| Case Report | ||
Open Vet. J.. 2023; 13(9): 1223-1227 Open Veterinary Journal, (2023), Vol. 13(9): 1223-1227 Case Report A spontaneous ovarian teratoma in an FVB/n female mouse: Case report and literature reviewPaula Alexandra Martins de Oliveira1,2,3*, Ana I. Faustino-Rocha1,2,4,5, Rui M. Gil da Costa1,2,6,7,8,9, Elisabete Nascimento Gonçalves1,2, Ana Margarida Calado3,10, Catarina Jota Baptista1,2,3,11,12, Adelina Gama3,10 and Fernanda Seixas3,101Centre for the Research and Technology of Agro-Environmental and Biological Sciences (CITAB), University of Trás-os-Montes and Alto Douro (UTAD), Vila Real, Portugal 2Institute for Innovation, Capacity Building and Sustainability of Agri-food Production (Inov4Agro), Vila Real, Portugal 3Department of Veterinary Sciences, School of Agrarian and Veterinary Sciences, UTAD, Vila Real, Portugal 4Department of Zootechnics, School of Sciences and Technology, University of Évora, Évora, Portugal 5Comprehensive Health Research Center, Évora, Portugal 6Molecular Oncology and Viral Pathology Group, Research Center of IPO Porto (CI-IPOP)/RISE [at] CI-IPOP (Health Research Network), Portuguese Oncology Institute of Porto (IPO Porto)/Porto Comprehensive Cancer Center (Porto.CCC), Porto, Portugal 7Biomedicine Research Center (CEBIMED), Faculty of Health Sciences, Fernando Pessoa University, Porto, Portugal 8Laboratory for Process Engineering, Environment, Biotechnology and Energy (LEPABE), Faculty of Engineering, University of Porto, Porto, Portugal 9Associate Laboratory in Chemical Engineering (ALiCE), Faculty of Engineering, University of Porto, Porto, Portugal 10Associate Laboratory for Animal and Veterinary Sciences (AL4AnimalS), Animal and Veterinary Research Centre (CECAV), UTAD, Vila Real, Portugal 11Institute of Biomedicine (IBIOMED), Universidad de León, León, Spain 12Faculty of Veterinary Medicine, Lusophone University of Humanities and Technologies, Lisbon, Portugal *Corresponding Author: Paula Alexandra Martins de Oliveira. Department of Veterinary Sciences, School of Agrarian and Veterinary Sciences, UTAD, Vila Real, Portugal. Email: pamo [at] utad.pt Submitted: 25/03/2023 Accepted: 25/08/2023 Published: 30/09/2023 © 2023 Open Veterinary Journal
AbstractBackground: Teratomas are rare types of germ cell neoplasms composed of various differentiated or undifferentiated tissues. Case Description: A 25-week-old female control FVB /n mouse in a 4-week toxicity study presented abdominal distension and poor body condition. It was euthanized, and the necropsy examination revealed a large mass connected to the tip of the right uterine horn, occupying the entire abdominal cavity. Microscopically, this mass showed areas of epidermal differentiation, with laminated keratin and sebaceous glands, differentiation into respiratory and digestive epithelium, cartilage, bone, and extensive areas of differentiation into the nervous tissue, being classified as an ovarian teratoma. Conclusion: As far as authors know, the occurrence of ovarian teratomas in the FVB/n mouse strain has never been previously described. Keywords: Mouse, Ovary, Rodent, Teratoma. IntroductionMice and rats make up approximately 95% of all laboratory animals, making the mice the most used animals in biomedical research (Hickman et al., 2017). The analysis of the published works regarding the development of spontaneous tumors in laboratory animals, particularly in the mouse, reveals their low incidence. These values may result from two different situations: either the incidence of spontaneous tumors is very low, or many small or microscopic tumors are not identified because tissues are not collected by routine. As a standard procedure, our research team performs a complete necropsy of all animals that die during experimental trials or those must be sacrificed before the scheduled date (due to humane endpoints). Thus, with the support of experienced pathologists, we have identified several undescribed tumors in rodents, such as a Zymbal tumor in a male Wistar rat (Faustino-Rocha et al., 2023) and a Schwannoma in a female Sprague–Dawley rat (Teixeira-Guedes et al., 2014). According to Gopinath (1994), the incidence of tumors in control animals during experimental protocols is influenced by the pathologist's experience. From our perspective, the incidence of spontaneous tumors in laboratory animals is also associated with the perspicacity of researchers and animal caretakers. Thus, we recommend that all animals in experimental trials, regardless of the cause of their death, should constantly be subjected to a careful and complete necropsy, and their organs should be analyzed by an experienced pathologist. With this approach, we will be able to understand the rate of spontaneous tumors in laboratory animals. Although spontaneous tumors are rare in laboratory animals, it is important to obtain data about their occurrence during their life, because spontaneous tumors observed before the end of a study may avoid a misinterpretation of the data (Son and Gopinath, 2004). Herein, for the first time, we describe a case of a spontaneous teratoma in a female FVB/n mouse. Case DetailsAnimalsTwenty female, 25-week-old FVB/n mice were used in a toxicity study for the safety assessment of a natural compound, the hydroethanolicc French Lavender extract (data not published). This animal belonged to the control group and was not exposed to the extract. These mice were housed in polycarbonate cages, under controlled conditions of humidity (50% ± 10%), temperature (23°C ± 2°C), air system filtration (10–20 ventilations/hour), and on a 12/12 hours light/dark cycle, and were allowed free access to standard laboratory diet (4RF21, Mucedola, Italy) and tap water. During the experimental assay, animals were observed and handled daily. On abdominal palpation, a large mass was detected in the abdomen of one mouse; its body weight was 50.1 g, while the average body weight of the other females was 25.2 g. The animal exhibited poor body condition, and, considering our humane endpoints, it was sacrificed by anesthetic overdose. The animal was subsequently subjected to a careful postmortem examination. Gross pathological examinationDuring the necropsy, a large mass connected to the right uterine horn was observed, occupying the entire abdominal cavity, measuring 5.5 × 3.5 × 2.8 cm in its largest dimensions and weighing 20.6 g (Figs. 1 and 2). This mass consisted of multiple cysts, the largest measuring 2.8 × 2.0 cm, with brownish content, and small whitish solid areas. The contralateral ovary showed no macroscopical alterations. The stomach was atrophied by compression, and the remaining organs did not show significant changes. The mass and all organs were fixed in 10% buffered formalin for 24 hours for histopathologic examination. Histopathological examinationThe specimens were processed for a routine histopathology examination. After sample fixation, they were cut using a microtome. Histological sections were then prepared and stained with hematoxylin and eosin (H&E).
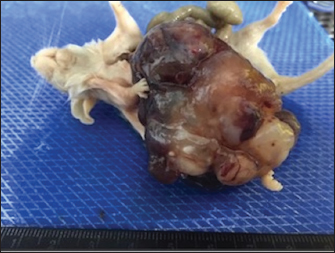
Fig. 1. Large cystic mass occupying the entire abdominal cavity.
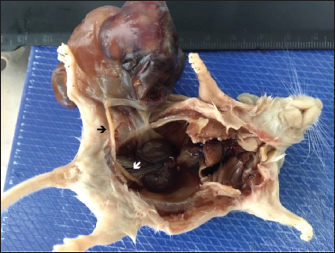
Fig. 2. Mass attached to the right uterine horn (black arrow); white arrow shows left uterine horn. The mass presented areas of epidermal differentiation with laminated keratin and sebaceous glands. Cell differentiation into digestive and respiratory epithelium, bone, cartilage, and vast areas of differentiation into nervous tissue were also present (Figs. 3–7). Moreover, the nervous tissue presented focal areas of ependymoma. Epithelial areas with hypercellularity, cell atypia, stromal microinvasion, hemorrhages, and necrosis were also observed. Metastatic epithelial cells were detected invading the pancreas, the small intestine serosa, and the mesenterium. Therefore, the mass was classified as a malignant teratoma with abdominal carcinomatosis. Ethical approvalAll animals were carefully handled following the European Guidelines for experimental studies (National Decree-law 113/2013 and European Directive 2010/63/EU). The study was approved by the National Competent Authority and by the University Ethics Committee.
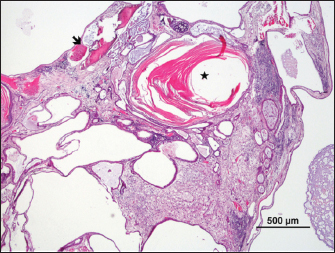
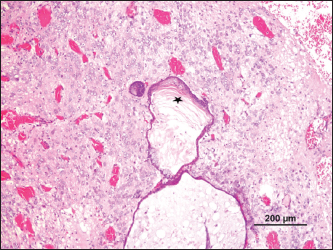
Fig. 3. Cystic mass with differentiation into stratified epithelium with accumulation of laminated keratin (*) and bone (arrow) (H&E).
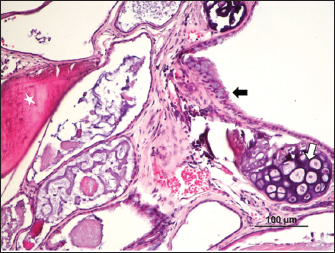
Fig. 4. Bone (*), cartilage (white arrow), and respiratory epithelium (black arrow) (H&E).
Fig. 5. Keratinized epithelium with an accumulation of laminated keratin (*), surrounded by well-differentiated nervous tissue (H&E).
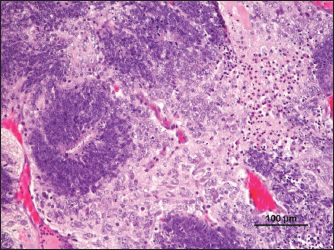
Fig. 6. Ependymoma (H&E).
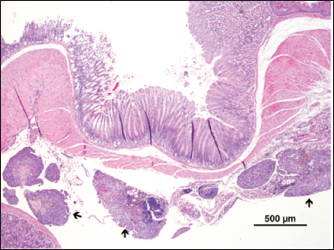
Fig. 7. Metastasis on gut serosa ( ) (H&E). DiscussionThe word “teratoma” is derived from the Greek word “teraton,” meaning monster, and it was coined by Virchow in 1863 (Comerci et al., 1994). Teratomas are complex neoplasms arising from more than one embryonic germ layer, composed of a variety of unorganized, differentiated, or undifferentiated tissues originating from ectoderm (hair, teeth, and nervous tissue), mesoderm (fibrous or adipose tissue, bone, muscle, and cartilage), and endoderm (respiratory tissue and salivary gland) (Sirivisoot et al., 2022). Most teratomas arise in the gonads, though occasionally, they may develop in extragonadal sites, such as the anterior mediastinum, retroperitoneal, and gastrointestinal tract. Extragonadal teratoma develops from primordial germ cells misplaced during their migration to gonads (De Felici et al., 2021; Sirivisoot et al., 2022). Two types of teratomas have been described: mature and immature. The mature teratoma is frequent, representing 50% of all ovary tumors, while the immature ones are scarce, representing only 1% (Saba et al., 2009). According to the ratio between dedifferentiated or incompletely differentiated and well-differentiated cell populations, biological behavior might range from benign to malignant (Sirivisoot et al., 2022). Mature teratomas are usually benign, but in 0.1%–0.2% of cases, they may undergo malignant transformation (Giustini et al., 1978). Teratomas are rare lesions in domestic animals. Nevertheless, gonadal teratomas are more common in bitches (Agnew and MacLachlan, 2016) and guinea pigs (Barthold et al., 2016), but rarely documented in other species, such as horses (Lefebvre et al., 2005), heifers (Carluccio et al., 2017), and cats (Sirivisoot et al., 2022). Considering laboratory animals, ovarian teratomas were defined as very rare in female rats (Tsubota et al., 2004). The first ovarian teratoma in mice was described by Slye et al. (1920). Afterward, ovarian teratomas were identified in a C3H female mouse by Jackson and Brues (1941); in Swiss albino mice by Fawcett (1950); and in C3HeB mice by Fekete and Ferrigno (1952). Thiery (1963) reported two cases of ovarian teratoma in C3H/N mice occurring in his colony: the first was identified in a 6-month-old breeding mouse and the second was reported in a virgin mouse. In this last case, the uterine cervix had been painted twice a week for 18 weeks with a 1% suspension of 3,4-benzopyrene in acetone to induce cervicovaginal cancer and had ingested 0.8 mg of estriol. According to Stevens and Varnum (1974), between 1920 and 1974, nine cases of teratomas were described in the following mice strains: C3H, Swiss Albino, C3HeB, C3H/N, CBA/J, and DBA/2J. Considering how relevant this tumor is in obstetrics and gynecology medicine, these researchers tried to develop an animal model to study ovarian teratoma in the LT mouse strain. However, ovarian teratomas of LT female mice were benign and composed of multiple types of well-differentiated tissues of embryonic and extraembryonic origin (Stevens and Varnum, 1974). Damjanov et al. (1975) described ovarian teratomas from 8, 9 to 10-week-old mice from a large colony of LT mice. Tumors were unilateral in all animals, except for one case that presented bilateral teratomas. In 1987, an analysis of the incidence of ovary teratoma in the B6C3F1 female mice was made and an incidence of 13.8% was reported (Alison and Morgan, 1987). More recently, Naser et al. (2021) crossed LT-ett1 and a previously established LT-Ter strain to develop the double-congenic strain LT-Ter-ett1. Furthermore, they also found a strain with a point mutation in the MC4R gene of the LT strain by genome editing, LT-MC4RG25S, and developed a double genetically modified strain LT-Ter-MC4RG25S to address the relation between Ter and MC4R genes. They observed the development of ovarian teratomas in all strains and concluded that MC4R is one of the genes responsible for forming ovarian teratomas (Naser et al., 2021). Although some reports of spontaneous ovarian teratomas in several strains of laboratory mice have appeared in the literature over the years, no reports were found in FVB/n mice. In the present case, for the first time, an ovarian teratoma was identified in a female laboratory FVB/n mouse, consisting of tissues originating from the three embryonic layers (endoderm, mesoderm, and ectoderm). The presence of invasive neoplasia and peritoneal carcinomatosis lesions confirms its malignant character. Though metastasis is rare, intra-abdominal metastasis was also reported to be associated with immature cat teratomas (Sirivisoot et al., 2022). AcknowledgmentNone. Conflict of interestThe authors declare that they have no conflict of interest. Author contributionsPaula A. Oliveira performed the experimental design, supervised the animal experiments, participated in animals’ sacrifice, and wrote the manuscript; Rui M. Gil da Costa made the histopathological diagnosis and wrote the manuscript; Elisabete Nascimento-Gonçalves conducted experiments with live animals and wrote the manuscript; Ana I. Faustino-Rocha conducted experiments with live animals and wrote the manuscript; Ana Margarida Calado made the histopathological diagnosis and wrote the manuscript; Adelina Gama made the histopathological diagnosis and wrote the manuscript; Catarina Jota Baptista wrote and revised the manuscript; Fernanda Seixas participated in animals’ sacrifice, made the histopathological diagnosis and wrote the manuscript. FundingThis work was supported by National Funds by the Portuguese Foundation for Science and Technology (FCT). The authors of the research unit CITAB received funding from FCT; reference of the projects UIDB/04033/2020 and LA/P/0126/2020. The authors of the research unit CECAV received funding from FCT; reference of the projects UIDB/CVT/00772/2020 and LA/P/0059/2020. ENG thanks FCT/MCTES and European Social Funding (EFS) through NORTE2020 for her Ph.D. fellowship grant ref. SFRH/BD/136747/2018. CJB is grateful to FCT for her Ph.D. grant (202104520.BD). Data availabilityAll data supporting the findings of this study are available within the manuscript. Any extra data needed are available from the corresponding author upon reasonable request. ReferencesAgnew, D.W. and MacLachlan, N.J. 2016. Tumors of the genital systems. In Tumors in domestic animals. Hoboken, NJ: John Wiley & Sons, pp: 689–722. Alison, R.H. and Morgan, K.T. 1987. Ovarian neoplasms in F344 rats and B6C3F1 mice. Environ. Health. Perspect. 73, 91–106. Barthold, S., Griffey, S. and Percy, D. 2016. Guinea pig. In Pathology of laboratory rodents and rabbits, 4th edn. Hoboken, NJ: John Wiley & Sons. Carluccio, A., Zedda, M.T., Contri, A., Gloria, A., Robbe, D., De Amicis, I. and Pau, S. 2017. Immature ovarian teratoma in two heifers. Vet. Ital. 53, 327–330. Comerci, J.T., Licciardi, F., Bergh, P.A., Gregori, C. and Breen, J.L. 1994. Mature cystic teratoma: a clinicopathologic evaluation of 517 cases and review of the literature. Obstet. Gynecol. 84, 22–28. Damjanov, I., Katić, V. and Stevens, L.C. 1975. Ultrastructure of ovarian teratomas in LT mice. Cancer. Res. Clin. Oncol. 83, 261–267. De Felici, M., Klinger, F.G., Campolo, F., Balistreri, C.R., Barchi, M. and Dolci, S. 2021. To be or not to be a germ cell: the extragonadal germ cell tumor paradigm. Int. J. Mol. Sci. 22, 5982. Faustino-Rocha, A.I., Oliveira, P.A., Caladom, A.M. and Seixas, F. 2023. Spontaneous Zymbal’s gland tumor in a 25-week old Wistar male rat: a case report. Vet. Stanica 54. Fawcett, D.W. 1950. Bilateral ovarian teratomas in a mouse. Cancer Res. 10, 705–707. Fekete, E. and Ferrigno, M.A. 1952. Studies on a transplantable teratoma of the mouse. Cancer Res. 12, 438–440. Giustini, F.G., Sohn, S. and Khosravi, H. 1978. Pelvic abscess and perforation of the sigmoid colon by a segment of benign cystic teratoma: an unusual complication of induced abortion. J. Reprod. Med. 20, 291–292. Gopinath, C. 1994. Spontaneous tumour rates: their use to support rodent bioassays. Toxicol. Pathol. 22, 160–164. Hickman, D.L., Johnson, J., Vemulapalli, T.H., Crisler, J.R. and Shepherd, R. 2017. Commonly used animal models. Princ. Anim. Res. Grad. Undergrad. Stud. 117–175. Jackson, E.B. and Brues, A.M. 1941. Studies on a transplantable embryoma of the mouse. Cancer Res. 1, 494–498. Lefebvre, R., Theoret, C., Doré, M., Girard, C., Laverty, S. and Vaillancourt, D. 2005. Ovarian teratoma and endometritis in a mare. Can. Vet. J. 46, 1029–1033. Naser, A.A., Miyazaki, T., Wang, J., Takabayashi, S., Pachoensuk, T. and Tokumoto, T. 2021. MC4R mutant mice develop ovarian teratomas. Sci. Rep. 11, 3483. Saba, L., Guerriero, S., Sulcis, R., Virgilio, B., Melis, G. and Mallarini G. 2009. Mature and immature ovarian teratomas: CT, US and MR imaging characteristics. Eur. J. Radiol. 72, 454–463. Sirivisoot, S., Siripara, N., Arya, N., Techangamsuwan, S., Rungsipipat, A. and Kasantikul, T. 2022. Case report: mature extragonadal teratoma at the proximal part of the tail in a kitten. Front. Vet. Sci. 9, 1003673. Slye, M., Holmes, H. and Wells, H. 1920. Primary spontaneous tumors of the ovary in mice.Stu dies on the incidence and inheritability of spontaneous tumors in mice. J. Cancer Res. 5, 205–226. Son, W.C. and Gopinath, C. 2004. Early occurrence of spontaneous tumors in CD-1 mice and Sprague-Dawley rats. Toxicol. Pathol. 32, 371–374. Stevens, L.C. and Varnum, D.S. 1974. The development of teratomas from parthenogenetically activated ovarian mouse eggs. Dev. Biol. 37, 369–380. Teixeira-Guedes, C.I., Faustino-Rocha, A.I., Talhada, D., Duarte. J.A., Ferreira, R., Seixas, F. and Oliveira, P.A. 2014. A liver schwannoma observed in a female Sprague-Dawley rat treated with MNU. Exp. Toxicol. Pathol. Pathol. 66, 125–128. Thiery, M. 1963. Ovarian teratoma in the mouse. Br. J. Cancer. 17, 231–234. Tsubota, K., Yoshizawa, K., Fujihira, S., Okazaki, Y., Matsumoto, M., Nakatsuji, S. and Oishi, Y. 2004. A spontaneous ovarian immature teratoma in a juvenile rat. J. Toxicol. Pathol. 17, 211–218. | ||
| How to Cite this Article |
| Pubmed Style De-oliveira PAM, Faustino-rocha AI, Da-costa RMG, Goncalves EN, Calado AM, Baptista CJ, Gama A, Seixas F. A spontaneous ovarian teratoma in an FVB/n female mouse: Case report and literature review. Open Vet. J.. 2023; 13(9): 1223-1227. doi:10.5455/OVJ.2023.v13.i9.19 Web Style De-oliveira PAM, Faustino-rocha AI, Da-costa RMG, Goncalves EN, Calado AM, Baptista CJ, Gama A, Seixas F. A spontaneous ovarian teratoma in an FVB/n female mouse: Case report and literature review. https://www.openveterinaryjournal.com/?mno=147336 [Access: January 14, 2026]. doi:10.5455/OVJ.2023.v13.i9.19 AMA (American Medical Association) Style De-oliveira PAM, Faustino-rocha AI, Da-costa RMG, Goncalves EN, Calado AM, Baptista CJ, Gama A, Seixas F. A spontaneous ovarian teratoma in an FVB/n female mouse: Case report and literature review. Open Vet. J.. 2023; 13(9): 1223-1227. doi:10.5455/OVJ.2023.v13.i9.19 Vancouver/ICMJE Style De-oliveira PAM, Faustino-rocha AI, Da-costa RMG, Goncalves EN, Calado AM, Baptista CJ, Gama A, Seixas F. A spontaneous ovarian teratoma in an FVB/n female mouse: Case report and literature review. Open Vet. J.. (2023), [cited January 14, 2026]; 13(9): 1223-1227. doi:10.5455/OVJ.2023.v13.i9.19 Harvard Style De-oliveira, P. A. M., Faustino-rocha, . A. I., Da-costa, . R. M. G., Goncalves, . E. N., Calado, . A. M., Baptista, . C. J., Gama, . A. & Seixas, . F. (2023) A spontaneous ovarian teratoma in an FVB/n female mouse: Case report and literature review. Open Vet. J., 13 (9), 1223-1227. doi:10.5455/OVJ.2023.v13.i9.19 Turabian Style De-oliveira, Paula Alexandra Martins, Ana I. Faustino-rocha, Rui M. Gil Da-costa, Elisabete Nascimento Goncalves, Ana Margarida Calado, Catarina Jota Baptista, Adelina Gama, and Fernanda Seixas. 2023. A spontaneous ovarian teratoma in an FVB/n female mouse: Case report and literature review. Open Veterinary Journal, 13 (9), 1223-1227. doi:10.5455/OVJ.2023.v13.i9.19 Chicago Style De-oliveira, Paula Alexandra Martins, Ana I. Faustino-rocha, Rui M. Gil Da-costa, Elisabete Nascimento Goncalves, Ana Margarida Calado, Catarina Jota Baptista, Adelina Gama, and Fernanda Seixas. "A spontaneous ovarian teratoma in an FVB/n female mouse: Case report and literature review." Open Veterinary Journal 13 (2023), 1223-1227. doi:10.5455/OVJ.2023.v13.i9.19 MLA (The Modern Language Association) Style De-oliveira, Paula Alexandra Martins, Ana I. Faustino-rocha, Rui M. Gil Da-costa, Elisabete Nascimento Goncalves, Ana Margarida Calado, Catarina Jota Baptista, Adelina Gama, and Fernanda Seixas. "A spontaneous ovarian teratoma in an FVB/n female mouse: Case report and literature review." Open Veterinary Journal 13.9 (2023), 1223-1227. Print. doi:10.5455/OVJ.2023.v13.i9.19 APA (American Psychological Association) Style De-oliveira, P. A. M., Faustino-rocha, . A. I., Da-costa, . R. M. G., Goncalves, . E. N., Calado, . A. M., Baptista, . C. J., Gama, . A. & Seixas, . F. (2023) A spontaneous ovarian teratoma in an FVB/n female mouse: Case report and literature review. Open Veterinary Journal, 13 (9), 1223-1227. doi:10.5455/OVJ.2023.v13.i9.19 |