| Research Article | ||
Open Vet. J.. 2023; 13(5): 654-662 Open Veterinary Journal, (2023), Vol. 13(5): 654–662 Original Research High prevalence of liver fluke infestation, Fasciola gigantica, among slaughtered cattle in Boyolali District, Central JavaDimas Ariyanto Prasetyo1, Andini Nurlaelasari1, Aisyah Retno Wulandari1, Muhammad Cahyadi1, April Hari Wardhana2, Heri Kurnianto2, Wahyu Kurniawan3, Yuli Purwandari Kristianingrum4, Tamara Muñoz-Caro5 and Penny Humaidah Hamid1*1Department of Animal Science, Faculty of Agriculture, Universitas Sebelas Maret, Indonesia 2National Research and Innovation Agency, Indonesia 3Agency of Livestock and Fishery Services, Boyolali District, Central Java, Indonesia 4Departement of Pathology, Veterinary Medicine, Universitas Gadjah Mada, Depok, Indonesia 5Escuela de Medicina Veterinaria, Facultad de Medicina Veterinaria Y Recursos Naturales, Universidad Santo Tomás, Talca, Chile *Corresponding Author: Penny Humaidah Hamid. Department of Animal Science, Faculty of Agriculture, Universitas Sebelas Maret, Indonesia. Email: pennyhumaidahhamid [at] staff.uns.ac.id Submitted: 14/02/2023 Accepted: 27/04/2023 Published: 23/05/2023 © 2023 Open Veterinary Journal
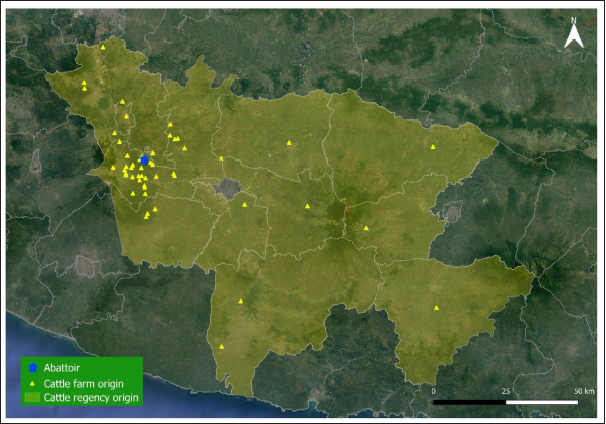
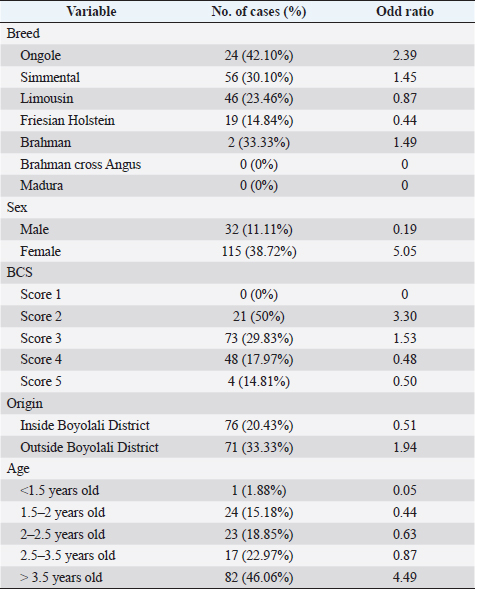
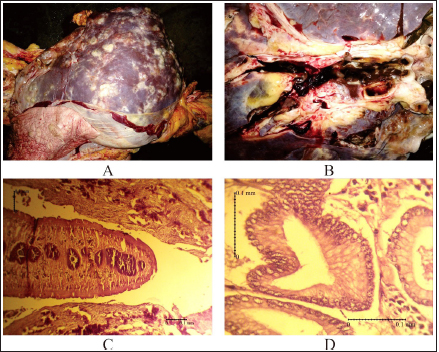
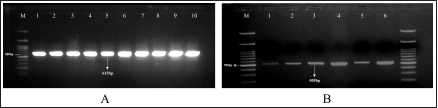
AbstractBackground: Fasciolosis is a foodborne disease caused by Fasciola sp. infecting ruminants, especially cattle. Fasciolosis remains a significant concern for Veterinary Public Health because of its zoonosis risk and transmission mode. Aim: This study aimed to determine the prevalence and risk factors associated with Fasciola gigantica infestation in cattle at Ampel abbatoir, Central Java, Indonesia. Methods: A cross-sectional study was performed on 585 cattle from February to August 2022. Visual observation postmortem was used to assess Fasciola infection based on adult flukes in liver parenchyma and ductuli biliferi. Results: The overall prevalence of fasciolosis in Ampel abbatoir is high, reaching 25.12% (147/585). The highest prevalence was observed in the Ongole breed, 42.1% (24/57), female cattle, 38.72% (115/297), body condition score criteria of 2 50% (21/42), cattle aged >3.5 years 46.06% (82/178), and cattle originated from outside of Boyolali district 33.33% (71/213). Conclusion: This study showed a high prevalence of fasciolosis in Ampel abbatoir, as shown in the correlation between the risk factors of breed, sex, body condition score (BCS), origin, and age. Because of the high prevalence of fasciolosis in the abattoirs, it is essential to continue performing epidemiology studies in more expansive areas. The subsequent plans are important to reduce the risk of fasciolosis as a threat to productive cattle husbandry and warrant its transmission to humans as a foodborne-zoonotic disease. Keywords: Cattle, Foodborne disease, F. gigantica, Prevalence, Risk factor. IntroductionFoodborne diseases are a significant public health problem and are the leading cause of global morbidity and mortality (Shonhiwa et al., 2019). Foodborne disease is caused by consuming food contaminated with pathogenic bacteria, viruses, or parasites (Mensah and Ofosu, 2020). Among pathogenic foodborne, fasciolosis caused by F. hepatica and F. gigantica is a major concern (Khademvatan et al., 2019; Bargues et al., 2021). Fasciola gigantica is endemic in Indonesia. The endemicity is mainly because of difficulty controlling the vectors (Yasin et al., 2018). The life cycle of F. gigantica begins when the adult worms infest the cattle body. Adult worms live in the liver and lay eggs in the intestines; then, the eggs are excreted along with the feces. The eggs hatch into miracidia and then swim to find snails. Different species of snails are vectors that play a key role in the life cycle of F. gigantica (Vázquez et al., 2018). The miracidium is in the snail’s body for 2 weeks, then it will turn into a sporocyst. Sporocyst will reproduce asexually to produce redia and become cercariae. Cercariae larva will have tail and become metacercariae, thereafter, leave the snail’s body to attach to aquatic plants. Cattle will consume plants contaminated with metacercariae, then the metacercariae penetrates the intestinal wall and reaching adult in the cattle liver (Bogitsh et al., 2013; Tenorio and Molina, 2021). The liver damage cause various symptoms in cattle such as suboptimal growth, decreased body weight, lower milk production in dairy cows, and end up with condemned liver in beef (Opio et al., 2021). Histopathologically, changes such as the presence of necrosis, hyperplasia, calcification, hemorrhagic, and fibrosis are found in the liver parenchyma (Chamuah et al., 2020). The direct impact on economic losses for farmers may be because of a reduced portion of liver tissue sold or consumed (Nyirenda et al., 2019). Indirectly, Fasciola sp. infection causes changes in the immune response, physiological function, and metabolism of the host, and thereafter, it has a significant effect on the purposes of livestock production (Nasreldin and Zaki, 2020). In countries with tropical climates, F. gigantica is the predominant causative agent of fasciolosis (Rokni et al., 2020) as documented across continents, e.g., in Ghana 10.27% (27/263) (Addy et al., 2020), Nigerian 74.9% (514/686) (Elelu et al., 2016), Bangladesh 66.14% (504/762) (Karim et al., 2015), Cambodia 7.14% (21/294) (Loeurng et al., 2019), and Thailand 52.94% (27/51) (Phalee and Wongsawad, 2014). Fasciolosis is also reported as one of the transboundary diseases transmitted across tropical geographic areas (Calvani and Šlapeta, 2021). The climate condition in Indonesia is characterized by high rainfall and humidity which are conducive to F. gigantica life cycle, including Java Island (Kusumarini et al., 2020). According to statistical data reported by Provincial Livestock Agency, the number of beef cattle slaughtered in Central Java Province slaughterhouses increased by 9.74% between 2020 and 2021, from 101,177 heads to 111,209 heads. Boyolali District in Central Java slaughtered the most cattle in slaughterhouses in 2021, with a total of 2,110 heads (Badan Pusat Statistik, 2021). Traditionally, some farmers in Java Province fed their livestock unprocessed rice straw, putting the livestock at high risk for infection by Fasciola sp. (Aini et al., 2021). Given that F. gigantica is endemic in Indonesia (Prasetyo et al., 2019), it is necessary to perform epidemiology studies periodically to help to understand disease dispersal. This study aimed to determine the prevalence and risk factors associated with F. gigantica infestation in slaughtered cattle in Ampel abattoir, in Boyolali of Central Java Province, Indonesia. The baseline information is beneficial for the control and prevention of F. gigantica infestation to further avoid economic losses. Materials and MethodsLocationThis study was performed from February to August 2022 at Ampel abbatoir, Boyolali District, Central Java Province, Indonesia. The study area was between 110°33ʹ east longitude and 7°28ʹ south latitude with an altitude of 631 m above sea level. Examination of cattleAntemortem examination was performed to determine risk factors, including breed, sex, age, origin, and body condition score (BCS). The breed and sex were assessed by observing the physical characteristics of the cattle. The age was evaluated by observing permanent incisivus (Tulloh, 1962). The BCS was estimated based on a 5-point scoring system. The assessment classes used were very thin (score 1), thin (score 2), moderate (score 3), fat (score 4), and very fat (score 5) (Wildman et al., 1982). Post-mortem observation and palpation were used to assess the presence of adult flukes in the liver parenchyma and ductuli biliferi. Molecular identification of Fasciola sp.Molecular identification was performed to determine the Fasciola species using duplex Polymerase Chain Reaction (PCR). The first PCR method was performed by targeting mitochondrial DNA (mtDNA) spanning the region of cox1-trnT tRNA-trnI tRNA (Le et al., 2012). The PCR composition was 12.5 µl My Taq Mix (Bioline, UK), 8.5 µl nuclease-free water (1st Base, Singapore), 1 µl of each 10 µM-primer pairs, and 1 µl DNA of each sample. The PCR reaction was performed with the following program: pre-denaturation 95°C for 3 minutes, then 35 cycles, including denaturation of 95°C for 30 seconds, annealing 52°C for 30 seconds, extension 72°C for 2 minutes, and the last step is final extension 72°C for 2 minutes. The second PCR was targeting the nuclear gene of phosphoenolpyruvate carboxykinase (pepck) (Calvani et al., 2020). The PCR composition was made up to 30 µl, including 1 µl of each 10 µM-primer pair, 15 µl MyTaq Red Mix (BioLine, UK), and 2 µl of template DNA. The reaction was run with an initial denaturation step at 95°C for 90 seconds, followed by 30 cycles of 95°C for 30 seconds, 61°C for 30 seconds, and 72°Cfor 60 seconds, and a final extension step of 72°C for 10 minutes. The amplicons were run onto 1.5% agarose gel with Gel Red (Biotium Inc., USA) for 45 minutes of 100 volts. PCR visualization was performed using the Glite UV Gel Doc System (Pacific Image Electronics Co., Taiwan), with the GeneRuler 1 kb DNA ladder (Thermo Fisher Scientific GmbH, Germany) as standard. Histopathology of infected liverInfected tissue was processed at the Anatomical Pathology Laboratory, Faculty of Medicine, Sebelas Maret University, Indonesia. The cross-sections of the liver samples were processed with hematoxylin-eosin staining. Statistical analysisMicrosoft Excel (Microsoft, USA) was used to input the data collected from the inspection at Ampel abbatoir. IBM SPSS Statistics 22 (IBM Corp., USA) was used for the statistical analysis of raw data. Map was created by using Quantum GIS version 3.10 Coruña. Ethical approvalThe animal experiments related to the collection of adult F. gigantica from cattle have been issued by the ethical committee of Ahmad Dahlan University with an approval letter no. 022206036. ResultsPrevalence of fasciolosis in slaughtered cattleFasciolosis were found in cattle originating from different areas both from inside Boyolali district and outside (Fig. 1). The examination of 585 cattle revealed that 25.12% (147/585) were infected with Fasciola sp. The Ongole breed showed the highest prevalence of infestation 42.10% (24/57) followed by Brahman 33.3% (2/6), Simmental 30.10% (56/186), Limousin 23.46% (46/196), and Friesian Holstein 14.84% (19/128), while none of Brahman cross Angus and Madura breeds were infested during the investigation. The female sex had a higher prevalence of 38.72% (115/297) than males 11.11% (32/288). The age group of cattle over 3.5 years had the highest prevalence 46.06% (82/178) compared to younger cattle aged 2.5–3.5 years 22.97% (17/74), 2–2.5 years 18.85% (23/122), 1.5– 2 years 15.18% (24/158), and less than 1.5 years 1.88% (1/53). BCS criteria of 2 had the highest prevalence 50% (21/42), compared to BCS 3 29.83% (74/248), BCS 4 17.97% (48/267), BCS 5 14.81% (4/27), and BCS 1 0% (0/1). Cattle originating from outside of Boyolali district had a higher prevalence 33.33% (71/213) than from inside 20.43% (76/372) (Table 1). Risk factors for F. gigantica infestationCompared to other cattle breeds investigated, Ongole breed showed a greater odds ratio (OR=2.39). Cattle over 3.5 years old at slaughter had a higher odds ratio (OR=4.49) for fasciolosis infestation compared to other younger cattle. Furthermore, our results revealed that cattle BCS 2 have the greatest odds (OR=3.30). The female cattle have a greater risk (OR=5.05) than the male. Cattle originating from outside Boyolali district had a higher odds ratio (OR=1.94) than cattle from inside (Table 1). Histopathology of infected liverThe gross pathology of the infected liver was shown in Figures 2A and B. The surface of the liver was shown to be 90% filled with white lesions. The edges of the liver underwent changes, which became blunt. When palpated, the consistency of the liver was hardened (Fig. 2A). Furthermore, in the transversal dissection, a total of 7 adult F. gigantica were found in the ductuli biliveri. The walls of the ductuli biliveri were thickened, hard, and white lesions dominated throughout the serous layer (Fig. 2B). The histopathological changes were shown in Figures 2C and B. An Adult worm section was observed in the liver parenchyma. Necrosis was found around the area which was irritated by the worm spina (Fig. 2C). Formation of the new duct occurs in the liver parenchyma. Necrotic tissue was shown around the new duct area and surrounded by an infiltration of inflammatory cells (Fig. 2D). Duplex PCR of Fasciola sp.The forward and reverse primers for F. hepatica amplified 1,031 bp PCR product, spanning the mitochondria region of cox1 to trnI tRNA. Whilst, the second forward primer with the reverse pair amplified a fragment of F. gigantica-specific trnI tRNA. All pooled samples were 80–100 Fasciola sp. tissue, resulting in 615 bp amplicon weight (Fig. 3A). The second PCR target in this study was the nuclear gene of pepck by using 2 forward primers specific to differentiate F. hepatica and F. gigantica, and 1 reverse primer for both. The forward and reverse primers for F. hepatica amplified 241 bp of PCR product in pepck gene which spanning pepck intron 1 to exon 2. The second pairs forward primer with reverse pair specific to amplified F. gigantica within pepck intron 1 to exon 2 as many 509 bp. All samples of pooled Fasciola sp. resulted in a single band of 509 bp amplicon weight (Fig. 3B).
Fig. 1. Map of cattle origins were slaughtered at the Ampel abattoir, Central Java, Indonesia. Table 1. Risk factors associated with F. gigantica infestation among slaughtered cattle in Ampel abattoir, Central Java, Indonesia.
DiscussionIn this study, F. gigantica was found in high prevalence 25.12% (147/585). This prevalence is lower rather than the previous report observed in Indonesia, i.e., in Aceh 56.3% (58/103) and West Papua 52.5% (252/480), but higher than in East Nusa Tenggara 17.19% (11/64) and East Kalimantan 21.65% (34/157) (Damayanti et al., 2019; Prasetya et al., 2019; Hambal et al., 2020; Purwaningsih et al., 2022). The higher infection in Aceh could be because of that mostly cattle be reared in an extensive farming system, leading to a higher risk of exposure to fasciolosis from consuming forage contaminated with metacercaria Fasciola sp. In addition, the climate in the study area was higher in rainfall as an environmental factor that supports the life cycle of Fasciola sp. (Hambal et al., 2013). Meanwhile, in West Papua, higher infection may be because most cattle were fed with the cut-carry forage, which was taken from waste plants or weeds living in rice fields or rivers, causing a high risk of contamination by Fasciola sp. metacercariae (Purwaningsih et al., 2018). In other Asia regions, this prevalence is lower than reported in Thailand 52.94% (27/51) and Philipines 93.3% (42/45), but higher than in Bangladesh 18.64% (343/1,840) and Malaysia 7.46% (5/67) (Phalee and Wongsawad, 2014; Gordon et al., 2015; Zainalabidin et al., 2015; Islam et al., 2016). High prevalence in Thailand and Philipines were reportedly due to its feeding management, which fed cattle with potentially contaminated plants, rice straw, and water from rice fields or grown beside the rivers or irrigations (Phalee and Wongsawad, 2014; Gordon et al., 2015). Meanwhile, in Boyolali District, most cattle were reared with an intensive farming system, which minimizes their grazing activity and risk of exposure to fasciolosis. However, the cattle are still fed by paddy straw and cut-carry forage as a combination of fed leads high risk of metacercaria contamination (Nugrahanto and Eviyanti, 2022). Feeding paddy straw to cattle could be a route of Fasciola sp. infection. The metacercaria could attach to plants living in watery areas such as paddy and waste plants in rivers (Rinca et al., 2019). The risk of infection can be minimized by processing the paddy straw before giving it to the cattle, i.e., drying the straw for 2–3 consecutive days before being given as feed, cutting the straw slightly above the water surface, and combining the straw with dry rice leaves (Martindah et al., 2005).
Fig. 2. Pathological changes of liver infected with F. gigantica among slaughtered cattle in Ampel abattoir, Central Java, Indonesia. (A): The proliferation of connective tissue. (B): Adult F. gigantica was found in the liver parenchyma. (C): Worms section in liver tissue. (D): Proliferation of new ducts.
Fig. 3. Result of duplex PCR from adult stage of F. gigantica targeting mtDNA spanning the region of cox1-trnT tRNA-18S rRNA (A) and nuclear gene of pepck (B). M: 100 bp DNA ladder. Cattle originating from outside Boyolali District had a higher risk (OR=1.94) for fasciolosis than inside. This case may have occurred because of unscheduled deworming activities, such as in Wonogiri and Magetan District (Mukmin and Lisnanti, 2019; Yanti et al., 2022) and feeding management, including unhygienic feed or giving waste plant as feed, which potentially as the reason for high parasite infection (Awaludin et al., 2018). Our study also revealed that Ongole, as a local breed, had a higher odds ratio (OR=2.39) to fasciolosis than other breeds. This result is in accordance with a previous study reported in Uganda and South Africa, which found that Zebu cattle, as a local breed, had higher infections than crossbred (Jaja et al., 2017; Opio et al., 2021). Local breeds are known to be reared with extensive farming systems, leading to exposure and more potential to consume pastures contaminated with Fasciola sp. metacercaria (Kudzai, 2018). In Indonesia, intensive farming is adopted mainly for crossbreed cattle husbandry, such as Simmental, Limousin, and Holstein–Friesian (Suretno et al., 2017; Efendi et al., 2020). Crossbreeds are more difficult to adapt in the tropical climate, therefore, almost all the farmers conside to choose intensive rearing for feedlot (Adhianto et al., 2015). The cattle aged 3.5 years old or more had a higher odds (OR=4.49) to fasciolosis than younger cattle. Our results showed that older animals experienced more grazing activities and, thus, more possibility of exposure to metasecaria-contaminated grass (Valinata et al., 2020). The higher cases of occurrence of older animals were also documented in Botswana (Mochankana and Robertson, 2018), Ethiopia (Yusuf et al., 2016), and Cambodia (Loeurng et al., 2016). We here showed that the female cattle in the investigation had a greater risk (OR=5.05) of fasciolosis than males. Almost all female cattle in this study had all permanent incisivus erupt which indicated ages at least 3.5 years old or more when being brought to the slaughterhouse. The farmers could not use young and productive females for consumption, since Indonesian government regulation UU/no. 41/2014 stated that female cattle for meat shall exceed 8 years old or evidently lack of reproduction performances. It is noteworthy that the local farmers usually give less attention to female cattle with low reproduction performances, since they were not profitable for feedlot purposes as males, and therefore, being fed with lesser nutritional supplies. This hypothesis was confirmed by our observations that female cattle at the Ampel abattoir had lower BCS than male cattle. The quality of nutritional intake would correspond to decreased immunity to disease infections (Abdelazeem et al., 2020), the same hold true was found with the case that occurred in Ethiopia with a higher risk of fasciolosis in females than males (Mohammed et al., 2018). Furthermore, in this study, cattle BCS 2 had higher odds (OR=3.30) of fasciolosis than other BCS. It is known that animals infected with Fasciola sp. would prioritize nutrition from intake feed for immunity and, thus, decreased energy storage leading to lower BCS (Levi et al., 2020). Association of low BCS to cattle fasciolosis was also reported in Nigeria (Shinggu et al., 2019), Kenya (Kipyegen et al., 2017), and Pakistan (Khan et al., 2020). Our results showed that Fasciola species that infected cattle processed at Ampel abattoir was solely F. gigantica as shown by duplex PCR targeting mitochondrial genome (Le et al., 2012) and nuclear gene of pepck (Hayashi et al., 2018). Both the first and second PCRs were used for pooled samples of 80–100 Fasciola sp. tissue and resulted in a single 615 and 509 bp amplicon weight, respectively. Therefore, we conclude that all Fasciola sp. populations in this study were F. gigantica according to these rapid screens (Hayashi et al., 2018; Le et al., 2012). The PCRs in this study were used in the screening of Fasciola sp. with DNA templates of F. gigantica and F. hepatica reportedly before i.e., Vietnam (Le et al., 2012), Malaysia (Ichikawa-Seki et al., 2022), and Australia (Calvani et al., 2020). It is shown that pathological changes which were pathognomonic occurred severely with white lesions covering 90% of the surface of livers almost in all infected cattle. The lesions were caused by the deposition of fibrocytes, leading to the excessive formation of connective tissue and whitish color all over the parenchyma (Salmo et al., 2014). Fibrosis causes structural changes in the liver hepatocytes, induces the hardened consistency, and the blunt edges of the liver lobuli (Belina et al., 2015). All these changes caused condemned liver, which has a direct impact on farmers, i.e., economic losses, because of a reduced portion of the liver to be sold or consumed. These losses are not felt huge for cattle individually, but when the sum is accumulated, the total values are beneficial. The economic losses, because of the condemned liver, reached USD 592,560 in Zambia (Nyirenda et al., 2019). Due to the high prevalence of fasciolosis in the abattoir observed postmortem, it is essential to continue performing epidemiology studies in wider areas with antemortem strategies, e.g., through fecal sampling and serological assays. The subsequent plannings are important to reduce the risk of fasciolosis as a threat to productive cattle husbandry to avoid huge accumulative losses. ConclusionThere was a significant correlation between the risk factors of breed, sex, BCS, origin, and age with the occurrence of F. gigantica infestations in cattle slaughtered in the Ampel abattoir. It is implied that the livestock management system also contributes to the prevalence of Fasciola infection. The increasing interest in organic farming by using livestock waste as fertilizer and raw vegetable consumption warrants the increasing potential for transmission to humans as a foodborne-zoonotic disease. AcknowledgmentsThe authors are grateful to Ampel abattoir staffs who helped during sample collection, as well as the Agency of Planning, Research and Development, Boyolali District, Indonesia, who gave permission to carry out this study in the region. This study was supported by Universitas Sebelas Maret Surakarta, HGR-A grant scheme 2023 to Research Group (RG) Tropical Animal Breeding, Health and Reproduction, Indonesia, and FONDECYT no.11200103 to T.M.C. from the National Research and Development Agency of Chile (ANID). Author contributionsConceptualization: P.H.H., W.K, A.H.W; Funding acquisition: P.H.H., T.M.C.; Sample collections and processing: D.A.P., A.N., M.C., and A.R.W.; Pathology analyses: Y.P.K. and D.A.P.; Statistical processing: H.K. and D.A.P; Writing: D.A.P., A.H.W., T.M.C. and P.H.H. All authors have read and agreed to the published version of the manuscript. Conflict of interestThe authors declare that there is no conflict of interest ReferencesAbdelazeem, A.G., Abdelaziz, A.R., Khalafalla, R.E. and Abushahba, M.F.N. 2020. Prevalence and phylogenetic analysis of Fasciola species in upper Egypt based on ribosomal ITS-2 gene sequencing. Egyptian Vet. Med. Soc. Parasitol. J. 16(1), 142–158. Addy, F., Gyan, K., Arhin, E. and Wassermann, M. 2020. Prevalence of bovine fasciolosis from the Bolgatanga abattoir, Ghana. Sci. Afr. 8(e00469), 1–6. Adhianto, K., Siswanto and Kesuma, C.N. 2015. Pengaruh frekuensi penyiraman air menggunakan sprinkler terhadap respon fisiologis dan pertumbuhan sapi peranakan simmental. Buletin Peternakan. 39(2), 109–115. Aini, F. N., Likah, S. and Nurlaili. 2021. Mapping the potential of food crop by-products as feed to support the increasing of beef cattle population in Malang District. Pastura: J. Trop. Forage Sci. 10(2), 101–106. Awaludin A. and Nusantoro S. 2018. Identify the diversity of helminth parasites in cattle in Jember district (East Java - Indonesia). IOP Conf. Ser. Earth Environ. Sci. 207(1), 1–5. Badan Pusat Statistik. 2021. Statistik Pemotongan Ternak Provinsi Jawa Tengah. Badan Pusat Statistik Provinsi Jawa Tengah. Bargues, M.D., Valero, M.A., Trueba, G.A., Fornasini, M., Villavicencio, A.F., Guamán, R., Elías-Escribano, A., De, Pérez-Crespo, I., Artigas, P. and Mas-Coma, S. 2021. DNA multi-marker genotyping and cias morphometric phenotyping of Fasciola gigantica-sized flukes from Ecuador, with an analysis of the radix absence in the new world and the evolutionary lymnaeid snail vector filter. Animals. 11(9), 2495. Belina, D., Demissie, T., Ashenafi, H. and Tadesse, A. 2015. Comparative pathological study of liver fluke infection in ruminants. Indian J. Vet. Pathol. 39(2), 113. Bogitsh, B.J., Carter, C.E. and Oeltmann, T.N. 2013. Human parasitology, 4th ed. Academic Press; doi:10.3989/arbor.1997.i623-624.1797 Calvani, N.E.D., Ichikawa-Seki, M., Bush, R.D., Khounsy, S. and Šlapeta, J. 2020. Which species is in the faeces at a time of global livestock movements: single nucleotide polymorphism genotyping assays for the differentiation of Fasciola spp. Int. J. Parasitol. 50(2), 91–101. Calvani, N.E.D. and Šlapeta, J. 2021. Fasciola species introgression: Just a fluke or something more? Trends Parasitol. 37(1), 25–34. Chamuah, J.K., Borkotoky, D., Amenti, Khate, K., Jacob, S.S., Lalchamliani, Raina, O.K., Khan, M.H. and Mitra, A. 2020. Molecular characterization and histopathological studies on Fasciola gigantica in Mithun (Bos frontalis). Indian J. Anim. Res. 54(8), 1012–1017. Damayanti, L.P.E., Almet, J. and Detha, A.I.R. 2019. Deteksi dan prevalensi fasciolosis pada sapi bali di Rumah Potong Hewan (RPH) Oeba Kota Kupang. J. Vet. Nusantara 2(1), 13–18. Efendi, Z., Ishak, A., Wulandari, W.A. and Afrizon, A. 2020. Sapi perah: masalah dan solusi peningkatan produksi susu (Kasus di kelompok tani Sepakat Ii, Desa Mojorejo, Kabupaten Rejang Lebong). AGRITEPA: J. Ilmu Teknologi Pertanian. 7(1), 41–50. Elelu, N., Ambali, A., Coles, G.C. and Eisler, M.C. 2016. Cross-sectional study of Fasciola gigantica and other trematode infections of cattle in Edu Local Government Area, Kwara State, north-central Nigeria. Parasit. Vectors. 9(1), 11–11. Gordon, C.A., Acosta, L.P., Gobert, G.N., Jiz, M., Olveda, R.M., Ross, A.G., Gray, D.J., Williams, G.M., Harn, D., Li, Y. and McManus, D.P. 2015. High prevalence of Schistosoma japonicum and Fasciola gigantica in bovines from Northern Samar, the Philippines. PLoS Negl. Trop. Dis. 9(2), 1–13. Hambal, M., Ayuni, R., Vanda, H., Amiruddin, A. and Athaillah, F. 2020. Occurrence of Fasciola gigantica and Paramphistomum spp infection in Aceh cattle. E3S Web of Conf. 151, 1–4. Hambal, M., Sayuti, A. and Darmawan, A. 2013. Tingkat kerentanan Fasciola gigantica pada sapi dan kerbau di Kecamatan Lhoong Kabupaten Aceh Besar. Medika Vet. 7, 49–53. Hayashi, K., Ichikawa-Seki, M., Mohanta, U.K., Shoriki, T., Chaichanasak, P. and Itagaki, T. 2018. Hybrid origin of Asian aspermic Fasciola flukes is confirmed by analyzing two single-copy genes, pepck and pold. J. Vet. Med. Sci. 80(1), 98–102. Ichikawa-Seki, M., Hayashi, K., Tashiro, M. and Khadijah, S. 2022. Dispersal direction of Malaysian Fasciola gigantica from neighboring southeast Asian countries inferred using mitochondrial DNA analysis. Infect. Genet. Evol. 105, 105373. Islam, K.M., Islam, M.S., Andhikary, G.N., Hossain, K.M.M., Rauf, S.M.A. and Rahman, M. 2016. Epidemiological studies of fascioliosis (Fasciola gigantica infection) in cattle. Adv. Parasitol. 3(1), 10–13. Jaja, I.F., Mushonga, B., Green, E. and Muchenje, V. 2017. Seasonal prevalence, body condition score and risk factors of bovine fasciolosis in South Africa. Vet. Anim. Sci. 4, 1–7. Karim, M.R., Mahmud, M.S. and Giasuddin, M. 2015. Epidemiological study of bovine fasciolosis: Prevalence and risk factor assessment at Shahjadpur Upazila of Bangladesh. Immunol. Infect. Dis. 3(3), 25–29. Khademvatan, S., Majidiani, H., Khalkhali, H., Taghipour, A., Asadi, N. and Yousefi, E. 2019. Prevalence of fasciolosis in livestock and humans: a systematic review and meta-analysis in Iran. Comp. Immunol. Microbiol. Infect. Dis. 65, 116–123. Khan, N.U., Sultan, S., Ullah, I., Ali, H., Sarwar, M.S., Ali, A., Usman, T., Khan, A.U., Hussain, M., Ali, M., Rabbani, F. and Rahman, A. 2020. Epidemiological study of bovine fasciolosis using coprological technique in district Mardan, Khyber Pakhtunkhwa, Pakistan. Pure Appl. Biol. 9(1), 455–463. Kipyegen, C.K., Muleke, C.I. and Otachi, E.O. 2017. Human and animal fasciolosis : coprological survey in Narok, Baringo and Kisumu counties, Kenya. Onderstepoort J. Vet. Res. 89(1), 1–6. Kudzai, M. 2018. Prevalence and risk factor associated with fasciolosis in the Southern Region of Masvingo Province. B.Sc. Thesis, Midlands State University. Kusumarini, S.R., Permata, F.S., Widyaputri, T. and Prasetyo, D. 2020. Prevalence of fasciolosis emphasis on age, origin, body condition and post mortem by geographic information systems on sacrificial examination in Malang District - East Java. J. Phys. Conf. Ser. 1430(1), 1–7. Le, T.H., Nguyen, K.T., Nguyen, N.T.B., Doan, H.T.T., Le, X.T.K., Hoang, C.T.M. and Van De, N. 2012. Development and evaluation of a single-step duplex PCR for simultaneous detection of Fasciola hepatica and Fasciola gigantica (family Fasciolidae, class Trematoda, phylum Platyhelminthes). J. Clin. Microbiol. 50(8), 2720–2726. Levi, K., Nigo, S., Agoth, L., Tom, Z.M., Mahmoud, M.A.M. and El-fadil, A.A.M. 2020. Epidemiological survey of bovine fasciolosis in Bor County, Jonglei State, South Sudan. J. Agric. Vet. Sci. 12(7), 27–31. Loeurng, V., Chea, B., Tum, S. and Seng, M. 2016. Challenge and prevalence of fasciolosis in cattle in Pursat Province, Cambodia. IJERD – Int. J. Environ. Rural. Dev. 7(1), 70–76. Loeurng, V., Ichikawa-Seki, M., Wannasan, A., Sothyra, T., Chaisowwong, W. and Tiwananthagorn, S. 2019. Genetic characterization of Cambodian Fasciola gigantica and dispersal direction of the species in Asia. Vet. Parasitol. 273, 45–51. Martindah, E., Widjajanti, S., Estuningsih, S.E. and Suhardono. 2005. Improvement of public awarness on fasciolosis as zoonosis disease. Wartazoa. 15(3), 143–154. Mensah, D.-J.F. and Ofosu, F.K. 2020. Emerging foodborne diseases: what we know so far. J. Food. Hyg. Safe. 35(1), 1–5. Mochankana, M.E. and Robertson, I.D. 2018. Cross-sectional prevalence of Fasciola gigantica infections in beef cattle in Botswana. Trop. Anim. Health Prod. 50(6), 1355–1363. Mohammed, C., Nigussie, L., Dugasa, J. and Seid, U. 2018. Prevalence of bovine fasciolosis and its associated risk factors in Eastern Shoa, Kuyu District Central Ethiopia Chala. Arch. Vet. Sci. Technol. 2018(1), 1–5. Mukmin, A. and Lisnanti, E.F. 2019. Pendampingan pengembangan kapasitas usaha berbasis klaster peternak sapi Kabupaten Magetan. Cendekia : J. Pengabdian Masyarakat. 1(1), 10. Nasreldin, N. and Zaki, R.S. 2020. Biochemical and immunological investigation of fascioliasis in cattle in Egypt. Vet. World 13(5), 923–930. Nugrahanto, W. and Eviyanti, A. 2022. Integrasi budidaya tanaman padi sehat dengan ternak berbasis pertanian berkelanjutan di Desa Ngleses, Boyolali. Prosiding Seminar Nasional IV, 1(1). Nyirenda, S.S., Sakala, M., Moonde, L., Kayesa, E., Fandamu, P., Banda, F. and Sinkala, Y. 2019. Prevalence of bovine fascioliasis and economic impact associated with liver condemnation in abattoirs in Mongu district of Zambia. BMC Vet. Res. 15(1), 1–8. Opio, L.G., Abdelfattah, E.M., Terry, J., Odongo, S. and Okello, E. 2021. Prevalence of fascioliasis and associated economic losses in cattle slaughtered at lira municipality abattoir in northern Uganda. Animals 11(3), 10–10. Phalee, A. and Wongsawad, C. 2014. Prevalence of infection and molecular confirmation by using ITS-2 region of Fasciola gigantica found in domestic cattle from Chiang Mai province, Thailand. Asian Pac. J. Trop. Med. 7(3). 207–211. Prasetya, M.R., Koesdarto, S., Lastuti, N.D.R., Suwanti, L.T., Kusnoto, and Yunus, M. 2019. Study on the morphology of Fasciola gigantica and economic losses due to fasciolosis in Berau, East Kalimantan. J. Biol. Biol. Educ. 11(1), 156–161. Prasetyo, M.R., Lasuti, N.D.R., Koesdarto, S., Suwanti, L.T., Kusnoto, and Yunus, M. 2019. Morfometri dan ultrastruktur cacing Fasciola gigantica pada sapi donggala dan sapi bali di Kabupaten Berau, Kalimantan Timur. J. Vet. 20(2), 171–178. Purwaningsih, P., Noviyanti, N. and Putra, R.P. 2018. Distribusi dan faktor risiko fasciolosis pada sapi bali di Distrik Prafi, Kabupaten Manokwari, Provinsi Papua Barat. Acta Vet. Indones. 5(2), 120–126. Purwaningsih, P., Palulungan, J.A., Tethool, A.N., Noviyanti, N., Satrija, F. and Murtini, S. 2022. Seasonal dynamics of Fasciola gigantica transmission in Prafi district, Manokwari Regency, West Papua, Indonesia. Vet. World 15, 2558–2564. Rinca, K.F., Prastowo, J., Widodo, D.P. and Nugraheni, Y.R. 2019. Trematodiasis occurrence in cattle along the Progo River, Yogyakarta, Indonesia. Vet. World. 12(4), 593–597. Rokni, M.B., Bashiri, H., Raeghi, S., Teimouri, A., Shojaeimotlagh, V., Shiee, M.R. and Bozorgomid, A. 2020. Molecular phylogenetic and genetic variability of Fasciola gigantica in Kermanshah province, western Iran with an overview to understand haplotypes distribution in Asia and Africa. Vet. Res. Forum. 11(3), 265–271. Salmo, N.A.M., Hassan, S.M.A. and Saeed, A.K. 2014. Histopathological study of chronic livers fascioliasis of cattle in Sulaimani abattoir. AL-Qadisiya J. Vet. Med. Sci. 13(2), 71–80. Shinggu, P.A., Olufemi, O.T., Nwuku, J.A., Baba-Onoja, E.B.T. and Iyawa, P.D. 2019. Liver flukes egg infection and associated risk factors in white fulani cattle slaughtered in Wukari, Southern Taraba State, Nigeria. Adv. Prev. Med. 2019, 1–5. Shonhiwa, A.M., Ntshoe, G., Essel, V., Thomas, J. and McCarthy, K. 2019. A review of foodborne diseases outbreaks reported to the outbreak response unit, national institute for communicable diseases, South Africa, 2013–2017. Int. J. Infect. Dis. 79, 73. Suretno, N.D., Purwanto, B.P., Priyanto, R. and Supriyatna, I. 2017. Evaluasi kesesuaian lingkungan berdasarkan penampilan produksi empat bangsa sapi pada ketinggian berbeda di Provinsi Lampung. J. Vet. 18(3), 478. Tenorio, J.C.B. and Molina, E.C. 2021. Monsters in our food: Foodborne trematodiasis in the Philippines and beyond. Vet. Integrative Sci. 19(3), 467–485. Tulloh, N.M. 1962. A study of the incisor teeth of beef cattle. Aust. J. Agric. Res. 13(2), 350–361. Valinata, S., Susilo, J., Karmil, T.F. and Hamzah, A. 2020. Incidency and fasciolosis risk factors in cows in Pringsewu District, November 2019-January 2020. J. Medika Vet. 14(1), 74–80. Vázquez, A.A., Alda, P., Lounnas, M., Sabourin, E., Alba, A., Pointier, J.P. and Hurtrez-Boussès, S. 2018. Lymnaeid snails hosts of Fasciola hepatica and Fasciola gigantica (Trematoda: Digenea): a worldwide review. CAB Rev. 13, 62. Wildman, E.E., Jones, G.M., Wagner, P.E., Boman, R.L., Troutt, H.F. and Lesch, T.N. 1982. A dairy cow body condition scoring system and its relationship to selected production characteristics. J. Dairy Sci. 65(3), 495–501. Yanti, Y., Pawestri, W. and Harjunowibowo, D. 2022. Penyuluhan penyakit parasiter pada ternak ruminansia dan pelatihan pembuatan pupuk organik di kelompok ternak putra rahayu, Kabupaten Wonogiri, Jawa Tengah. Warta Pengabdian Andalas, 29(3), 231–238. Yasin, M.G., Alim, M.A., Anisuzzaman, M., Ahasan, S.A., Munsi, M.N., Chowdhury, E.H., Hatta, T., Tsuji, N. and Mondal, M.M.H. 2018. Trematode infections in farm animals and their vector snails in Saint Martin’s Island, the Southeastern offshore area of Bangladesh in the Bay of Bengal. J. Vet. Med. Sci. 80(4), 684–688. Yusuf, M., Ibrahim, N., Tafese, W. and Deneke, Y. 2016. Prevalence of bovine fasciolosis in municipal abattoir of Haramaya, Ethiopia. Food Sci. Quality Manag. 48(1), 38–43. Zainalabidin, F.A., Noor Azmi, M.S.N., Wan Omar Bakri, W.N., Sathaya, G. and Ismail, M.I. 2015. Screening for zoonotic fascioliasis in slaughtered large ruminants in abattoirs in Perak, Malaysia. Trop. Life Sci. Res. 26(2), 121–124. | ||
| How to Cite this Article |
| Pubmed Style Prasetyo DA, Nurlaelasari A, Wulandari AR, Cahyadi M, Wardhana AH, Kurnianto H, Kurniawan W, Kristianingrum YP, Muñoz-caro T, Hamid PH. High prevalence of liver fluke infestation, Fasciola gigantica, among slaughtered cattle in Boyolali District, Central Java. Open Vet. J.. 2023; 13(5): 654-662. doi:10.5455/OVJ.2023.v13.i5.19 Web Style Prasetyo DA, Nurlaelasari A, Wulandari AR, Cahyadi M, Wardhana AH, Kurnianto H, Kurniawan W, Kristianingrum YP, Muñoz-caro T, Hamid PH. High prevalence of liver fluke infestation, Fasciola gigantica, among slaughtered cattle in Boyolali District, Central Java. https://www.openveterinaryjournal.com/?mno=143738 [Access: November 23, 2025]. doi:10.5455/OVJ.2023.v13.i5.19 AMA (American Medical Association) Style Prasetyo DA, Nurlaelasari A, Wulandari AR, Cahyadi M, Wardhana AH, Kurnianto H, Kurniawan W, Kristianingrum YP, Muñoz-caro T, Hamid PH. High prevalence of liver fluke infestation, Fasciola gigantica, among slaughtered cattle in Boyolali District, Central Java. Open Vet. J.. 2023; 13(5): 654-662. doi:10.5455/OVJ.2023.v13.i5.19 Vancouver/ICMJE Style Prasetyo DA, Nurlaelasari A, Wulandari AR, Cahyadi M, Wardhana AH, Kurnianto H, Kurniawan W, Kristianingrum YP, Muñoz-caro T, Hamid PH. High prevalence of liver fluke infestation, Fasciola gigantica, among slaughtered cattle in Boyolali District, Central Java. Open Vet. J.. (2023), [cited November 23, 2025]; 13(5): 654-662. doi:10.5455/OVJ.2023.v13.i5.19 Harvard Style Prasetyo, D. A., Nurlaelasari, . A., Wulandari, . A. R., Cahyadi, . M., Wardhana, . A. H., Kurnianto, . H., Kurniawan, . W., Kristianingrum, . Y. P., Muñoz-caro, . T. & Hamid, . P. H. (2023) High prevalence of liver fluke infestation, Fasciola gigantica, among slaughtered cattle in Boyolali District, Central Java. Open Vet. J., 13 (5), 654-662. doi:10.5455/OVJ.2023.v13.i5.19 Turabian Style Prasetyo, Dimas Ariyanto, Andini Nurlaelasari, Aisyah Retno Wulandari, Muhammad Cahyadi, April Hari Wardhana, Heri Kurnianto, Wahyu Kurniawan, Yuli Purwandari Kristianingrum, Tamara Muñoz-caro, and Penny Humaidah Hamid. 2023. High prevalence of liver fluke infestation, Fasciola gigantica, among slaughtered cattle in Boyolali District, Central Java. Open Veterinary Journal, 13 (5), 654-662. doi:10.5455/OVJ.2023.v13.i5.19 Chicago Style Prasetyo, Dimas Ariyanto, Andini Nurlaelasari, Aisyah Retno Wulandari, Muhammad Cahyadi, April Hari Wardhana, Heri Kurnianto, Wahyu Kurniawan, Yuli Purwandari Kristianingrum, Tamara Muñoz-caro, and Penny Humaidah Hamid. "High prevalence of liver fluke infestation, Fasciola gigantica, among slaughtered cattle in Boyolali District, Central Java." Open Veterinary Journal 13 (2023), 654-662. doi:10.5455/OVJ.2023.v13.i5.19 MLA (The Modern Language Association) Style Prasetyo, Dimas Ariyanto, Andini Nurlaelasari, Aisyah Retno Wulandari, Muhammad Cahyadi, April Hari Wardhana, Heri Kurnianto, Wahyu Kurniawan, Yuli Purwandari Kristianingrum, Tamara Muñoz-caro, and Penny Humaidah Hamid. "High prevalence of liver fluke infestation, Fasciola gigantica, among slaughtered cattle in Boyolali District, Central Java." Open Veterinary Journal 13.5 (2023), 654-662. Print. doi:10.5455/OVJ.2023.v13.i5.19 APA (American Psychological Association) Style Prasetyo, D. A., Nurlaelasari, . A., Wulandari, . A. R., Cahyadi, . M., Wardhana, . A. H., Kurnianto, . H., Kurniawan, . W., Kristianingrum, . Y. P., Muñoz-caro, . T. & Hamid, . P. H. (2023) High prevalence of liver fluke infestation, Fasciola gigantica, among slaughtered cattle in Boyolali District, Central Java. Open Veterinary Journal, 13 (5), 654-662. doi:10.5455/OVJ.2023.v13.i5.19 |