| Case Report | ||
Open Vet. J.. 2022; 12(2): 182-187 Open Veterinary Journal, (2022), Vol. 12(2): 182–187 Case Report Monopolar electrocautery use in minimally invasive urosurgery: Case report of ectopic ureter management in a bitchEvelina Burbaitė1*, Vytautas Sabūnas1,2, Vytautas Stankus1, Ernest Kostenko2 and Rūta Karalienė21Vetamicus Ltd. Private Veterinary Clinic, Klaipėda, Lithuania 2Veterinary Department, Faculty of Agrotechnology, Vilnius College, Vilnius, Lithuania *Corresponding Author: Evelina Burbaitė. Vetamicus Ltd. Private Veterinary Clinic, Klaipėda, Lithuania. Email: evelina.burbaite.vet [at] gmail.com Submitted: 14/11/2021 Accepted: 23/02/2022 Published: 15/03/2022 © 2022 Open Veterinary Journal
AbstractBackground: Ureteral ectopy is a congenital anomaly, affecting young dogs, predominantly bitches. The main complication of the disease is urinary incontinence, which leads to low life quality for both animals and their owners. However, only two less invasive surgical management options are reported. Laser ablation is quite popular, while monopolar electrocautery use is very rare. Case Description: A 3-month and 1-week-old, 1.7 kg female Poodle was admitted to the Vetamicus clinic with severe urinary incontinence and moisture-associated dermatitis. After diagnosing intramural ectopy type during computed tomography scan and video cystoscopy, a search for surgical management options began. We proceeded with the monopolar cauterization technique, where a semirigid Karl Storz monopolar coagulating ball electrode is used to cut a mucous membrane partition between the ureter and urinary bladder. No complications occurred during or after the surgery. Ureter successfully healed and full continence was achieved together with sporadically using phenylpropanolamine syrup. Conclusion: The present case indicates that monopolar electrocautery use in intramural ectopy type management and possibly other urinary tract pathologies might be strongly beneficial. Keywords: Ectopic ureter, Monopolar electrocautery, Minimally invasive surgery. IntroductionUreters are a part of the urinary system and are a junction carrying urine from the renal pelvis of the kidney to the bladder. Normally, ureters are opening into the vesical trigone as an orifice obliquely and slightly intramurally (Evans and De Lahunta, 2013). Any other ureteric opening is considered an ectopic ureter which is a congenital anomaly (Berent et al., 2012; Volstad et al., 2014; Ferreira et al., 2019; Hoey et al., 2021; Soares de Sousa et al., 2021). It is categorized as extramural and intramural. Extramural ectopic ureters bypass the urinary bladder and open distally into the urethra. Intramural ureters enter the bladder wall in the correct anatomical location but extend through a tunnel distally before opening into the urethra (Reichler et al., 2012; Volstad et al., 2014). Minimally invasive surgery techniques are only suitable for the intramural ureter ectopy type (Smith et al., 2010). The incidence rate of this anomaly is significantly higher in females; breeds such as Siberian Husky, Newfoundland, Bulldog, West Highland White Terrier, Fox Terrier, and Toy Poodle seem to be overrepresented (Hayes, 1984; McLoughlin and Chew, 2000; Reichler et al., 2012). Anomaly-related symptoms involve urinary incontinence, infections, or result in severe outcomes like hydronephrosis, hypoplastic bladder, dilated ureters, primary urethral sphincter mechanism incompetence, or vestibulovaginal malformations (McLoughlin and Chew, 2000; Berent et al., 2012; Reichler et al., 2012). Multimodal diagnostics are used to confirm ectopic ureters. Diagnostic imaging such as ultrasound, contrast radiography (excretory urography, retrograde vaginourethrography), computed tomography (CT), and cystoscopy are widely used (Hoey et al., 2021; Soares de Sousa et al., 2021). Ureteral ectopy is traditionally managed surgically, performing ureteroneocystostomy, neoureterostomy, reconstruction of the urethra and trigone, or nephrectomy (McLaughlin and Miller, 1991; McLoughlin and Chew, 2000; Mayhew et al., 2006; Reichler et al., 2012; Volstad et al., 2014). Recently, a few minimally invasive surgery techniques have been published in the veterinary literature. Intramural ectopy might be treated by performing cystoscopy-controlled laser ablation or monopolar electrocautery (Smith et al., 2010; Berent et al., 2012; Hoey et al., 2021). Laser ablation is gaining more popularity among veterinary surgeons, but the use of monopolar electrodes is still rare. One veterinary case has been reported so far and two human medicine-related studies were conducted (Ortiz et al., 2017; Fernández-Bautista et al., 2019; Ferreira et al., 2019). Medical treatment is possible to further lessen the possibility of urinary incontinence (Noël et al., 2017). Case DetailsA 3-month and 1-week-old, 1.7 kg female Poodle presented to the private veterinary surgery and diagnostic center Vetamicus in Klaipeda town, Lithuania. The reason of the appointment was urinary incontinence, which has never improved since getting the dog in Belarus. The urine leaked constantly but she was also able to urinate voluntarily. Around the vulva, there was moisture-associated dermatitis. Blood work showed eosinophilia and leukocytosis, yet C-reactive protein was within the normal range. To confirm the possibility of an ectopic ureter, we proceeded with CT scan and cystoscopy. Patient was anesthetized with dexmedetomidine (Dexdomitor ® 0.5 mg/ml–0.04 ml i.m.) and butorphanol (Butomidor ® 10 mg/ml–0.04 ml i.m.). Propofol (Propofol Fresenius ® 10 mg/ml–0.2 ml i.v.) and midazolam (Fulsed ® 5 mg/ml–0.1 ml i.v.) were used for induction. The CT scan was performed on a GE BrightSpeed 8 CT machine. The patient was positioned with the head pointing to the gantry in dorsal recumbency. Anesthesia was maintained with isoflurane gas flowing through a fourth-size endotracheal tube. A helical scan acquisition was performed through points of I (eight thoracic vertebra) through S (the caudal portion of os pubis) with slice gaps of 1.25 mm. A total of 448 images were taken twice—pre and post-contrast medium infusion (iohexol, Omnipaque ® 647 mg/ml–2.4 ml i.v.). Analyzing soft tissue reconstructions in transverse, sagittal, and dorsal planes of the CT tomograms, we found that the left ureter opening is pathological (Fig. 1). An orifice is found immediately where the urethra is formed; therefore, urine flows outward bypassing the urinary bladder and sphincter. Ectopy type is thought to be intramural due to visible thin mucous membrane partition between the bladder and ureter. It extends from the urethra to the anatomical ureter opening location. As seen in other ectopy cases, we observed a complex of pathologies: the urinary bladder was localized more caudally, and the urinary tract was shorter than usual (Fig. 2). Cystoscopy was performed with the instrument shown in Figure 3. During the cystoscopy, a 1,000 ml injectable water bottle with disposable pressure infuser was used to expand and rinse the urinary tract. Findings were consistent with CT results. The urethra and bladder contained a mucous membrane tunnel which prevented the left ureter to open in a normal anatomic position. Instead, its orifice was shifted caudally straight into the urethra and not the trigone of the bladder. One-and-a-half month after the diagnosis, surgery using monopolar electrocautery was performed. For that, we only needed a semirigid Karl Storz monopolar coagulating ball electrode (3 Fr × 53 cm), LED Surtron 120 high-frequency electrosurgery unit in a monopolar mode, and a grounding steel plate prepared with a thick ultrasonographic gel layer in contact with shaved patient’s skin (Figs. 3-5).
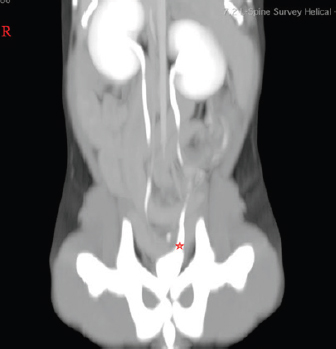
Fig. 1. Post-contrast dorsal reconstructed CT study image. Red star indicates the caudal portion of the left ureter, which is expanded and might indicate a forming mild hydroureter. The dog was anesthetized using dexmedetomidine (Dexdomitor ® 0.5 mg/ml–0.04 ml i.m.), butorphanol (Butomidor ® 10 mg/ml–0.04 ml i.m.), and ketamine (Ketamidor ® 10%–0.01 ml i.m.). Propofol (Propofol Fresenius ® 10 mg/ml–0.5 ml i.v.) and midazolam (Fulsed ® 5 mg/ml–0.1 ml i.v.) were used for induction. Maintenance was achieved with inhalation of isoflurane anesthetic gasses (2% minimum alveolar concentration (MAC)). A 0.5 liter counterlung was used and we needed no artificial lung ventilation as the patient was stable during the procedure. We infused 50 ml of 0.9% NaCl solution intravenously to prevent hypovolemia and kept the surgical table warm to avert hypothermia. The procedure was carried out aseptically; trichotomy was made around the vulva and inner thighs. The skin was scrubbed in with a soapy povidone-iodine solution and finished off with Cutasept F® antiseptic skin solution. Sterile drapes (size 150 × 200 cm), camera covers, and gowns were used. Optic light cable and telescope were coldly disinfected in a Sekusept Aktiv® solution. The surgery aimed to cut the outer ureteral membrane and redirect left kidney-produced urine from the urethra to the bladder. Before cutting, a hydrophilic 3 Fr catheter was introduced through a pathologic left ureter opening. It worked as a guide to fully visualize the intramural ureteral tunnel. After that, a Karl Storz monopolar coagulating ball electrode (3 Fr × 53 cm) was introduced through the working channel of the cystoscope sheath. The electrode cut the medial aspect of the ureteral wall up to the bladder trigone, where the contralateral normal ureter opens. The cut membrane did not bleed during the surgery (Figs. 6–8).
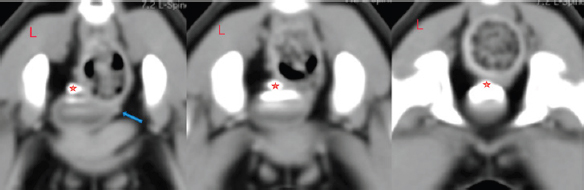
Fig. 2. Post-contrast transverse reconstructed CT study images. Red stars indicate the left ureter opening into the urinary bladder. The blue arrow shows the contralateral ureter position. They both cross the wall of the bladder in a normal position, but the left one is excreting urine through a channel into the urethra. An orifice is found immediately where the urethra is formed; therefore, urine flows outward bypassing the urinary bladder and sphincter.
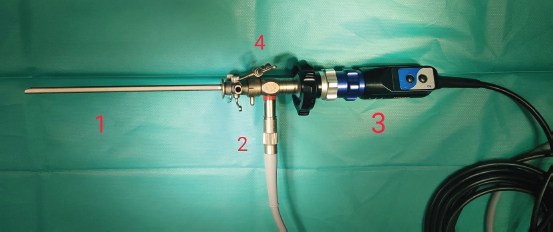
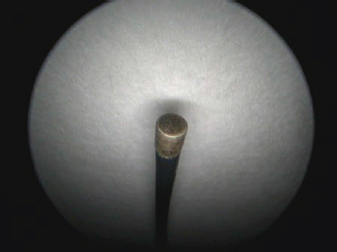
Fig. 3. Cystoscopy set. Numbers indicate certain equipment. 1, Karl Storz rigid cysto-urethroscope sheath, 14 Fr size; 2, Karl Storz fiber optic light cable; 3, Karl Storz telecam camera head with a connected Hopkins 30-degree, 2.7 mm diameter, and 18 cm length rigid telescope; 4, sheath has three separate channels: one is used as a working channel and the other two for irrigation and suction.
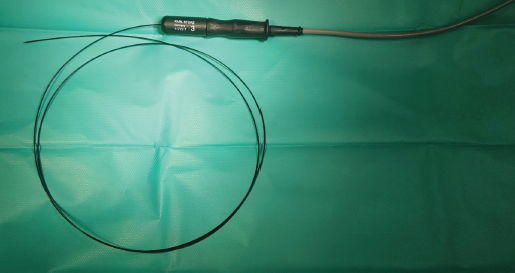
Fig. 4. Semirigid Karl Storz monopolar coagulating ball electrode (3 Fr × 53 cm) connected to a monopolar 4 mm Ø HF connection cable. Another hydrophilic 3 Fr pigtail-type catheter was introduced through the working channel with one end opening into the left kidney pelvis and another into the bladder. It was used to prevent ureteral tunnel healing and was left in for 7 days. Amoxicillin and clavulanic acid solution (Synulox RTU® 140 mg/ml–0.2 ml s.c.) was injected after the procedure and tablets were given at home (1/2 BID). NSAIDs were given s.c. once (Meloxidyl ® 5mg/ml solution–0.09 ml s.c.) and prescribed (Meloxidyl® 1.5 mg/ml solution–0.15 ml p.o.) to use 3 days SID, and relieve postoperative irritation and pain.
Fig. 5. A semirigid Karl Storz monopolar coagulating ball electrode tip.
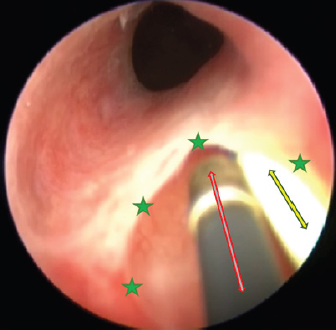
Fig. 6. Cystoscopy picture where the ureteral membrane (see the green stars) is seen before cutting with the semirigid Karl Storz monopolar coagulating ball electrode (marked with a red arrow). A bright yellow 3 Fr guide catheter is seen on the right side of the picture (marked with a yellow arrow). One week postoperation, a control cystoscopy was performed. The ureter looked well healed; therefore, we took out the pigtail catheter. The owner stated that the dog experienced no pain or discomfort after the surgery, apart from catheter-caused stranguria. Dermatitis resolved quicker than expected because the urine stopped leaking immediately after the procedure. Two weeks later, the owner reported that urine still leaks at night; ergo, a medical treatment was started by prescribing phenylpropanolamine (Propalin® 40 mg/ml syrup–0.1 ml TID 30 minutes before meals, p.o.). Nine months after the surgery, we formed a telephone questionnaire and evaluated owner’s answers about the dog. Urine incontinence level was reported to be 0/3 (3 being constant leak and 0 being no leak whatsoever). Dermatitis has never returned. Urination is normal, just like in a healthy dog. We found out that the animal still gets phenylpropanolamine syrup occasionally; hence, we repeated the questionnaire 2 weeks after stopping the medication. The results showed that 3 days after stopping the drug, she had a mild leakage at night. But after that, full continence was achieved. It is important to note that even being a grown-up dog now (1 year and 2 months), she never had her first heat, which might be due to the complex urogenital tract pathologies. The questionnaire also sought to evaluate the burden of having a dog that cannot urinate properly. It was stated that dealing with this sort of problem is a huge affliction. Extra time, expenses, and efforts must be taken into consideration. In the last question, the owner evaluated the diagnostics, surgery, and healing processes to be 5/5 (with 5 being very satisfactory and 0 being not satisfactory at all). They would repeat it if needed and recommend it to other animal owners. Ethical approvalAll clinical procedures with the animal followed in this study were in accordance with Lithuanian (The Republic of Lithuania Law on Welfare and Protection of Animals No. XI-2271) and European legislation for the protection of animals. DiscussionIntramural ectopy type is a fortunate pathology in a way that there are multiple options of surgical management for it. We believe that a minimally invasive approach is best suitable for the patients and their owners. Unfortunately, laser ablation surgery, which is known for decent outcomes, requires a lot of specific and expensive equipment. Diode or Holmium: yttrium aluminum garnet laser fibers are usually used. Apart from it being very specific hardware, certain knowledge must be attained to use it (Smith et al., 2010). What is more, in our area, the estimated price for laser ablation equipment was five times higher than for monopolar cauterization. Studies report that laser ablation surgery resulted in urine continence without any additional treatment in 47% and 63% of the patients (Berent et al., 2012; Hoey et al., 2021). Monopolar cautery is still a new concept, and the only case report in veterinary literature resulted in a comeback of urinary incontinence (Ferreira et al., 2019). But a few human studies using this tool were successful. First, ectopic ureter management with monopolar electrocautery in human infants was used to create a new ureteral outlet into the bladder (Ortiz et al., 2017). Second, monopolar heat probe electrode (2 Fr=2/3 mm, 20 V) was used to successfully manage human ureteral stump syndrome after nephrectomy (Fernández-Bautista et al., 2019).
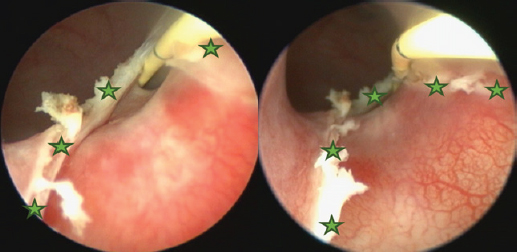
Fig. 7. Cystoscopy pictures where green stars indicate edges of the cut ureteral membrane. A yellow 3 Fr guide catheter is seen on the right side of both pictures.
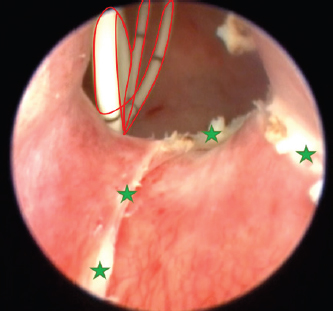
Fig. 8. Cystoscopy picture taken after cutting the ureteral membrane (see the green stars). A 3 Fr pigtail type catheter is outlined in red; it is curled inside the urinary bladder. Complications in ectopic ureter surgical management are not rare whatsoever. Continued postoperative incontinence is the most frequent as well as disappointing, and might result in euthanasia later in life (McLoughlin and Chew, 2000; Noël et al., 2017). Intramural ureter recanalization after ligation or cauterization is another possible malady (Smith et al., 2010; Ferreira et al., 2019). To prevent that, the authors suggest double ligation (McLaughlin and Miller, 1991). We assumed that cauterized ureteral walls might recanalize too; therefore, we used a pigtail catheter for them to not have any contact. From our experience, 6 days are enough for the mucosa to fully heal. [Ferreira et al. (2019) reported leaving the catheter in for 11 days. The reason for insertion was to prevent ureteral stenosis, possibly due to cautery-related edema.] Abdominal surgery resulted in a higher rate of more severe complications. 50% of the dogs after ureteral reimplantation developed worsening hydroureter or hydronephrosis; 16% of the dogs after the intravesicular transplantation technique had dysuria; and 8% of the dogs with ureteronephrectomy developed renal failure (Berent et al., 2012). A complication rate of 26% occurred after surgical correction by modified neoureterostomy or ureteroneocystostomy (Reichler et al., 2012). Severe laser ablation procedure complications involve uroretroperitoneum or uroabdomen, which we successfully avoided. It is reported that the overall complication rate in a minimally invasive approach is rare, which is the reason why professionals might prefer modern surgical solutions (Smith et al., 2010). Carrying out a minimally invasive urosurgery in a 1.7 kg toy dog was risky and challenging, especially considering that this has been seldom described. The smallest weight was reported to be 2.7 kg. The same study suggested that female dogs who weigh more than 20 kg have worse continence outcomes (Berent et al., 2012). McLoughlin and Chew (2000) stated that cystoscopy is safe in puppies above 3 kg of weight. Although our patient was almost twice as small, we achieved satisfactory results. The poodle is now fully capable to urinate voluntarily; no serious complications have been noticed. The patient succeeded in full continence after monopolar cauterization and medical treatment with phenylpropanolamine syrup. We speculate that as the animal matures, the urethral muscles will tend to strengthen. Additionally, prescribed phenylpropanolamine could have further developed the sphincter muscles. This case report is a proof that monopolar electrocautery is safe if used wisely and with caution. We recommend continuing further research in the use of monopolar electrosurgery. More studies should be conducted to fully evaluate complication rates and outcomes. We also recommend using a monopolar electrode to manage ureterocele. AcknowledgmentThe authors thank Vilnius College for supporting this research. Authors’ ContributionsAll authors contributed to the study. All authors read and approved the final manuscript. Conflict of interestThe authors declare that there is no conflict of interest. ReferencesBerent, A.C., Weisse, C., Mayhew, P.D., Todd, K., Wright, M. and Bagley, D. 2012. Evaluation of cystoscopic-guided laser ablation of intramural ectopic ureters in female dogs. J. Am. Vet. Med. Assoc. 240(6), 716–725. Evans, H.E. and De Lahunta, A. 2013. Miller’s anatomy of the dog. Elsevier Health Sciences, Amsterdam, Netherlands. Fernández-Bautista, B., Hernández, A.P., Rodríguez, R.O., Lucena, L.B. and Madero, J.A. 2019. Tratamiento endourológico del muñón ureteral sintomático posnefrectomía. Actas Urol. Esp. 43(1), 39–43. Ferreira, A.A., Tortato, N.N.G., Teixeira, W.T., Albernaz, V.G.P., Carareto, R., Froes, T.R., Castro, J.L.C. and Dornbusch, P.T. 2019. Use of monopolar cauterization by cystoscopy for ectopic intramural ureter correction in a bitch. Acta Sci. vet. 47(Suppl. 1), 426. Hayes Jr, H.M. 1984. Breed associations of canine ectopic ureter: a study of 217 female cases. J. Small Anim. Pract. 25(8), 501–504. Hoey, C.S., Friend, E., Meakin, L.B. and Chanoit, G.P. 2021. Long-term outcome of female dogs treated for intramural ectopic ureters with cystoscopic-guided laser ablation. Vet. Surg. 50(7), 1449–1462. Mayhew, P.D., Lee, K.C., Gregory, S.P. and Brockman, D.J. 2006. Comparison of two surgical techniques for management of intramural ureteral ectopia in dogs: 36 cases (1994–2004). J. Am. Vet. Med. Assoc. 229(3), 389–393. Mclaughlin Jr, R.O.N. and Miller, C.W. 1991. Urinary incontinence after surgical repair of ureteral ectopia in dogs. Vet. Surg. 20(2), 100–103. McLoughlin, M.A. and Chew, D.J. 2000. Diagnosis and surgical management of ectopic ureters. Clin. Tech. Small Anim. Pract. 15(1), 17–24. Noël, S.M., Claeys, S. and Hamaide, A.J. 2017. Surgical management of ectopic ureters in dogs: clinical outcome and prognostic factors for long-term continence. Vet. Surg. 46(5), 631–641. Ortiz, R., Parente, A., Burgos, L. and Angulo, J.M. 2017. Endoscopic urinary diversion as initial management of symptomatic obstructive ectopic ureter in infants. Front. Pediatr. 5, 208. Reichler, I.M., Eckrich Specker, C., Hubler, M., Alois, B., Haessig, M. and Arnold, S. 2012. Ectopic ureters in dogs: clinical features, surgical techniques and outcome. Vet. Surg. 41(4), 515–522. Smith, A.L., Radlinsky, M.G. and Rawlings, C.A. 2010. Cystoscopic diagnosis and treatment of ectopic ureters in female dogs: 16 cases (2005–2008). J. Am. Vet. Med. Assoc. 237(2), 191–195. Soares de Sousa, C.V.S., Rocha, C.C., da Silva, R.S.B., Dutra, A.A., Rocha, B.Z.L.L., Pacó, T.R. and de Paula Antunes, J.M.A. 2021. Ultrasonographic and radiographic diagnosis of ectopic ureter in a dog. Acta Sci. Vet. 49(Suppl. 1), 613. Volstad, N.J., Beck, J. and Burgess, D.M. 2014. Correction of intramural ureteral ectopia by ureteral transection and neoureterostomy with the distal ureter left in situ. Aust. Vet. J. 92(3), 81–84. | ||
| How to Cite this Article |
| Pubmed Style Burbaite E, Sabunas V, Stankus V, Kostenko E, Karaliene R. Monopolar electrocautery use in minimally invasive urosurgery: case report of ectopic ureter management in a bitch. Open Vet. J.. 2022; 12(2): 182-187. doi:10.5455/OVJ.2022.v12.i2.4 Web Style Burbaite E, Sabunas V, Stankus V, Kostenko E, Karaliene R. Monopolar electrocautery use in minimally invasive urosurgery: case report of ectopic ureter management in a bitch. https://www.openveterinaryjournal.com/?mno=138297 [Access: January 12, 2026]. doi:10.5455/OVJ.2022.v12.i2.4 AMA (American Medical Association) Style Burbaite E, Sabunas V, Stankus V, Kostenko E, Karaliene R. Monopolar electrocautery use in minimally invasive urosurgery: case report of ectopic ureter management in a bitch. Open Vet. J.. 2022; 12(2): 182-187. doi:10.5455/OVJ.2022.v12.i2.4 Vancouver/ICMJE Style Burbaite E, Sabunas V, Stankus V, Kostenko E, Karaliene R. Monopolar electrocautery use in minimally invasive urosurgery: case report of ectopic ureter management in a bitch. Open Vet. J.. (2022), [cited January 12, 2026]; 12(2): 182-187. doi:10.5455/OVJ.2022.v12.i2.4 Harvard Style Burbaite, E., Sabunas, . V., Stankus, . V., Kostenko, . E. & Karaliene, . R. (2022) Monopolar electrocautery use in minimally invasive urosurgery: case report of ectopic ureter management in a bitch. Open Vet. J., 12 (2), 182-187. doi:10.5455/OVJ.2022.v12.i2.4 Turabian Style Burbaite, Evelina, Vytautas Sabunas, Vytautas Stankus, Ernest Kostenko, and Ruta Karaliene. 2022. Monopolar electrocautery use in minimally invasive urosurgery: case report of ectopic ureter management in a bitch. Open Veterinary Journal, 12 (2), 182-187. doi:10.5455/OVJ.2022.v12.i2.4 Chicago Style Burbaite, Evelina, Vytautas Sabunas, Vytautas Stankus, Ernest Kostenko, and Ruta Karaliene. "Monopolar electrocautery use in minimally invasive urosurgery: case report of ectopic ureter management in a bitch." Open Veterinary Journal 12 (2022), 182-187. doi:10.5455/OVJ.2022.v12.i2.4 MLA (The Modern Language Association) Style Burbaite, Evelina, Vytautas Sabunas, Vytautas Stankus, Ernest Kostenko, and Ruta Karaliene. "Monopolar electrocautery use in minimally invasive urosurgery: case report of ectopic ureter management in a bitch." Open Veterinary Journal 12.2 (2022), 182-187. Print. doi:10.5455/OVJ.2022.v12.i2.4 APA (American Psychological Association) Style Burbaite, E., Sabunas, . V., Stankus, . V., Kostenko, . E. & Karaliene, . R. (2022) Monopolar electrocautery use in minimally invasive urosurgery: case report of ectopic ureter management in a bitch. Open Veterinary Journal, 12 (2), 182-187. doi:10.5455/OVJ.2022.v12.i2.4 |