella shedding in free-range egg production systems. Vet. Res. 50, 58. Meijerink, N., van den Biggelaar, R.H.G.A., van Haarlem, D.A., Stegeman, J.A., Rutten, V.P.M.G. and Jansen, C.A. 2021. A detailed analysis of innate and adaptive immune responsiveness upon infection with Salmonella enterica serotype Enteritidis in young broiler chickens. Vet. Res. 52, 109. Mogana, D.M. and Patchamuthu, R. 2016. Sample preparations for scanning electron microscopy – life sciences. In Modern electron microscopy in physical and life sciences. Eds., Milos, J. and Robert, K. Rijeka, Croatia: Books on Demand. Mohammed, M. 2017. Phage typing or CRISPR typing for epidemiological surveillance of Salmonella typhimurium? BMC Res. Notes 10, 578. Mshelbwala, F.M., Ibrahim, N.D., Saidu, S.N., Azeez, A.A., Akinduti, P.A., Kwanashie, C.N., FakilahyelKadiri, A.K., Muhammed, M., Fagbamila, I.O. and Luka, P.D. 2017. Motile Salmonella serotypes causing high mortality in poultry farms in three South-Western States of Nigeria. Vet. Rec. 4(1), e000247. Nygård, K., de Jong, B., Guerin, P.J., Yvonne, A., Agneta O. and Giesecke, J. 2004. Emergence of new Salmonella Enteritidis phage types in Europe? Surveillance of infections in returning travellers. BMC Med. 2, 32. Rankin, S. and Platt, D.J. 1995. Phage conversion in Salmonella enterica serotype Enteritidis: implications for epidemiology. Epidemiol. Infect. 114(2), 227–236. Rebollada-Merino, A., Ugarte-Ruiz, M., Hernández, M., Miguela-Villoldo, P., Abad, D., Rodríguez-Lázaro, D., de Juan, L., Domínguez, L. and Rodríguez-Bertos, A. 2020. Reduction of Salmonella typhimurium cecal colonisation and improvement of intestinal health in broilers supplemented with fermented defatted ‘Alperujo’, an olive oil by-product. Animals 10, 1931. Vandepitte, J., Verhaegen, J., Engbaek, K., Rohner, P., Piot, P. and Heuck, C.C. 2003. Basic laboratory procedures in clinical bacteriology, 2nd ed. Geneva, Switzerland: World Health Organization, pp: 42–59. Vencia, W., Gariano, G.R., Bianchi, D.M., Zuccon, F., Sommariva, M., Nguon, B., Malabaila, A., Gallina, S. and Decastelli, L. 2015. A Salmonella Enterica Subsp. Enterica Serovar Enteritidis foodborne outbreak after consumption of homemade Lasagne. Ital. J. Food Saf. 4(4), 5127. Ward, L.R., de Sa, J.D.H. and Rowe, B. 1987. A phage-typing scheme for Salmonella enteritidis. Epidemiol. Infect. 99(2), 291–294. Yamauchi, K., Kamisoyama, H. and Isshiki, Y. 1996. Effects of fasting and refeeding on structures of the intestinal villi and epithelial cells in white leghorn hens. Br. Poult. Sci. 37(5), 909–921. Yan, S., Zhang, W., Li, C., Liu, X., Zhu, L., Chen, L. and Yang, B. 2021. Serotyping, MLST, and core genome MLST analysis of Salmonella enterica from different sources in China during 2004-2019. Front. Microbiol. 12, 688614. | ||
| How to Cite this Article |
| Pubmed Style Ahmad S, Hair-bejo M, Hussein EA, Awad EA, Saeed MI, Sze LP, Zakaria Z. Pathogenicity of Salmonella enterica subspecies enterica serovar Enteritidis phage type 1 in one-day-old specific pathogen free chicks. Open Vet J. 2022; 12(6): 839-850. doi:10.5455/OVJ.2022.v12.i6.8 Web Style Ahmad S, Hair-bejo M, Hussein EA, Awad EA, Saeed MI, Sze LP, Zakaria Z. Pathogenicity of Salmonella enterica subspecies enterica serovar Enteritidis phage type 1 in one-day-old specific pathogen free chicks. https://www.openveterinaryjournal.com/?mno=89495 [Access: July 01, 2025]. doi:10.5455/OVJ.2022.v12.i6.8 AMA (American Medical Association) Style Ahmad S, Hair-bejo M, Hussein EA, Awad EA, Saeed MI, Sze LP, Zakaria Z. Pathogenicity of Salmonella enterica subspecies enterica serovar Enteritidis phage type 1 in one-day-old specific pathogen free chicks. Open Vet J. 2022; 12(6): 839-850. doi:10.5455/OVJ.2022.v12.i6.8 Vancouver/ICMJE Style Ahmad S, Hair-bejo M, Hussein EA, Awad EA, Saeed MI, Sze LP, Zakaria Z. Pathogenicity of Salmonella enterica subspecies enterica serovar Enteritidis phage type 1 in one-day-old specific pathogen free chicks. Open Vet J. (2022), [cited July 01, 2025]; 12(6): 839-850. doi:10.5455/OVJ.2022.v12.i6.8 Harvard Style Ahmad, S., Hair-bejo, . M., Hussein, . E. A., Awad, . E. A., Saeed, . M. I., Sze, . L. P. & Zakaria, . Z. (2022) Pathogenicity of Salmonella enterica subspecies enterica serovar Enteritidis phage type 1 in one-day-old specific pathogen free chicks. Open Vet J, 12 (6), 839-850. doi:10.5455/OVJ.2022.v12.i6.8 Turabian Style Ahmad, S., M. Hair-bejo, Elawad A. Hussein, E. A Awad, Mohamed Ibrahim Saeed, Liew Pit Sze, and Zunita Zakaria. 2022. Pathogenicity of Salmonella enterica subspecies enterica serovar Enteritidis phage type 1 in one-day-old specific pathogen free chicks. Open Veterinary Journal, 12 (6), 839-850. doi:10.5455/OVJ.2022.v12.i6.8 Chicago Style Ahmad, S., M. Hair-bejo, Elawad A. Hussein, E. A Awad, Mohamed Ibrahim Saeed, Liew Pit Sze, and Zunita Zakaria. "Pathogenicity of Salmonella enterica subspecies enterica serovar Enteritidis phage type 1 in one-day-old specific pathogen free chicks." Open Veterinary Journal 12 (2022), 839-850. doi:10.5455/OVJ.2022.v12.i6.8 MLA (The Modern Language Association) Style Ahmad, S., M. Hair-bejo, Elawad A. Hussein, E. A Awad, Mohamed Ibrahim Saeed, Liew Pit Sze, and Zunita Zakaria. "Pathogenicity of Salmonella enterica subspecies enterica serovar Enteritidis phage type 1 in one-day-old specific pathogen free chicks." Open Veterinary Journal 12.6 (2022), 839-850. Print. doi:10.5455/OVJ.2022.v12.i6.8 APA (American Psychological Association) Style Ahmad, S., Hair-bejo, . M., Hussein, . E. A., Awad, . E. A., Saeed, . M. I., Sze, . L. P. & Zakaria, . Z. (2022) Pathogenicity of Salmonella enterica subspecies enterica serovar Enteritidis phage type 1 in one-day-old specific pathogen free chicks. Open Veterinary Journal, 12 (6), 839-850. doi:10.5455/OVJ.2022.v12.i6.8 |