| Research Article | ||
Open Vet. J.. 2023; 13(5): 604-612 Open Veterinary Journal, (2023), Vol. 13(5): 604–612 Original Research Plasma N-terminal pro-atrial natriuretic peptide concentrations are affected by dehydration in healthy dogsMizuki Ogawa*, Yuki Kojima, Mio Ishizaka, Hirosumi Miyakawa, Huai-hsun Hsu, Yuichi Miyagawa and Naoyuki TakemuraLaboratory of Veterinary Internal Medicine II, School of Veterinary Medicine, Nippon Veterinary and Life Science University, Tokyo, Japan *Corresponding Author: Mizuki Ogawa. Laboratory of Veterinary Internal Medicine II, School of Veterinary Medicine, Nippon Veterinary and Life Science University, Tokyo, Japan. Email: mogawa0319 [at] gmail.com. Submitted: 26/01/2023 Accepted: 12/04/2023 Published: 14/05/2023 © 2023 Open Veterinary Journal
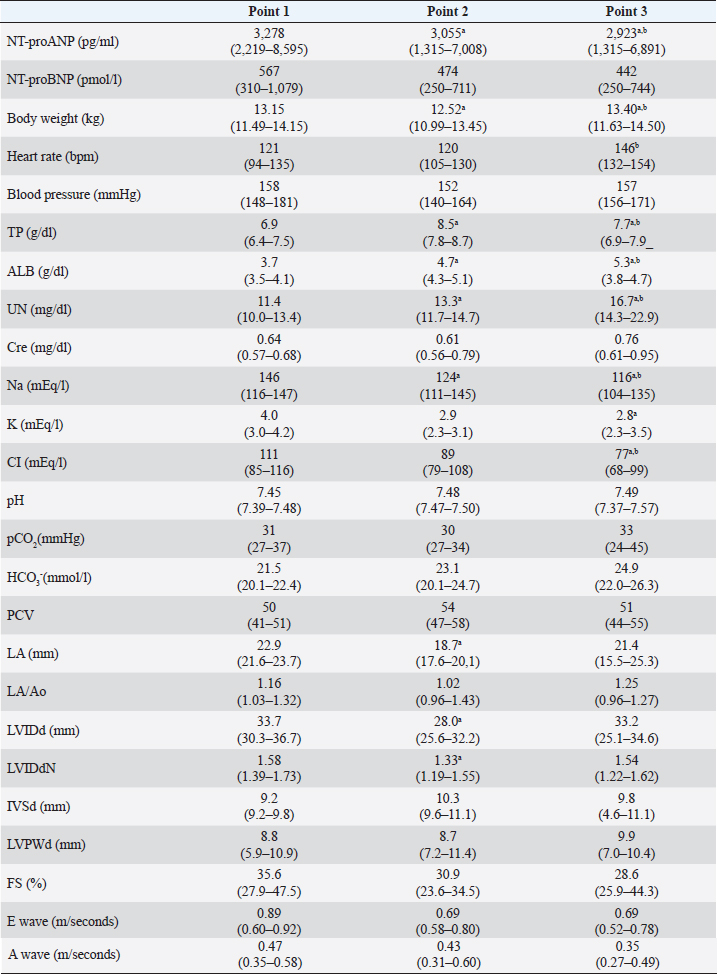
AbstractBackground: Plasma N-terminal pro-atrial natriuretic peptide (NT-proANP) and plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP) concentrations may be affected by the hydration status. Aim: This study aimed to evaluate the effect of dehydration on plasma NT-proANP and NT-proBNP concentrations in healthy dogs. Methods: This prospective study included five clinically healthy dogs. Furosemide was administered intravenously at 2–4 mg/kg every 1–2 hours until completion of the dehydration model. The dehydration model was considered complete when weight loss was ≥5% and findings of dehydration on physical examination were observed. Plasma NT-proANP and NT-proBNP concentrations were compared at three-time points: before the dehydration model was created (point 1), at the completion of the dehydration model (point 2), and when dehydration was judged to have improved (point 3). Association between plasma NT-proANP and NT-proBNP concentrations, and each clinical variable (physical examination, blood pressure, blood chemistry, blood gases, and echocardiography) was assessed using linear regression analysis. Results: Plasma NT-proANP concentration decreased significantly from point 2 to point 1 (p < 0.05), whereas plasma NT-proBNP concentration showed a decreasing trend but did not differ significantly between points 1 and 2. Plasma NT-proANP concentration correlated significantly with body weight (R2=0.178) and plasma NT-proBNP concentration (R2=0.284) (p < 0.05, respectively), and plasma NT-proBNP concentration correlated significantly with electrolytes (sodium, R2=0.439; potassium, R2=0.444; and chloride, R2=0.419), and echocardiographic parameters [diastolic left ventricular internal diameter (LVIDd) R2=0.519; weight-standardized LVIDd, R2=0.535] (p < 0.01, respectively). Conclusion: The plasma NT-proANP concentrations decreased with dehydration. However, the plasma NT-proBNP concentration did not change with mild dehydration and reflected left ventricular morphology. Keywords: Cardiac biomarkers, Dehydration, Dogs, Fluid volume, N-terminal pro-atrial natriuretic peptide. IntroductionPlasma N-terminal pro-atrial natriuretic peptide (NT-proANP) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) are cardiac biomarkers that increase with the progression of heart disease in dogs (Eriksson et al., 2014; De Lima and Ferreira, 2017; Klein et al., 2022). These cardiac biomarkers are considered to be more reflective of the clinical stages of heart disease. In previous studies, we demonstrated that in dogs with myxomatous mitral valve disease, both cardiac biomarkers have a high discriminatory ability, especially for cardiac dilatation [stage B2 in the most recent consensus statement from the American College of Veterinary Internal Medicine (ACVIM); Keene et al., 2019] and pulmonary oedema (Ogawa et al., 2021). However, cardiac biomarkers may be affected by salt intake, exercise, hydration status, and renal function, and these factors may cause abnormal values, even in the absence of cardiac problems (Engle and Watson, 2016; Joubert et al., 2018). Atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP) are sensitive to hydration status because of their inhibitory action on the renin-angiotensin-aldosterone system, natriuretic activity, and vasodilator action (Daniels and Maisel, 2007; Tapolyai et al., 2013). During dehydration, plasma ANP and BNP concentrations show a decreasing trend (Daniels and Maisel, 2007). A decrease in plasma ANP concentration due to dehydration has been reported in dogs, mice, and humped camels (Vollmar et al., 1994; Toyoshima et al., 1996; Adem et al., 2013). Since NT-proANP is released into the blood along with ANP (Ruskoaho, 2003), the plasma NT-proANP concentration may also decrease during dehydration in dogs. No studies have investigated the effect of dehydration on plasma NT-proANP concentration in either humans or dogs. In humans, it has been reported that plasma NT-proBNP concentration decreases during dehydration owing to a decrease in ventricular extensibility caused by a decrease in ventricular volume (Tanaka et al., 2017). As with plasma NT-proANP concentration, no studies have investigated the effect of dehydration on plasma NT-proBNP concentration in dogs. Diuretics such as furosemide are administered for the treatment of congestive heart failure in dogs (Keene et al., 2019), which can result in a fluid loss (hypotonic dehydration) with sodium (Na) deficiency (Plumb, 2018; Verbrugge, 2018). If plasma NT-proANP and NT-proBNP concentrations decrease during dehydration, these cardiac biomarkers may underestimate the severity of dogs in ACVIM stage C or higher, such as those receiving diuretics (Keene et al., 2019). This prospective study aimed to evaluate the effects of dehydration on plasma NT-proANP and NT-proBNP concentrations in dogs. Materials and MethodsDogsThis study was performed using five colony-sourced beagle dogs that were regarded as clinically healthy based on physical examination, blood pressure, complete blood count, blood chemistry, blood gases, echocardiography, electrocardiography, and thoracic radiography. The dogs were housed individually in cages, and fed commercial maintenance dry food twice daily with free access to water. Study protocolFurosemide (Lasix 20 mg injection; Nichiiko Sanofi Inc., Tokyo, Japan) was administered intravenously at 2–4 mg/kg every 1–2 hours until a 5% decrease in body weight was observed (Campbell and Kittleson, 2007). After fasting for >12 hours, all dogs had urinary catheters placed and bladders emptied; the dogs were weighed before furosemide administration. Food and water were not provided from the beginning of the study. The dehydration model was considered complete when >5% weight reduction was observed, and if the physical examination indicated mild dehydration. If skin turgor and capillary refill time were prolonged for more than 1 second, and mucous membrane moistness was dry on physical examination, the patient was considered mildly dehydrated (Stephen, 2012). After the dehydration model was completed, water was provided to improve the hydration status until body weight was regained, and no dehydration findings were observed on physical examination. We defined point 1 as, before the completion of the dehydration model; point 2 as, at the completion of the dehydration model; and point 3 as, when the dehydration improved. Physical examination, blood pressure measurement, blood sampling, and echocardiography were performed at each point. Furthermore, body weight and degree of dehydration were checked every 30 minutes between points by physical examination. All dogs were fasted in stainless-steel cages during the dehydration protocol. Each examination was conducted with the dogs outside the cage. Blood pressure measurementSystolic blood pressure was measured using a doppler sphygmomanometer (Vet Dop2. Vmed Technology, Mill Creek, WA). Blood pressure measurements were based on the guidelines for systemic hypertension of the ACVIM (Acierno et al., 2018). Cardiac biomarker measurementBlood was drawn from the external jugular vein of each dog using direct venipuncture. Blood samples were placed in a heparin tube (Fuji heparin tube 1.5 ml, Fujifilm, Tokyo, Japan) to perform plasma biochemical tests and blood gas analysis. Plasma biochemical test markers were measured using an automated biochemical analyzer (DRI-CHEM 4000V. FUJIFILM Medical, Tokyo, Japan). For blood gas analysis markers, a machine blood gas analysis apparatus (GEM premier 3500. I. L. Japan, Tokyo, Japan) was used. To measure plasma NT-proANP and NT-proBNP concentrations, blood samples were placed in 2-ml vacutainer tubes containing Ethylenediaminetetraacetic acid dipotassium salt. The dispensed blood samples were centrifuged at 1,187 g for 5 minutes at 4°C (Ogawa et al., 2021). The plasma was stored at −80°C. Both cardiac biomarkers were measured at commercial laboratories: plasma NT-proANP concentration at Kyoritsu Seiyaku, Tokyo, Japan, and plasma NT-proBNP concentration at IDEXX Laboratories, Tokyo, Japan. The plasma NT-proANP concentration was measured by a sandwich enzyme-linked immunosorbent assay (ELISA) using two monoclonal antibodies (KS1-6 and biotinylated KS2-2). Streptavidin with horseradish peroxidase-conjugated enzyme was used as a detection reagent. The antibodies targeted proANP31-67, a fragment of NT-proANP that showed the same optical density as NT-proANP and had the same affinity in terms of molarity. Antibodies against proANP31-67 were generated in mice, but cross-reactivity was observed. Based on the above observation, plasma NT-proANP concentration was calculated by multiplying the measured value of proANP31-67 by the molecular weight of NT-proANP (10466) divided by the molecular weight of proANP31-67 (3815). The upper limit of measurement for plasma NT-proANP concentration was 2,000 pg/ml. When this value was exceeded, the plasma was diluted 1/4 to 1/512 with 1% bovine serum albumin in phosphate-buffered saline solution, and the value obtained by multiplying the measured value (mean value) by the dilution factor of each serial dilution was taken as the plasma concentration of the sample. The plasma NT-proBNP concentration was measured using a sandwich ELISA. The upper limit of the plasma NT-proBNP concentration was 10,000 pmol/l. If the upper limit was exceeded, they were measured using serial dilution. The dilution linearity of these cardiac biomarkers was analyzed in both cases. EchocardiographyEchocardiography was performed by experienced echocardiographers following the conventional method, using an ultrasonographic unit fitted with a 6–12-MHz probe [SSA-660A (Xario). Canon Medical Systems; Tochigi, Japan]. Electrocardiogram (lead II) was also recorded during echocardiography. Each dog was manually restrained first in the right and subsequently in the left lateral recumbency. The left atrium (LA) and aorta (Ao) dimensions were obtained from the right parasternal short-axis view during early diastole, and the LA/Ao ratio (LA/Ao) was calculated (Boswood et al., 2016). The Ao dimension was measured by placing the first caliper on the midpoint of the convex curvature of the right coronary aortic sinus wall, and the second caliper on the point where the aortic wall and non-coronary and left coronary aortic cusps merged. The LA dimension was then measured from this point by extending the Ao line to the blood–tissue interface of the LA wall. The left ventricular internal diameter in diastole (LVIDd), interventricular septal wall in diastole (IVSd), LV free wall in diastole (LVFWd), and fractional shortening were measured from the right parasternal short-axis view at the chordae tendinae level using M-mode echocardiography; the leading-edge method was used for these measurements. Measured LVIDd was normalized for body weight (LVIDdN) (Hansson et al., 2002; Cornell et al., 2004; Keene et al., 2019): LVIDdN=LVIDd [cm]/body weight [kg]0.294 The echocardiographic measurements were accorded to the ACVIM guidelines (Keene et al., 2019). Statistical analysisStatistical analyses were performed using a commercial software (SPSS Statistics version 24.0. IBM, Tokyo, Japan). Data normality was assessed using the Shapiro–Wilk test. Each clinical variable was compared between points 1, 2, and 3 using the Mann–Whitney U test and Bonferroni correction. The association between plasma NT-proANP and NT-proBNP concentrations, and each clinical variable [body weight, heart rate, blood pressure, total protein, albumin, urea nitrogen, creatinine, Na, potassium (K), chloride (Cl), pH, partial pressure of carbon dioxide, bicarbonate ion, packed cell volume, LA/Ao, LVIDd, LVIDdN, IVSd, LVFWd, fractional shortening, and mitral inflow velocity (E wave, A wave)] were assessed using linear regression analysis. Ethical approvalThis study followed the Guidelines for Institutional Laboratory Animal Care and Use, and was approved by the Ethics Committee of the Nippon Veterinary and Life Science University (Approval number: 2019s-46). ResultsCharacteristics of the dehydration modelThe five healthy dogs consisted of four males and one female. The group's age (mean ± SD) was 4.2 ± 2.4 years, and body weight was 13.1 ± 0.9 kg. The median time from the start of the dehydration protocol to the creation of the dehydration model was 270 minutes (min-max, 222–275 minutes), and the median time from the completion of the dehydration protocol to the time when dehydration was judged to have improved was 95 minutes (70–100 minutes). The total dose of furosemide required to complete the dehydration model was 5 mg/kg (4–7 mg/kg). Comparison of clinical variables across pointsThe results of the clinical variables at each point are shown in Table 1. The plasma NT-proANP concentration decreased significantly at points 2 and 3 compared to that at point 1 (p < 0.05). The plasma NT-proBNP concentration showed a decreasing trend at points 2 and 3 compared to that at point 1; however, no significant difference was observed between the two points (Fig. 1). Correlation between cardiac biomarkers and clinical variablesLinear regression analysis indicated that plasma NT-proANP and NT-proBNP concentrations (R2=0.284), and body weight before and after dehydration (R2=0.178) had a significant positive correlation (p < 0.05, respectively; Fig. 2). Plasma NT-proBNP concentration and plasma Na (R2=0.439), K (R2=0.444), Cl concentrations (R2=0.419), LVIDd (R2=0.519), and LVIDdN (R2=0.535) had significant positive correlation (p < 0.01, respectively; Fig. 3). Notably, plasma NT-proANP concentrations tended to decrease with decreasing body weight (Fig. 2B). DiscussionPlasma NT-proANP concentration reduced significantly owing to dehydration. This result is similar to that of a previous investigation on the effect of dehydration on plasma ANP concentration in dogs (Vollmar et al., 1994). Therefore, plasma NT-proANP concentration may underestimate the severity of heart failure in dehydrated dogs, and the results should be interpreted with caution. Unlike a previous study in humans (Tanaka et al., 2017), plasma NT-proBNP concentration did not differ significantly before and after dehydration. In dogs, plasma NT-proBNP concentration may be used as a cardiac biomarker in mild dehydration. When dehydration was judged to have improved, the plasma NT-proANP concentration did not regress to the value at point 1 and remained decreased. Plasma NT-proANP concentration did not correlate significantly with plasma Na concentration. However, the plasma Na concentration also showed a decreasing trend when the dehydration model was completed and remained significantly decreased when body weight increased, and dehydration was judged to have improved based on physical examination (Chalifoux et al., 2021). In the point diagram, the plasma NT-proANP concentration decreased significantly with body weight loss. However, there were discrepancies between plasma NT-proANP concentrations and body weight when body weight increased and dehydration was judged to have improved. Based on a previous study, a dehydration model was created by administering furosemide to dogs that fasted for >12 hours; subsequently providing them water, and dehydration was judged to have improved when their body weight had recovered and there were no findings of dehydration on physical examination (Campbell and Kittleson, 2007). However, based on the above results, it was considered that at the time when dehydration was judged to have improved, although the total fluid volume increased owing to the provision of water, the lack of salt intake accompanied by fasting for >12 hours could not be compensated. A lower interstitial hydrostatic pressure by hypovolaemia drives fluid from the intravascular space into the interstitial space (Burkitt Creedon, 2014; Goucher et al., 2019). Moreover, physical examination abnormalities of dehydration in dogs are only detected after a weight loss of >5% (Harrison et al., 1996). The dogs may have been in a state of hypotonic dehydration even without physical examination abnormalities when dehydration was judged to have improved. The effect of hypotonic dehydration could be the reason why the plasma NT-proANP concentration remained low even when the dehydration was judged to have improved. Table 1. The clinical variables of point 1, 2 and 3.
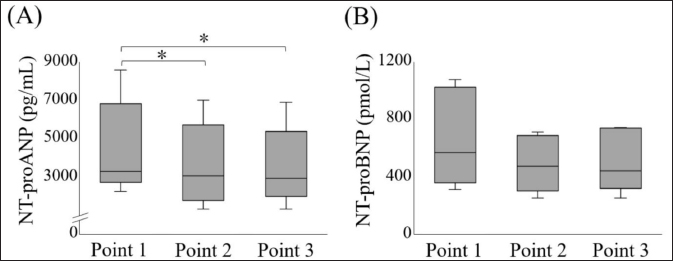
Fig. 1. Comparison of plasma NT-proANP and NT-proBNP concentrations at each point. (A) Plasma NT-proANP concentration, (B) Plasma NT-proBNP concentration. The box represents the IQR and the line within the median. The whiskers reflect minimum and maximum values. *p < 0.05. IQR, interquartile range; NT-proANP, N-terminal pro-atrial natriuretic peptide; NT-proBNP, N-terminal pro-brain natriuretic peptide.
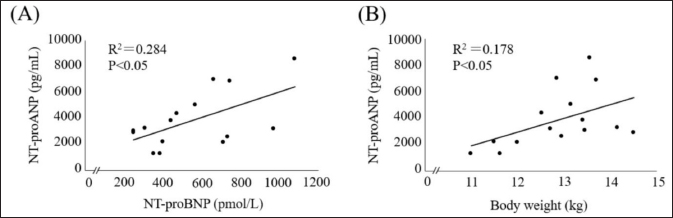
Fig. 2. Scatterplot of clinical variables significantly correlated with plasma NT-proANP concentration. (A) versus plasma NT-proBNP concentration, (B) versus body weight. NT-proANP, N-terminal pro-atrial natriuretic peptide; NT-proBNP, N-terminal pro-brain natriuretic peptide.
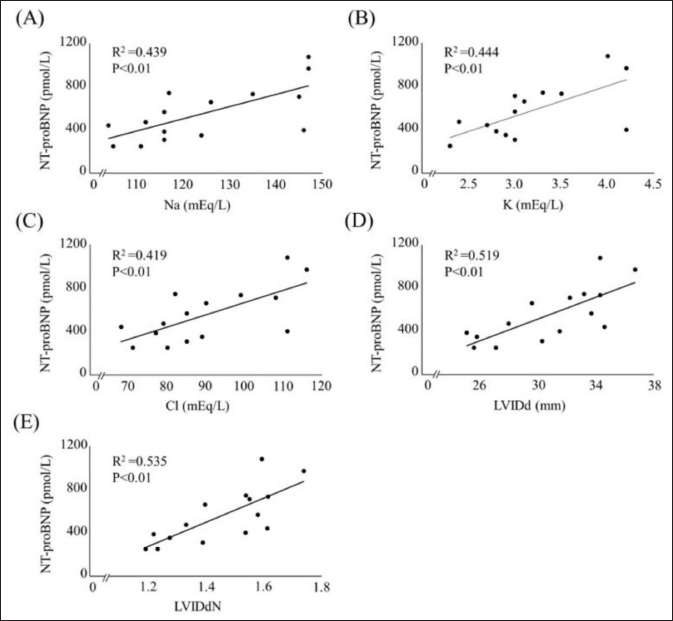
Fig. 3. Scatterplot of clinical variables significantly correlated with plasma NT-proBNP concentration. (A) versus plasma Na concentration; (B) versus plasma K concentration; (C) versus plasma Cl concentration; (D) versus LVIDd; (E) versus LVIDdN. NT-proBNP, N-terminal pro-brain natriuretic peptide; LVIDd, left ventricular internal diameter in diastole; LVIDdN, LVIDd normalized for body weight. Plasma NT-proBNP concentration correlated significantly with LVIDd and LVIDdN, indicating the left ventricular diastolic diameter. In humans, the plasma NT-proBNP concentration has been reported to decrease during dehydration due to a decrease in ventricular volume, and a concomitant decrease in ventricular extensibility (Tanaka et al., 2017). In healthy dogs, most plasma NT-proBNP concentration is produced in the ventricles (Van Kimmenade and Januzzi, 2009). In this study, plasma NT-proBNP concentration significantly correlated with plasma Na, K, and Cl concentrations. Hypokalemia can also affect cardiac function, including arrhythmias (Hanton et al., 2007). In the present study, no arrhythmias associated with decreased potassium were observed. Therefore, the electrolyte decrease may not associate directly with plasma NT-proBNP concentration. The loop diuretic furosemide inhibits the Na+-K+-2Cl− co-transporter in the ascending Henle loop; therefore, plasma Na, K, and Cl concentrations are reduced after furosemide administration (Plumb, 2018). The decreasing trend in plasma NT-proBNP concentrations during dehydration may be due to the decrease in the LVIDd associated with a reduction in circulating blood volume due to dehydration. Plasma NT-proANP concentration, unlike plasma NT-proBNP concentration, did not correlate with any echocardiographic parameter. In a previous study, a reduction in circulating blood volume due to dehydration, and a consequent reduction in atrial muscle elasticity led to a decrease in plasma ANP concentration in dogs (Vollmar et al., 1994). NT-proANP is released from the atrium in response to an atrial wall stretching along with ANP (Ruskoaho, 2003). The decrease in plasma NT-proANP concentration during dehydration may be related to a mechanism different from that of atrial muscle stretching. In humans, nitric oxide has been reported to act as a factor inhibiting the release of ANP into the blood (Ruskoaho, 2003). Besides, a previous study showed elevated nitric oxide levels during dehydration in mice (Gharbi et al., 2004; Fellet et al., 2011). The systolic blood pressure at completion of the dehydration model was almost the same as that at point 1. This suggests that blood vessels may have attempted to maintain blood pressure by contracting in response to a decrease in the circulating blood volume. Nitric oxide has vasodilatory effects, and has been reported to increase during vasoconstriction associated with dehydration (Gharbi et al., 2004). Therefore, the decrease in plasma NT-proANP concentration during dehydration might have been due to mechanisms maintaining fluid homeostasis, such as inhibition of the release of NT-proANP into the blood by elevated nitric oxide. This study had some limitations. First, the sample size was small concerning animal welfare; this study included a minimal number of dogs that can be statistically analyzed. Second, dehydration improved with an increase in water intake. Therefore, there was a time lag between the time taken for ingested water to be absorbed from the gut, and the time taken to enter the extracellular fluid and recover fluid volume. More research is needed on the changes in cardiac biomarkers after dehydration improved. Third, dehydration improvement was assessed based only on body weight and physical examination findings. The improvement in dehydration was the same as in previous studies (Campbell and Kittleson, 2007; Chalifoux et al., 2021). However, the results of the plasma Na concentrations in this study, and the physical examination findings may have underestimated the actual dehydration state after the administration of water. Therefore, whether the plasma Na concentration regressed to the value at baseline should have been considered when investigating cardiac biomarkers after dehydration was improved. In addition, plasma NT-proBNP concentration showed a decreasing trend at the completion of the dehydration model and an improvement in dehydration compared with that before the dehydration model was created. Plasma NT-proBNP concentration correlated with plasma Na and NT-proANP concentrations in this study; plasma NT-proBNP concentrations might also decrease during more severe dehydration. However, this study did not investigate changes in cardiac biomarkers in severe dehydration. The effect of dehydration on plasma NT-proBNP concentration should have been researched in dogs with severe dehydration. In conclusion, this study showed that plasma NT-proANP concentration decreased with mild dehydration by furosemide. However, the plasma NT-proBNP concentration did not change with mild dehydration and reflected left ventricular morphology. AcknowledgmentsWe would like to thank the students of the Laboratory of Veterinary Internal Medicine II at the Nippon Veterinary and Life Science University for supporting our study. We would also like to thank Editage (www.editage.com) for the English language editing. Conflict of interestThe authors declare that there is no conflict of interest. FundingThe authors received no financial support for the research, authorship, or publication of this article. Authors’ contributionMizuki Ogawa: Conception and Design of the Study, Acquisition, Analysis, Interpretation of Data, and Drafting of the Article. Yuki Kojima: Acquisition, Analysis, and Interpretation of Data. Mio Ishizaka: Review of the article. Hirosumi Miyakawa: Review of the article. Huai-hsun Hsu: Review of the article. Yuichi Miyagawa: Review of the article. Naoyuki Takemura: Review of the article. ReferencesAcierno, M.J., Brown, S., Coleman, A.E., Jepson, R.E., Papich, M., Stepien, R.L. and Syme, H.M. 2018. ACVIM consensus statement: guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J. Vet. Intern. Med. 32, 1803–1822. Adem, A., Al Haj, M., Benedict, S., Yasin, J., Nagelkerke, N., Nyberg, F., Yandle, T.G., Frampton, C.M., Lewis, L.K., Nicholls, M.G. and Kazzam, E. 2013. ANP and BNP responses to dehydration in the one-humped camel and effects of blocking the renin-angiotensin system. PLoS One 8, e57806. Boswood, A., Häggström, J., Gordon, S.G., Wess, G., Stepien, R.L., Oyama, M.A., Keene, B.W., Bonagura, J., MacDonald, K.A., Patteson, M., Smith, S., Fox, P.R., Sanderson, K., Woolley, R., Szatmári, V., Menaut, P., Church, W.M., O'Sullivan, M.L., Jaudon, J.P., Kresken, J.G., Rush, J., Barrett, K.A., Rosenthal, S.L., Saunders, A.B., Ljungvall, I., Deinert, M., Bomassi, E., Estrada, A.H., Fernandez Del Palacio, M.J., Moise, N.S., Abbott, J.A., Fujii, Y., Spier, A., Luethy, M.W., Santilli, R.A., Uechi, M., Tidholm, A. and Watson, P. 2016. Effect of pimobendan in dogs with preclinical myxomatous mitral valve disease and cardiomegaly: the EPIC study-a randomized clinical trial. J. Vet. Intern. Med. 30, 1765–1779. Burkitt Creedon, J.M. 2014. Sodium disorders. In Small animal critical care medicine, 2nd ed. Eds., Silverstein, D.C. and Hopper, K. St Louis, MO: Elsevier Saunders, pp: 263–267. Campbell, F.E. and Kittleson, M.D. 2007. The effect of hydration status on the echocardiographic measurements of normal cats. J. Vet. Intern. Med. 21, 1008–1015. Chalifoux, N.V., Spielvogel, C.F., Stefanovski, D. and Silverstein, D.C. 2021. Standardized capillary refill time and relation to clinical parameters in hospitalized dogs. J. Vet. Emerg. Crit. Care (San Antonio). 31, 585–594. Cornell, C.C., Kittleson, M.D., Della Torre, P., Häggström, J., Lombard, C.W., Pedersen, H.D., Vollmar, A. and Wey, A. 2004. Allometric scaling of M-mode cardiac measurements in normal adult dogs. J. Vet. Intern. Med. 18, 311–321. Daniels, L.B. and Maisel, A.S. 2007. Natriuretic peptides. J. Am. Coll. Cardiol. 50, 2357–2368. De Lima, G.V. and Ferreira, F.D.S. 2017. N-terminal-pro brain natriuretic peptides in dogs and cats: a technical and clinical review. Vet. World. 10, 1072–1082. Engle, S.K. and Watson, D.E. 2016. Natriuretic peptides as cardiovascular safety biomarkers in rats: comparison with blood pressure, heart rate, and heart weight. Toxicol. Sci. 149, 458–472. Eriksson, A.S., Häggström, J., Pedersen, H.D., Hansson, K., Järvinen, A.K., Haukka, J. and Kvart, C. 2014. Increased NT-proANP predicts risk of congestive heart failure in Cavalier King Charles spaniels with mitral regurgitation caused by myxomatous valve disease. J. Vet. Cardiol. 16, 141–154. Fellet, A.L., Arza, P.R., Nuñez, M., Arranz, C.T. and Balaszczuk, A.M. 2011. Hypovolemic state: age-related influence of water restriction on cardiac nitric oxide synthase in rats. Eur. J. Nutr. 50, 657–664. Gharbi, N., Mornagui, B., El-Fazaâ, S., Kamoun, A. and Gharib, C. 2004. Effect of dehydration on nitric oxide, corticotropic and vasopressinergic axis in rat. C. R. Biol. 327, 12–20. Goucher, T.K., Hartzell, A.M., Seales, T.S., Anmuth, A.S., Zanghi, B.M. and Otto, C.M. 2019. Evaluation of skin turgor and capillary refill time as predictors of dehydration in exercising dogs. Am. J. Vet. Res. 80, 123–128. Hansson, K., Häggström, J., Kvart, C. and Lord, P. 2002. Left atrial to aortic root indices using two-dimensional and M-mode echocardiography in Cavalier King Charles spaniels with and without left atrial enlargement. Vet. Radiol. Ultrasound. 43, 568–575. Hanton, G., Yvon, A., Provost, J.P., Racaud, A. and Doubovetzky, M. 2007. Quantitative relationship between plasma potassium levels and QT interval in beagle dogs. Lab. Anim. 41, 204–217. Harrison, J.B., Sussman, H.H. and Pickering, D.E. 1996. Fluid and electrolyte therapy in small animals. J. Am. Vet. Med. Assoc. 137, 637–645. Joubert, D.P., Granados, J.Z., Oliver, J.M., Noack, B.L., Grandjean, P.W., Woodman, C.R., Riechman, S.E. and Crouse, S.F. 2018. An acute bout of aquatic treadmill exercise induces greater improvements in endothelial function and postexercise hypotension than land treadmill exercise: a crossover study. Am. J. Phys. Med. Rehabil. 97, 578–584. Keene, B.W., Atkins, C.E., Bonagura, J.D., Fox, P.R., Häggström, J., Fuentes, V.L., Oyama, M.A., Rush, J.E., Stepien, R. and Uechi, M. 2019. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J. Vet. Intern. Med. 33, 1127–1140. Klein, S., Nolte, I., Granados-Soler, J.L., Lietz, P., Sehn, M., Raue, J.F., Rohn, K., Packeiser, E.M. and Bach, J.P. 2022. Evaluation of new and old biomarkers in dogs with degenerative mitral valve disease. BMC. Vet. Res. 18, 256. Ogawa, M., Hori, Y., Kanno, N., Iwasa, N., Toyofuku, T., Isayama, N., Yoshikawa, A., Akabane, R., Sakatani, A., Miyakawa, H., Hsu, H.H., Miyagawa, Y. and Takemura, N. 2021. Comparison of N-terminal pro-atrial natriuretic peptide and three cardiac biomarkers for discriminatory ability of clinical stage in dogs with myxomatous mitral valve disease. J. Vet. Med. Sci. 83, 705–715. Plumb, D.C. 2018. Furosemide. Plumb's veterinary drug handbook, 9th ed. Hoboken, NJ: Wiley-Blackwell, pp: 638–642. Ruskoaho, H. 2003. Cardiac hormones as diagnostic tools in heart failure. Endocr. Rev. 24, 341–356. Stephen, P.D. 2012. Monitoring fulid therapy and complications of fluid therapy. Fluid, electrolyte, and acid-base disorders in small animal practice, 4th ed. Amsterdam, The Netherlands: NLD Elsevier, pp: 386–404. Tanaka, H., Takano, K., Iijima, H., Kubo, H., Maruyama, N., Hashimoto, T., Arakawa, K., Togo, M., Inagaki, N. and Kaku, K. 2017. Factors affecting canagliflozin-induced transient urine volume increase in patients with type 2 diabetes mellitus. Adv. Ther. 34, 436–451. Tapolyai, M., Faludi, M., Réti, V., Lengvárszky, Z., Szarvas, T., Fülöp, T., Bekő, G. and Berta, K. 2013. Volume estimation in dialysis patients: the concordance of brain-type natriuretic peptide measurements and bioimpedance values. Hemodial. Int. 17, 406–412. Toyoshima, Y., Suzuki, S., Awal, M.A., Matsumoto, M., Nishinakagawa, H., Mifune, H. and Honda, J. 1996. Atrial natriuretic peptide (ANP)-granules of auricular cardiocytes in dehydrated and rehydrated mice. Exp. Anim. 45, 135–140. Van Kimmenade, R.R. and Januzzi, J.L. 2009. The evolution of the natriuretic peptides—current applications in human and animal medicine. J. Vet. Cardiol. 11(Suppl 1), 9–21. Verbrugge, F.H. 2018. Editor's choice-diuretic resistance in acute heart failure. Eur. Heart. J. Acute. Cardiovasc. Care. 7, 379–389. Vollmar, A.M., Montag, C., Preusser, U., Kraft, W. and Schulz, R. 1994. Atrial natriuretic peptide and plasma volume of dogs suffering from heart failure or dehydration. Zentralbl. Veterinarmed. A. 41, 548–557. | ||
| How to Cite this Article |
| Pubmed Style Ogawa M, Kojima Y, Ishizaka M, Miyakawa H, Hsu H, Miyagawa Y, Takemura N. Plasma N-terminal pro-atrial natriuretic peptide concentrations are affected by dehydration in healthy dogs. Open Vet. J.. 2023; 13(5): 604-612. doi:10.5455/OVJ.2023.v13.i5.13 Web Style Ogawa M, Kojima Y, Ishizaka M, Miyakawa H, Hsu H, Miyagawa Y, Takemura N. Plasma N-terminal pro-atrial natriuretic peptide concentrations are affected by dehydration in healthy dogs. https://www.openveterinaryjournal.com/?mno=141919 [Access: January 12, 2026]. doi:10.5455/OVJ.2023.v13.i5.13 AMA (American Medical Association) Style Ogawa M, Kojima Y, Ishizaka M, Miyakawa H, Hsu H, Miyagawa Y, Takemura N. Plasma N-terminal pro-atrial natriuretic peptide concentrations are affected by dehydration in healthy dogs. Open Vet. J.. 2023; 13(5): 604-612. doi:10.5455/OVJ.2023.v13.i5.13 Vancouver/ICMJE Style Ogawa M, Kojima Y, Ishizaka M, Miyakawa H, Hsu H, Miyagawa Y, Takemura N. Plasma N-terminal pro-atrial natriuretic peptide concentrations are affected by dehydration in healthy dogs. Open Vet. J.. (2023), [cited January 12, 2026]; 13(5): 604-612. doi:10.5455/OVJ.2023.v13.i5.13 Harvard Style Ogawa, M., Kojima, . Y., Ishizaka, . M., Miyakawa, . H., Hsu, . H., Miyagawa, . Y. & Takemura, . N. (2023) Plasma N-terminal pro-atrial natriuretic peptide concentrations are affected by dehydration in healthy dogs. Open Vet. J., 13 (5), 604-612. doi:10.5455/OVJ.2023.v13.i5.13 Turabian Style Ogawa, Mizuki, Yuki Kojima, Mio Ishizaka, Hirosumi Miyakawa, Huai-hsun Hsu, Yuichi Miyagawa, and Naoyuki Takemura. 2023. Plasma N-terminal pro-atrial natriuretic peptide concentrations are affected by dehydration in healthy dogs. Open Veterinary Journal, 13 (5), 604-612. doi:10.5455/OVJ.2023.v13.i5.13 Chicago Style Ogawa, Mizuki, Yuki Kojima, Mio Ishizaka, Hirosumi Miyakawa, Huai-hsun Hsu, Yuichi Miyagawa, and Naoyuki Takemura. "Plasma N-terminal pro-atrial natriuretic peptide concentrations are affected by dehydration in healthy dogs." Open Veterinary Journal 13 (2023), 604-612. doi:10.5455/OVJ.2023.v13.i5.13 MLA (The Modern Language Association) Style Ogawa, Mizuki, Yuki Kojima, Mio Ishizaka, Hirosumi Miyakawa, Huai-hsun Hsu, Yuichi Miyagawa, and Naoyuki Takemura. "Plasma N-terminal pro-atrial natriuretic peptide concentrations are affected by dehydration in healthy dogs." Open Veterinary Journal 13.5 (2023), 604-612. Print. doi:10.5455/OVJ.2023.v13.i5.13 APA (American Psychological Association) Style Ogawa, M., Kojima, . Y., Ishizaka, . M., Miyakawa, . H., Hsu, . H., Miyagawa, . Y. & Takemura, . N. (2023) Plasma N-terminal pro-atrial natriuretic peptide concentrations are affected by dehydration in healthy dogs. Open Veterinary Journal, 13 (5), 604-612. doi:10.5455/OVJ.2023.v13.i5.13 |