Open Veterinary Journal, (2021), Vol. 11(3): 517–524
Original Research
10.5455/OVJ.2021.v11.i3.25
Molecular characterization and in vivo pathogenicity study of Listeria monocytogenes isolated from fresh and frozen local and imported fish in Jordan
Yaser Tarazi1* , Saeb El-Sukhon1, Adil Al-Rahbi2 and Zuhair Bani Ismail3
, Saeb El-Sukhon1, Adil Al-Rahbi2 and Zuhair Bani Ismail3
1Department of Basic Veterinary Sciences, Faculty of Veterinary Medicine, Jordan University of Science and Technology, Irbid, Jordan
2Ministry of Agriculture, Fisheries and Water Resources, Mascat, Oman
3Department of Clinical Veterinary Medicine, Faculty of Veterinary Medicine, Jordan University of Science and Technology, Irbid, Jordan
*Corresponding Author: Yaser Tarazi. Department of Basic Veterinary Sciences, Faculty of Veterinary Medicine, Jordan University of Science and Technology, Irbid, Jordan. Email: tarazi [at] just.edu.jo
Submitted: 21/06/2021 Accepted: 01/09/2021 Published: 30/09/2021
© 2021 Open Veterinary Journal
This is an Open Access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives License (http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited, and is not altered, transformed, or built upon in any way.
Abstract
Background: Listeria monocytogenes (L. monocytogenes) is a serious zoonotic and food transmitted human pathogen causing meningitis and abortions. Several outbreaks of listeriosis have been associated with the consumption of ready-to-eat food products; dairy, meat, fish, and contaminated fruits and vegetables worldwide.
Aim: This study was designed to detect and characterize L. monocytogenes isolated from local and imported fish in Jordan.
Methods: A total of 170 fish (70 local and 100 imported), of which 140 fresh and 30 frozen samples were used in this study. Listeria monocytogenes was cultured and initially identified using conventional microbiological methods. For confirmation and serotyping of the L. monocytogenes isolates, PCR techniques were used. Using oral and intraperitoneal administration, mice were used to determine the pathogenicity and LD50 of the isolated L. monocytogenes.
Results: A total of 72 Listeria spp. isolates were cultured from fish. Of those, 24 were positively identified as L. monocytogenes. Other strains of Listeria spp. were L. ivanovii (21), L. innocua (11), and L. grayi (16). Serotyping of the L. monocytogenes indicated that 14 isolates belonged to the 1/2b, 3b serotypes whereas 10 isolates belonged to the 4a and 4c serotypes. All isolates were virulent to mice with an LD50 dose ranging from 3 × 1010 CFU/ml to 3 × 107.5 CFU/ml. All the virulent isolates belonged to the serotype 1/2b. Histopathologically, dead mice showed multiple necrotic lesions in the liver and spleen.
Conclusion: Results of this study showed the presence of potentially pathogenic L. monocytogenes in fresh and frozen, local, and imported fish in Jordan. Strict monitoring and quality control regulatory measures must be adopted to prevent future outbreaks of food poisoning associated with fish consumption.
Keywords: L. monocytogenes, Fish, Serotypes, Virulence, Nested PCR.
Introduction
Members of the genus Listeria are widely distributed in the environment and consist of eight species; L. monocytogenes, L. ivanovii, L. seeligeri, L. innocua, L. welashimari, L. grayi, L. marthii and L. rocourtiae (Skowron et al., 2019). Listeria monocytogenes survive under food preservation conditions, including pH, salinity, and refrigeration (Swaminathan and Gerner-Smidt, 2007; Buchanan et al., 2017; Jordan and McAuliffe, 2018; Cabal et al., 2019). It can also withstand the high pressure and light therapy commonly used to control the growth of organisms in food (Buchanan et al., 2017; Jordan and McAuliffe, 2018; Cabal et al., 2019).
Listeria monocytogenes is an important zoonotic and food-borne human pathogen causing listeriosis. Listeriosis is a serious disease characterized by acute meningitis and spontaneous abortions commonly affecting pregnant women, neonates, adults, and immunocompromised patients (Wang et al., 2015; Jensen et al., 2016; Moura et al., 2016; Skowron et al., 2019; Chen et al., 2020; Li et al., 2020). In recent years, several outbreaks were reported worldwide, most often associated with consuming ready-to-eat food (RTE) products (Jami et al., 2014; European Centre for Disease Prevention and Control and European Food Safety Authority, 2018, 2019). Recently, 22 cases of food-borne listeriosis were reported in 5 EU countries: Denmark (9 cases), Estonia (6), Finland (2), France (1), and Sweden (4) (European Centre for Disease Prevention and Control and European Food Safety Authority, 2019). In eight patients, infection was positively linked to the consumption of cold-smoked fish products (European Centre for Disease Prevention and Control and European Food Safety Authority, 2019).
In Jordan, fresh and frozen fish are commonly consumed by people, surprisingly no outbreaks of listeriosis associated with fish consumption have been reported. However, Listeria exist in fish and fish products may cause significant public health concern. Therefore, this study aimed to isolate, characterize, and serotype Listeria species from fresh and frozen, local and imported fish in Jordan and to determine their virulence factors and in vivo pathogenicity in mice.
Materials and Methods
Fish sample collection
A total of 170 fish samples were used in this study (40 local and 130 imported) of which 140 were fresh and 30 frozen fish. Imported fish were from Yemen, Egypt, Qatar, Saudi Arabia, and Oman. Samples belonged to various fish types, including Nemipterus tambuloides, Lutjanus malabaricus, Lethrinus lentjan, Lutjanus monostigma, and Nemipterus nematophorus. Whole fish were collected and placed in an ice box and transported to the laboratory for immediate processing.
Bacterial culture and identification
All processing techniques were conducted under strict aseptic conditions. Each fish sample was divided into three tissue portions: the gills, intestine, and scales/muscles (in total 510 portions). From each portion, 50 g were homogenized (Karl Kolb, Germany) in 150 ml sterile saline. Then, 0.1 ml of the homogenate was cultured on sheep blood agar (Oxoid, UK), tryptic soy agar (Oxoid, UK), Fraser broth (Conda, Spain), and Listeria selective chromogenic base supplemented with polymyxin B, ceftazidime, nalidixic acid, and cycloheximide (Conda, Spain). All the inoculated media were incubated aerobically at 37°C for 24–48 hours. The inoculated broth tubes were kept in the refrigerator for 2–4 weeks (cold enrichment). Suspected L. monocytogenes isolates were identified initially based on culture and Gram stain characteristics. Biochemical characterization was followed using the esculin hydrolysis, hemolysis assay, and Microgen Listeria 12L Kit (Microgen, USA) according to the manufacturer’s instructions. Finally, the identified L. monocytogenes isolates were frozen and stored in brain-heart infusion broth (BHI, Merck) with 15% glycerol at −80°C until further analysis was performed. This study used the reference bacterial strain L. monocytogenes (ATCC 19116) as positive control.
Molecular identification of L. monocytogenes
The genomic DNA was extracted from isolated strains of L. monocytogenes using i-genomic BYF DNA Extraction Mini Kit (Boca Scientific, USA) according to the manufacture guidelines. Nested PCR was used to positively identify L. monocytogenes targeting the universal hlyA gene of L. monocytogenes (Mojgani et al., 2006) using commercially primers (Intron Biotechnology, USA). Nested PCR of two rounds were performed. In the first round, the primer pair LM1 (5ʹ-CCTAAGACGCCAATCGAA- 3ʹ) and LM2 (5ʹ-AAGCGCTTGCAACTGCTC-3ʹ) derived from the listeriolysin O gene were used for PCR products target hlyA gene of L. monocytogenes. The amplification conditions were initial denaturation at 95°C for 3 minutes, followed by 30 cycles denaturation at 94°C for 1 minute, primer annealing at 60°C for 1 minute, DNA extension at 72°C for 1 minute. A final extension completed the reaction at 72°C for 5 minutes, and the PCR product of 702 bp fragment of the hlyA gene was cooled at 4°C. The second round of nested PCR was conducted using the nested primers: LL5 (5ʹ-AACCTATCCAGGTGCTC-3ʹ) and LL6 (5ʹ- CTGTAAGCAATTTCGTC-3ʹ) targeting the hlyA gene. The amplification conditions were described in round one, except primer annealing was at 55°C for 1 minute. The PCR products were viewed under UV light after being run in agarose gel electrophoresis stained with ethidium bromide. Real time PCR was used to amplify L. monocytogenes specific gene prfA using the PowerChek Kit (Kogene Biotech, South Korea). The PCR products were viewed under UV light after being run in agarose gel electrophoresis stained with ethidium bromide.
Serotyping of L. monocytogenes
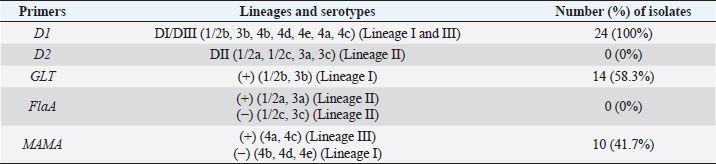
All L. monocytogenes were subjected to serotyping using a PCR (Bio-Rad, USA). The D1, D2, GLT, FlaA, and MAMA primers (Table 1) have been used at different PCR conditions (Table 2). The serotyping procedure was performed according to the study reported by Borucki and Call (2003).
Mouse lethality assay
To determine the lethality and discriminate between virulent and avirulent isolates, 0.5 ml of 3 × 109 CFU/ml of each isolate was injected intraperitoneally into mice (Swiss Albino, strain BALB/C), reared, and reproduced at the Animal House unit at the Faculty of Veterinary Medicine/JUST. The L. monocytogenes (ATCC 19116) reference strain was used as a positive control. Monitoring of mice was extended for 2 weeks (Liu, 2004).
In vivo determination of LD50 was performed using 5 mice groups (n=8). Mice in each group were inoculated intraperitoneally with 0.5 ml of different dilutions of 24 hours old culture of L. monocytogenes (100, 10−1, 10−2, 10−3, and 10−4). Mice were monitored daily, and after 2 weeks, all mice have died. The death time and the mortalities were recorded and compared with the number and time of deaths induced by the reference strain. All dead mice were subjected to necropsy and re-isolation of L. monocytogenes (Liu, 2004).
In vivo pathogenicity study of fish tissue contaminated with L. monocytogenes
Twelve mice were used in this experiment. Two mice were fed 100 g (50 g each) of sterile fish tissue (that did not show any bacterial growth when inoculated on different bacteriological media) after being fasted for 12 hours. Another two mice were injected intraperitoneally with 0.5 ml of sterile fish tissue homogenate (which was prepared by homogenizing 50 g fish tissue (Karl Kolb homogenizer, Germany) in 150 ml sterile normal saline). The two mice fed orally with sterile fish tissue, and the two mice injected intraperitoneally with sterile tissue homogenate were considered as control groups. The same protocols were repeated using contaminated fish tissues with 3 × 109 CFU/ml of L. monocytogenes (4 mice were fed 50 g each, and 2 mice were injected intraperitoneally with 0.5 ml of contaminated fish tissue homogenate). The last group (two mice) was injected intraperitoneally with 3 × 109 CFU/ml of the L. monocytogenes. All of the mice were monitored daily for 2 weeks.
Table 1. Primers used to serotype L. monocytogenes isolated from fish samples in Jordan.
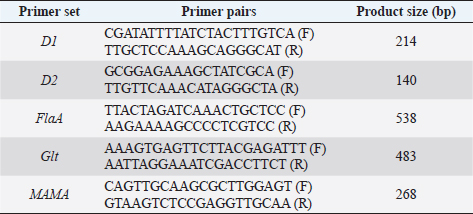
Table 2. Primers and PCR conditions used to serotype L. monocytogenes isolated from fish samples in Jordan.
Histopathology
All dead mice were subjected to a thorough necropsy procedure. Tissue samples from the liver, spleen, brain, and heart were collected in 10% buffered formalin, dehydrated in alcohol, embedded in paraffin wax, cut at 5 microns (Microtome, Thermo Scientific, USA), and stained using hematoxylin and eosin stain. The stained sections were examined under a light microscope.
Ethical approval
Treatment and the maintenance of the animals were in accordance with the Jordan University of Science and Technology Animal Care and Use Committee which follows the international animal care and use guidelines (Institute of Laboratory Animal Resources (ILAR), 1996).
Results
The prevalence of Listeria spp. in fresh and frozen fish in Jordan are presented in Tables 3 and 4. A total of 72 Listeria isolates were obtained from 170 examined fish. Eighteen L. monocytogenes isolates were positively identified from fresh fish and six isolates from frozen fish. Additionally, 16, 9, and 10 Listeria species isolated from fresh fish, and other 5, 2, and 6 isolates from the frozen fish were diagnosed as L. ivanovii, L. innocua, and L. grayi, respectively.
Listeria monocytogenes were isolated and confirmed using PCR. Figure 1 shows bands corresponding to the 454 bp target prfA gene specific for the L. monocytogenes. Lanes representing L. grayi, L. innocua, and L. ivanovii were negative for this gene. In Figure 2, lanes 1–27 lanes (except lanes 5, 14, and 26) represent L. monocytogenes isolates with bands of 267 bp of the target hlyA gene. Lanes 5, 14, and 26 represent L. grayi, L. innocua, and L. ivanovii, respectively, which were negative for this gene.
Results of the serotyping of L. monocytogenes are presented in Figure 3 and Table 5. Fourteen isolates (58%) revealed bands of 483 bp that match the Glt primer band, which indicates that these isolates fall within the spectrum of 1/2b, 3b serotyping. The rest 10 (42%) isolates that were found negative with the Glt primer were run using the 268 bp MAMA primer. They all showed bands corresponding to that of the MAMA primer, indicating that these isolates fall within the 4a and 4c serotypes. Serotyping of the L. monocytogenes indicated that 14 isolates belonged to the 1/2b, 3b serotypes whereas 10 isolates belonged to the 4a and 4c serotypes.
Table 3. Number of Listeria spp. isolates from different portions of fresh and frozen fish in Jordan.
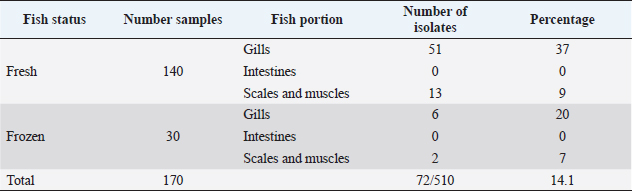
Table 4. Number and percentages of Listeria species isolated from fresh and frozen fish in Jordan.
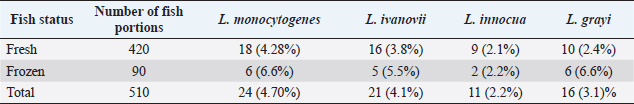
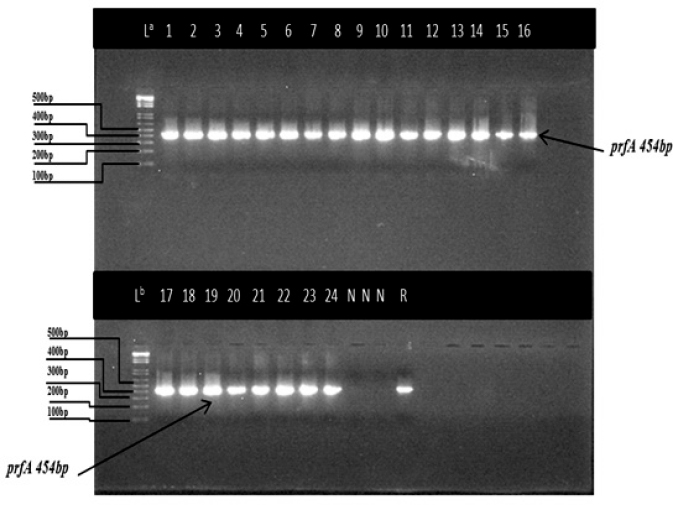
Fig. 1. Agarose gel electrophoresis (2%) image representing the prfA gene of L. monocytogenes isolates from fresh and frozen fish. Lanes: La and Lb: 100 bp molecular weight marker, Lanes: 1–24 prfA 454 bp specific for L. monocytogenes, Lanes N: no bands, negative for (L. grayi, L. innocua, and L. ivanovii), Lanes (R) positive reference strain (ATCC 1932).
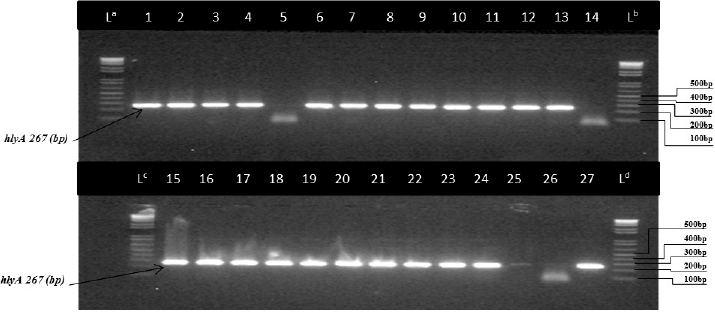
Fig. 2. Image of the nested PCR product on agarose gel electrophoresis (2%) targeting the hlyA gene of L. monocytogenes isolates from fresh and frozen fish. Lanes: (La, Lb, Lc, and Ld): 100 bp molecular weight marker. Lanes:1, 2, 3, 4, 6, 7, 8, 9, 10, 11, 12, 13, 15, 16, 17, 18, 19, 20, 22, 23, 24, 25, and 27 represent L. monocytogenes while lanes 5, 14, and 26 represent L. grayi, L. innocua, and L. ivanovii, respectively.
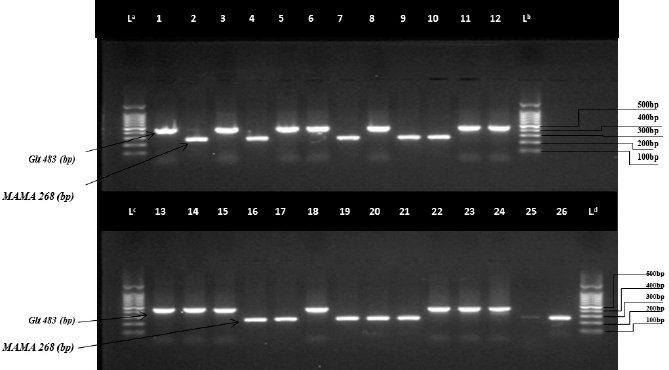
Fig. 3. PCR results of serotyping by use of Glt and MAMA primers. Agarose gel electrophoresis (1.5%) of L. monocytogenes isolated from fresh and frozen fish. Lanes: (La, Lb, Lc, and Ld) 100 bp molecular weight markers. Lanes: 1, 3, 5, 6, 8, 11, 12, 13, 14, 15, 18, 22, 23, and 24 (+) product amplified by the primer sets of the amplicon size product 483 (bp) Glt gene for all L. monocytogenes isolates. Lanes: 2, 4, 7, 9, 10, 16, 17, 19, 20, 21, and 25 (+) product amplified by the primers sets of the amplicon size product 268 (bp) MAMA gene for all L. monocytogenes isolates. Lane 26: positive control for the (ATCC 19116) MAMA gene.
All Listeria isolates were used in the mouse lethality assay. Only 8 out of 24 L. monocytogenes isolates were lethal to mice within 3–6 days. Mice inoculated with other Listeria species (21 L. ivanovii, 11 L. grayi, and 16 L. innocua) survived the entire length of the experiment (14 days).
Eight isolates of L. monocytogenes were used to determine the LD50 in mice. The results showed that 0.5 ml of 3 × 1010 CFU/ml of the reference strain resulted in 100% mortality while 3 × 109 CFU/ml of the bacteria resulted in 50% mortality. The LD50 of the eight isolates were 3 × 1010, 3 × 109.5, 3 × 107.5, 3 × 109, 3 × 109.5, 3 × 109.5, 3 × 107.5, and 3 × 108.5 CFU/ml.
Table 5. PCR serotyping of L. monocytogenes isolates (n =24) from fresh and frozen fish in Jordan.
In the in vivo pathogenicity assay, one isolate was found lethal after intraperitoneal inoculation without using the fish tissue as a vector. None of the two mice fed with sterile fish tissue homogenate had died whereas one out of the four mice fed with contaminated fish tissue had died. On the other side, the two mice that were inoculated intraperitoneally with sterile fish tissue survived over the entire period of the experiment, whereas the two mice that were inoculated intraperitoneally with contaminated fish tissue had died. All the virulent isolates were belonging to the serotype 1/2b.
Histopathologically, mice infected with LD50 of L. monocytogenes showed multiple areas of necrotic hepatocytes that are replaced with a large number of inflammatory cells as compared with normal liver. The spleen showed depletion and necrosis of lymphoid follicles as compared with the normal spleen. No lesions were seen in the brain.
Discussion
Jordan imports large quantities of fresh and frozen fish products (3,080.588 tons) for human consumption (Jordan Ministry of Agriculture (MOA) Annual Reports, 2008, 2010, 2013). Domestic fish production reached about 1,040 tons in 2010. About half of this production comes from farms in Jordan Valley. In 2008, about 160 tons were caught from the Gulf of Aqaba (Jordan Ministry of Agriculture (MOA) Annual Reports, 2008, 2010). In the last 3 years, fish consumption has increased as a reflection of a remarkable jump in the price of red meat (Jordan Ministry of Agriculture (MOA) Annual Reports, 2013). Although no outbreaks of listeriosis associated with fish consumption have been reported in Jordan, Listeria spp. exist in fish and fish products which may cause significant public health concerns. In fact, L. monocytogenes have previously been recovered from cheese, beef, and chickens in Jordan (El-Sukhon, 1993; Awaisheh, 2010). In this study, several species of Listeria, other than L. monocytogenes were recovered from fresh and frozen fish in Jordan for the first time that may need pathogenicity study.
In this study, 17% of sea fish samples collected from the fish selling markets in different locations were contaminated with Listeria 12–24 hours post fishing. This may reflect the unhygienic environment and the high climatic temperatures of the study locations. However, fresh fish samples originated from rivers and dams in Jordan and collected from fish selling markets showed a very high (77.5%) Listeria contamination. This may reflect water contamination of rivers and dams in Jordan (Fandi et al., 2009; Nwachukwu et al., 2010). In addition to transport factor and storing conditions of fish in dirty polyester boxes commonly used to store and display fish on the ground in the public markets, which increases the vulnerability to microbial contamination of fish. Circumstantial factors, such as handling, selling environment, and using unclean utensils and surfaces may influence the hygienic status of fresh fish and cross contamination after being in contact with raw foods that may increase Listeria contamination. These findings are similar to other studies where a high (81%) incidence of Listeria was recovered from freshwater fish than that (30%) in seawater fish (Nwachukwu et al., 2010).
In this study, Listeria contamination in the imported fresh sea fish samples was 22.5% and in the frozen fish was 26.6%. Listeria monocytogenes may naturally be present in the soil adjacent to the riverbanks as Listeria is frequently found in soil and water environments (Schaffter and Parriaux, 2002). The widespread of L. monocytogenes in soil has often been attributed to contamination from decaying plant and fecal material, with damp surface soil providing a cool, moist protective environment and the decaying material that act as substrate, which together enables the survival of L. monocytogenes from season to season (Gahan and Hill, 2014).
In this study, the incidence of L. monocytogenes was relatively high (22.5%) and (26.6%) in fresh and frozen fish, respectively. In Northern Spain, 25% of RTE smoked fish were contaminated with L. monocytogenes (Garrid et al., 2009). Variations in the rate of Listeria recovery from fish and fish products in different parts of the world are expected due to different methods used to collect samples, different standards in the handling and hygiene of fish during storage, transportation, and market displaying conditions.
No previous studies to characterize and determine the pathogenicity of L. monocytogenes isolated from fish could be found in Jordan. The virulent strains of L. monocytogenes determined in this study were belonging to the 1/2b serotype. However, most virulent isolates from beef and poultry in Jordan belonged to the 1/2a, 1/2b, 1/2c, and 4b serotypes (Awaisheh, 2010). It was reported that serotype 1/2b is responsible for clinical listeriosis in humans (Heymann, 2008).
In the mouse lethality assay, seven L. monocytogenes isolates were lethal to mice with LD50 ranging between 3 × 1010 and 3 × 107.5 CFU/ml. Such diversity in LD50 values has been reported by others (Liu, 2004). The virulence and lethality assay results are in accordance with recorded serotyping results where the seven virulent isolates belonged to the serotype 1/2b. The serotypes 1/2b along with the 1/2a, and 4b serotypes are responsible for more than 96% of human listeriosis (Heymann, 2008). All the 24 L. monocytogenes isolates were found to belong to lineage I and III. Of those, 14 belonged to serotypes 1/2b, 3b, 7 of them were lethal to mice. These data raise an important alarm indicating potential human health hazard.
In the pathogenicity assay, none of the orally fed mice or intraperitoneally injected with sterile fish tissue or sterile fish tissue homogenate were died. While two of two mice injected intraperitoneally with fish tissue homogenate contaminated with 3 × 109 CFU/ml L. monocytogenes were died. Also, one of two mice injected intraperitoneally with 3 × 109 CFU/ml L. monocytogenes without fish tissue vector were died, and one of four mice orally fed with contaminated fish tissue was died. All these results confirm that L. monocytogenes are virulent and lethal to mice. This pathogenicity assay demonstrates the presence of virulent L. monocytogenes strains in fish in Jordanian markets. In Jordan, no standards on the allowable numbers of L. monocytogenes in seafood products are available. Therefore, the conduction of further studies becomes crucial to determine L. monocytogenes impact on public health, depending on international standards.
To control and reduce L. monocytogenes hazard in Jordan, strict implementation of the Hazard Analysis and Critical Control Point system and the Sanitation Standard Operation Procedure should be adopted at the retail level, fish markets, and storage conditions (Holah et al., 2004). On the other hand, educating consumers and fish market dealers minimizes the risk of listeria infection (Buchanan et al., 2017; Kosa et al., 2007).
Conclusion
Virulent strains of L. monocytogenes and other strains of Listeria spp. have been recovered from fresh and frozen, local and imported fish in Jordan. Strict monitoring and quality control regulatory measures must be adopted to prevent future outbreaks of food poisoning associated with fish consumption.
Acknowledgements
The authors would like to thank the Deanship of Research at Jordan University of Science and Technology for their support.
Author contributions
YHT and SE conceived, designed, and supervised the project, performed analysis and/or interpretation of data. AA collected laboratory samples, conducted the practical part of the study, conducted biochemical analyses, and drafted the manuscript. ZBI carried out final writing and critical review/revision of the manuscript. YHT and SE had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of interest
The authors declare that they have no conflicts of interests.
Funding
This work was supported by a grant from Deanship of Research at Jordan University of Science and Technology, Jordan (grant number 15/2012). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
Awaisheh, S.S. 2010. Incidence and contamination level of Listeria monocytogenes and other Listeria spp. in ready-to-eat meat products in Jordan. J. Food Prot. 73, 535–540.
Borucki, M.K. and Call, D.R. 2003. Listeria monocytogenes serotype identification by PCR. J. Clin. Microbiol. 41(12), 5537–5540.
Buchanan, R.L., Gorris, L.G.M, Hayman, M.M., Jackson, T.C. and Whiting, R.C. 2017. A review of Listeria monocytogenes: an update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control 75, 1–13.
Cabal, A., Pietzka, A., Huhulescu, S., Allerberger, F., Ruppitsch, W. and Schmid, D. 2019. Isolate-based surveillance of Listeria monocytogenes by whole genome sequencing in Austria. Front. Microbiol. 10, 2282.
Chen, S., Meng, F., Sun, X., Yao, H., Wang, Y., Pan, Z., Yin, Y. and Jiao, X. 2020. Epidemiology of human listeriosis in China during 2008–2017. Foodborne Pathog. Dis. 7(2), 119–125.
El-Sukhon, S.N. 1993. Bacteriological analysis of white brined (Nabulsian) cheese with reference to Listeria monocytogenes in Jordan. J. Egypt. Vet. Med. Assoc. 53, 139–144.
European Centre for Disease Prevention and Control and European Food Safety Authority. 2018. Multicounty outbreak of Listeria monocytogenes sequence type 8 infections linked to consumption of salmon products– 25 October 2018. Stockholm, Sweden and Parma, Italy: ECDC/EFSA.
European Centre for Disease Prevention and Control and European Food Safety Authority. 2019. Multicounty outbreak of Listeria monocytogenes clonal complex 8 infections linked to consumption of cold-smoked fish products – 4 June 2019. Stockholm and Parma: ECDC/EFSA.
Fandi, K.G., Qudsieh, I.Y., Muyibi, S.A. and Massadeh, M. 2009. Water Pollution Status Assessment of King Talal Dam, Jordan. Adv. Enviro. Biol. 3(1), 92-100.
Gahan, C.G.M. and Hill, C. 2014. Listeria monocytogenes: Survival and adaptation in the gastrointestinal tract. Front. Cell. Infect. Microbiol. 4, 9.
Garrido, V., Vitas, A.I. and Garcia-Jalon, I. 2009. Survey of Listeria monocytogenes in ready-to-eat products: prevalence by brands and retail establishments for exposure assessment of listeriosis in Northern Spain. Food Control 78, 1–6.
Heymann, D.L. 2008. Listeriosis. Control of communicable diseases manual, 19th ed. Washington, DC: American Public Health Association.
Holah, J.T., Bird, J. and Hall, K.E. 2004. The microbial ecology of high-risk, chilled food factories; evidence for persistent Listeria spp. and Escherichia coli strains. J. Appl. Microbiol. 97, 68–77.
Institute of Laboratory Animal Resources (ILAR). 1996. Guide for the care and use of laboratory. Washington, DC: National Academy Press
Jami, M., Ghanbari, M., Zunabovic, M., Doming, K.J. and Kneifel, W. 2014. Listeria monocytogenes in aquatic food products-a review. Compr. Rev. Food Sci. Food Saf. 13, 798–813.
Jensen, A.K., Björkman, J.T., Ethelberg, S., Kiil, K., Kemp, M. and Nielsen, E.M. 2016. Molecular typing and epidemiology of human listeriosis cases, Denmark, 2002–2012. Emerg. Infect. Dis. 22(4), 625–633.
Jordan, K. and McAuliffe, O. 2018. Listeria monocytogenes in foods. Adv. Food Nutr. Res. 86, 181–213.
Jordan Ministry of Agriculture (MOA) Annual Reports. 2008, 2010, 2013. Available via https://www.agriculture.gov.fj/documents/annualreport/AR2013.pdf
Kosa, K.M., Cates, S.C., Karns, S., Godwin, S.L. and Chambers, D. 2007. Consumer home refrigeration practices. Results of a web-based survey. J. Food Prot. 174(70), 1640–1649.
Li, C., Zeng, H., Ding, X., Chen, Y., Liu, X., Zhou, L., Wang, X., Cheng, Y., Hu, S., Cao, Z., Liu, R. and Yin, C. 2020. Perinatal listeriosis patients treated at a maternity hospital in Beijing, China, from 2013–2018. BMC Infect. Dis. 20(1), 601.
Liu, D. 2004. Listeria monocytogenes: comparative interpretation of mouse virulence assay. FEMS Microbiol. Lett. 233, 159-164.
Mojgani, N., Taqizadeh, M. and Ashtiani, M.P. 2006. Rapid detection of Listeria monocytogenes in clinical samples by targeting hlyA gene in a PCR reaction and its comparison with routine culture method. Eur. J. Sci. Res. 11(3), 151–157.
Moura, A., Criscuolo, A., Pouseele, H., Maury, M.M., Leclercq, A., Tarr, C., Björkman, J.T., Dallman, T., Reimer, A., Enouf, V., Larsonneur, E., Carleton, H., Bracq-Dieye, H., Katz, L.S., Jones, L., Touchon, M., Tourdjman, M., Walker, M., Stroika, S., Cantinelli, T., Chenal-Francisque, V., Kucerova, Z., Rocha, E.P., Nadon, C., Grant, K., Nielsen, E.M., Pot, B., Gerner-Smidt, P., Lecuit, M. and Brisse, S. 2016. Whole genome-based population biology and epidemiological surveillance of Listeria monocytogenes. Nat. Microbiol. 2, 16185.
Nwachukwu, N.C., Orji, F.A., Iheukwumere, I. and Ekeleme, U.G. 2010. Antibiotic Resistant Environmental Isolates of Listeria monocytogenes from Anthropogenic Lakes in Lokpa-Ukwu, Abia State of Nigeria. Aust. J. Basic Appl. Sci. 4(7), 1571-1576.
Schaffter, N. and Parriaux, A. 2002. Pathogenic-bacterial water contamination in mountainous catchments. Water Res. 36, 131–139.
Skowron, K., Wiktorczyk, N., Grudlewska, K., Wałecka-Zacharska, E., Paluszak, Z., Kruszewski, S. and Gospodarek-Komkowska, E. 2019. Phenotypic and genotypic evaluation of Listeria monocytogenes strains isolated from fish and fish processing plants. Ann. Microbiol. 69, 469–482.
Swaminathan, B. and Gerner-Smidt, P. 2007. The epidemiology of human listeriosis. Microbes Infect. 9, 1236–1243.
Wang, P., Chen, Y., Wang, H., Yang, S., Xu, Y. and Li, T. 2015. A clinical analysis of 16 patients with maternal listeriosis. Zhonghua Nei Ke Za Zhi 54(9), 763–767.
















