| Case Report | ||
Open Vet. J.. 2023; 13(9): 1205-1211 Open Veterinary Journal, (2023), Vol. 13(9): 1205–1211 Case Report Complete remission of two canine cases with precursor-targeted immune-mediated anemia after combination therapy with prednisolone, cyclosporine, and oclacitinibMasashi Yuki1,2*, Hiroto Taira1, Momoko Narita1, Takanori Inden1, Shunya Yokota1,2, Eiji Naito1 and Sadatoshi Maeda21Yuki Animal Hospital, Minato-ku, Japan 2The United Graduate School of Veterinary Sciences, Gifu University, Gifu, Japan *Corresponding Author: Raushan Rychshanova. Research Institute of Applied Biotechnology, A. Baitursynov Kostanay Regional University, Kostanay, Republic of Kazakhstan. Email: rychshanovaraushan [at] gmail.com Submitted: 20/05/2023 Accepted: 16/08/2023 Published: 30/09/2023 © 2023 Open Veterinary Journal
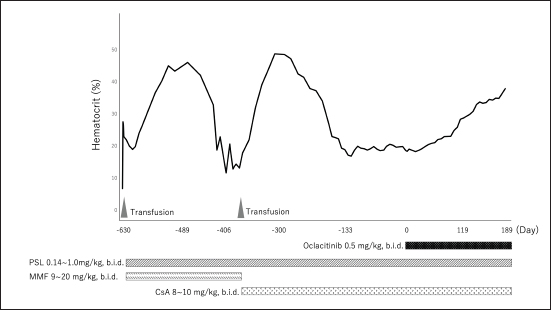
AbstractBackground: Precursor-targeted immune-mediated anemia (PIMA) has been described in dogs presenting with nonregenerative anemia and evidence of ineffective erythropoiesis. Although it has been suggested that its occurrence may be related to the immune targeting of erythroid precursors, this pathogenesis has not been established. PIMA is mainly treated with glucocorticoids, and in cases where glucocorticoids alone are not effective, immunosuppressants are also used as combination therapy. However, not all cases of PIMA go into remission after these treatments. Case Description: Two dogs with severe nonregenerative anemia diagnosed as PIMA based on the results of clinical pathological examinations, including bone marrow examination, were treated with whole-blood transfusion and immunosuppressive doses of prednisolone, mycophenolate mofetil, and cyclosporine. However, these treatments failed to achieve remission of PIMA. Therefore, concomitant administration of oclacitinib, which is a Janus kinase-1 inhibitor that has been applied recently to the treatment of immune-mediated diseases, was performed; this combined regimen improved the anemia and achieved complete remission of PIMA. Conclusion: Oclacitinib may be an option for the treatment of PIMA in dogs failing to achieve remission with conventional immunosuppressive therapy. Keywords: Cyclosporine, Dog, Oclacitinib, Prednisolone, Precursor-targeted immune-mediated anemia. IntroductionPrecursor-targeted immune-mediated anemia (PIMA) has been described in dogs presenting with nonregenerative anemia and evidence of ineffective erythropoiesis; moreover, although it has been suggested that its occurrence may be related to the immune targeting of erythroid precursors, this pathogenesis has not been established (Weiss, 2008; Lucidi et al., 2017; Assenmacher et al., 2019). However, 5 of 14 dogs with PIMA exhibited binding of immunoglobulin (Ig)G to erythrocyte precursors (Lucidi et al., 2021). It is also hypothesized that IgM, complement protein 3, IgA, and other mediators of phagocytosis and cell-mediated immunity, as well as immune-mediated hemolytic anemia, are involved in the pathogenesis of this disease (Lucidi et al., 2021). In fact, phagocytosis of intact erythroid precursors (rubriphagocytosis) by normal-appearing macrophages is found in bone marrow samples from most dogs with PIMA (Lucidi et al., 2017; Assenmacher et al., 2019). Moreover, many cases of PIMA respond to immunosuppressive therapy (Stokol et al., 2000; Assenmacher et al., 2019; Morishita et al., 2023), which supports these pathological hypotheses. Dogs presenting with nonregenerative anemia for >5 days accompanied by cytological or histological evidence of ineffective bone marrow erythropoiesis are diagnosed with PIMA (Lucidi et al., 2017; Assenmacher et al., 2019). In addition, findings suggestive of immune-mediated mechanisms, such as the rubriphagocytosis of intact erythrocyte precursors and a response to glucocorticoid therapy, are used to further confirm the diagnosis (Lucidi et al., 2017; Assenmacher et al., 2019). Although PIMA is mainly treated with glucocorticoids, in cases in which glucocorticoids alone are not effective, immunosuppressants (such as azathioprine, mycophenolate mofetil [MMF], cyclosporine A [CsA], human gamma-globulin, and biological agents) are used as combination therapy (Scott-Moncrieff et al., 1995; Stokol et al., 2000; Yuki, 2011; Assenmacher et al., 2019; Morishita et al., 2023). However, not all cases of PIMA go into remission after these treatments. Janus kinases (JAKs) are intracellular cytoplasmic tyrosine kinases that transduce the cytokine-mediated activation of membrane receptors via the phosphorylation of signal transducers and activators of transcription (STATs) (Schwartz et al., 2016). There are four known JAK subtypes (JAK1, JAK2, JAK3, and tyrosine kinase2), and the blockage of cytokine signaling via inhibition of the JAK–STAT pathway is a promising therapeutic option for immune-mediated disease (Kim, 2020). In recent years, JAK1 inhibitors have begun to be clinically applied in human medicine to treat rheumatoid arthritis (Kim and Keam, 2022), inflammatory bowel disease (Harris and Cummings, 2021), and aplastic anemia (AA) (Rosenberg et al., 2022). In veterinary medicine, a JAK1 inhibitor (oclacitinib) has been developed and has been clinically applied to inhibit the interleukin (IL)-31-mediated intracellular signaling that induces itching and inflammation in canine atopic or allergic dermatitis (Gonzales et al., 2014). Recently, oclacitinib was reported to be effective against immune-mediated skin diseases in dogs, such as ischemic dermatopathy, autoimmune subepidermal blistering dermatosis, hyperkeratotic erythema multiforme, pemphigus vulgaris, and cutaneous lupus erythematosus (Aymeric and Bensignor, 2017; Levy et al., 2019; High et al., 2020; Martinez et al., 2022; Harvey et al., 2023). An in vitro study of the effects of oclacitinib on T-cell proliferation and cytokine production found that this drug significantly reduced the secretion of clonal activator cytokines (IL-2 and IL-15), proinflammatory cytokines (interferon-γ and IL-18), and the regulatory cytokine IL-10; whereas the production of the tumor necrosis factor α and IL- 6 was mildly inhibited (Banovic et al., 2019). These actions are thought to be involved in the therapeutic effects of oclacitinib on immune-mediated skin diseases; however, the details of the underlying mechanisms have not been clarified. We report two canine cases in which complete PIMA remission was achieved after the administration of a combination therapy of oclacitinib in addition to prednisolone (PSL) and CsA, in anticipation of its immunosuppressive or modulatory effects of JAK1 inhibitor on these cases of PIMA that had failed to achieve remission previously. Case DetailsIn Japan, there are no ethics committees for private-practice veterinary hospitals. Nevertheless, this research was conducted following the ethical guidelines of the Japan Veterinary Medical Association. The samples were collected after obtaining written consent from the dog’s owner. Case 1An 8-year-old male spayed mix dog presented with vomiting to a family doctor 18 days before the visit to our institution, and was noted to have a heart murmur and mucosal membrane pallor; however, no detailed examination was performed. Eighteen days later, a blood test performed by a family doctor revealed the presence of severe anemia, and the dog was referred to our hospital for treatment. On physical examination, this dog weighed 6.3 kg, its rectum temperature was 38.6°C, its heart rate was 160 beats/min, and its respiratory rate was 40 breaths/min. The mucous membranes were pale, and the dog’s heart murmur was a systolic murmur with a Levine grade of 3/6. The dog’s general appearance was quiet, alert, and responsive. There was no history of drug administration. A hemogram revealed severe anemia (hematocrit (Ht), 6.8% (reference interval [RI], 37.3–61.7%); hemoglobin concentration was 2.2 g/dl (RI, 13.1–20.5 g/dl)). The mean corpuscular volume (MCV) (81.0 fl [RI, 60–77 fl]) and mean corpuscular hemoglobin concentration (MCHC) (32.4 g/dl [RI, 32–36 g/dl]) indicated macrocytic normochromic anemia. The reticulocyte count was 36,000/μl (RI, 10,000–110,000/μl) (ProCyte Dx Hematology Analyzer; IDEXX, Tokyo, Japan). Autoaggregation, as assessed using the saline agglutination test (four drops of saline per drop of blood), was negative. Biochemical analyses revealed various abnormalities, including increased activity of alkaline phosphatase (331 U/l [RI, 47–254 U/l]); an increased concentration of glucose (147 mg/dl [RI, 75–128 mg/dl]); and an increased level of C-reactive protein (CRP) (6.9 mg/dl [RI, 0–1.0 mg/dl]) (FUJI DRI-CHEM 7000V; FUJIFILM Corporation, Tokyo, Japan). The results of the urinalysis were unremarkable. Radiography of the thorax and abdomen revealed no abnormalities. The results of echocardiography were unremarkable; however, abdominal ultrasonography revealed a slight biliary sludge. Additional tests, including the direct antiglobulin test (DAT) (Animal Medical Technology, Nagoya, Japan) and polymerase chain reaction (PCR), were used to detect a panel of vector-borne hemopathogens (Anaplasma spp., Babesia spp., Bartonella spp., Ehrlichia spp., Hepatozoon spp., Leishmania spp., Neorickettsia risticii, and Rickettsia rickettsii) (IDEXX Laboratories, Tokyo, Japan). The tests were performed in a commercial veterinary medical laboratory. DAT was negative at both 4°C and 37°C. Furthermore, the PCR for both vector-borne pathogens was negative. Whole-blood transfusion from a single appropriate donor, as identified based on blood type and the results of cross-matching tests, was performed. Ht after whole-blood transfusion was 25.9%. On the following day, a bone marrow biopsy was performed under general anesthesia. The findings of the bone marrow examination indicated ineffective erythropoiesis with rubriphagocytosis and myelofibrosis. Even after an additional 5 days, the reticulocyte count was 49,600/μl; thus, a diagnosis of nonregenerative anemia was established. These clinicopathological findings met the diagnostic criteria of Assenmacher et al. (2019) and a diagnosis of PIMA, as follows: (a) unexplained persistent nonregenerative anemia (on the day of bone marrow collection, all had Ht ≤30% and reticulocyte count <76,000 reticulocytes/μl and documented anemia or anemia-related clinical signs for at least 5 days); (b) ineffective erythropoiesis was defined as the present of one of several erythropoietic response patterns with or without evidence of rubriphagocytosis, as evidenced by persistent anemia with erythroid hyperplasia or an increase in early-stage erythroid precursors with maturation arrest; and (c) a clinical diagnosis of PIMA. Treatment with PSL (PREDONINE Tablets, Shionogi & Co., Ltd, Osaka, Japan) (1 mg/kg, bis in die [b.i.d.], per os [p.o.]), MMF (CELLCEPT capsules, CHUGAI PHARMACEUTICAL CO., LTD., Tokyo, Japan) (18 mg/kg, b.i.d., p.o.), and lansoprazole (Takepron OD Tablets, Takeda Pharmaceutical Co., Ltd., Tokyo, Japan) (0.5 mg/kg, semel in die [s.i.d.], p.o.) was initiated. Twenty-one days after the onset of treatment, the dog’s clinical signs improved, the reticulocyte counts increased to 113,100/μl, and the Ht level (19%) began to indicate recovery (Fig. 1). Three months after the onset of treatment, the dog’s Ht level recovered to RI and complete remission was obtained. PSL could be tapered down to 0.15 mg/kg, b.i.d., p.o., and MMF was maintained at the same dose (Fig. 1). After about 4 months of remission, anemia progressed again, and PSL was increased to 1 mg/kg, b.i.d., p.o., albeit with no effect. Moreover, Ht decreased to 11.7%; thus, a second whole-blood transfusion was performed. In addition, the combination therapy for PSL was changed from MMF to CsA (Atopica, Elanco Japan, Tokyo, Japan) (8 mg/kg, b.i.d., p.o.). Two months after the switch to CsA, Ht recovered to within the RI and a second remission was obtained; therefore, PSL was tapered down to 0.15 mg/kg, b.i.d., p.o., and CsA was maintained at the same dose (Fig. 1). Although this second remission was maintained for 5 months, anemia progressed again at this time point. PSL was increased to 1 mg/kg, b.i.d., p.o., but remission was not obtained. Even when PSL was tapered to 0.15 mg/kg, b.i.d., p.o., the Ht level remained at around 20% (Fig. 1). After determining that complete remission was not possible, the owner of the dog consented to the initiation of combination therapy with oclacitinib (Apoquel, Zoetis Japan, Tokyo, Japan) (0.5 mg/kg, b.i.d., p.o.), in addition to PSL, and CsA. Two weeks after the onset of the combination therapy with oclacitinib, the reticulocyte count (from 61,700/μl to 81,500/μl) increased, and the level of Ht (from 18.3% to 19.2%) tended to recover. After 6 months, Ht returned to the RI and the dog achieved complete remission (Fig. 1). Complete remission (CR) is still maintained with the administration of the combination therapy of PSL (0.14 mg/kg, b.i.d., p.o.), CsA (7 mg/kg, b.i.d., p.o.), and oclacitinib (0.5 mg/kg, b.i.d., p.o.) at 11 months after its initiation. Since then, CsA has started to taper. Case 2A 10-year-old male spayed Maltese presented to our hospital with appetite loss. On physical examination, the dog weighed 3.55 kg, its rectum temperature was 38.7°C, its heart rate was 150 beats/min, and its respiratory rate was 30 breaths/min. The mucous membranes were pale, and the dog’s heart murmur was a systolic murmur with a Levine grade of 2/6. The dog’s general appearance was quiet, alert, and responsive. There was no history of drug administration. A hemogram revealed the presence of anemia (Ht, 24.3%); hemoglobin concentration, 7.9 g/dl). The MCH (67.3 fl) and MCHC (32.5 g/dl) values were indicative of normocytic normochromic anemia. The reticulocyte count was 21,300/μl. Autoaggregation, as assessed using the saline agglutination test, was negative. Biochemical analyses revealed a mildly increased CRP (1.1 mg/dl) level. Radiography of the abdomen revealed microhepatia. Moreover, the results of echocardiography revealed slight mitral regurgitation, whereas abdominal ultrasonography revealed slight biliary sludge. The measurement of the total bile acid concentration, as an additional test, yielded high pre (16.2 μmol/l) and post (41.4 μmol/l) values (RI, 7.9 μmol/l).
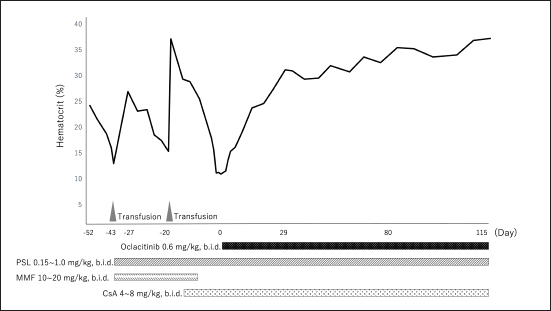
Fig. 1. Dynamic change in the hematocrit (case 1). PSL=prednisolone; MMF=mycophenolate mofetil; CsA=cyclosporine A. The concentration of serum iron, total iron-binding capacity, total-T4, free-T4, and thyroid-stimulating hormone were measured at a commercial laboratory, which revealed that total-T4 (0.7 μg/dl [RI, 0.9–2.9]) was low, thyroid-stimulating hormone (0.46 ng/mL [RI, 0.01–0.33]) was high, and serum iron and total iron-binding capacity was normal. Based on these results, hepatic failure and hypothyroidism were suspected, and administration of levothyroxine (Thyro-Tabs, LLOYD, Inc. Iowa, IA) (14 μg/kg, b.i.d., p.o.) was started. However, 10 days later, the dog’s Ht was 13%, MCV was 66.3 fl, MCHC was 30.8 g/dl, and reticulocyte count was 26,900/μl, indicating the progression of nonregenerative anemia with normocytic normochromic anemia. Additional tests, including DAT and PCR, were used to detect a panel of vector-borne hemopathogens. DAT was negative at both 4°C and 37°C. Furthermore, the PCR of both vector-borne pathogens was negative. A whole-blood transfusion was performed, and the post-transfusion Ht was 27%. Moreover, a bone marrow examination was performed, and its findings indicated ineffective erythropoiesis with erythroid hyperplasia. These clinicopathological findings met the diagnostic criteria of Assenmacher et al. (2019), and the dog was diagnosed with PIMA. Treatment with PSL (1 mg/kg, b.i.d., p.o.), MMF (10 mg/kg, b.i.d., p.o.), and lansoprazole (0.5 mg/kg, s.i.d., p.o.) was initiated (Fig. 2). However, 7 days after the onset of the treatment, Ht decreased to 19.7%; therefore, MMF was increased to 20 mg/kg, b.i.d. Nine days after increasing the MMF dose, Ht decreased to 15.4%, and a second whole-blood transfusion was performed (Fig. 2). Subsequently, 19 days after the initiation of MMF administration, the combination therapy with PSL was changed from MMF to CsA (7 mg/kg, b.i.d., p.o.). After the whole-blood transfusion, Ht was 34%, but decreased to 14.9%, 12 days after starting CsA administration (Fig. 2). In addition, lethargy, anorexia, and diarrhea were observed, and platelet count (31,000/μl [RI, 148,000–484,000/μl]) and alanine transaminase (349 U/l [RI, 17–78 U/l]), alkaline phosphatase (3,272 U/l), γ-glutamyltransferase (102 U/l [RI, 5–14 U/l]), total bilirubin (1.5 mg/dl [RI, 0.1–0.5 mg/dl]), and CRP (14.0 mg/dl) levels were increased. Moreover, prolongation of the activated partial thromboplastin time (39.2 s [RI, 12.0–24.0 s]) was detected, and the results requested from a commercial veterinary medical laboratory revealed low antithrombin (93% [RI, 116–161%]) and a high concentration of thrombin-antithrombin Ⅲ complex (0.767 ng/mL [RI, <0.251 ng/mL]) and D-dimer (141 μg/dl [RI, <1 μg/dl]). Abdominal ultrasonography indicated thickening of the gallbladder wall, suggesting predisseminated intravascular coagulation caused by bacterial cholangiohepatitis, or a thromboembolic event. The dog was immediately hospitalized, and intensive care with antibiotic therapy (ampicillin (20 mg/kg, b.i.d. subcutaneous injection [s.c.]) (LANOAX, Fujita Pharmaceutical Co., Ltd. Tokyo, Japan) and enrofloxacin (10 mg/kg, s.i.d., s.c.) (Baytril, Elanco Japan, Tokyo, Japan)) and intravenous infusion was initiated. PSL tapered immediately to 0.6 mg/kg, every other day. The administration of rivaroxaban (0.6 mg/kg, s.i.d., p.o.) (Xarelto, Bayer Japan, Osaka, Japan) was started, for predisseminated intravascular coagulation or a thromboembolic event. Because we could not find a blood-transfusion donor for PIMA, and because we could not increase the dose of prednisolone because of the dog’s suspected bacterial infection or a thromboembolic event, we started a combination therapy with PSL, CsA, and oclacitinib (0.6 mg/kg, b.i.d., p.o.) with the consent of the owner (Fig. 2). Ten days after hospitalization, the clinical signs of the dog improved, indicating a recovery trend and the biochemical analyses that showed abnormal values at the time of admission improved almost to the respective RIs; therefore, the patient was discharged. At 13 days after the onset of the combination therapy with oclacitinib, the reticulocyte count (from 41,500/μl to 146,700/μl) increased, and the level of Ht (from 17.9% to 19.2%) tended to recover. At 17 days after, Ht was 23.8%, and Ht was 31% by day 31, with complete remission achieved approximately 4 months later (Fig. 2). At 5 months after the onset of the combination therapy with oclacitinib, CsA tapering began, and 11 months later, CsA was successfully withdrawn, and CR is maintained with PSL (0.25 mg/kg, s.i.d., p.o.) and oclacitinib (0.45 mg/kg, b.i.d., p.o.). DiscussionThe two cases presented here were diagnosed with PIMA based on the diagnostic criteria proposed by Assenmacher et al. (2019). Those authors reported that clinical remission was achieved in 73% of patients with PIMA after the administration of a combination treatment of PSL and immunosuppressants, such as azathioprine, CsA, and MMF (Assenmacher et al., 2019). Based on that report, similar treatments were performed in the current study; however, although case 1 reached a temporary remission with these treatments, it was of short duration and could not be maintained. In turn, case 2 did not respond to any of these treatments and had to undergo repeated blood transfusions. In human medicine, JAK inhibitors inhibit the JAK1–STAT pathway and are used to suppress inflammatory cytokines, such as interferon-α and IL-6, in patients with conditions such as rheumatoid arthritis (Kim and Keam, 2022) and inflammatory bowel disease (Harris and Cummings, 2021). In recent years, because of concerns about adverse reactions (such as cytopenia triggered by pan-JAK inhibition), upadacitinib (Harris and Cummings, 2021), filgotinib (Kavanaugh et al., 2017), and itacitinib (Avci et al., 2021), which selectively JAK1, have been developed and approved. In human hematologic diseases, based on a case in which remission was obtained after the administration of the JAK1 inhibitor itacitinib to patients with AA with gain-of-function variants in STAT1, the dysregulation of the JAK1–STAT1 pathway was shown to be associated with the development of the condition (Rosenberg et al., 2022). In addition, the efficacy of the JAK1/2 inhibitor ruxolitinib in murine immune AA supports the clinical trials of JAK1/2 inhibitors in human AA and other immune-mediated myelopathies (Groarke et al., 2023). PIMA resembles AA (AA is an autoimmune disease that is caused by the overactivation of T cells primarily targeting the bone marrow), in that, the immune system mistargets and destroys blood cell precursors in the bone marrow (Lucidi et al., 2017; Young, 2018). Considering the possibility that the pathology of PIMA also involves the JAK1–STAT pathway, we administered oclacitinib to the two cases of dogs with PIMA, and both patients achieved complete remission. Although the detailed mechanism underlying this effect could not be clarified, these findings suggest that the JAK1–STAT pathway is involved in the pathogenesis of PIMA, as observed in AA, and that blocking JAK1 prevents the targeted destruction of blood cell progenitors. However, the mechanism of action of oclacitinib in PIMA warrants further research.
Fig. 2. (a) Dynamic Change in the hematocrit (case 2). PSL=prednisolone; MMF=mycophenolate mofetil; CsA=cyclosporine A. Oclacitinib administered at a licensed dose of 0.4–0.6 mg/kg twice daily for 2 weeks and then once daily as required is recommended for the treatment of canine atopic or allergic dermatitis (Cosgrove et al., 2013). Recently, a study of the safety of long-term administration of oclacitinib twice daily for canine atopic disease was reported, which described adverse events including pyoderma, gastrointestinal signs, and otitis externa, whereas blood laboratory tests showed slight decreases in white blood cell, neutrophil, eosinophil, and monocyte counts; however, the frequency was consistent with the approved protocol (Denti et al., 2022). In addition, the doses used to date to treat immune-mediated skin diseases have been 0.5–0.7 mg/kg twice daily for long periods of 6 weeks to 2 years or longer, and no adverse reactions were reported (Aymeric and Bensignor, 2017; Levy et al., 2019; High et al., 2020; Martinez et al., 2022; Harvey et al., 2023). In the two cases included in this study, long-term administration of 0.5–0.6 mg/kg of oclacitinib twice a day was performed, but no adverse reactions were observed. Moreover, administration of oclacitinib to treat immune-mediated skin disease has been performed using oclacitinib alone or in combination with PSL (Aymeric and Bensignor, 2017; Levy et al., 2019; High et al., 2020; Martinez et al., 2022; Harvey et al., 2023); in contrast, in the present study, combined therapy of PSL and CsA with oclacitinib was administered to the two dogs until complete remission was achieved. During the period leading up to CR, we closely monitored the patients for adverse events, but no adverse reactions were observed in either of the two cases. Long-term combination therapy with CsA and tofacitinib for 6 months has also been reported for steroid-resistant ulcerative colitis in humans, with no adverse reactions being reported (Yang et al., 2021). The two cases in this study did not receive oclacitinib alone; therefore, it remains unclear whether its concomitant use with prednisolone or CsA is required for PIMA remission. The combination of PSL, CsA, and oclacitinib may cause severe adverse reactions, and the need for the administration of these combinations should be carefully considered. This case report has a limitation. A recent study reported a 5% increase in median time in Ht after CsA administration in nonregenerative anemia of 55.5 days span (Morishita et al., 2023). In case 1 of this study, Ht increased by 5% after the first administration of CsA after 56 days. Therefore, in case 2, administration of oclacitinib was initiated after 14 days of CsA, but it can be assumed that CsA alone might have been effective without additional administration of oclacitinib. However, due to the unavailability of a continuous donor, we were unable to wait for more than 14 days for the effects of CsA to appear. Also, in case 2, Ht increased by 5% in 17 days after the additional administration of oclacitinib; hence, we assumed that the additional administration of oclacitinib was effective. ConclusionDogs with PIMA who failed to achieve remission with PSL and CsA achieved CR when combined with oclacitinib. Therefore, oclacitinib may be an option for the treatment of PIMA in dogs failing to achieve remission with conventional immunosuppressive therapy. However, as this study was a case report that included only two patients, the effect of oclacitinib on PIMA warrants further investigation. AcknowledgmentsNone. Author contributionsM. Yuki contributed in writing—original draft preparation and supervision. H. Taira, M. Narita, and T. Inden is the primary clinician. H. Taira, M. Narita, S. Yokota, and E. Naito collected and analyzed the data. M. Yuki and S. Maeda supervised the final manuscript. All authors have read and agreed to the published version of the manuscript. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Data availabilityThe datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request. ReferencesAssenmacher, T.D., Jutkowitz, L.A., Koenigshof, A.M., de, A.L.C. and Scott, M.A. 2019. Clinical features of precursor-targeted immune-mediated anemia in dogs: 66 cases (2004–2013). J. Am. Vet. Med. Assoc. 255, 366–376. Avci, A.B., Feist, E. and Burmester, G.R. 2021. Early phase studies of JAK1 selective inhibitors in rheumatoid arthritis. Rheumatology 60, ii11–ii16. Aymeric, E. and Bensignor, E. 2017. A case of presumed autoimmune subepidermal blistering dermatosis treated with oclacitinib. Vet. Dermatol. 28, 512-e123. Banovic, F., Tarigo, J., Gordon, H., Barber, J.P. and Gogal, R.M., Jr. 2019. Immunomodulatory in vitro effects of oclacitinib on canine T-cell proliferation and cytokine production. Vet. Dermatol. 30, 17-e16. Cosgrove, S.B., Wren, J.A., Cleaver, D.M., Walsh, K.F., Follis, S.I., King, V.I., Tena, J.K. and Stegemann, M.R. 2013. A blinded, randomized, placebo-controlled trial of the efficacy and safety of the Janus kinase inhibitor oclacitinib (Apoquel®) in client-owned dogs with atopic dermatitis. Vet. Dermatol. 24, 587–597, e141-582. Denti, D., Caldin, M., Ventura, L. and De Lucia, M. 2022. Prolonged twice-daily administration of oclacitinib for the control of canine atopic dermatitis: a retrospective study of 53 client-owned atopic dogs. Vet. Dermatol. 33, e149–e142. Gonzales, A.J., Bowman, J.W., Fici, G.J., Zhang, M., Mann, D.W. and Mitton-Fry, M. 2014. Oclacitinib (APOQUEL(®)) is a novel Janus kinase inhibitor with activity against cytokines involved in allergy. J. Vet. Pharmacol. Ther. 37, 317–324. Groarke, E.M., Feng, X., Aggarwal, N., Manley, A.L., Wu, Z., Gao, S., Patel, B.A., Chen, J. and Young, N.S. 2023. Efficacy of JAK1/2 inhibition in murine immune bone marrow failure. Blood 141, 72–89. Harris, C. and Cummings, J.R.F. 2021. JAK1 inhibition and inflammatory bowel disease. Rheumatology (Oxf. Engl.). 60, ii45–ii51. Harvey, R.G., Olivri, A., Lima, T. and Olivry, T. 2023. Effective treatment of canine chronic cutaneous lupus erythematosus variants with oclacitinib: seven cases. Vet. Dermatol. 34, 53–58. High, E.J., Linder, K.E., Mamo, L.B., Levy, B.J., Herrmann, I. and Bizikova, P. 2020. Rapid response of hyperkeratotic erythema multiforme to oclacitinib in two dogs. Vet. Dermatol. 31, e330–e386. Kavanaugh, A., Kremer, J., Ponce, L., Cseuz, R., Reshetko, O.V., Stanislavchuk, M., Greenwald, M., Van der Aa, A., Vanhoutte, F., Tasset, C. and Harrison, P. 2017. Filgotinib (GLPG0634/GS-6034), an oral selective JAK1 inhibitor, is effective as monotherapy in patients with active rheumatoid arthritis: results from a randomised, dose-finding study (DARWIN 2). Ann. Rheum. Dis. 76, 1009–1019. Kim, E.S. and Keam, S.J. 2022. Correction to: filgotinib in rheumatoid arthritis: a profile of its use. Clin. Drug Investig. 42, 101. Kim, H.O. 2020. Development of JAK inhibitors for the treatment of immune-mediated diseases: kinase-targeted inhibitors and pseudokinase-targeted inhibitors. Arch. Pharm. Res. 43, 1173–1186. Levy, B.J., Linder, K.E. and Olivry, T. 2019. The role of oclacitinib in the management of ischaemic dermatopathy in four dogs. Vet. Dermatol. 30, 201-e263. Lucidi, C.A., de Rezende, C.L.E., Jutkowitz, L.A. and Scott, M.A. 2017. Histologic and cytologic bone marrow findings in dogs with suspected precursor-targeted immune-mediated anemia and associated phagocytosis of erythroid precursors. Vet. Clin. Pathol. 46, 401–415. Lucidi, C.A., Gerlach, J.A., Jutkowitz, A. and Scott, M.A. 2021. Immunoglobulin G and phosphatidylserine in regenerative and nonregenerative immune-mediated anemias of dogs. J. Vet. Intern. Med. 35, 2713–2721. Martinez, N., McDonald, B. and Crowley, A. 2022. A case report of the beneficial effect of oclacitinib in a dog with pemphigus vulgaris. Vet. Dermatol. 33, 237-e265. Morishita, K., Sugawara-Suda, M., Yamazaki, J., Sasaki, N., Nakamura, K., Ohta, H. and Takiguchi, M. 2023. Evaluation of responses to immunosuppressive therapy in dogs with suspected non-regenerative immune-mediated anaemia: 11 cases (2012–2018). J. Small. Anim. Pract. Online ahead of print. Rosenberg, J.M., Peters, J.M., Hughes, T., Lareau, C.A., Ludwig, L.S., Massoth, L.R., Austin-Tse, C., Rehm, H.L., Bryson, B., Chen, Y.B., Regev, A., Shalek, A.K., Fortune, S.M. and Sykes, D.B. 2022. JAK inhibition in a patient with a STAT1 gain-of-function variant reveals STAT1 dysregulation as a common feature of aplastic anemia. Med 3, 42–57.e45. Schwartz, D.M., Bonelli, M., Gadina, M. and O’Shea, J.J. 2016. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat. Rev. Rheumatol. 12, 25–36. Scott-Moncrieff, J.C., Reagan, W.J., Glickman, L.T., DeNicola, D.B. and Harrington, D. 1995. Treatment of nonregenerative anemia with human gamma-globulin in dogs. J. Am. Vet. Med. Assoc. 206, 1895–1900. Stokol, T., Blue, J.T. and French, T.W. 2000. Idiopathic pure red cell aplasia and nonregenerative immune-mediated anemia in dogs: 43 cases (1988–1999). J. Am. Vet. Med. Assoc. 216, 1429–1436. Weiss, D.J. 2008. Bone marrow pathology in dogs and cats with non-regenerative immune-mediated haemolytic anaemia and pure red cell aplasia. J. Comp. Pathol. 138, 46–53. Yang, Q., Chen, L., Feng, L., Liu, C., Fang, L., Liu, Z. and Sun, X. 2021. Success of cyclosporin and tofacitinib combination therapy in a patient with severe steroid-refractory ulcerative colitis. Inflam. Bowel. Dis. 27, e157–e158. Young, N.S. 2018. Aplastic anemia. N. Engl. J. Med. 379, 1643–1656. Yuki, M. 2011. A case of non-regenerative immune-mediated anemia treated by combination therapy of human immune globulin and mycophenolate mofetil in a dog. Open. Vet. J. 1, 46–49. | ||
| How to Cite this Article |
| Pubmed Style Yuki M, Taira H, Narita M, Inden T, Yokota S, Naito E, Maeda S. Complete remission of two canine cases with precursor-targeted immune-mediated anemia after combination therapy with prednisolone, cyclosporine, and oclacitinib. Open Vet. J.. 2023; 13(9): 1205-1211. doi:10.5455/OVJ.2023.v13.i9.16 Web Style Yuki M, Taira H, Narita M, Inden T, Yokota S, Naito E, Maeda S. Complete remission of two canine cases with precursor-targeted immune-mediated anemia after combination therapy with prednisolone, cyclosporine, and oclacitinib. https://www.openveterinaryjournal.com/?mno=153759 [Access: January 15, 2026]. doi:10.5455/OVJ.2023.v13.i9.16 AMA (American Medical Association) Style Yuki M, Taira H, Narita M, Inden T, Yokota S, Naito E, Maeda S. Complete remission of two canine cases with precursor-targeted immune-mediated anemia after combination therapy with prednisolone, cyclosporine, and oclacitinib. Open Vet. J.. 2023; 13(9): 1205-1211. doi:10.5455/OVJ.2023.v13.i9.16 Vancouver/ICMJE Style Yuki M, Taira H, Narita M, Inden T, Yokota S, Naito E, Maeda S. Complete remission of two canine cases with precursor-targeted immune-mediated anemia after combination therapy with prednisolone, cyclosporine, and oclacitinib. Open Vet. J.. (2023), [cited January 15, 2026]; 13(9): 1205-1211. doi:10.5455/OVJ.2023.v13.i9.16 Harvard Style Yuki, M., Taira, . H., Narita, . M., Inden, . T., Yokota, . S., Naito, . E. & Maeda, . S. (2023) Complete remission of two canine cases with precursor-targeted immune-mediated anemia after combination therapy with prednisolone, cyclosporine, and oclacitinib. Open Vet. J., 13 (9), 1205-1211. doi:10.5455/OVJ.2023.v13.i9.16 Turabian Style Yuki, Masashi, Hiroto Taira, Momoko Narita, Takanori Inden, Syunya Yokota, Eiji Naito, and Sadatoshi Maeda. 2023. Complete remission of two canine cases with precursor-targeted immune-mediated anemia after combination therapy with prednisolone, cyclosporine, and oclacitinib. Open Veterinary Journal, 13 (9), 1205-1211. doi:10.5455/OVJ.2023.v13.i9.16 Chicago Style Yuki, Masashi, Hiroto Taira, Momoko Narita, Takanori Inden, Syunya Yokota, Eiji Naito, and Sadatoshi Maeda. "Complete remission of two canine cases with precursor-targeted immune-mediated anemia after combination therapy with prednisolone, cyclosporine, and oclacitinib." Open Veterinary Journal 13 (2023), 1205-1211. doi:10.5455/OVJ.2023.v13.i9.16 MLA (The Modern Language Association) Style Yuki, Masashi, Hiroto Taira, Momoko Narita, Takanori Inden, Syunya Yokota, Eiji Naito, and Sadatoshi Maeda. "Complete remission of two canine cases with precursor-targeted immune-mediated anemia after combination therapy with prednisolone, cyclosporine, and oclacitinib." Open Veterinary Journal 13.9 (2023), 1205-1211. Print. doi:10.5455/OVJ.2023.v13.i9.16 APA (American Psychological Association) Style Yuki, M., Taira, . H., Narita, . M., Inden, . T., Yokota, . S., Naito, . E. & Maeda, . S. (2023) Complete remission of two canine cases with precursor-targeted immune-mediated anemia after combination therapy with prednisolone, cyclosporine, and oclacitinib. Open Veterinary Journal, 13 (9), 1205-1211. doi:10.5455/OVJ.2023.v13.i9.16 |