| Research Article | ||
Open Vet. J.. 2023; 13(10): 1251-1258 Open Veterinary Journal, (2023), Vol. 13(10): 1251–1258 Original Research Morphological and histological structure of hepatopancreas in rock goby Gobius paganellus on the western coast of LibyaSalma Aribe Abusrer*, and Hanan Husain ShtewiZoology Department, Faculty of Sciences, University of Tripoli, Tripoli, Libya *Corresponding Author: Salma Aribe Abusrer. Zoology Department, Faculty of Sciences, University of Tripoli, Tripoli, Libya. Email: salma_Abusrer [at] hotmail.com Submitted: 10/06/2023 Accepted: 06/09/2023 Published: 31/10/2023 © 2023 Open Veterinary Journal
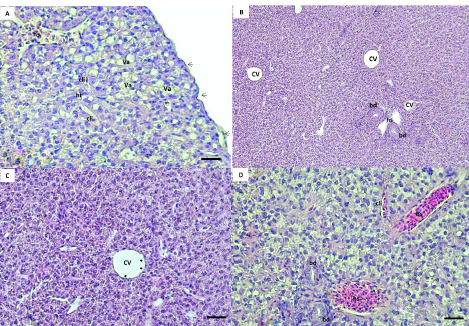
AbstractBackground: The rock goby, Gobius paganellus, is not a commercial species. This species has an essential role in the coastal ecosystem as a biological indicator. Therefore, it has been selected as the study’s model species. Aim: Due to the insufficient studies that have described the hepatopancreas of G. paganellus, this study aimed to provide information on the anatomical and histological structure of the hepatopancreas of the alimentary canal of this species on the western coast of Libya. Methods: Fifty mature G. paganellus specimens were collected from the northwest of Libya (Tajoura, Jodaem, and Farwa Island). Total length and total weight of the samples were measured and performed by using gross anatomy and histology. Then, the histological sections (3–5 µm) were stained with hematoxylin and eosin (H and E). Results: Morphologically, the liver has a large pyriform lobe. The dorsal surface of the liver is shiny and smooth, and the ventral surface contains shallow sulci; each sulus has a large blood vessel. Histologically, the liver is wrapped with a thin capsule of fibro-connective tissue. The hepatic parenchyma is made of hepatocytes with blood sinusoids. The hepatocytes are polygonal-shaped cells and have no hepatic lobules or portal triads. Melano-macrophage centers are distributed next to the blood vessels and bile ducts. The bile ducts are lined by columnar epithelial cells. The exocrine pancreatic tissue was observed in the liver parenchyma, and it consists of acini that are composed of pyramidal cells and contain zymogen granules. Conclusion: The liver of this species has both pancreatic and liver tissue, which was discovered in this investigation for the first time. Keywords: G. paganellus, Hepatopancreas, Architecture, Libya. IntroductionThe liver is a large vital organ connected to a vertebrate’s digestive system. The size, shape, and volume of the liver are adapted to the available space between other visceral organs in the general cavity, and it has a wide range of functions in all vertebrates (Faccioli et al., 2014). Fish organ systems vary to some degree from that of mammals due to the aquatic environment (Ferguson, 1989). The liver regulates the fish metabolism during anabolism and catabolism (Bruslé and Anadon, 1996; Lall and Kaushik, 2021). In addition, it plays a significant role in storing the metabolites, as well as producing most of the plasma proteins. It breaks down old red blood cells, and one of its products and gall secretion is eliminated through a duct into the duodenum (Bertolucci et al., 2008; Faccioli et al., 2014; Stori et al., 2014). However, the liver is a prominent organ that is well-recognized for being sensitive to a wide range of environmental factors. Because of the liver’s capacity for detoxification and storage of harmful components, which affect its morphological characteristics, it is often used as an environmental biomarker (Al-Yousuf et al., 2000; Brusle and Anadon, 1996; Gochfeld, 2003; Petcoff et al., 2006; Rocha et al., 1994). Nevertheless, there are variations in the number of liver lobs among the various fish species (Sales et al., 2017). The predominant cellular type of the liver, hepatocytes are arranged as cords to form cellular plates that separate a network of biliary canaliculi and many vascular sinusoids (Eurell and Haensly, 1982; Bruslé and Anadon 1996; Bombonato et al., 2007; Faccioli et al., 2014). The melano-macrophages are visible in the parenchyma of the fish liver, and unique clusters of pigment-containing cells are often arranged in melano-macrophage centers (Hartley et al., 1996). Melano-macrophage centers enlarged in conditions of environmental stress and have been proposed as accurate biomarkers for water quality in terms of both deoxygenation and iatrogenic chemical pollution (Agius and Roberts, 2003). The liver is a target organ for research on how the environment affects hepatic structure and/or physiological function Bruslé and Anadon (1996). The rock goby, Gobius paganellus, is one of the most abundant among all fish species. This species lives in Mediterranean Sea, Black Sea, Gulf of Eilat, and the Red Sea, as well as the northwest of Atlantic Ocean (Amores et al., 1990; Engin and Seyhan, 2009; Louiz et al., 2016; Ragheb et al., 2019), Portuguese rocky shores (Henriques et al., 1999), Western Scotland to Senegal, and the Atlantic islands of the Azores, Madeira, and Canary Islands (Miller, 1986). G. paganellus exists on the subtidal rocky areas at about five meters on Ireland’s coast and fifteen meters depth in the Azores (Dunne, 1978; Miller, 1984; Patzner et al., 1992; Miller, 1986). Family Gobiidae includes the benthic species Tripterygion delaisi, Parablennius incognitus, and P. ruber, it is considered one of the biggest marine families (Azevedo and Simas, 2000). In British Island; goby individuals reach maturity in their second or third year (Manuel et al., 2000). However, Gobiidae plays a significant role in the ecosystems of temperate and tropical coral reefs (Louiz, et al., 2016). Although many Gobiidae species are poorly understood in their environments and have limited descriptions of their eggs and larvae, the beginning of reproduction is also influenced by the water temperature throughout the winter (Miller, 1984; Ruple, 1984; Compaire et al., 2018). However, G. paganellus started the reproductive period in October, and the spawning season was from January to March. (Hajji et al., 2012). Despite G. paganellus species being adapted to tidal environments, they can be stressed at high temperatures of 30°C and above, which may damage the normal biological functions of organisms (Madeira et al., 2014). In addition, they may be particularly sensitive to high thermal waves that happen in low tide, which happens to be the warmest time of the day in the summer. However, the biological component of rock pond fish like G. paganellus, is of considerable importance in evaluations of environmental stress since it will help to create more plausible scenarios when dealing with the consequences of stress on tidal pond animals (Barton, 2002; Portner and Farrell, 2008; Sokolova, 2013; Pörtner et al., 2017; Ruple, 1984; Louiz et al., 2016; Paul et al., 2018). Therefore, this study investigates the liver of G. paganellus to test the hypotheses that the liver has a distinct structure. Materials and MethodsAnimalsFifty mature G. paganellus specimens were collected from the northwest of Libya (Tajoura, Jodaem, and Farwa Island). The total length and total weight of the samples were measured in grams and millimeters, respectively. A longitudinal incision was made a longitudinal incision along the ventral region of the fish. Gross anatomyThe liver’s features were examined and photographed alongside digestive tract organs. Samples were dissected, and the liver was then preserved in 4% formaldehyde for examination and documentation using a Leica M50 stereomicroscope (Germany). Histological proceduresAfter fish dissection, liver pieces from ten random samples were fixed in a 4% formaldehyde solution, and then the samples were washed with 70% ethanol, dehydrated in graded ethanol solutions, and then embedded in paraffin. Histological sections (3–5 µm) were stained with hematoxylin and eosin stain (H&E) (Suvarna et al., 2012). The sections were analyzed and photodocumented using an Olympus BX50 microscope (Japan). Ethical approvalNot needed for this study. ResultsGross anatomyThe results of this study showed the range of total length and total weight of the samples are 81–196 mm and 6.13–101.6 gm, respectively. The liver of G. paganellus lies ventral to the stomach and is located cranially to the stomach and posterior of the esophagus on the left side in the general cavity. The liver is pale reddish-brown (Fig. 1A and B). It is pyriform-shaped, with one lobe that is wide in the anterior portion and narrows in the posterior portion; a thin layer of serous membrane covers it and is completely suspended by mesenteries. The dorsal surface of the liver is smooth, whereas the ventral surface has a shallow fissure surrounding the large blood vessels (Fig. 1C). There is a main shallow fissure that appears along the ventral surface of the liver and branches to smaller on both sides of the liver, but it does not penetrate deep into the liver (Fig. 1D). Therefore, the lobs are less evident on both surfaces (Fig. 1C and D) and there is no appearance of a separate pancreas organ in the body cavity. Histological studiesThe liver parenchyma is encapsulated by a thin, delicate layer of loose connective tissues and squamous epithelial cells. Branches of the connective tissues were dispatched to the liver parenchyma, and no lobulation was present. However, the loose connective tissue was primarily composed of collagen fibers. The liver parenchyma was not divided into distinct hexagonal lobules. The hepatic parenchyma consists of polygonal to rounded hepatocytes with fat droplets (Fig. 2A). The parenchyma hepatocytes are arranged in acinus-like aggregation or cords that are organized in two cells thick with small canaliculi between a double face of the hepatocyte cords and no portal triads as in higher vertebrates (Fig. 2B and D). Hepatocytes were usually polygonal in shape and had spherical nuclei, eosinophilic cytoplasm, fat droplets, transparent cytoplasm, and large clear vacuolization. These vacuoles are glycogen and lipid droplets during the preparation of histologic specimens (Fig. 2A and D).
Fig. 1. (A, B) liver of G. paganellus lies cranially ventral to the stomach (st), on the left side in the general cavity, in a pale reddishbrown color. (C, D) Pyriform-shaped, one lobe with a smooth concave dorsal surface (ds) and a convex ventral surface (vs) with shallow fissure (arrowed) surrounding the large blood vessels. The liver is wide from the cranial region and, narrow from the tail and covered with a thin layer of serous membrane.
Fig. 2. (A, B, C, D) Histological sections of the liver show the hepatic tissue (ht) with discrete hepatocyte, central vein (cv), hepatic sinusoids (hs). A and C a thin layer of simple squamous epithelium with lamina propria encapsulated the liver parenchyma (black arrow). The presence of vacuolated hepatocyte (va). hs and cv lined with endothelial cells (black and white arrow), respectively. B and D bile ducts (bd) lined with simple cuboidal. The canaliculi (cli) between the hepatocytes. (Bar: B: 0.001 mm; A, C, D: 0.0005 mm). The blood sinusoidal is lined with simple squamous epithelial tissue (elongated endothelial cells with flattened nuclei) and discontinuous basement membrane forming a very thin epithelial sheet (Fig. 2D). No portal triads as in higher vertebrates or lobule division; instead, it has a structure of sinusoids that converge at the central vein (Fig. 2B). The biliary system consists of ducts and bile canaliculi (Fig. 2B and D). The bile ducts and portal vein are located surrounded mainly by the parenchyma of the liver tissue. The bile duct is lined by simple columnar epithelium and structurally consists of three distinct layers: tunica mucosa of epithelial cells that are simple cuboidal with large spherical nucleus and brush border, tunica muscularis of some smooth muscles, and very thin tunica serosa of dense connective tissues. The apical border of the adjacent hepatocytes faces toward the bile canaliculi direction, where the bile ducts exist (Fig. 2D).
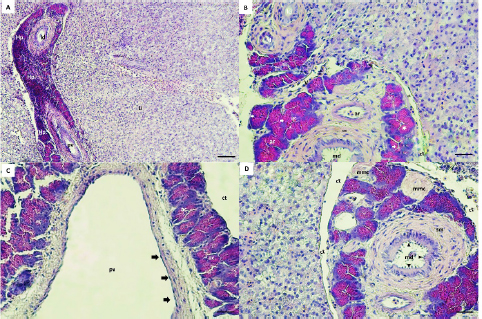
Fig. 3. Hepaticpancreas tissue (hp) architectures are diffused in the liver parenchyma (Li), Hematoxylin–eosin (H&E) staining, exocrine acini (ac) readily recognized around the portal vein (pv), vein (v), artery (ar) and ducts (A, B, C, D). The lobes are surrounded by a thin layer of connective tissue (ct) (B, C, D). The main duct (md) is lined by a simple columnar with a brush border (black arrow heads), surrounded with smooth muscle (sm) and collagen fibers (B, D). The lateral ducts (ld) are lined by simple cuboidal epithelial cells (B). The acini cells are pyramidal in shape with eosinophilic zymogen granules, dark basophilic cytoplasm, and distinct basal nuclei (B). The pv is lined with endothelial cells (black arrow) (C). The melanomacrophage centers (mmc) in Hepaticpancreas are located near the blood vessels and ducts (D). (Bar: A: 0.001 mm; B, C, D: 0.0005 mm). Microscopic structure results showed that hepatopancreatic tissue of G. paganellus was located in the internal surface of the liver and diffused in the liver parenchyma, arranged around the branches of the portal vein, arteries, and ducts in the portal area. Hepatopancreatic tissues were readily discernible (Fig. 3A). The hepatopancreatic tissues represented about 6% of the liver tissue. It has a cluster of an exocrine acinar arrangement, separated from hepatic parenchyma by a thin layer of connective tissues; each acinus consists of mostly organized tall pyramidal cells. The acinus cells have apical eosinophilic zymogen granules, which vary in density, as well as a dark basophilic cytoplasm and distinct basal nuclei that contact the basal membrane (Fig. 3B). Moreover, the pancreatic acinar cells are distinguished from hepatic tissue by their basophilic basal pole and eosinophilic apical cytoplasm and there was no pancreatic endocrine tissue observed among exocrine hepatopancreatic tissue. Thus, the hepatopancreatic portion was clearly shown that it was the exocrine pancreas (Fig. 3B and C). The exocrine hepatopancreas ductular system of G. paganellus is composed of a large main duct and small lateral ducts (Fig. 3A, B, and D). The main duct opens in the middle portion of intestinal lobules (Fig. 3B and C), surrounded by smooth muscle cells, collagenous fibers present between the pancreatic lobules, and lined by the mucosa consisting of simple columnar epithelium with an apical brush border. At the same time, the mucosa of the lateral ducts consists of simple cuboidal cells (Fig. 3B). The melanomacrophage centers (MMCs) of G. paganellus, which are often nodular in appearance, are located near the blood vessels and bile ducts and are lined by a thin connective tissue that enters the organ as septa; some dark pigments have been found in the cytoplasm of these cells, primarily along the edges of the melano-macrophage centers (Fig. 3D). DiscussionThis study describes for the first time the normal anatomy and histology of the liver of G. paganellus. The anatomical description of the liver organ of G. paganellus revealed a pyriform shape, with one lobe that is wide in the anterior portion and narrow in the posterior portion. The liver is a pale yellowish-brown, similar to G. brasiliensis (Bruslé and Anadon, 1996), while the color’s liver and the number of lobes of Hoplias aff. malabaricus and Hypostomus francisci were different (Sales et al., 2017). Maybe the liver color is related to different habits. Whereas the adult individuals of G. paganellus and G. brasiliensis prefer areas with rocks or coral and live on the reef inhabitant of seawater, while H. aff. malabaricus and H.francisci are freshwater fish and pelagic fish (Sales et al., 2017). The liver of G. paganellus has a smooth dorsal surface and a shallow fissure ventral surface surrounded by large blood vessels. While the digestive glands of Neurergus microspilotus and N. kaiseri consist of a big liver and a small pancreas, it is divided into two separated lobes, right and left of both species (Vaissi et al., 2017). However, these results agree with the findings of Vaissi et al. (2017) and Akou (2019) that the gall bladder is situated just dorsal to the right side of the liver lobe of N. microspilotus, N. kaiseri, C. carpiolinnaeus, and Mesopotamichthys sharpeyi. Histologically, this result showed that a thin layer of serous membrane of mesothelium and fibro-connective tissue-rich collagen fibers surround the liver organ of G. paganellus; the covering epithelium may play a role in the protection of the liver fraction during movement by secreting peritoneum fluid in the abdominal cavity. The same appearance was described by El-Shammaa et al. (2008) of gilthead sea bream and Vaissi et al. (2017) of N. microspilotus, and N. kaiseri. In addition, glycogen and fat storage dissolve regularly throughout the histopathologic process, leading to substantial histological variety. The hepatic parenchyma of G. paganellus’ liver consists of hepatocytes spread out as irregular cords arranged radially around a central vein. These cords are not divided into distinct hexagonal lobules, nor do they have portal triads, as in higher vertebrates, agreed with the results shown by Akoul and AL-Jowari (2019) and Akiyoshi and Inoue (2004). Each plate has polygonal-shaped cells with spherical nuclei usually centrally located, eosinophilic cytoplasm with presents a large amount of vacuolated; this arrangement was detected in trout (Anderson and Mitchum, 1974), in tilapia (Abd El-Fatah, 1999), and gilthead sea bream (El-Shammaa et al., 2008). Hepatocytes face the biliary apically and the sinusoidal at the base. Hepatocyte cords can be organized in two cells thick or branch and/or consolidated between two neighboring sinusoids. The hepatocyte arrangement in G. paganellus resembles that recognized in other studies as grey mullets (Biagianti-Risbourg, 1991) and in the Atlantic croaker (Eurell and Hanesly, 1982); the former authors showed that hepatocytes surrounded a sinusoid that was arranged as tubules of hepatocytes. The blood sinusoidal is lined with simple squamous epithelial tissue. Endothelial cells are elongated with dark nuclei and a discontinuous basement membrane, forming a very thin epithelial sheet. The sinusoidal structure of G. paganellus corresponded with that of other fishes. While endothelial cells rest on a basal lamina, there are no portal triads as in higher vertebrates or lobule division; instead, they have a structure of sinusoids that converge at the central vein. The same appearance was described in flatfish sea bream (EL-Shammaa, 2008), teleosts (Ferri and Sesso, 1981), and catfish (Hinton and Pool, 1976). The bile duct and portal vein are surrounded by G. paganellus liver tissue parenchyma. The biliary system consists of ducts and bile tubules. Structurally, the bile duct consists of three distinct layers: tunica mucosa, tunica muscularis, and tunica serosa. The apical border of adjacent hepatocytes points towards the biliary tubules, where the bile ducts are present. The bile duct mucosa was lined by a simple cuboid to the columnar epithelium, consistent with Vicentini et al. (2005) and Faccioli et al. (2014), who observed that the bile ducts in the three species had a simple cuboid epithelium, which becomes columnar in large passages similar to other bony fish. In this study, the MMCs located near the blood arteries and bile ducts also existed in the hepatic and pancreatic tissue. Many dark pigments were found in the cytoplasm of these cells, mainly along the edges of the MMCs. Similar to Mela et al. (2013) and Sales et al. (2017) in bony fish. The number of MMCs, their size, and distribution vary with species, organ, age, nutritional level, and stress conditions, as shown by Fishelson (2006) and Viana et al. (2021). Moreover, Van der Oost et al. (2003) and Steinel and Bolnick (2017) recognized the function and potential of MMCs as a histological biomarker of the immune response and to destroy, detoxify, or recycle foreign materials. G. paganellus has intrahepatic exocrine pancreatic tissue consisting of exocrine acini. The acinus cells were arranged in clusters of columnar epithelial cells with a distinct basal nucleus and basophilic cytoplasm in the base, but the apical cytoplasm is acidophilic because of the presence of many eosinophilic zymogen secretory granules; it produces digestive enzymes. Nejedli and Gajger (2013) and Rocha et al. (1997) noticed that in some teleost fishes, the largest portion of the liver is liver parenchyma tissue, about 95%, and a smaller proportion of tissue about 5% is the hepatopancreas. It was not in conflict with G. paganellus’ liver, where 6% of the hepatopancreatic tissue was present. In addition, Akoul and AL-Jowari (2019) indicated that exocrine pancreatic tissue was diffused intrahepatic in C. carpiolinnaeus, and M. sharpeyi mainly consists of acini. Furthermore, the hepatopancreas has been reported in other teleosts (Vicentini et al., 2005), and its percentage varies by species (Nejedli and Gajger, 2013; Faccioli et al., 2014). ConclusionThere is no unique model described for Teleostei liver, despite the fact that morphological and histological features of fish liver vary across species. For this reason, more research and in-depth analysis of each Teleostei species is needed. Overall, research on G. paganellus can help expand the local database and enhance Libyan markets, making it a crucial component of the country’s coastal ecology, food chain, and ecological biomarker. AcknowledgmentsThe authors are very grateful to the University of Tripoli for the support. The authors also thank the Marine Biology Research Center for the assistance in obtaining the desired results. Authors contributionsSAA and HHS planned the experimental design and executed the in-vitro and in-vivo study. SAA and HHS conducted the literature review, drafted and revised the literature, and SAA wrote the final manuscript. SAA provided a hand in the histological examination and comments. HHS provided and dissected the fish, measured the length and organ weights of the fish, and calculated tissue presence ratios of the liver with ImageJ. All authors read and approved the article. Data availabilityAll data supporting the findings of this study are available within the manuscript, and no additional data sources are required. Funding The authors did not receive any external fund. The authors funded the study. Conflict of interest The authors declare that there is no conflict of interest. ReferencesAbd El-Fatah, M. 1999. Histological and histochemical studies of the liver in fishes. Assiut Vet. Med. J. 40(80), 56–71. Agius, C. and Roberts, R. J. 2003. Melano-macrophage centers and their role in fish pathology. J. Fish Dis. 26(9), 499–509. Akiyoshi, H. and Inoue, A. 2004. Comparative histological study of teleost livers in relation to phylogeny. Zool. Sci. 21(8), 841–850. Akoul, M.A. and AL-Jowari, S.A.K. 2019. Comparative anatomical and histological study of some organs in two fish species Cyprinus carpio Linnaeus, 1758 and Mesopotamichthys sharpeyi (Günther, 1874) (Cypriniformes, Cyprinidae). Bull. Iraq Nat. Hist. Mus. 15(4), 425–441. Al-Yousuf, M.H., El-Shahawi, M.S. and Al-Ghais, S.M. 2000. Trace metals in liver, skin and muscle of Lethrinus lentjan fish species in relation to body length and sex. Sci. Total Environ. 256(2–3), 87–94. Amores, A., Giles, V., Thode, G. and Alvarez, M.C. 1990. Adaptative character of a Robertsonian fusion in chromosomes of the fish Gobius paganellus (Pisces, Perciformes). Heredity, 65(2), 151–155. Anderson, D.P. and Mitchum, D.L. 1974. Atlas of trout histology textbook, Wyoming game fish department Cheyenne, WY 82009, USA. Azevedo, J.M.N. and Simas, A.M.V. 2000. Age and growth, reproduction and diet of a sublittoral population of the rock goby Gobius paganellus (Teleostei, Gobiidae). Hydrobiologia. 440, 129–135. Barton B.A. 2002. Stress in fishes: a diversity of responses with particular references to changes in circulating corticosteroids. Integr. Comp. Biol. 42, 517–525. Bertolucci, B., Vicentini, C.A., Franceschini-Vicentini, I.B. and Bombonato, M.T.S. 2008. Light microscopy and ultrastructure of the liver of Astyanax altiparanae Garutti and Britski, (Teleostei, Characidae). Acta. Sci. Biol. Sci. 30(1), 73–76. Biagianti-Risbourg, S. 1991. Fine structure of hepatocytes in juvenile grey mullets: Liza saliens Risso, L. ramada Risso and L. aurata Risso (Teleostei: Mugillidae). J. Fish Biol. 39, 687–703. Bombonato, M.T.S., Rochel, S.S., Vicentini, C.A. and Franceschini-Vicentini, I.B. 2007. Estudo morfológico do tecido hepático de Leporinus macrocephalus. Acta Sci. Biol. Sci. 29(1), 81–85. Bruslé, J. and Anadon, G.G. 1996. The structure and function of fish liver. In: Munshi, J.S.D. and Dutta, H.M. (Eds.). Fish Morphology. North-Holland: Science Publishers. Compaire, J.C., Casademont, P., Gómez-Cama, C. and Soriguer, M.C. 2018. Reproduction and recruitment of sympatric fish species on an intertidal rocky shore. J. Fish Biol. 92(2), 308–329. Dunne, J. 1978. January. Littoral and benthic investigations on the west coast of Ireland: IX. Section A (Faunistic and Ecological Studies). The biology of the rock-goby, Gobius paganellus L., at Carna. In Proceedings of the Royal Irish Academy. Section B: Biological, Geological, and Chemical Science, pp: 179–191. El-Shammaa, M.A., El-Shafey S.M., El-Bargeesy, G.H. and Abd-Rabou, M.I. 2008. Sea Bream Liver: histological and ultrastructure studies (I) hepatocytes. Kafrelsheikh Vet. Med. J. 6(1), 1–31. Engin, S. and Seyhan, K. 2009. Biological characteristics of rock goby, Gobius paganellus (Actinopterygii: Perciformes: Gobiidae), in the south-eastern Black Sea. Acta. Ichthyol. Piscat. 39(2), 111–118. Eurell, J.A. and Haensly, W.E. 1982. The histology and ultrastructure of the liver of Atlantic croaker Micropogon undulatus L. J. Fish Biol. 21(1), 113-125. Faccioli, C.K., Chedid, R.A., Bombonato, M., Vicentini, C.A. and Vicentini, I. 2014. Morphology and histochemistry of the liver of carnivorous fish Hemisorubim platyrhynchos. Int. J. Morphol. 32(2), 715–720. Ferguson, H.W. 1989. Systemic pathology of fish. A text and atlas of comparative tissue responses in diseases of teleosts. Ames, IA: Iowa State University Press. Ferri, S. and Sesso, A. 1981. Ultrastructural study of the endothelial cells in teleost liver sinusoids, under normal and experimental conditions. Cell Tissue Res. 219, 649–657. Fishelson, L. 2006. Cytomorphological alterations of the thymus, spleen, head-kidney, and liver in cardinal fish (Apogonidae, Teleostei) as bioindicators of stress. J. Morphol. 267, 57–69. Gochfeld, M. 2003. Cases of mercury exposure, bioavailability, and absorption. Ecotoxicol. Environ. Saf. 56(1), 174–179. Hajji, F., Ouannes-Ghorbel, A., Ghorbel, M. and Jarboui, O. 2012. Reproductive biology of the rock goby, Gobius paganellus (Actinopterygii: Perciformes: Gobiidae), on the southern Tunisian coast (Gulf of Gabes). Cienc. Mar. 38(3), 505–515. Hartley, W.R., Thiyagarajah, A. and Treinies, A.M. 1996. Liver lesions in the gar fish (Lepisosteidae) as biomarkers of exposure. Mar. Environ. Res. 42(1–4), 217–221. Henriques, M., Goncalves, E.J. and Almada, V.C. 1999. The conservation of littoral fish communities: a case study at the Arrabida coast (Portugal). Behaviour and conservation of littoral fishes, pp: 473–519. Hinton, D.E. and Pool, C.R. 1976. Ultrastructure of the liver in channel catfish Ictalurus punctatus (Rafinesque). J. Fish Biol. 8, 209–219. Lall, S.P. and Kaushik, J.S. 2021. Nutrition and metabolism of minerals in fish. Animals, 11(9), 2711. Louiz, I., Hassine, O.K.B., Palluel, O., Ben-Attia, M. and Aït-Aïssa, S. 2016. Spatial and temporal variation of biochemical biomarkers in Gobius niger (Gobiidae) from a southern Mediterranean lagoon (Bizerta lagoon, Tunisia): Influence of biotic and abiotic factors. Mar. Pollut. Bull. 107(1), 305–314. Madeira, D., Mendonc, V., Dias, M., Roma, J., Costa, P.M., Diniz, M.S. and Vinagre, C. 2014. Physiological and biochemical thermal stress response of the intertidal rock goby Gobius paganellus. Ecol. Indic. 46, 232–239. Manuel, J., Azevedo, N., Maria, A. and Simas, V. 2000. Age and growth, reproduction and diet of a sublittoral population of the rock goby Gobius paganellus (Teleostei, Gobiidae). Hydrobiologia. 440, 129–135. Mela, M., Guiloski, I.C., Doria, H.B., Randi, M.A.F., de Oliveira Ribeiro, C.A., Pereira, L., Maraschi, A.C., Prodocimo, V., Freire, C.A. and De Assis, H.S. 2013. Effects of the herbicide atrazine in neotropical catfish (Rhamdia quelen). Ecotoxicol. Environ. Saf. 93, 13–21. Miller, P.J. 1986. Gobiidae. Fishes of the North-eastern Atlantic and Mediterranean, pp: 1019–1085. Miller, P. 1984. The gobiid fishes of temperate Macaronesia (Eastern Atlantic). J. Zool. Lond. 204(3), 363–412. Nejedli, S. and Gajger, I. 2013. Hepatopancreas in some sea fish from different species and the and the structure of the liver in teleost fish, common pandora, Pagellus erythinus (Linnaeus, 1758) and whiting, and whiting, Merlangius merlangus euxinus (Nordmann, 1840). Vet. Arh. 83(4), 441–452. Patzner, R.A., Santos, R.S., Ré, P. and Nash, R.D., 1992. Littoral fishes of the Azores: An annotated checklist of fishes observed during the “Expedition Azores 1989”. Arquipélago-Life Earth Sci. 10, 101–111. Paul, N., Novais, S.C., Lemos, M.F., and Kunzmann, A. 2018. Chemical predator signals induce metabolic suppression in rock goby (Gobius paganellus). PLoS One. 13(12), e0209286. Petcoff, G.M., Díaz, A.O., Escalante, A.H. and Goldemberg, A.L. 2006. Histology of the liver of Oligosarcus jenynsii (Ostariophysi, Characidae) from Los Padres Lake, Argentina. Iheringia Ser. Zool. 96, 205–208. Pörtner, H.O., Bock, C. and Mark, F.C. 2017. Oxygen-and capacity-limited thermal tolerance: bridging ecology and physiology. J. Exp. Biol., 220(15), 2685–2696. Portner, H.O. and Farrell, A.P. 2008. Physiology and climate change. Sci. 322, 690–692. Ragheb, E., Khamis Akel, E. and Rizkalla, S. 2019. Analyses of the non-target catch from the Egyptian Mediterranean trawlers off Port Said. Egypt. J. Aquat. Res. 45, 239–246. Rocha, E., Monteiro, R.A. and Pereira, C.A. 1994. The liver of the brown trout, Salmo trutta fario: a light and electron microscope study. J. Anat. 185(Pt. 2), 241–249. Rocha, E., Monteiro, R. and Pereira, C. 1997. Liver of the Brown Trout, Salmo trutta (Teleostei, Salmonidae): a stereological study at light and electron microscopic levels. Anat. Rec. 247, 317–328. Ruple, D. 1984. Gobioidei: development. In Moser, H. G. (ed.), Ontogeny and systematics of fishes. ASIH. 1, 582–587. Sales, C.F., Silva, R.F., Amaral, M.G.C., Domingos, F.F.T., Ribeiro, R.I.M.A., Thomé, R.G. and Santos, H.B. 2017. Comparative histology in the liver and spleen of three species of freshwater teleost. Neotrop. Ichthyol. 15(1), e160041. Sokolova, I.M. 2013. Energy-limited tolerance to stress as a conceptual framework to integrate the effects of multiple stressors. ICB, 53(4), 597–608. Steinel, N.C. and Bolnick, D.I. 2017. Melanomacrophage centers as a histological indicator of immune function in fish and other Poikilotherms. Front. Immunol. 8, 827. Stori, E.M., Rocha, M.L.C.F., Dias, J.F., dos Santos, C.E.I., de Souza, C.T., Amaral, L. and Dias, J.F. 2014. Elemental characterization of injuries in fish liver. Nucl. Instrum. Methods Phys. Res. 318, 83–87. Suvarna, K.S., Layton, C. and Bancroft, J.D. 2012. Bancroft’s theory and practice of histological techniques. 7th ed. China, Churchill Livingstone. Vaissi, S., Parto, P. and Sharifi, M. 2017. Anatomical and histological study of the liver and pancreas of two closely related mountain newts Neurergus microspilotus and N. kaiseri (Amphibia: Caudata: Salamandridae). Zool. 34, 1–8. Van der Oost, R., Beyer, J. and Vermeulen, N.P.E. 2003. Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ. Toxicol. Pharmacol. 13, 57–149. Viana, H.C., Jesus, W.B., Silva, S.K.L., Jorge, M.B., Santos, D.M.S. and Carvalho Neta, R.N.F. 2021. Aggregation of hepatic melanomacrophage centers in S. herzbergii (Pisces, Ariidae) as indicators of environmental change and well-being. Arq. Bras. Med. Vet. Zootec. 73(4), 868–876. Vicentini C.A., Franceschini-Vicentini I.B., Bombonato M.T.S., Bertolucci B., Lima S.G. and Santos A.S. 2005. Morphological study of the liver in the teleost Oreochromis niloticus. Int. J. Morphol. 23(3), 211–216. | ||
| How to Cite this Article |
| Pubmed Style Abusrer SA, Shtewi HH. Morphological and histological structure of hepatopancreas in rock goby Gobius paganellus in the western coast of Libya. Open Vet. J.. 2023; 13(10): 1251-1258. doi:10.5455/OVJ.2023.v13.i10.3 Web Style Abusrer SA, Shtewi HH. Morphological and histological structure of hepatopancreas in rock goby Gobius paganellus in the western coast of Libya. https://www.openveterinaryjournal.com/?mno=156500 [Access: November 23, 2025]. doi:10.5455/OVJ.2023.v13.i10.3 AMA (American Medical Association) Style Abusrer SA, Shtewi HH. Morphological and histological structure of hepatopancreas in rock goby Gobius paganellus in the western coast of Libya. Open Vet. J.. 2023; 13(10): 1251-1258. doi:10.5455/OVJ.2023.v13.i10.3 Vancouver/ICMJE Style Abusrer SA, Shtewi HH. Morphological and histological structure of hepatopancreas in rock goby Gobius paganellus in the western coast of Libya. Open Vet. J.. (2023), [cited November 23, 2025]; 13(10): 1251-1258. doi:10.5455/OVJ.2023.v13.i10.3 Harvard Style Abusrer, S. A. & Shtewi, . H. H. (2023) Morphological and histological structure of hepatopancreas in rock goby Gobius paganellus in the western coast of Libya. Open Vet. J., 13 (10), 1251-1258. doi:10.5455/OVJ.2023.v13.i10.3 Turabian Style Abusrer, Salma Aribe, and Hanan Husain Shtewi. 2023. Morphological and histological structure of hepatopancreas in rock goby Gobius paganellus in the western coast of Libya. Open Veterinary Journal, 13 (10), 1251-1258. doi:10.5455/OVJ.2023.v13.i10.3 Chicago Style Abusrer, Salma Aribe, and Hanan Husain Shtewi. "Morphological and histological structure of hepatopancreas in rock goby Gobius paganellus in the western coast of Libya." Open Veterinary Journal 13 (2023), 1251-1258. doi:10.5455/OVJ.2023.v13.i10.3 MLA (The Modern Language Association) Style Abusrer, Salma Aribe, and Hanan Husain Shtewi. "Morphological and histological structure of hepatopancreas in rock goby Gobius paganellus in the western coast of Libya." Open Veterinary Journal 13.10 (2023), 1251-1258. Print. doi:10.5455/OVJ.2023.v13.i10.3 APA (American Psychological Association) Style Abusrer, S. A. & Shtewi, . H. H. (2023) Morphological and histological structure of hepatopancreas in rock goby Gobius paganellus in the western coast of Libya. Open Veterinary Journal, 13 (10), 1251-1258. doi:10.5455/OVJ.2023.v13.i10.3 |