| Research Article | ||
Open Vet. J.. 2023; 13(9): 1135-1140 Open Veterinary Journal, (2023), Vol. 13(9): 1135–1140 Original Research Fecal microbiota transplant for treatment of diarrhea in adult hospitalized horses—111 cases (2013–2018)Camilla Quattrini1*, Rana Bozorgmanesh2,3, Patricia Egli2 and K. Gary Magdesian41William R. Pritchard Veterinary Medical Teaching Hospital, School of Veterinary Medicine, University of California Davis, Davis, CA 95650, USA 2Hagyard Equine Medical Institute, Lexington, KY 40511, USA 3Steinbeck Peninsula Equine Clinics, Menlo Park, CA 94028, USA 4Department of Medicine and Epidemiology, School of Veterinary Medicine, University of California Davis, Davis, CA 95650, USA *Corresponding Author: Camilla Quattrini. William R. Pritchard Veterinary Medical Teaching Hospital, School of Veterinary Medicine, University of California Davis, Davis, CA 95650, USA. Email: cquattrini [at] ucdavis.edu Submitted: 26/04/2023 Accepted: 13/08/2023 Published: 30/09/2023 © 2023 Open Veterinary Journal
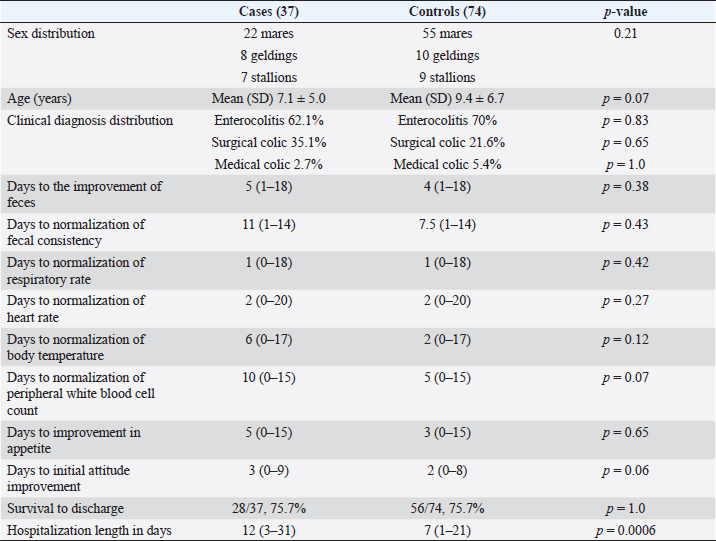
AbstractBackground: Fecal microbiota transplant (FMT) is increasingly administered as part of the treatment of colitis in horses, yet there is little data as to its effectiveness. Aim: Retrospective evaluation of the effects of FMT on discharge status, fecal consistency, length of hospitalization, and improvement in clinical signs in horses hospitalized for diarrhea. Methods: Retrospective case-control study. Medical records of adult horses (>1 year old) that received at least one transfaunation treatment (2013–2018) in two referral hospitals were identified through a medical records database search. Medical records of contemporary adult horses with diarrhea who did not receive FMT at the same study centers were used as controls. Results: Control horses had statistically significant shorter hospitalization [7 (1–21)] as compared to the transfaunation group [12 (3–31)] ( p =0.0006). There were no significant differences between groups in the number of days to the improvement of feces (p =0.38), or in days to normalization of fecal consistency (p =0.43), respiratory rate (p =0.42), heart rate (p =0.27), body temperature (p =0.12), peripheral white blood cell count (p =0.37), improvement in appetite (p =0.81), or attitude (p =0.06). There was also no significant difference in survival to discharge (transfaunation 28/37, 75.7%; control 56/74, 75.7%, p =1.0). Conclusion: There were no significant advantages of performing FMTs in horses with diarrhea in this retrospective study. This highlights the need for prospective, randomized studies to evaluate the efficacy of FMT, as well as different formulations, in horses with colitis before this can become standard practice. Keywords: Colitis, Diarrhea, Equine, Transfaunation, Treatment. IntroductionGastrointestinal (GI) disease is one of the leading causes of critical illness in horses (Costa et al., 2015), with equine colitis having an estimated mortality rate of 25%–35% (McKinney et al., 2021). There are multiple causes of colitis in horses, including infectious etiologies, parasites, antimicrobial or nonsteroidal anti-inflammatory drugs, carbohydrate overload, sand enteropathy, inflammatory bowel disease, and GI toxins, among others. Colitis is also a potential postoperative complication in horses undergoing colic surgery (Dias et al., 2018). Many cases remain undiagnosed as to specific etiology. Based on the inciting cause and severity of the disease, colitis can manifest with a spectrum of clinical signs, ranging from moderate illness to severe endotoxic shock. Diarrhea is a common clinical sign in adult horses, but abdominal discomfort, signs of systemic inflammatory response syndrome, and fever are also frequently reported. Due to the complex pathophysiology of enterocolitis in horses, and the lack of a specific diagnosis in up to 50% of cases (McKinney et al., 2020), treatment is often supportive and includes fluid therapy, nutritional support, and control of systemic inflammation secondary to endotoxemia and sepsis. The use of antimicrobial medications is controversial due to the risks of inducing or potentiating dysbiosis, except for cases where it is specifically indicated, such as Potomac horse fever or clostridial enteritis (Feary and Hassel, 2006). Broad-spectrum antimicrobials are often used in horses with marked neutropenia and fever, to prevent bacteremia or organ colonization by enteric organisms. Medications that decrease colonic fluid secretion or line the inflamed colon, such as bismuth subsalicylate, activated charcoal, and kaolin, are frequently used in horses with colitis, but their effectiveness remains to be studied. Di-tri-octahedral smectite (bio-sponge) is also used to absorb bacterial toxins and endotoxin (Smith et al., 2019). Fecal microbiota transplant (FMT) has been an effective and safe treatment for recurrent Clostridioides difficile infections (CDIs) and other GI disorders in humans (Van Nood et al., 2013; Cammarota et al., 2014); this finding, together with some evidence that alterations in the equine fecal microbiome are associated with several pathological conditions (Mullen et al., 2018) including colitis (Costa et al., 2012), has led to the use of FMT as adjunctive, empirical treatment in horses with diarrhea. Recently and for the first time, the FDA has authorized a fecal microbiota product for the treatment of C. difficile in humans (Rebyota, Ferring Pharmaceuticals, Parsippany, NJ). FMT or transfaunation in horses consists of nasogastric administration of feces or fecal extracts collected from a healthy equine donor, to restore normal GI flora in the recipient horse. A healthy GI microbiome is crucial for immune system function and resistance against colonization with pathogens (Kamada et al., 2013), as well as for normal cellulose digestion and subsequent production of volatile short-chain fatty acids, and normal GI physiology. There are limited reports and small case series describing FMT in horses (Dias et al., 2018; Muller et al., 2018; McKinney et al., 2021) and clinical results have been variable. A recent prospective study showed more rapid improvement of diarrhea score in horses receiving FMT compared to FMT-untreated cases (McKinney et al., 2021); however, transfaunated and control groups were treated in two different referral hospitals which precluded blinding, adding confounders unique to each hospital center, and the number of affected animals was small. In another report, FMT failed to cause significant changes in the microbiota of treated diarrheic horses (Costa et al., 2021). The objective of this study was to retrospectively evaluate the effects of FMT in a population of horses hospitalized for treatment of diarrhea. Outcome, fecal consistency, length of hospitalization, and speed of improvement of clinical signs were compared between adult horses that received FMT as a treatment for diarrhea and control horses treated with conventional methods and hospitalized at the same institutions and during the same period. We hypothesized that transfaunated horses would have a higher survival rate, shorter hospitalization period, and more rapid improvement in fecal consistency and clinical signs compared to control horses. Materials and MethodsAnimals/case selectionA retrospective study of medical records of horses ≥1 year of age that received at least one fecal transplant for diarrhea from Hagyard Equine Medical Institute in Lexington, KY (Institution 1), and from the University of California, Davis William R. Pritchard Veterinary Medical Teaching Hospital (Institution 2), from 2013 to 2018, was performed. Equids <1 year of age were excluded. To control for the time and effect of hospitalization, two control adult equids were selected for each transfaunated horse. Control cases were horses with diarrhea that were presented temporally just before and after each study animal at the same institution. The inclusion criteria for control horses was a complaint of diarrhea or development of diarrhea during hospitalization and no fecal transplantation administered. Data collectionData collected included signalment, diagnosis, physical examination findings at admission and during hospitalization (heart rate, temperature, respiratory rate, and attitude/mentation), appetite, fecal consistency, total white blood cell count at admission and during hospitalization, antimicrobial administration, and outcome (survived to discharge, euthanasia, and death). For the study cases, data regarding when transfaunation was performed, what protocol was used, how many treatments were administered, and concurrent use of gastro protectants and antibiotics were compiled. Horses were considered febrile if rectal temperature was increased >101.5°F (38.6°C). Animals were classified as tachycardic if the heart rate was >48 beats/minute and tachypneic if the respiratory rate was >20 breaths/minute. Horses were considered leukopenic if white blood cell count was decreased (<5,000/μl); leukocytosis at Institution 1 was defined as white blood cell count >11,600/μl, whereas at Institution 2 it was considered >12,000/μl based on institution laboratory reference ranges. Fecal consistency was described as diarrhea (anything looser than “cow pie”), “cow pie” consistency, or formed. Appetite was classified as good, fair, or poor based on medical records; horses with gastric reflux were grouped separately. Attitude was described as bright alert and responsive, quiet alert and responsive, and dull or painful based on medical records. Survival was described as survival to discharge from the hospital. FMT protocolsAt Institution 1, all horses received 3 l of fecal fluid. Feces were collected from donors and mixed with water. The mixture was strained in water, and fecal fluid was administered via nasogastric intubation; for all of the horses at Institution 1, fecal fluid was mixed with 1–3 l of bismuth subsalicylate (Pepto Bismol®, Procter and Gamble, Cincinnati, 45202) before administration. All horses had been treated with omeprazole by mouth (GastroGard®, Boehringer Ingelheim, Duluth, 30096; 4.4 mg/kg once a day) before and on the day of first transfaunation. In this population, 18/24 horses received systemic antimicrobials on the day of transfaunation. At Institution 2, all horses received 2–5 l of fecal fluid as the FMT. As per Institution 1, fresh feces were collected from the donors, wrapped in muslin cloth, and soaked in water for approximately 1 hour. All horses had been treated with omeprazole by mouth (4.4 mg/kg once a day) on the day prior and on the day of the first transfaunation. In this population, nasogastric intubation with fecal fluid only was performed in 8/13 horses; 5/13 horses received either bismuth subsalicylate, a feed supplement (FORCO® feed supplement digestive fortifier, Forco LLC, Canon City, 81212) or di-tri-octahedral smectite (Bio-Sponge® Platinum Performance, Buellton 93427) through nasogastric intubation at the time of transfaunation. In this group, 8/13 horses received systemic antimicrobials on the day of transfaunation. At both Institutions, fecal donors were adult horses, which were permanent residents at the facility and were routinely screened for Salmonella spp. Data analysisThe normality of the data was tested using the Kolmogorov–Smirnov test. Descriptive statistics were performed. Mean ± standard deviation (SD) was reported when data were normally distributed, whereas median and range were reported when data were not normally distributed. Fisher’s exact test, with calculation of odds ratios and 95% confidence intervals, was used to compare categorical data between groups. Continuous data were compared between cases and controls using Mann–Whitney tests, as data were nonparametric. Commercial statistical software was used (GraphPad InStat version 3.10, GraphPad software, San Diego, CA) and p < 0.05 was considered significant. Ethical approvalNot needed as this is a retrospective study. ResultsOne hundred and eleven horses were included in the study. Thirty-seven adult horses met the inclusion criteria for cases and 74 met the inclusion criteria for controls. Of the 37 cases, 24 were from Institution-1 and 13 were from Institution-2. Of the 74 controls, 48 were from Institution-1 and 26 from Institution-2. Horses represented 13 breeds, which included Thoroughbreds (75), Quarter Horses (14), Friesians (8), Mustangs (3), Warmbloods (3), Appaloosa (1), Andalusian (1), Clydesdale (1), Arabian (1), Morgan (1), American miniature (1), Norwegian Fjord (1), and Welsh Pony (1). The case distribution was 67.5% Thoroughbreds, 13.5% Quarter horses, 5.4% Friesians, 5.4% Mustangs, 2.7% and one each individual of a variety of breeds. The breed distribution in the controls was similar, with 67.5% Thoroughbreds, 12.1% Quarter horses, 8.1% Friesians, 2.7% Warmbloods, with one horse of a variety of other breeds. There were 77 mares, 18 geldings, and 16 stallions included in the study. The controls included 74.3% mares (55), 13.5% geldings (10), and 12.1% stallions (9). The cases included 61.1% mares (22), 21.6% geldings (8), and 18.9% stallions (7). There was no statistically significant difference in the sex distribution of males and females between groups (p =0.21). Mean (SD) and median (range) ages in the cases were 7.1 ± 5.0 and 6.0 (1.0–24.0) years, while they were 9.4 ± 6.7 and 8.0 (1.0–29.0) in the control horses, respectively. There was not a statistically significant difference in the ages between groups (p =0.07). Reasons for admission to the hospital included enterocolitis (73), surgical colic (31), medical colic (6), postfoaling peritonitis (1), and gastric ulcers (1). The cases included enterocolitis (62.1%), surgical colic (35.1%), and medical colic (2.7%). The controls included enterocolitis (70%), surgical colic (21.6%), medical colic (5.4%), equine gastric ulcer syndrome (1.3%), and postfoaling peritonitis (1.3%). There were no statistically significant differences in clinical diagnosis distribution between groups (enterocolitis, p =0.83; surgical colic, p =0.65; medical colic, p =1.0). Fecal transfaunation was first initiated on day 6 of hospitalization (range, 0–14, with one horse having initiated transfaunation on the farm). The number of transfaunations was a median of 2 (1–5). There were no statistically significant differences between groups in number of days to improvement of feces (p =0.38), days to normalization of fecal consistency (p =0.43), days to normalization of respiratory rate (p =0.42), heart rate (p =0.27) or body temperature (p =0.12), days to normalization of peripheral white blood cell count (p =0.37), or improvement in appetite (p =0.81) or attitude (p =0.06). While days to initial attitude improvement was not statistically significant, there was a trend for the control horses to achieve initial improvement in a shorter period [2 (0–8) vs. 3 (0–9) days, p =0.06]. There was no statistically significant difference in survival to discharge (transfaunation 28/37, 75.7%; control 56/74, 75.7%, p =1.0). There was a statistically significant difference in hospitalization length, with controls having a statistically significant shorter [7 (1–21)] duration as compared to the transfaunation group [12 (3–31)] ( p =0.0006) (Table 1). DiscussionResults of this retrospective study showed that the horses in the control group had a shorter hospitalization duration as compared to those in the transfaunation group, as well as a trend toward a shorter interval to the improvement of attitude. We hypothesize that this finding may reflect the use of FMT in horses with more severe disease at these hospitals, those that had failed to respond to conventional treatments. This would have resulted in a bias toward horses being treated with FMT when they had not improved with standard therapy, with a resultant longer hospitalization period and longer duration to attitude improvement. Table 1. Summary of clinical variables evaluated in 37 adult transfaunated horses (cases) and 74 adult control horses.
No statistically significant differences in time to improvement of vitals, fecal consistency, or laboratory data were found between the two groups. These findings are in contrast to results from studies in other species. Fecal microbiota transplantation is an effective treatment of human clostridial colitis and canine parvoviral gastroenteritis (Pereira et al., 2018). In the canine study, FMT was administered rectally, therefore, bypassing the possible effect of gastric acidity on microbial availability. In humans, FMT is often performed rectally as well, and this was recently approved as a rectally administered product by the FDA (Rebyota® Ferring Pharmaceuticals). This is the first fecal microbiota therapy approved by the FDA for recurrent CDI after patients have completed antibiotic therapy for recurrent CDI. The equine GI tract has major anatomical differences compared to humans and small companion animals; it is still uncertain how many viable bacteria can reach the cecum and colon of horses when FMT is administered intragastrically. When using nasogastric intubation, fecal fluid is deposited into the stomach and bacteria must survive the acidic environment to reach the large intestine. Sodium bicarbonate and proton pump inhibitors (PPIs, omeprazole in the horses of this study) are commonly administered to decrease gastric acidity before FMT (Dias et al., 2018), but their value as part of the transfaunation protocol has not been studied and remains speculative. Furthermore, the administration of PPIs has been listed as one of the factors associated with increased risk of CDI in humans (Barletta and Sclar, 2014). In accordance with our results, a recent experimental study in horses evaluating the efficacy of FMT in the prevention of metronidazole-induced dysbiosis did not find transfaunation with nasogastric intubation to be effective in preventing the microbiome changes induced by this antibiotic (Kinoshita et al., 2022). Even though the results of this work question the use of FMT from a prophylactic, rather than therapeutic, point of view further investigation on the effectiveness of fecal transplant protocols in horses is needed. Fecal storage methods, frequency of FMT administration, and amount of feces used, as well as the optimal handling of the feces, require study to fully investigate the potential utility of FMT in horses in the clinical setting. In the study evaluating metronidazole, donor feces were stored temporarily at −20°C while shipped and then frozen at −80°C (Kinoshita et al., 2022). A recent study compared different protocols for the preservation of equine fecal material and results suggested that fresh feces may be superior to frozen for this purpose. The bacterial viability significantly decreased when samples were stored at −20°C for more than 90 days (Costa et al., 2021). In our retrospective study, all FMTs were performed within 2 hours of collection of fecal material. Potential reasons for the lack of significant differences, aside from hospitalization length, in our study are the retrospective nature, which precludes a randomized, controlled trial (which would reduce confounders such as severity or etiology of disease, as well as additional therapeutics that may have been variably administered). It is possible, and likely, that horses treated with FMT were more severely ill or poorly responsive to traditional therapeutics. The longer hospitalization length suggests this may be the case. In addition, the viability and diversity of the microbial population of the fecal material used for transplantation were not assessed. Another likely reason for the lack of differences in the clinical efficacy of FMT in our study is that most of the studies reporting efficacy in humans target very specific diseases or etiologic agents, such as C. difficile, whereas our study had a wide heterogeneity of causes of diarrhea. A study of FMT in horses diagnosed with C. difficile may be warranted. This study has several limitations, primarily due to its retrospective nature. Description of the transfaunation protocols and assessments was subject to data available in the medical records. Subjective data such as the description of fecal consistency and demeanor of the horse relied on notes made in the clinical records and it is possible that assessments differed between personnel evaluating the patient. Furthermore, a perfect match between control and transfaunated horses was not always possible, due to the variety of medical conditions affecting the population of patients enrolled in the study. Treatments administered to the patients varied based on disease and clinician preference. Transfaunation was often administered as a second-line therapy with only 9/37 horses receiving fecal transplants within the first 2 days of hospitalization. This most likely influenced the results of our study. Patients were only followed to discharge from the hospital and any reoccurrence of diarrhea, change in longer-term mortality, or deterioration in clinical status after this time point would have not been accounted for. Most of the horses received antibiotics at the time of transfaunation (75% in Institution-1 and 65% in Institution-2, respectively). Studies have shown that several classes of antimicrobials can potentially reach high intestinal concentrations and induce changes in the luminal bacterial populations (Costa et al., 2015). Consequently, discontinuation of antibiotic therapy before FMT is recommended in the proposed guidelines for transfaunation in horses (Feary and Hassel, 2006; Mullen et al., 2018). Conversely, in a retrospective case series of four horses undergoing colic surgery and receiving FMT as treatment of postoperative acute colitis, 3/4 horses were transfaunated immediately after diarrhea and fever onset without discontinuation of antimicrobials; these horses showed prompt resolution of colitis within 24 hours (Dias et al., 2018). In 1/4 of cases, FMT was postponed to avoid administering it while the horse was receiving antibiotic medication; the authors felt that the delay of the procedure could potentially have caused prolongation of diarrhea and development of sepsis. The authors of that case series suggested that discontinuation of antibiotic therapy to perform FMT in colic cases might not be necessary. The intestinal microbiota was not evaluated in the donor horses. In human medicine, several studies suggest that the success of transfaunation is at least partially dependent on the microbial composition and diversity of the donor’s stool (Wilson et al., 2019). This concept led to the definition of “super donor” as a person whose microbiome is more likely to colonize a recipient’s GI tract. With the advancement of microbiota analysis techniques, the identification of these types of donors will likely be important in the equine population as well, requiring microbiome analysis in horses considered suitable donors based on conventional criteria. ConclusionIn conclusion, the results of this retrospective study did not show improvement in clinical parameters or outcomes for horses with diarrhea that received FMT as compared to controls. This highlights the need for prospective controlled clinical trials evaluating the effects of FMT in adult horses with colitis before it becomes established as a standard treatment. Further areas of research should focus on the intestinal microbiota composition of donors, as well as protocols that maximize the survival of the microbes. AcknowledgmentsNone. Conflict of interestThe authors declare that there is no conflict of interest. Authors contributionsCQ: conceptualization, data collection, methodology, writing, editing, and reviewing. RB: conceptualization, data collection, editing, and reviewing. PE: data collection and review. KGM: conceptualization, supervision, analyzing and interpreting data, methodology, writing, editing, and reviewing. FundingThere was no funding source for this study. Data availabilityAny extra data needed are available from the corresponding author upon reasonable request. ReferencesBarletta, J.F. and Sclar, D.A. 2014. Proton pump inhibitors increase the risk for hospital-acquired Clostridium difficile infection in critically ill patients. Crit. Care 18(1), 714–717. Cammarota, G., Ianiro, G. and Gasbarrini, A. 2014. Fecal microbiota transplantation for the treatment of Clostridium difficile infection a systematic review. J. Clin. Gastroenterol. 48(8), 693–702. Costa, M.C., Arroyo, L.G., Allen-Vercoe, E., Stämpfli, H.R., Kim, P.T., Sturgeon, A. and Weese, S.J. 2012. Comparison of the fecal microbiota of healthy horses and horses with colitis by high throughput sequencing of the V3-V5 region of the 16s rRNA gene. PLoS One 7(7), e41484. Costa, M.C., Stämpfli, H.R., Arroyo, L.G., Allen-Vercoe, E., Gomes, R.G. and Weese, J.S. 2015. Changes in the equine fecal microbiota associated with the use of systemic antimicrobial drugs. BMC Vet. Res. 11(1), 11–19. Costa, M., Di Pietro, R., Bessegatto, J.A., Fajardo Valente Pereira, P., Stievani, F.C., Gomes, R.G., Lisbôa, J.A.N. and Weese, J.S. 2021. Evaluation of changes in microbiota after fecal microbiota transplantation in 6 diarrheic horses. Can. Vet. J. 62(10), 1123–1130. Dias, D.P.M., Sousa, S.S., Molezini, F.A., Ferreira, H.S.D. and De Campos, R. 2018. Efficacy of faecal microbiota transplantation for treating acute colitis in horses undergoing colic surgery. Pesq. Vet. Bras. 38(8), 1564–1569. Feary, D.J. and Hassel, D.M. 2006 Enteritis and colitis in horses. Vet. Clin. North Am. 22(2), 437–479. Kamada, N., Chen, G.Y., Inohara, N. and Núñez, G. 2013. Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol. 14(7), 685–690. Kinoshita, Y., Niwa, H., Uchida-Fujii, E., Nukada, T. and Ueno, T. 2022. Simultaneous daily fecal microbiota transplantation fails to prevent metronidazole-induced dysbiosis of equine gut microbiota. J. Equine Vet. Sci. 114, 104004. McKinney, C.A., Oliveira, B.C.M., Bedenice, D., Paradis, M., Mazan, M., Sage, S., Sanchez, A. and Widmer, G. 2020. The fecal microbiota of healthy donor horses and geriatric recipients undergoing fecal microbial transplantation for the treatment of diarrhea. PLoS One 15(3), e0230148. McKinney, C.A., Bedenice, D., Pacheco, A.P., Oliveira, B.C.M., Paradis, M., Mazan, M. and Widmer, G. 2021. Assessment of clinical and microbiota responses to fecal microbial transplantation in adult horses with diarrhea. PLoS One 16(1), e0244381. Mullen, K.R., Yasuda, K., Divers, T.J. and Weese, J.S. 2018. Equine faecal microbiota transplant: current knowledge, proposed guidelines and future directions. Equine Vet. Educ. 30(3), 151–160. Pereira, G.Q., Gomes, L.A., Santos, I.S., Alfieri, A.F., Weese, J.S. and Costa, M.C. 2018. Fecal microbiota transplantation in puppies with canine parvovirus infection. J. Vet. Intern. Med. 32(2), 707. Smith, B., Van Metre, D. and Pusterla, N. 2019. Large animal internal medicine, 6th ed. Elsevier. Van Nood, E., Vrieze, A., Nieuwdorp, M., Fuentes, S., Zoetendal, E.G., De Vos, W.M., Visser, C.E., Kuijper, E.J., Bartelsman, J.F.W.M., Tijseen, J.G.P., Speelman, P., Dijkgraaf, M.G.W. and Keller, J.J. 2013. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 368(5), 407–415. Wilson, B.C., Vatanen, T., Cutfield, W.S. and O’Sullivan, J.M. 2019. The super-donor phenomenon in fecal microbiota transplantation. Front. Cell Infect. Microbiol. 9, 2. | ||
| How to Cite this Article |
| Pubmed Style Quattrini C, Bozorgmanesh R, Egli P, Magdesian KG. Fecal microbiota transplant for treatment of diarrhea in adult hospitalized horses – 111 cases (2013 – 2018). Open Vet. J.. 2023; 13(9): 1135-1140. doi:10.5455/OVJ.2023.v13.i9.9 Web Style Quattrini C, Bozorgmanesh R, Egli P, Magdesian KG. Fecal microbiota transplant for treatment of diarrhea in adult hospitalized horses – 111 cases (2013 – 2018). https://www.openveterinaryjournal.com/?mno=151125 [Access: January 25, 2026]. doi:10.5455/OVJ.2023.v13.i9.9 AMA (American Medical Association) Style Quattrini C, Bozorgmanesh R, Egli P, Magdesian KG. Fecal microbiota transplant for treatment of diarrhea in adult hospitalized horses – 111 cases (2013 – 2018). Open Vet. J.. 2023; 13(9): 1135-1140. doi:10.5455/OVJ.2023.v13.i9.9 Vancouver/ICMJE Style Quattrini C, Bozorgmanesh R, Egli P, Magdesian KG. Fecal microbiota transplant for treatment of diarrhea in adult hospitalized horses – 111 cases (2013 – 2018). Open Vet. J.. (2023), [cited January 25, 2026]; 13(9): 1135-1140. doi:10.5455/OVJ.2023.v13.i9.9 Harvard Style Quattrini, C., Bozorgmanesh, . R., Egli, . P. & Magdesian, . K. G. (2023) Fecal microbiota transplant for treatment of diarrhea in adult hospitalized horses – 111 cases (2013 – 2018). Open Vet. J., 13 (9), 1135-1140. doi:10.5455/OVJ.2023.v13.i9.9 Turabian Style Quattrini, Camilla, Rana Bozorgmanesh, Patricia Egli, and K. Gary Magdesian. 2023. Fecal microbiota transplant for treatment of diarrhea in adult hospitalized horses – 111 cases (2013 – 2018). Open Veterinary Journal, 13 (9), 1135-1140. doi:10.5455/OVJ.2023.v13.i9.9 Chicago Style Quattrini, Camilla, Rana Bozorgmanesh, Patricia Egli, and K. Gary Magdesian. "Fecal microbiota transplant for treatment of diarrhea in adult hospitalized horses – 111 cases (2013 – 2018)." Open Veterinary Journal 13 (2023), 1135-1140. doi:10.5455/OVJ.2023.v13.i9.9 MLA (The Modern Language Association) Style Quattrini, Camilla, Rana Bozorgmanesh, Patricia Egli, and K. Gary Magdesian. "Fecal microbiota transplant for treatment of diarrhea in adult hospitalized horses – 111 cases (2013 – 2018)." Open Veterinary Journal 13.9 (2023), 1135-1140. Print. doi:10.5455/OVJ.2023.v13.i9.9 APA (American Psychological Association) Style Quattrini, C., Bozorgmanesh, . R., Egli, . P. & Magdesian, . K. G. (2023) Fecal microbiota transplant for treatment of diarrhea in adult hospitalized horses – 111 cases (2013 – 2018). Open Veterinary Journal, 13 (9), 1135-1140. doi:10.5455/OVJ.2023.v13.i9.9 |