| Original Article | ||
Open Vet J. 2022; 12(5): 744-753 Open Veterinary Journal, (2022), Vol. 12(5): 744–753 Original Research Subconjunctival use of allogeneic mesenchymal stem cells to treat chronic superficial keratitis in German shepherd dogs: Pilot studyAlexandre Luiz Pereira1*, Maura Krähembuhl Wanderley Bittencourt1, Michele Andrade Barros2, Rodolfo Malago1, João Flavio Martins Panattoni2, Bruna Pereira de Morais2, Fabiano Montiani-Ferreira3 and Jose Paulo Cabral Vasconcellos11Department of Ophthalmology, Faculty of Medical Sciences, UNICAMP, Campinas, Brazil 2Regenera Stem Cells Laboratory, Campinas, Brazil 3Department of Veterinary Medicine, Federal University of Paraná, Curitiba, Brazil *Corresponding Author: Alexandre Luiz Pereira. Department of Ophthalmology, Faculty of Medical Sciences, UNICAMP, Campinas, Brazil. Email: vet.arcadenoe [at] hotmail.com Submitted: 21/03/2022 Accepted: 18/09/2022 Published: 07/10/2022 © 2022 Open Veterinary Journal
AbstractBackground: Chronic superficial keratitis (CSK) is an ocular condition in dogs characterized by corneal opacification leading to visual function impairment. Control of this chronic condition requires the use of topical immunomodulators or corticosteroids daily. Regenerative medicine has shown promising results in several fields of medicine. Aim: The aim of this study was to evaluate the clinical effect of allogeneic mesenchymal stem cells (MSCs) of adipose tissue applied via subconjunctival in dogs with CSK. Methods: A series of cases of eight dogs diagnosed with CSK were divided into two groups, four dogs each; the conventional treatment group received prednisolone 1% as topical eye drops and the experimental group (EG) received allogeneic MSCs transplantation. The dogs had not previously been treated for CSK. Systemic and ophthalmologic examinations were performed to exclude other abnormalities. An administered amount of MSC (1 × 106 cells each time) was injected via subconjunctival in the peri-limbal region at 0 and 30 days. The animals were followed for 110 days for clinical evaluation, and, at the same time, the images of the corneal abnormalities were obtained and analyzed in the ImageJ software. The statistical analysis was performed in the GrandPrism 7.0 software. Results: Initial and final images revealed that areas with neovascularization, inflammatory infiltrate, and opacity regressed in most eyes in both groups (7/8 eyes in each group) at the end of the 110 days, p=0.0391 and p=0.0078 respectively, but this response was minor in the EG comparing to conventional group (CG) (p=0.026). No local or systemic side effects were observed. Conclusions: Despite the small melioration, MSCs treatment suggests clinical improvement in patients with CSK after 110 days without any local or systemic side effects. However, the improvement achieved was significantly less than the observed within CG. Further studies still are needed to evaluate the use and benefits of stem cells as an adjunct treatment for CSK. Keywords: Autoimmune diseases, Chronic superficial keratitis, Cornea, Mesenchymal stem cells, Regenerative medicine. IntroductionChronic superficial keratitis (CSK) or Uberreiter Syndrome (Überreiter, 1961) represents a chronic, inflammatory non-ulcerative disease of the superficial corneal stroma, which causes significant damage and consequent visual impairment in dogs (Balicki, 2012). This condition is particularly common in German shepherds, Greyhounds, and Belgian Shepherd dogs, but other breeds may also be affected (Williams, 2005; Drahovska et al., 2014). The disease also affects humans (Espana et al., 2004; Nicholas and Mysore, 2021) and affected dogs may serve as a model species for this disease. The CSK diagnoses are usually made through typical clinical appearance and breed association. Clinical signs of the disease are conjunctival redness and corneal pigmentation bilaterally. Corneal lesion development, usually in its ventrolateral region, has a typical pattern of cellular infiltration from the limbus into the cornea and the conjunctiva adjacent to the cornea-affected region presents signs of inflammation. In the early stages of CSK, epithelial cells proliferate and the superficial stroma is infiltrated by plasma cells and lymphocytes (Slatter, 2005; Balicki et al., 2021). As the disease progresses, melanocytes, histiocytes, and fibrocytes enter the cornea, leading to edema and neovascularization. In the advanced stages, the epithelium and anterior stroma are heavily pigmented and vascularized. It has also been suggested that an autoimmune mechanism is involved in the pathophysiology of this condition that shows a predominance of CD4+ lymphocytes in the infiltrate (Williams, 1999). Recent studies have shown a genetic basis for CSK (Jokinen et al., 2011; Barrientos et al., 2013; Drahovska et al., 2014; Cheng et al., 2016). Corticosteroids are used as anti-inflammatory and immunosuppressive agents and are generally accepted as a form of medical management for CSK (Slatter, 2005). The need for treatment throughout the lifetime exposes the patients to important side effects from corticosteroids. Thus, efforts are being made to develop alternative therapies to inhibit the immune response and chronic inflammatory processes in order to reduce the suffering of animals with CSK and the need for long periods of care. Many experimental tests have been done with therapies including contact lenses (Cheng et al., 2016), radiotherapy with surgical procedures (Denk et al., 2011), and immunosuppressive drugs (Williams et al., 1995; Nell et al., 2005; Balicki and Trbolova, 2010; Balicki, 2012). Recently, the use of tacrolimus topically has been considered to be the therapy of choice (Balicki and Trbolova, 2010; Balicki, 2012). Mesenchymal stem cells (MSCs) are a type of multipotent cell originally isolated from bone marrow which have subsequently been isolated from other tissues (Kerkis et al., 2006), such as adipose tissue (Zakirova et al., 2019). As stem cells can be induced to become a particular cell type, they have the potential to be used in several diseases such as osteoarthritis, diabetes, neurodegenerative diseases, and macular degeneration in order to replace damaged tissues (Zakrzewski et al., 2019). Recently, MSCs are a therapeutic approach with have been performed to treat ophthalmologic diseases in dogs with keratoconjunctivitis sicca (Villatoro et al., 2015; Bittencourt et al., 2016; Sgrignoli et al., 2019) and eosinophilic keratitis in cats (Villatoro et al., 2018). In these studies, MSCs transplantation was effective in reducing inflammation of the lacrimal gland in dogs and recovering normal lacrimal production and controlling corneal infiltration in cats. Additionally, there are many studies in animal models of corneal diseases showing the ability of these cells to influence and modulate excessive immune response (Yao et al., 2012; Lee et al., 2015). This potent action of MSCs as regulators of the immune response is related to the release of soluble bioactive factors PGE2, IL-1, IL-4, IL-6, TGF-β, and IDO in tissue (Nagaya et al., 2005; Caplan and Dennis, 2006; Sgrignoli et al., 2019). The cornea is an immune-privileged tissue (Stern et al., 2010) and its anatomical characteristics allow easy treatment assessment and evaluation of the therapeutic efficacy in live animals after MSCs transplantation. Additionally, dogs share corneal characteristics with humans leading to the translation of basic mechanistic models opening new potential therapeutic approaches in humans. In another hand, the advances in animal models could be applied directly in clinical veterinary medicine as well. This study aims to evaluate the treatment of CSK in dogs using subconjunctival allogeneic MSCs transplantation. At the same time, the short-term safety of subconjunctival transplantation of allogeneic MSCs will be assayed. This study, therefore sought, first, to investigate and describe the initial results of MSCs therapy in dogs with CSK without the concomitant use of conventional drug therapy and second, to compare with the conventional treatment (CT) with corticosteroids. Materials and MethodsThis is a prospective interventional case-control study comprising a series of eight cases of dogs with CSK diagnosis in German Shepherds. The inclusion criteria were: the presence of chronic, non-ulcerative keratitis, mainly in the lower lateral limbus with a combination of neovascularization and pigmentation that extends to the center of the cornea.; the dogs must have the phenotypic characterization of the breed "German shepherd" as shown in official sites of cynophilia: https://clubepastoralemao.com.br. The exclusion criteria were: the presence of other ocular conditions, such as corneal ulceration, dry keratoconjunctivitis, glaucoma, infectious keratitis or conjunctivitis, use of any medication including immunosuppressive agents, and any other ocular or systemic diseases. Serious adverse reactions that could compromise the animal's vision permanently or endanger his or her life were also considered criteria for stopping the study. A total of 16 eyes, of seven females and one male of the German shepherd breed, aged 4 to 10 years, were analyzed in this study. Clinical evaluationThe dogs were undergone to systemic and ophthalmologic evaluations which included abdominal ultrasound and chest X-ray, complete blood count, and serum biochemistry. The ophthalmologic evaluation consisted of a neuro-ophthalmological examination (dazzle reflex, photomotor pupillary reflex, and menace response test), Schirmer tear test-1 (STT-I characterized by measurement without the use of topical anesthesia; Schirmer Test, Ophthalmos®, São Paulo), applanation tonometry (Tono-Pen, Reichert Inc®, USA).), fluorescein test (Fluorescein strips, Ophthalmos®, São Paulo), slit lamp biomicroscopy (Kowa, Japan®), indirect ophthalmoscopy (Ocular Instruments®, USA), and corneoconjunctival cytology were also performed to identify different types of ocular surface cells to exclude neoplasia. The dogs were monitored clinically at 0, 30, and 110 days after diagnosis and treatment. The day of the first transplant was considered day 0. Comprehensive clinical and ophthalmologic examinations were performed for each visit including corneal surface evaluation with fluorescein, measurement of intraocular pressure, indirect ophthalmoscopy, and Schirmer's test. Normal values in dogs were STT-1 15–25 mm/minutes and normal tonometry of 10–25 mmHg. The clinical manifestation of pain and ocular discomfort in dogs is characterized by an increase in the natural rhythm of the blinking to the point of keeping the eyelids closed (blepharospasm), associated with increased tear production. The assessment of vision in dogs was based on ambulation tests in known and unknown environments in light and dark conditions observing their ability to deviate from obstacles. A menace response test was performed to evaluate the vision with the cognitive capacity of interpretation and several neurological pathways related to the cranial nerves. These signs were monitored on the days of follow-up and the owners were trained to observe and report the occurrence of variations of them at home. A subjective scale was standardized in relation to cornea transparency from 0 to 4, with being 0 the equivalent of a normal and 4 of a totally opaque cornea. The corneal surface was divided into four quadrants and evaluated how many quadrants were involved. The areas of corneal vascularization added to the areas with inflammatory infiltration defined the affected area, similar to that described by Balicki (2012). Pigmented areas were assessed separately comprising areas where the disease was in the inactive or scarring phase. ImageJ (ImageJ -http://imagej.nih.gov/ij/) was used to measure affected and pigmented areas based on photographs taken at each evaluation time, at 0, 30, and 110 days. The photographs were all taken by the same operator and produced under the same conditions, with the same lighting and equipment to ensure the consistency of the results. The images were taken by OLYMPUS CAMERA model S1030SW (resolution of 3648x2736 PIXELS (10Mp), at 10 cm eye distance. In the evaluation criteria in ImageJ, the total area of the cornea in a given image was defined as 100%. The affected area was a percentage of the total cornea from the summed areas of the neovascularization and opacity (inflammatory infiltrate). The same criterion was used to estimate the pigmented area. Ocular surface measurements were carried out by three examiners who were masked for the treatment (experimental treatment and CT) and the period of follow-up. Four dogs were included for each group of treatment: experimental with MSCs or CT with topical anti-inflammatories. The selection criteria were based on the owners’ preference, without the interference of the examiners, following given instructions on the pathology of their companion animals, and available treatment models. The dogs in the experimental treatment group received two applications of 1 million MSCs, with a 30-day interval between applications. The volume of injection of MSCs applied was 0.2 ml subconjunctivally near the limbus. The amount of MSCs applied was 1 × 106 as reported by Bittencourt et al. (2016) and Sgrignoli et al. (2019). The dogs in the CT group received topical administration of prednisone (Predfort® eye drops, 1 drop 3× daily, continuous use) and oral prednisolone 1mg/kg 1× daily until symptom remission (ranging from 10 to 20 days) followed by 50% of the dose for another 5 days (protocol recommendation of Brazilian Veterinary Committee). MSC and transplantationThe MSCs derived from dog’s adipose adipose tissue were obtained from the stem cell bank of the company Regenera Stem Cells® (Campinas/SP, Brazil), cryopreserved in individual flasks containing doses of 1 × 106 cells, ready for use. According to company information, the origin of the adipose tissue is peri-ovarian (10–15 g) collected during the castration of young donors (≤2 years) and healthy (negative for distemper, parvovirus, and leishmania). The collected adipose tissue was washed, fragmented, and incubated for 1 h in a saline solution buffered with Dulbecco’s phosphate with a 0.1% type I collagenase, and centrifuged. Subsequently, the cell precipitate was resuspended in culture (modified Eagle medium with high glucose concentration (LGC Biotechnology, São Paulo, Brazil), 15% fetal bovine serum (Hyclone Inc., Logan/UT), 1% penicillin/streptomycin, 1% of non-essential amino acids, and 1% L-glutamine (LGC Biotechnology) and incubated at 37°C and 5% CO2. When it reached approximately 70% confluence, the cells were trypsinized and expanded. Part of the material was separated for characterization, where the potential for differentiation of MSCs was assessed through its ability to differentiate into three different cell lines (osteogenic, adipogenic, and chondrogenic) under specific culture conditions. After the expansion process, the immunophenotypic profile of MSCs was evaluated through flow cytometry by expression of CD44, CD73, CD90, CD29, and the absence of CD45. All cells used were between passage 3 and passage 4. Furthermore, during and after the expansion process, the cells were also screened for the absence of pathogens and contaminants (e.g., bacteria, fungi, mycoplasma, and endotoxins). When the adipose tissue has already been sufficiently expanded, to obtain enough MSCs to carry out the protocol, the cells are collected, counted, and cryopreserved in a freezer at −80°C in duly labeled aliquots containing a bar code, which enables the traceability of the sample. For the thawing and washing of the cells, a thawing kit from the company was used, which guarantees viability above 70%. For clinical use, cryopreserved MSCs are supplied packed in dry ice along with a kit for three sequential washing. The result is a cell button at the bottom of the test tube with one million MSCs that were finally resuspended in 0.2 ml of sterile saline solution (0.9%; Eurofarma, São Paulo, SP, Brazil) and injected in the subconjunctival and lower lateral peri-limbal region using a 25 × 0.7 mm needle in a 1 ml syringe. For the MSCs transplantation procedure, the animals were sedated with propofol (5 mg/kg, Ourofino, Cravinhos, SP, Brazil) and tramadol (2 mg/kg, União Química, São Paulo, SP, Brazil) followed by topical eye anesthesia using proxymetacaine hydrochloride 1–2 drops (0.5%; Anestalcon; Alcon, São Paulo, SP, Brazil), and then, both eyes and the surrounding skin were prepared aseptically. The second transplant, with the same technical rigor, was performed 30 days after the first one. Groups comparison regarding ocular surfaceComparisons were performed regarding affected and pigmented areas. A baseline comparison (day 0) was made intergroup (EG, and conventional group [CG]; Figs. 1–5). Posteriorly, an evaluation was performed for each group (intragroup) at baseline 30 and 110 days as well as for EG and CG (intergroup) considering the same parameters and visits. For statistical analyses, the non-normal data of the clinical evolution of the affected and pigmentation areas were performed by the Wilcoxon test within each group. The Mann–Whitney test was used for comparisons between the groups. In all analyses, p < 0.05 was set as statistical significance.
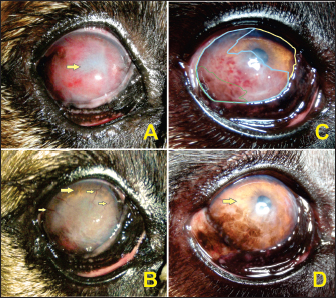
Fig. 1. Participants of the EG. A and B present the right eye of participant #1 on days 0 and 110, respectively. C and D present the right eye of participant #2 on days 0 and 110, respectively. In A, the neovascularization blocked anterior chamber visibility along with poor visual ability behavior evidenced by the negative result in the menace response test (arrow). In B, neovascularization declined to the point where only a small number of blood vessels were visible (small arrows), the periphery of the cornea became more transparent (large arrows), and visual behavior was clinically normal. In C, the disease stage was just the lateral portion of the cornea affected. As a model, the color lines represent the measurements analyzed: yellow represents the total area of the cornea, blue is the affected area, and green is the pigmented area. In D, at the end of the follow-up, the cornea had no blood vessels with the cornea becoming clear (arrow) but the pigmentation clinically occupied the same area as the initial blood vessels. Ethical approvalAll procedures with animals were conducted according to the guidelines approved by the Ethics Committee of Care and Animal Use of the State University of Campinas (UNICAMP), Brazil, under protocol number 3636-1. The owners of the animals signed the free and informed consent form. ResultsSafety of allogeneic MSCs transplantationNo side effects such as ocular pain, discomfort, or irritation of the conjunctival tissue secondary to MSCs transplantation were observed. The ophthalmologic examination showed that tear production, corneal epithelial integrity, and intraocular pressure were maintained within the range of normality at all moments during the follow-up. In addition, no changes were detected in appetite, defecation/urination frequency, body weight, or body temperature and no allergic reaction was observed. Participants remained healthy throughout follow-up, showing that treatment did not produce detectable systemic changes. The owners' adherence proved to be sufficient and efficient, as evidenced by the frequency of clinical returns for evaluations and second applications of MSCs, as well as by the reported descriptions of the subjective values of pain and ocular discomfort and descriptions of the daily behavior patterns of the animals. Corneal cytology revealed no signs of neoplasia (data not shown).
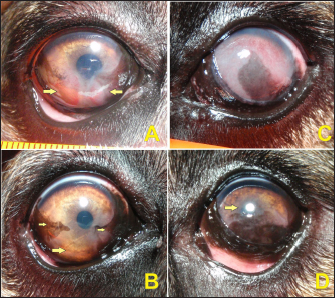
Fig. 2. Participants of the CG. A and B present the right eye of participant #6 on days 0 and 110, respectively. C and D present the left eye of participant #7 on days 0 and 110, respectively. In A, the disease is at the initial stage with only two lower quadrants affected (arrows). In B, the neovascularized area had regressed without becoming pigmented (large arrow) and the initial pigmented area had established peripherally (small arrows). In C, the disease was in a more advanced stage with a chronic pattern due to the presence of extensive visible pigmentation associated with neovascularization. In D, the cornea showed the dorsal area cleaned (arrow) and the pigmented area had regressed in the central area allowing visual menace response and visual capability.
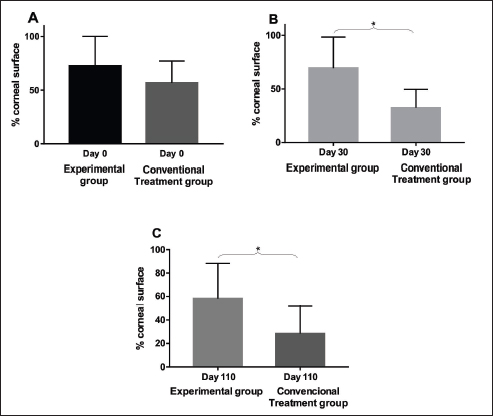
Fig. 3. A. Comparison between groups on day 0, with no statistical difference between their values (p=0.28). Columns represent mean and standard deviation, n=8. B. Comparison between groups on day 30, with statistical difference (*) among their values (p=0.04). Columns represent mean and standard deviation, n=8. C. Comparison between groups on day 110, with statistical difference (*) among their values (p=0.03). Columns represent mean and standard deviation, n=8.
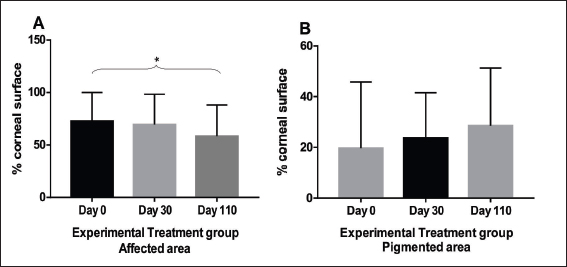
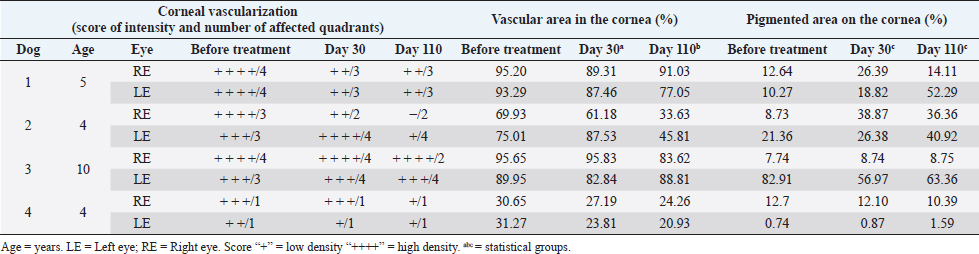
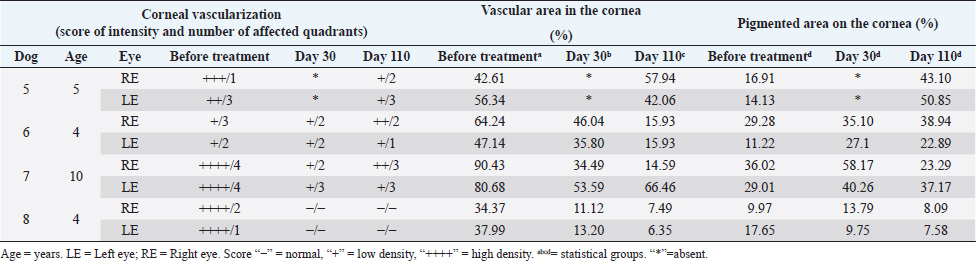
Fig. 4. Experimental group: A. Comparison between follow-up days: affected areas. There was no statistical difference between day 0 and day 30 (p=0.25) and with statistical difference (*) between day 0 and day 110 (p=0.008). Columns represent the mean and standard deviation, n=8. B. Comparison between follow-up days: pigmented areas. Comparison between follow-up days. No statistical difference between day 0, 30 and day 110 (p=0.25). Columns represent the mean and standard deviation, n=8. Evaluation of the ocular surfaceThere was no statistically significant difference between the readings of ocular surface alterations among the examiners ( p=0.5474). Comparisons in terms of the extent of the affected corneal area between the two groups (EG and CG) in relation to pre-treatment moment (day 0), day 30, and day110 are represented in Figure 3. A comparison between the initial data of the ocular surface changes of the two groups (EG and CG) showed no significant difference on day 0 (p=0.2786; Fig. 3A). The mean values of the readings of ocular surface alterations for the EG are expressed in Table 1 and for the CG in Table 2. In the EG, there was no significant difference for the affected areas comparing the initial (day 0) and intermediate (day 30) days (p=0.25). But, it reached a significant difference ( p=0.0078) comparing the initial and last follow-up (day 110) with the reduction of the area with active inflammation in 7/8 eyes (Fig. 4A). Whereas for pigmented areas there was no significant difference (p=0.25) comparing the initial and final days (Fig. 4B). In CG, there was a significant difference for the affected areas comparing the initial and intermediate follow-up ( p=0.0313). Also, there was a significant difference ( p=0.0391) comparing the initial and final evaluation with a reduction in 7/8 eyes (Fig. 5A). Whereas for pigmented areas, there was no significant difference ( p=0.3828) comparing the initial and final days (Fig. 5B). When a comparison between EG and CG was performed, a significant difference ( p=0.0426) comparing the values for the affected areas of the intermediate follow-up were favorable to CG (Fig. 3B) and this difference remained significant ( p=0.0263) at the last follow-up (Fig. 3C). DiscussionThis study, therefore sought, first, to investigate and describe the initial results of MSCs therapy in dogs with CSK without the concomitant use of conventional topical immunomodulating therapy and second, to compare with the CT with corticosteroids (Williams et al., 1995; Balicki et al., 2021). In this series of cases, no side effects were observed among dogs treated with MSCs transplantation through the subconjunctival peri-limbal application. There was a significant improvement in neovascularization in the EG, as well as in clinical visual tests and visual behavior, according to the owner’s reports. Considering that in this pathology there is no spontaneous regression of inflammation, the results show that the MSCs were able to reduce neovascularization. After 110 days of follow-up, the reduction in the affected areas in relation to neovascularization varied up to 36% of the total corneal area and corresponded to the mean values obtained in several studies using conventional therapies. The understanding of these actions requires new studies to elucidate which are the mechanisms involved. Several studies have used different products applied daily, including cyclosporin A, dexamethasone (Williams et al., 1995), pimecrolimus (Nell et al., 2005), tacrolimus with (Balicki, 2012) and without (Balicki and Trbolova, 2010) the combination of Dimethyl sulfoxide (DMSO), and recently, dexamethasone and 0.75% cyclosporine in combination with 30% DMSO (Balicki et al., 2021). These medications often lead to a reduction of neovascularization and corneal opacities within 4–6 weeks, only pimecrolimus presented a reduction of cicatricial pigmentation (Nell et al., 2005). However, in discontinuing such medications used to control the disease, the pathological condition relapses in a short time (Williams et al., 1995). In the literature, currently, the clinical response to these drugs is satisfactory, but daily use may interfere with adherence to treatment (Balicki and Trbolova, 2010). One of the possible advantages of the MSCs therapy would be its profile of lower dose application, increasing treatment adherence. Other approaches that have been described include that suggested by Allgoewer and Hoechst (2010), who using radiotherapy in combination with superficial keratectomy, demonstrated that the reduction in pigmentation and vascularization was maintained over the 24-week observation period. Another treatment protocol was the use of contact lenses with ultraviolet radiation, but no desired therapeutic effect was achieved (Denk et al., 2011).
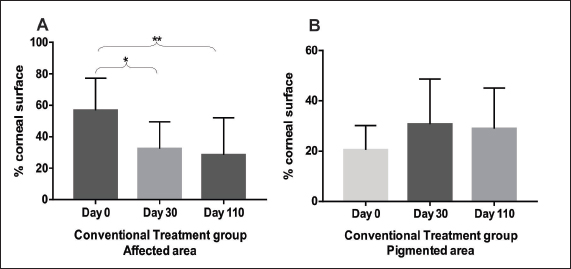
Fig. 5. Conventional Treatment Group: A. Comparison between follow-up days: affected areas. With statistical difference (*) between day 0 and day 30 (p=0.03) and with statistical difference (**) between day 0 and day 110 (p=0.04). Columns represent the mean and standard deviation, n=8. B. Comparison between follow-up days: pigmented areas. Comparison between follow-up days. No statistical difference between day 0 and day 110 (p=0.4). Columns represent the mean and standard deviation, n=8. Table 1. Experimental group. Quantitative measures of the affected and pigmented areas and qualitative measures of density and area.
Table 2. CG. Quantitative measures of the affected and pigmented areas and qualitative measures of density and area.
In EG, there was no statistical variation in neovascularization areas between days 0 and 30. One possible explanation would be that the first transplantation of stem cells failed to produce a significant result. However, with the second application, a clinical improvement in the affected areas can be noted at the end of the follow-up. Otherwise, there was no significant change in corneal pigmentation in our EG. The pigmentation increased in six eyes and reduced in two eyes. This could be due to camouflage by the inflammation and only became visible once the vascularization had receded (Slatter, 2005). It is also possible that stem cells do not affect pigment production, or that the pigmented tissue does not provide a suitable microenvironment for the immunomodulatory action of stem cells. The same findings also were observed for the CG as has been reported in the literature Balicki (2012). The etiology of CSK is still unknown, despite the genetics basis (Jokinen et al., 2011; Barrientos et al., 2013), phenotypically it has been observed that although the disease occurs in the stromal layer of the cornea, the cytology of the corneal surface has indicated the presence of conjunctivalization of the cornea in all the patients with CSK, it is possible to suppose that these patients may have some type of impairment of the barrier function (Sanchez and Daniels, 2016). Recent studies have shown the importance of limbus stem cells in preserving the specialized characteristics of the cornea. When there is a deficiency of limbus stem cells, the adjacent cornea becomes opaque as a result of conjunctivalization, with irregular appearance and the presence of blood vessels and lymphocytic infiltrate (Espana et al., 2004). Chronic inflammatory conditions such as CSK may be associated with this deficiency (Sanchez and Daniels, 2016). Although the subconjunctival administration of MSCs has produced a clinical improvement in CSK in dogs, the process by which the MSCs act has not yet been elucidated. In parallel, the CG demonstrated that the anti-inflammatory action of corticosteroids is more efficient for suppression of CSK and promoted regression of neovascularization at a similar time to that described by Williams et al. (1995). The result of the CG was more efficient in time response and remission intensity of the active inflammatory process when compared to the EG. In addition, the literature has reported that MSCs, at a certain dose, could also have an anti-inflammatory property (Villatoro et al., 2018). Other studies should be conducted to investigate the beneficial and undesirable effects of this potential new treatment. To this end, the number of cells administered, the number of applications, and the interval between applications can be better determined and standardized in order to obtain even greater control of the therapeutic response. These variations may be better defined if the paracrine mechanisms of MSCs in the microenvironment of the ocular surface are elucidated. MSCs secrete interleukins with anti-inflammatory action, for example, interleukins IL-4, IL-10, and IL-13, and also the growth factor of hepatocytes, TGF-B and PGE2, which are capable of suppressing IFN-γ production from Th11 cells. Investigating whether these mechanisms occurred in the eyes of the participating dogs is an important part of the next studies (Novak et al., 2003; Machado et al., 2013; Balicki and Sobczyńska-Rak, 2014; Sanchez and Daniels, 2016; Sgrignoli et al., 2019). New challenges must therefore be overcome, including investigating the intrinsic mechanisms by which MSCs act by identifying the cytokines found on the surface of the eye at various stages of the disease and identifying the membrane receptors where these cytokines act, whether in the various cells involved in the immune response or in the native cells on the ocular surface. Once this information is available, CSK and other ocular surface diseases may be better understood and it may even be possible for veterinarians to provide the optimal combination of treatment for each patient at each stage of the disease. It is important to emphasize that MSCs treatment has been evaluated in other corneal conditions with promising results (Lee et al., 2015; Villatoro et al., 2015, 2018). This study has limitations, mainly non-randomization between CT and MSCs, although the groups were comparable in relation to the clinical parameters evaluated at the beginning of the study. Despite the small number of participants, this was sufficient to indicate a clinical improvement in both groups and to suggest that the CT group presents a better clinical response to the treatment. The hypothesis of the mechanisms by which there was a small clinical improvement with the use of MSCs in CSK is beyond the scope of this study. In addition, if MSCs treatment becomes feasible, it must be considered that the need for sedative procedures to administer MSCs may be one of the limiting factors for high-risk patients. The other would be the cost of treatment with MSCs compared to conventional therapy. ConclusionThe subconjunctival use of adult allogeneic MSCs was clinically safe for use in dogs during the follow-up period. It promoted a little improvement in the clinical manifestation of CSK in naturally affected German shepherd dogs, as demonstrated by the reduction of the intensity and extension of the affected corneal areas without the use of any drug combination. Although this improvement was significantly less than the conventional topical treatment with a corticosteroid. Further studies are still needed to assess the use and benefits of stem cells as an adjunct treatment for CSK. AcknowledgmentsThe stem cells used in this study were kindly provided by Regenera Laboratory of Campinas, São Paulo, Brazil. Conflict of interestThe authors declare that they have no competing interests. FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Authors’ contributionsALP: conceived the study, carried out the cell implantation, participated in clinical evaluation and post-implantation monitoring and drafted the manuscript, and responded to the reviewers; MKWB: participated in clinical evaluation and post-implantation monitoring; MAB: developed the cell implantation protocol and participated in its coordination; RM: participated in clinical evaluation and post-implantation monitoring; JFPM: developed the cell implantation protocol; BM: carried out the cell differentiation experiments and drafted the manuscript; FMF: participated in the design of the study and helped to draft the manuscript; JPCV: conceived the study, participated in its coordination, and helped to draft the manuscript. All authors read and approved the final version of the manuscript. Availability of data and materialsAll data generated or analyzed during this study are included in this published article and its supplementary information file. The dissertation is available at the link: https://doi.org/10.47749/T/UNICAMP.2018.1060881 ReferencesAllgoewer, I. and Hoecht, S. 2010. Radiotherapy for canine chronic superficial keratitis using soft X-rays (15 kV). Vet. Ophthalmol. 13, 20–25. Balicki, I. 2012. Clinical study on the application of tacrolimus and DMSO in the treatment of chronic superficial keratitis in dogs. Pol. J. Vet. Sci. 15, 667–676. Balicki, I. and Trbolova, A. 2010. Clinical evaluation of tacrolimus eye drops for chronic superficial keratitis treatment in dogs. Bull. Vet. Inst. Pulawy. 54, 251–258. Balicki, I. and Sobczyńska-Rak, A. 2014. Serum vascular endothelial growth factor concentration in dogs diagnosed with chronic superficial keratitis. Acta. Vet. Hung. 62(1), 22–32. Balicki, I., Szadkowski, M., Balicka, A. and Zwolska, J. 2021. Clinical study on the application of dexamethasone and cyclosporine/dimethyl sulfoxide combination eye drops in the initial therapy of chronic superficial keratitis in dogs. Pol. J. Vet. Sci. 24(3), 415–423. Barrientos, L.S., Zapata, G., Crespi, J.A., Posik, D.M., Díaz, S., It, V., Peral-García, P. and Giovambattista, G. 2013. A study of the association between chronic superficial keratitis and polymorphisms in the upstream regulatory regions of DLA-DRB1, DLA-DQB1 and DLA-DQA1. Vet. Immunol. Immunopathol. 156, 205–210. Bittencourt, M.K.W., Barros, M.A., Martins, J.F.P., Vasconcellos, J.P.C., Morais, B.P., Pompeia, C., Bittencourt, M.D., Evangelho, K.S., Kerkis, I. and Wenceslau, C. 2016. Allogeneic mesenchymal stem cell transplantation in dogs with keratoconjunctivitis sicca. Cell. Med. 8, 63–77. Caplan, A.I. and Dennis, J.E. 2006. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 98, 1076–1084. Cheng, S., Wigney, D., Haase, B. and Wade, C.M. 2016. Inheritance of chronic superficial keratitis in Australian greyhounds. Anim. Genet. 47(5), 629. Denk, N., Fritsche, J. and Reese, S. 2011. The effect of UV-blocking contact lenses as a therapy for canine chronic superficial keratitis. Vet. Ophthalmol. 4(3), 86–94. Drahovska, Z., Balicki, I., Trbolova, A., Mihalova, M. and Holickova, M. 2014. A retrospective study of the occurrence of chronic superficial keratitis in 308 German shepherd dogs: 1999-2010. Pol. J. Vet. Sci. 17(3), 543–546. Espana, E.M., Di Pascuale, M.A., He, H., Kawakita, T., Raju, V.K., Lui, C.Y. and Tseng, S.C.G. 2004. Characterization of corneal pannus removed from patients with total limbal stem cell deficiency. Invest. Ophthalmol. Vis. Sci. 45(9), 2961–2966. Jokinen, P., Rusanen, E.M., Kennedy, L.J. and Lohi, H. 2011. MHC class II risk haplotype associated with canine chronic superficial keratitis in German shepherd dogs. Vet. Immunol. Immunopathol. 140(1–2), 37–41. Kerkis, I., Kerkis, A., Dozortsev, D., Stukart-Parsons, G.C., Massironi, S.G.M., Pereira, L.V., Caplan, A.I. and Cerruti, H.F. 2006. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells. Tissues. Organs. 184(3–4), 105–116. Lee, M.J., Ko, A.Y., Ko, J.H., Lee, H.J., Kim, M.K., Wee, W.R., Khwarg, S.I. and Oh, J.Y. 2015. Mesenchymal stem/stromal cells protect the ocular surface by suppressing inflammation in an experimental dry eye. Mol. Ther. 23(1), 139–146. Machado, C.V., Telles, P.D.S. and Nascimento, I.L.O. 2013. Immunological characteristics of mesenchymal stem cells. Rev. Bras. Hematol. Hemoter. 35(1), 62–67. Nagaya, N., Kangawa, K., Itoh, T., Iwase, T., Murakami, S., Miyahara, Y., Fujii, T., Uematsu, M., Ohgushi, H., Yamagishi, M., Tokudome, T., Mori, H., Miyatake, K. and Kitamura, S. 2005. Transplantation of mesenchymal stem cells improve cardiac function in a rat model of dilated cardiomyopathy. Circulation 112(8), 1128–1135. Nell, B., Walde, I., Billich, A., Vit, P. and Meingassner, J.G. 2005. The effect of topical pimecrolimus on keratoconjunctivitis sicca and chronic superficial keratitis in dogs: results from an exploratory study. Vet. Ophthalmol. 8(1), 39–46. Nicholas, M.P. and Mysore, N. 2021. Corneal neovascularization. Exp. Eye. Res. 202, 108363. Novak, N., Siepmann, K., Zierhut, M. and Bieber, T. 2003. The good, the bad and the ugly – APCs of the eye. Trends. Immunol. 24(11), 570–574. Sanchez, R.F. and Daniels, J.T. 2016. Mini-review: limbal stem cells deficiency in companion animals: time to give something back? Curr. Eye. Res. 41(4), 425–432. Slatter, D. 2005. Cornea and esclera. In Fundamentals of veterinary ophthalmology. Eds., Slatter D. São Paulo, Brazil: Roca, pp: 283–338. Sgrignoli, M.R., Silva, D.A., Nascimento, F.F., Sgrignoli, D.A.M., Nai, G.A., da Silva, M.G., de Barros, M.A., Bittencourt, M.K.W., de Morais, B.P., Dinallo, H.R., Foglia, B.T.D., Cabrera, W.B., Fares, E.C. and Andrade, S.F. 2019. Reduction in the inflammatory markers CD4, IL-1, IL-6 and TNFα in dogs with keratoconjunctivitis sicca treated topically with mesenchymal stem cells. Stem. Cell. Res. 39, 101525; doi: 10.1016/j.scr.2019.101525. Stern, M.E., Schaumburg, C.S., Dana, R., Calonge, M., Niederkorn, J.Y. and Pflugfelder, S.C. 2010. Autoimmunity at the ocular surface: pathogenesis and regulation. Mucosal. Immunol. 3(5), 425–442. Überreiter, O. 1961. Eine besondere keratitisform (keratitis superficialis chronica) beim hunde. Wien. Tieraerztl. Monatsschr. 2, 65–77. Villatoro, A.J., Fernández, V., Claros, S., Rico-Llanos, G.A., Becerra, J. and Andrades, J.A. 2015. Use of adipose-derived mesenchymal stem cells in keratoconjunctivitis sicca in a canine model. Biomed. Res. Int. 2015, 527926; doi: 10.1155/2015/527926. Villatoro, A.J., Claros, S., Fernandez, V., Alcoholado, C., Farinas, F., Moreno, A., Becerra, J. and Andrades, A.J. 2018. Safety and efficacy of the mesenchymal stem cell in feline eosinophilic keratitis treatment. BMC. Vet. Res. 14, 116. Williams, D.L., Hoey, A.J. and Smitherman, P. 1995. Comparison of topical cyclosporine and dexamethasone for the treatment of chronic superficial keratitis in dogs. Vet. Rec. 137(25), 635–639. Williams, D.L. 1999. Histological and immunohistochemical evaluation of canine chronic superficial keratitis. Res. Vet. Sci. 67(2), 191–195. Williams, D.L. 2005. Major histocompatibility class II expression in the normal canine cornea and in canine chronic superficial keratitis. Vet. Ophthalmol. 8(6), 395–400. Yao, L., Li, Z.R., Su, W.R., Li, Y.P., Lin, M.L., Zhang, W.X., Liu, Y., Wan, Q. and Liang, D. 2012. Role of mesenchymal stem cells on cornea wound healing induced by acute alkali burn. PLoS One. 7(2), e30842 Zakirova, E.Y., Valeeva, A.N., Aimaletdinov, A.M., Nefedovskaya, L.V., Akhmetshin, R.F., Rutland, C.S. and Rizvanov, A.A. 2019. Potential therapeutic application of mesenchymal stem cells in ophthalmology. Exp Eye Res. 189, 107863. doi: 10.1016/j.exer.2019.107863. Zakrzewski, W., Dobrzyński, M., Szymonowicz, M. and Rybak, Z. 2019. Stem cells: past, present, and future. Stem. Cell. Res. Ther. 10(1), 68. | ||
| How to Cite this Article |
| Pubmed Style Pereira AL, Bittencourt MKW, Barros MA, Malagó RL, Panattoni JFM, Morais B, Montiani-ferrreira F, Vasconcellos JPC. Subconjunctival use of allogeneic mesenchymal stem cells to treat chronic superficial keratitis in German shepherd dogs: pilot study. Open Vet J. 2022; 12(5): 744-753. doi:10.5455/OVJ.2022.v12.i5.20 Web Style Pereira AL, Bittencourt MKW, Barros MA, Malagó RL, Panattoni JFM, Morais B, Montiani-ferrreira F, Vasconcellos JPC. Subconjunctival use of allogeneic mesenchymal stem cells to treat chronic superficial keratitis in German shepherd dogs: pilot study. https://www.openveterinaryjournal.com/?mno=91165 [Access: July 01, 2025]. doi:10.5455/OVJ.2022.v12.i5.20 AMA (American Medical Association) Style Pereira AL, Bittencourt MKW, Barros MA, Malagó RL, Panattoni JFM, Morais B, Montiani-ferrreira F, Vasconcellos JPC. Subconjunctival use of allogeneic mesenchymal stem cells to treat chronic superficial keratitis in German shepherd dogs: pilot study. Open Vet J. 2022; 12(5): 744-753. doi:10.5455/OVJ.2022.v12.i5.20 Vancouver/ICMJE Style Pereira AL, Bittencourt MKW, Barros MA, Malagó RL, Panattoni JFM, Morais B, Montiani-ferrreira F, Vasconcellos JPC. Subconjunctival use of allogeneic mesenchymal stem cells to treat chronic superficial keratitis in German shepherd dogs: pilot study. Open Vet J. (2022), [cited July 01, 2025]; 12(5): 744-753. doi:10.5455/OVJ.2022.v12.i5.20 Harvard Style Pereira, A. L., Bittencourt, . M. K. W., Barros, . M. A., Malagó, . R. L., Panattoni, . J. F. M., Morais, . B., Montiani-ferrreira, . F. & Vasconcellos, . J. P. C. (2022) Subconjunctival use of allogeneic mesenchymal stem cells to treat chronic superficial keratitis in German shepherd dogs: pilot study. Open Vet J, 12 (5), 744-753. doi:10.5455/OVJ.2022.v12.i5.20 Turabian Style Pereira, Alexandre Luiz, Maura Krähembuhl Wanderley Bittencourt, Michele Andrade Barros, Rodolfo Luiz Malagó, João Flavio Martins Panattoni, Bruna Morais, Fabiano Montiani-ferrreira, and Jose Paulo Cabral Vasconcellos. 2022. Subconjunctival use of allogeneic mesenchymal stem cells to treat chronic superficial keratitis in German shepherd dogs: pilot study. Open Veterinary Journal, 12 (5), 744-753. doi:10.5455/OVJ.2022.v12.i5.20 Chicago Style Pereira, Alexandre Luiz, Maura Krähembuhl Wanderley Bittencourt, Michele Andrade Barros, Rodolfo Luiz Malagó, João Flavio Martins Panattoni, Bruna Morais, Fabiano Montiani-ferrreira, and Jose Paulo Cabral Vasconcellos. "Subconjunctival use of allogeneic mesenchymal stem cells to treat chronic superficial keratitis in German shepherd dogs: pilot study." Open Veterinary Journal 12 (2022), 744-753. doi:10.5455/OVJ.2022.v12.i5.20 MLA (The Modern Language Association) Style Pereira, Alexandre Luiz, Maura Krähembuhl Wanderley Bittencourt, Michele Andrade Barros, Rodolfo Luiz Malagó, João Flavio Martins Panattoni, Bruna Morais, Fabiano Montiani-ferrreira, and Jose Paulo Cabral Vasconcellos. "Subconjunctival use of allogeneic mesenchymal stem cells to treat chronic superficial keratitis in German shepherd dogs: pilot study." Open Veterinary Journal 12.5 (2022), 744-753. Print. doi:10.5455/OVJ.2022.v12.i5.20 APA (American Psychological Association) Style Pereira, A. L., Bittencourt, . M. K. W., Barros, . M. A., Malagó, . R. L., Panattoni, . J. F. M., Morais, . B., Montiani-ferrreira, . F. & Vasconcellos, . J. P. C. (2022) Subconjunctival use of allogeneic mesenchymal stem cells to treat chronic superficial keratitis in German shepherd dogs: pilot study. Open Veterinary Journal, 12 (5), 744-753. doi:10.5455/OVJ.2022.v12.i5.20 |