| Research Article | ||
Open Vet. J.. 2023; 13(10): 1318-1325 Open Veterinary Journal, (2023), Vol. 13(10): 1318–1325 Original Research Molecular detection of Spirochetes and Borrelia burgdorferi in stray dogs of Nineveh province, IraqEva Ayser Ajaj*, and Zahraa Mustafa Al-Jumaa1Department of Internal and Preventive Medicine, College of Veterinary Medicine, University of Mosul, Nineveh, Iraq *Corresponding Author: Eva Ayser Ajaj. Department of Internal and Preventive Medicine, College of Veterinary Medicine, University of Mosul, Nineveh, Iraq. Email: evaaisser2012 [at] uomosul.edu.iq Submitted: 20/06/2023 Accepted: 20/09/2023 Published: 31/10/2023 © 2023 Open Veterinary Journal
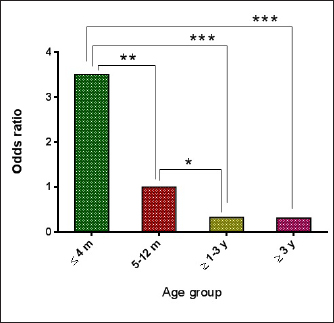
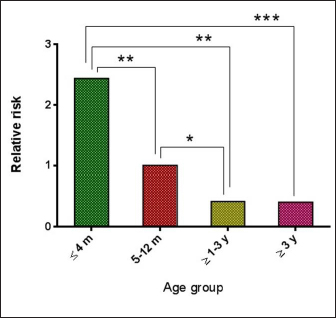
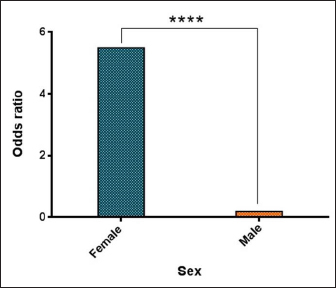
AbstractBackground: Borrelia burgdorferi is a Gram-negative bacterium that causes Lyme disease or borreliosis in domestic and wild animals, including dogs, with the possible transmission to humans. Aim: This study was conducted to investigate the infection rate of Spirochetes and B. burgdorferi in stray dogs in Nineveh province, Iraq. Methods: During the period from May to October (2022), a total of 55 stray dogs were selected randomly from different areas in Nineveh province, Iraq. Blood samples were collected from cephalic venous and tested molecularly using the conventional polymerase chain reaction technique. Results: The present study revealed that the total infection rates of Spirochetes and B. burgdorferi were 41.82% and 27.27%, respectively. Concerning age, values of infection rate, odds ratio, and relative risk of B. burgdorferi were increased significantly in dogs aged ? 4 months (42.86%, 3.505%, and 2.438%, respectively), while decreased in dogs of ? 1–3 (12.5%, 0.337% and 0.42%, respectively) and ? 3 (13.33%, 0.32% and 0.409%) years old when compared to dogs aged 5–12 months (27.27%, 1% and 1%, respectively). While concerning dogs sex, a significantly higher infection rate, odds ratio, and relative risk of B. burgdorferi were shown in females (32.56%, 5.495% and 6.792%, respectively) compared to males (8.33%, 0.182% and 0.147%, respectively). Conclusion: To the best of our knowledge, this represents the first Iraqi study on the prevalence of spirochetes, in particular B. burgdorferi, in stray dogs in Nineveh province (Iraq). However, additional studies of B. burgdorferi infection in other animals as well as vectors such as ticks in different geographic areas, appear necessary to detect variation in the distribution patterns of infection. In addition, owners and veterinarians should be aware of zoonotic diseases transmitted from wild and domestic animals, in particular those with tick-bite histories. Keywords: Spirochetes, Lyme disease, Borrelia burgdorferi, Tick-transmitted diseases, Iraq. IntroductionSpirochetes are the etiological agents of several important animal and human diseases such as Leptospirosis (Leptospira spp.), Lyme disease (LM) (Borrelia burgdorferi), and syphilis (Treponema pallidium), which exhibit unique morphological and physiological features (San Martin et al., 2022). Borrelia burgdorferi, a Gram-negative bacterium belonging to the Borreliaceae Family in the Spirochaetia Class, is a tick-borne multi-systemic zoonotic pathogen that causes (LM or Lyme Borreliosis (Borreliosis) in many countries, worldwide (Mafra and Montandon, 2017). Horizontal transmission is the main method of B. burgdorferi transmission within wild animals and between ticks (Milkovičová et al., 2023). In most cases, B. burgdorferi invades salivary glands as well as the midgut of ticks (Ixodes pacificus, Ixodes scapularis, Ixodes ricinus, and Ixodes persulcatus called (deer tick, or black-legged tick), and delivered routinely through the transstadial the transstadial transmission (Başbulut et al., 2012; Divers et al., 2018). In dogs of all ages, early infection is characterized by high temperature, loss of appetite, dullness, lymphadenopathy, and acute onset of pain or stiffness, while dogs that are infected acutely generally do not exhibit swollen joints and are usually difficult to estimate the origin of pain (Ebani et al., 2014; Bajer et al., 2022). In addition, lameness might be intermittent and shift from one leg to another (Divers et al., 2018). At the same time, advanced manifestations of Lyme borreliosis are recurrent and non-erosive arthritis, recurrent episodes of lameness with involvement of multiple joints, particularly the carpus joint (Verbsky, 1997; Clausen et al., 2020). The association between these lesions and erythema chronicum migrans has not been established. In addition, dogs living in endemic areas have displayed increasing erythematous rashes on the abdomen or other sparsely hairy areas. These migrans are distinct from a limited response to a tick bite due to their spreading nature (O’Connell, 2014; Quarsten et al., 2017). Myocardial dysfunctions, including atrio-ventricular heart block, myocardial necrosis, and vegetative endocarditis (Saunders et al., 2013; Adaszek et al., 2020), as well as renal (Rolla et al., 2014) and neurologic (Han et al., 2015; Borys et al., 2019) diseases, have been noted in some experimentally and naturally infected dogs for B. burgdorferi. In stray dogs, the problems with the interpretation of different diseases have stemmed from the lack of data concerning the clinical symptoms after being naturally infected. Without a pathognomonic marker for canine B. burgdorferi infections, definitive advanced tests such as serological and molecular methods have been proposed to investigate and confirm active and subclinical infections, but with variable findings (Gettings et al., 2019; Bajer et al., 2022). However, valuable sensitivity and specificity of molecular diagnostic techniques such as polymerase chain reaction (PCR) have proven useful in the identification and differentiation of Borrelia species over the serological assays (Barth et al., 2012; Maggi et al., 2014; Koetsveld et al., 2016; Das et al., 2020). In Iraq, few studies were conducted to detect the prevalence rate of B. burgdorferi in humans (Ameen et al., 2013; Madhi et al., 2019), but not in dogs. Hence, this study aimed to investigate Spirochetes and B. burgdorferi in stray dogs in Iraq. Materials and MethodsAnimals and samples collectionsThe current study involved 55 stray dogs selected randomly from various regions in Nineveh province, Iraq, during the period between May and October (2022). Under the sedative effect of xylazine (1 mg/Kg BW), 2.5 ml of cephalic venous blood was sampled from each dog using a disposable syringe, then the blood was transferred into an EDTA tube and kept frozen at 4°C until tested. Data regarding the sex of the study animal were determined based on observations of external sex organs and physical characteristics of the abdomen, while age was evaluated according to the dental formula for deciduous and permanent teeth of dogs (Gracis, 2018). Molecular diagnosisBlood samples were prepared and processed following the blood protocol procedure of gSYNC™ DNA Extraction Kit (Geneaid Biotech, Korea). The concentration (ng/ml) and purity of obtained DNAs were checked using the NanoDrop spectrophotometer (Thermo Scientific, UK) at an absorbance of A260/280. At a final volume of 20 ml, the MasterMix tubes were prepared following the manufacturer’s instructions of the AccuPower PCR PreMix Kit (Bioneer, Korea) targeting the 16S rRNA gene detect the Spirochetes [SPIRO: F (5′-GGC GGC GCT ATT AAG-3′) and SPIRO: R (5′-TAC CTT GTT ACG ACT TCA-3′)] (Campbell and Cary, 2001), and B. burgdorferi [BOR: F (5′-GCT GTC AGT GCG TCT TAA G-3′) and BOR: R (5′-CTT AGC TGC TGC CTC CGT A-3′)] (Skotarczak et al., 2005). For DNAs amplification, conventional PCR-reaction was carried out in the Thermocycler System (BIO-RAD, USA) for both Spirochetes and B. burgdorferi, respectively, as 1 cycle for initial denaturation (95°C/5 minutes), 30 cycles for denaturation (95°C/20 seconds), annealing (51°C and 56°C/1 minute), and extension (72°C/1 minute); and 1 cycle for final extension (72°C/10 minute). Electrophoresis of 1.5% agarose gel stained with SYBR Safe DNA Gel Stain (ThermoFisher Scientific, USA) at 100 V, 80 Am for 1 hour was performed to analyze the PCR products. Finally, the product sizes of amplified DNAs were visualized using the UV illuminator (Clinx Science, China) and photographed by a digital camera (Nikon, Japan). The positive samples for Spirochetes were identified at product 650 bp while, for B. burgdorferi of 230 bp. Statistical analysisData risk was analyzed using the chi-square test and odds ratio in the GraphPad Prism Software (GraphPad Software Inc., USA) to determine variation between specific groups for each age, and sex factors were considered significant at p < 0.05 (Gharban, 2023). Ethical approvalThe current study was authorized and conducted by the license of the Scientific Committee of the College of Veterinary Medicine, University of Mosul, Nineveh province, Iraq. (UM.VET.2022.064) on 17th of April 2022. ResultsAmong a total of 55 stray dogs tested for detection of Spirochetes and B. burgdorferi by PCR assay, the total positive rate was 23 (41.82%) and 15 (27.27%), respectively (Figs. 1 and 2). Concerning age factor, B. burgdorferi increased significantly ( p < 0.0487) in dogs aged £4 months [9/21 (42.86%)] and decreased significantly in dogs aged 31–3 years [1/8 (12.5%)] and 33 years [2/15 (13.33%)] when compared to dogs aged 5–12 months [3/11 (27.27%)]. Higher odds ratio and relative risk (0.0002 and 0.0003, respectively) values were seen in dogs aged £4 months (3.505 and 2.438, respectively) than 5–12 months old (1.523 and 1.333, respectively), 3 1–3 years old (0.305 and 0.392, respectively), and 33 years old (0.286 and 0.381, respectively), (Figs. 3 and 4). Moreover, the association of sex to B. burgdorferi showed a significant elevation (p < 0.0354) in positive females [14/43 (32.56%)] in comparison with males [1/12 (8.33%)]. In addition, values of odds ratio and relative risk were significantly higher in females (5.495 and 6.792, respectively) than in males (0.182 and 0.147, respectively) (Fig. 5).
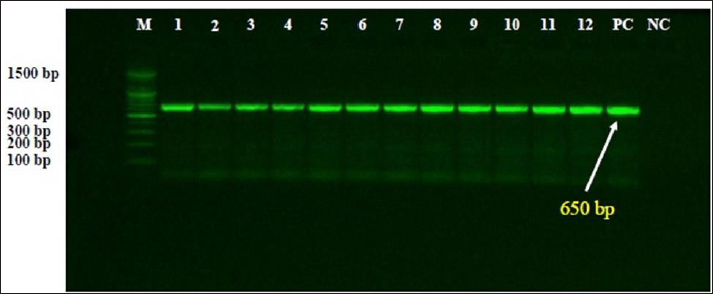
Fig. 1. Representative image for electrophoresis of PCR products in 1.5% agarose gel to detect amplified DNAs of Spirochetes targeting 16S rRNA gene. While, Lane M: Ladder marker (100–1,500 bp); Lane PC: Positive control, Lane NC: Negative control, Lanes 1–12: Positive samples of Spirochetes at 650 bp.
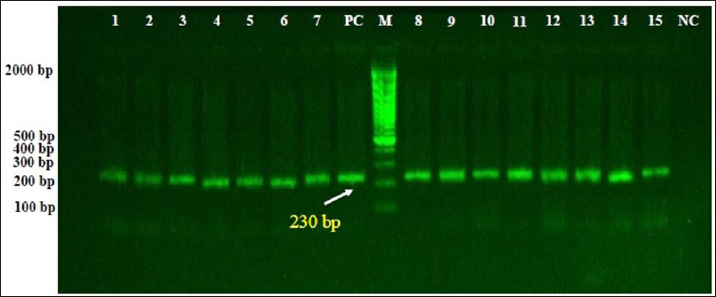
Fig. 2. Electrophoresis of PCR products in 1.5% agarose gel to detect amplified DNAs of B. burgdorferi targeting 16S rRNA gene. While Lane M: Ladder marker (100–2,000 bp); Lane PC: Positive control, Lane NC: Negative control, Lanes 1–12: Positive samples of B. burgdorferi at 230 bp. DiscussionSeveral worldwide studies attempted to identify or isolate Spirochetes and B. burgdorferi from different samples (blood or joint and cerebrospinal fluids) of patients, which often failed due to the low number of organisms found in tested samples, lack of ideal culture conditions, long time consumed, and excessive laboratory steps in addition to the risk of infection of transmission for workers. In the last decades, molecular techniques have been provided highly sensitive, specific, and safe alternative tools for the diagnosis of agents such as Spirochetes and B. burgdorferi in humans and animals (Straubinger et al., 1997; Straubinger, 2000; Coulter et al., 2005; Anthony et al., 2013; Bil-Lula et al., 2015). Duhamel et al. (1998) confirmed that the large intestines of dogs, like those of humans and other animals, including birds, are colonized by more than one species of spirochete. The present study reported that the prevalence rate of B. burgdorferi in dogs by PCR assay was 29.09%. In other studies, there were 0.1%–17.3% in Mexico (Solís-Hernández et al., 2018; Bedoya et al., 2023), 0.4%–40% in Croatia (Poljak et al., 2000; Jurković et al., 2019), 0.6% in Romania (Cazan et al., 2020), 0.9% in China (Wang et al., 2018), 1.09% in France (Pantchev et al., 2009), 1.47% in Italy (Ebani et al., 2014), 2.049% in Latvia (Berzina and Matise, 2013), 2.4%–74.5% in Bulgaria (Angelov et al., 1993; Pantchev et al., 2015), 2.8%–45.3% in Slovakia (Stefancikova et al., 1996; Čabanová et al., 2015), 3.75%–33.7% in Poland (Skotarczak et al., 2005; Cardoso et al., 2012), 6.4% in South Korea (Lee et al., 2020), 6.5%–53.7% in Czech Republic (Sýkora et al., 1990; Pejchalová et al., 2006), 9.52% in Iran (Mosallanejad et al., 2015), 10.2%–27.3% in Japan (Arashima, 1991; Uesaka et al., 2016), 16.8% in Sweden (Egenvall et al., 2001), 17% in Netherlands (Goossens et al., 2001), 21% in Spain (Amusategui et al., 2008), 38.22% in Turkey (Altug et al., 2022), 23–56.9% in USA (Guerra et al., 2000; Wagner and Erb, 2012), 25.81% in Serbia (Savić et al. 2010), 31.3% in Brazil (Nascimento et al., 2016), and 35.5% in Germany (Barth et al., 2012). This variation confirmed that the prevalence rate of B. burgdorferi in dogs is different between regions, countries, and years, which might be attributed to the diagnostic method used, the presence of vectors as ticks, geographical characteristics, consequence of environmental factors, and diversity in the epidemiology of infection.
Fig. 3. Results of odds ratio in different age groups.
Fig. 4. Results of relative risk in different age groups.
Fig. 5. Results of odds ratio in different sex groups. Concerning age, we found that there was an increase in the prevalence of B. burgdorferi in dogs aged £4 and 5–12 months when compared to older age groups, indicating that younger dogs are more susceptible to B. burgdorferi infection than older ones. Skotarczak et al. (2005) reported that the highest percentage of positive dogs was in groups of 2–5 and 5–8 years old, while the lowest was in groups of 0.5–1.5 and >8 years old. Several authors demonstrated that the threshold for dogs developing a stable immune response is their age over 1 year (Stefancikova et al., 1996; Merino et al., 2000). Other researchers indicate that seropositive response in dogs stabilizes itself from their second year of life (Schulze et al., 1987; Lindenmayer et al., 1991; Hovius et al., 1999; Goossens et al., 2001). In contrast, Mosallanejad et al. (2015) showed that seropositivity against B. burgdorferi was significantly higher in adult dogs above 5 years compared with dogs 1–5 years and <1-year old while, Jung et al. (2012) revealed a lack of significant variation between different age groups (<2, 2–5 and >5 years old). We observed female stray dogs have a higher infection rate and relative risk than males, which might be caused by the greater exposure of females to ticks and pathogens, physiological burden due to pregnancy and suckling (stress factor), and larger sample size of females than males (Bowman et al., 2009). According to many reports, sex factors do not have an effect on the frequency of disease in dogs infected naturally with B. burgdorferi (Stefancikova et al., 1996; Jung et al., 2012; Merino et al., 2000; Skotarczak et al., 2003). In contrast, Mosallanejad et al. (2015) showed that seropositivity against B. burgdorferi was significantly higher in male dogs than in females, significantly. ConclusionTo the best of our knowledge, this represents the first Iraqi study on the prevalence of spirochetes, in particular B. burgdorferi, in stray dogs in Nineveh province (Iraq). However, additional studies of B. burgdorferi infection in other animals and vectors, such as ticks in different geographic areas, appear necessary to detect variation in the distribution patterns of infection. In addition, owners and veterinarians should be aware of zoonotic diseases transmitted from wild and domestic animals, in particular those with tick-bite histories. AcknowledgmentsThe authors have appreciated all the facilities provided by the College of Veterinary Medicine at the University of Mosul (Nineveh province, Iraq) to complete this study. Authors contributionsBoth authors contributed to conceptualization. Eva Aisser Ajaj and Zahraa Mustafa Al-Jumaa: Collection of blood samples, molecular testing of blood samples, and statistical analysis of data with approval of the final copy of the manuscript. Conflict of interestThe authors declare no conflicts of interest. FundingThe authors have not received any funds for this work. Data availabilityAll data is available in this manuscript. ReferencesAdaszek, L., Gatellet, M., Mazurek, P., Debiak, P., Skrzypczak, M. and Winiarczyk, S. 2020. Myocarditis secondary to Borrelia infection in a dog: a case report. Ann. Parasitol. 66(2), 255–257. Altug, N., Muz, M.N., Dilek, M. and Yipel, F.A. 2022. The molecular prevalence of Borrelia burgdorferi, Babesia spp., and Anaplasma spp. In shelter dogs of the Thrace Region in Turkey. Turk. J. Vet. Anim. Sci. 46(3), 14. Ameen, K.A.H., Abdullah, B.A. and Abdul-Razaq, R.A. 2013. Lyme disease in Iraq: first detection of IgM antibodies to Borrelia burgdorferi in human sera. J. Life. Sci. 7(11), 1143–1146. Amusategui, I., Tesouro, M.A., Kakoma, I. and Sainz, Á. 2008. Serological reactivity to Ehrlichia canis, Anaplasmaphagocytophilum, Neorickettsiaristicii, Borrelia burgdorferi, and Rickettsia conorii in dogs from northwestern Spain. Vector. Borne. Zoonotic. Dis. 8(6), 797–804. Angelov, L., Arnaudov, D., Rakajieva, T. and Kostova, E. 1993. Studies of epizootiological process with lyme borreliosis in Bulgaria. Eur. J. Epidemiol. 5, 12–14. Anthony, N.E., Blackwell, J., Ahrens, W., Lovell, R. and Scobey, M.W. 2013. Intestinal spirochetosis: an Enigmatic disease. Dig. Dis. Sci. 58(1), 202–208. Arashima, Y. 1991. Anti-Borrelia burgdorferi antibody in dogs: lyme disease as zoonosis. Rinshobyori. Jpn. J. Clin. Pathol. 39(8), 869–874. Bajer, A., Kowalec, M., Levytska, V.A., Mierzejewska, E.J., Alsarraf, M., Poliukhovych, V. and Dwużnik-Szarek, D. 2022. Tick-borne pathogens, Babesia spp. and Borrelia burgdorferi sl, in sled and companion dogs from Central and North-Eastern Europe. Pathogens 11(5), 499. Barth, C., Straubinger, R.K., Sauter-Louis, C. and Hartmann, K. 2012. Prevalence of antibodies against Borrelia burgdorferi sensulato and Anaplasmaphagocytophilum and their clinical relevance in dogs in Munich, Germany. Berl. Munch. Tierarztl. Wochenschr. 125(7–8), 337–344. Başbulut, E.A., Gözalan, A., Sönmez, C., Çöplü, N., Körhasan, B., Esen, B. and Ertek, M. 2012. Seroprevalence of Borrelia burgdorferi and tick-borne encephalitis virus in a rural area of Samsun, Turkey. Mikrobiyol. Bul. 46(2), 247–256. Bedoya, F., Beugnet, F., Tobias, E., Garcia-Mendizabal, E., Hay-Parker, S., Montes, N. and Mondaca, E. 2023. Geographical analysis of seroprevalence of Ehrlichia spp., Anaplasma spp., Borrelia burgdorferi and Dirofilariaimmitis, in clinics and dog shelters in different Mexican states. Curr. Res. Parasitol. Vector. Borne. Dis. 3, 100112. Berzina, I. and Matise, I. 2013. Seroprevalence against Borrelia burgdorferi sensulato and occurrence of antibody co-expression with Anaplasma phagocytophilum in dogs in Latvia. Ir. Vet. J. 66, 1–3. Bil-Lula, I., Matuszek, P., Pfeiffer, T. and Woźniak, M. 2015. Lyme borreliosis–the utility of improved real-time PCR assay in the detection of Borrelia burgdorferi infections. Adv. Clin. Exp. Med. 24(4), 663–670. Borys, M.A., Kass, P.H., Mohr, F.C. and Sykes, J.E. 2019. Differences in clinicopathologic variables between Borrelia C6 antigen seroreactive and Borrelia C6 seronegative glomerulopathy in dogs. J. Vet. Intern. Med. 33(5), 2096–2104. Bowman, D., Little, S.E., Lorentzen, L., Shields, J., Sullivan, M.P. and Carlin, E.P. 2009. Prevalence and geographic distribution of Dirofilaria immitis, Borrelia burgdorferi, Ehrlichia canis, and Anaplasma phagocytophilum in dogs in the United States: results of a national clinic-based serologic survey. Vet. Parasitol. 160, 138–148. Čabanová, V., Pantchev, N., Hurníková, Z. and Miterpáková, M. 2015. Recent study on canine vector-borne zoonoses in southern Slovakia-serologic survey. Acta. Parasitol. 60, 749–758. Campbell, B.J. and Cary, S.C. 2001. Characterization of a novel spirochete associated with the hydrothermal vent polychaete annelid, Alvinella pompejana. Appl. Environ. Microbiol. 67(1), 110–117. Cardoso, L., Mendão, C. and Madeira de Carvalho, L. 2012. Prevalence of Dirofilariaimmitis, Ehrlichia canis, Borrelia burgdorferi sensulato, Anaplasma spp. and Leishmania infantum in apparently healthy and CVBD-suspect dogs in Portugal-a national serological study. Parasit. Vectors. 5(1), 1–9. Cazan, C.D., Ionică, A.M., Matei, I.A., D’Amico, G., Muñoz, C., Berriatua, E. and Dumitrache, M.O. 2020. Detection of Leishmania infantum DNA and antibodies against Anaplasma spp., Borrelia burgdorferi sl and Ehrlichia canis in a dog kennel in South-Central Romania. Acta. Vet. Scand. 62(1), 1–4. Clausen, A.S., Ørbæk, M., Pedersen, R.R., Jensen, P.O., Lebech, A.M. and Kjaer, A. 2020. 64Cu-DOTATATE positron emission tomography (PET) of Borrelia burgdorferi infection: in vivo imaging of macrophages in experimental model of lyme arthritis. Diagnostics 10(10), 790. Coulter, P., Lema, C., Flayhart, D., Linhardt, A.S., Aucott, J.N., Auwaerter, P.G. and Dumler, J.S. 2005. Two-year evaluation of Borrelia burgdorferi culture and supplemental tests for definitive diagnosis of lyme disease. J. Clin. Microbiol. 43(10), 5080–5084. Das, S., Hammond-McKibben, D., Guralski, D., Lobo, S. and Fiedler, P.N. 2020. Development of a sensitive molecular diagnostic assay for detecting Borrelia burgdorferi DNA from the blood of lyme disease patients by digital PCR. PLoS One 15(11), e0235372. Divers, T.J., Gardner, R.B., Madigan, J.E., Witonsky, S.G., Bertone, J.J., Swinebroad, E.L. and Johnson, A.L. 2018. Borrelia burgdorferi infection and lyme disease in North American horses: a consensus statement. J. Vet. Intern. Med. 32(2), 617–632. Duhamel, G.E., Trott, D.J., Muniappa, N., Mathiesen, M.R., Tarasiuk, K., Lee, J.I. and Hampson, D.J. 1998. Canine intestinal spirochetes consist of Serpulina pilosicoli and a newly identified group provisionally designated “Serpulina canis” sp. nov., J. Clin. Microbiol. 36(8), 2264–2270. Ebani, V.V., Bertelloni, F., Torracca, B. and Cerri, D. 2014. Serological survey of Borrelia burgdorferi sensulato, Anaplasmaphagocytophilum, and Ehrlichia canis infections in rural and urban dogs in Central Italy. Ann. Agric. Environ. Med. 21(4), 671–675. Egenvall, A., Franzén, P., Gunnarsson, A., Engvall, E.O., Vågsholm, I., Wikström, U.B. and Artursson, K. 2001. Cross-sectional study of the seroprevalence to Borrelia burgdorferi sensulato and granulocytic Ehrlichia spp. and demographic, clinical and tick-exposure factors in Swedish horses. Prev. Vet. Med. 49(3–4), 191–208. Gettings, J.R., Lopez, J.E., Krishnavajhala, A., Armstrong, B.A., Thompson, A.T. and Yabsley, M.J. 2019. Antibodies to Borrelia turicatae in experimentally infected dogs cross-react with Borrelia burgdorferi serologic assays. J. Clin. Microbiol. 57(9), e00628–19. Gharban, H.A. 2023. Molecular prevalence and phylogenetic confirmation of bovine trichomoniasis in aborted cows in Iraq. Vet. World. 16(3), 580–587. Goossens, H.A.T., Van Den Bogaard, A.E. and Nohlmans, M.K.E. 2001. Dogs as sentinels for human lyme borreliosis in the Netherlands. J. Clin. Microbiol. 39(3), 844–848. Gracis, M. 2018. Dental anatomy and physiology. In BSAVA manual of canine and feline dentistry and oral surgery. Gloucester, UK: BSAVA Library, pp: 6–32. Guerra, M.A., Walker, E.D. and Kitron, U. 2000. Quantitative approach for the serodiagnosis of canine lyme disease by the immunoblot procedure. J. Clin. Microbiol. 38(7), 2628–2632. Han, J.I., Chang, D.W. and Na, K.J. 2015. A multiplex quantitative real-time polymerase chain reaction panel for detecting neurologic pathogens in dogs with meningoencephalitis. J. Vet. Sci. 16(3), 341–347. Hovius, K.E., Rijpkema, S.G., Westers, P., Van der Zeijst, B.A.M., Van Asten, F.J.A.M. and Houwers, D.J. 1999. A serological study of cohorts of young dogs, naturally exposed to Ixodes ricinus ticks, indicates seasonal reinfection by Borrelia burgdorferi sensulato. Vet. Q. 21(1), 16–20. Jung, B.Y., Gebeyehu, E.B., Seo, M.G., Byun, J.W., Kim, H.Y. and Kwak, D. 2012. Prevalence of vector-borne diseases in shelter dogs in Korea. Vet. Rec. 171(10), 249. Jurković, D., Beck, A., Huber, D., Mihaljević, Ž., Polkinghorne, A., Martinković, F. and Beck, R. 2019. Seroprevalence of vector-borne pathogens in dogs from Croatia. Parasitol. Res. 118, 347–352. Koetsveld, J., Tijsse-Klasen, E., Herremans, T., Hovius, J.W. and Sprong, H. 2016. Serological and molecular evidence for spotted fever group Rickettsia and Borrelia burgdorferi sensulato co-infections in the Netherlands. Ticks. Tick. Borne. Dis. 7(2), 371–377. Lee, S., Lee, H., Park, J.W., Yoon, S.S., Seo, H.J., Noh, J. and So, B.J. 2020. Prevalence of antibodies against Anaplasma spp., Borrelia burgdorferi sensulato, Babesia gibsoni, and Ehrlichia spp. in dogs in the Republic of Korea. Ticks. Tick. Borne. Dis. 11(4), 101412. Lindenmayer, J.M., Marshall, D. and Onderdonk, A.B. 1991. Dogs as sentinels for lyme disease in Massachusetts. Am. J. Public. Health. 81(11), 1448–1455. Madhi, D.K., Issa, A.H., Al-Luaibi, Y.Y. and Al-Maliki, Q.N. 2019. First report of Borrelia spp. and Borreliella spp. in blood, urine, and buccal cavity of patients with psychiatric disorder in Basra Province, Iraq. Drug. Invent. Today. 11(11), 2927–2932. Mafra, C. and Montandon, C.E. 2017. Borreliosis. Arthropod Borne Diseases. pp: 193–204. Maggi, R.G., Birkenheuer, A.J., Hegarty, B.C., Bradley, J.M., Levy, M.G. and Breitschwerdt, E.B. 2014. Comparison of serological and molecular panels for diagnosis of vector-borne diseases in dogs. Parasit. Vectors. 7, 1–9. Merino, F.J., Serrano, J.L., Saz, J.V., Nebreda, T., Gegundez, M. and Beltran, M. 2000. Epidemiological characteristics of dogs with lyme borreliosis in the province of Soria (Spain). Eur. J. Epidemiol. 16(2), 97–100. Milkovičová, M., Šimková, J., Valko-Rokytovská, M., Očenáš, P., Salayová, A. and Bhide, M.R. 2023. Lyme borreliosis in dogs: background, epidemiology, diagnostics, treatment and prevention. Folia. Vet. 67(1), 75–90. Mosallanejad, B., Avizeh, R., Jalali, M.H.R. and Pourmahdi, M. 2015. A serological survey on Borrelia burgdorferi infection among companion dogs in Ahvaz district, southwestern Iran. Comp. Clin. Pathol. 24, 1559–1563. Nascimento, D.A.G., Vieira, R.F.D.C., Vieira, T.S.W.J., Toledo, R.D.S., Tamekuni, K., Santos, N.J.R.D. and Vidotto, O. 2016. Serosurvey of Borrelia in dogs, horses, and humans exposed to ticks in a rural settlement of southern Brazil. Rev. Bras. Parasitol. Vet. 25, 418–422. O’Connell, S. 2014. Lyme borreliosis. Medicine 42(1), 14–17. Pantchev, N., Schaper, R., Limousin, S., Norden, N., Weise, M. and Lorentzen, L. 2009. Occurrence of Dirofilariaimmitis and tick-borne infections caused by Anaplasma phagocytophilum, Borrelia burgdorferi sensulato and Ehrlichia canis in domestic dogs in France: results of a countrywide serologic survey. Parasitol. Res. 105, 101–114. Pantchev, N., Schnyder, M., Vrhovec, M.G., Schaper, R. and Tsachev, I. 2015. Current surveys of the seroprevalence of Borrelia burgdorferi, Ehrlichia canis, Anaplasmaphagocytophilum, Leishmania infantum, Babesia canis, Angiostrongylus vasorum and Dirofilariaimmitis in dogs in Bulgaria. Parasitol. Res. 114(Suppl. 1), 117–130. Pejchalová, K., Žákovská, A., Fučik, K. and Schánilec, P. 2006. Serological confirmation of Borrelia burgdorferi infection in dogs in the Czech Republic. Vet. Res. Commun. 30, 231–238. Poljak, I., Trošelj-Vukiæ, B., Miletiæ, B., Moroviæ, M., Ružiæ-Sabljiæ, E., Vuèemiloviæ, A., and Materljan, E. 2000. Low sero-prevalence of lyme borreliosis in the forested mountainous area of Gorski Kotar, Croatia. Public. Health. 41(4), 433–436. Quarsten, H., Grankvist, A., Høyvoll, L., Myre, I.B., Skarpaas, T., Kjelland, V. and Noraas, S. 2017. Candidatus Neoehrlichiamikurensis and Borrelia burgdorferi sensulato detected in the blood of Norwegian patients with erythema migrans. Ticks. Tick. Borne. Dis. 8(5), 715–720. Rolla, D., Conti, N., Ansaldo, F., Panaro, L. and Lusenti, T. 2014. Post-infectious glomerulonephritis presenting as acute renal failure in a patient with Lyme disease. J. Ren. Inj. Prev. 3(1), 17. San Martin, F., Fule, L., Iraola, G., Buschiazzo, A. and Picardeau, M. 2022. Diving into the complexity of the spirochetal endoflagellum. Trends. Microbiol. 31(3), 294–307. Saunders, A.B., Gordon, S.G., Rector, M.H., DeMaster, A., Jackson, N., Clubb, F.J. and Miller, M.W. 2013. Bradyarrhythmias and pacemaker therapy in dogs with Chagas disease. J. Vet. Intern. Med. 27(4), 890–894. Savić, S., Vidić, B., Lazić, S., Lako, B., Potkonjak, A. and Lepšanović, Z. 2010. Borrelia burgdorferi in ticks and dogs in the province of Vojvodina, Serbia. Parasite 17(4), 357–361. Schulze, T.L., Bosler, E.M., Shisler, J.K., Ware, I.C., Lakat, M.F. and Parkin, W.E. 1987. Prevalence of canine lyme disease from an endemic area as determined by serosurvey. Zentralblatt für Bakteriologie, Mikrobiologie und Hygiene. Series. A. Med. Microbiol. Infect. Dis. Virol. Parasitol. 263(3), 427–434. Skotarczak, B., Koś, W., Wodecka, B., Rymaszewska, A., Sawczuk, M., Zajkowska, J. and Świerzbińska, R. 2003. Domestic dog as a reservoir of Borrelia burgdorferi sensulato spirochetes from endemic areas of lyme disease in north-western Poland. Arthropods. Hosts. 12(2), 231–240. Skotarczak, B., Wodecka, B., Rymaszewska, A., Sawczuk, M., Maciejewska, A., Adamska, M. and Swierzbinska, R. 2005. Prevalence of DNA and antibodies to Borrelia burgdorferi sensulato in dogs suspected of borreliosis. Ann. Agric. Environ. Med. 12(2), 199–205. Solís-Hernández, A., Rodríguez-Vivas, R.I., Esteve-Gasent, M.D. and Villegas-Pérez, S.L. 2018. Detection of Borrelia burgdorferi sensulato in dogs and its ticks in rural communities of Yucatán, Mexico. Rev. Biol. Trop. 66(1), 428–437. Stefancikova, A., Skardova, I., Pet’ko, B., Janovska, D. and Cyprichova, V. 1996. IgG antibodies to Borrelia in dogs in the area of Kosice. Vet. Med. 41(3), 83–86. Straubinger, R.K. 2000. PCR-based quantification of Borrelia burgdorferi organisms in canine tissues over a 500-day postinfection period. J. Clin. Microbiol. 38(6), 2191–2199. Straubinger, R.K., Summers, B.A., Chang, Y.F. and Appel, M.J. 1997. Persistence of Borrelia burgdorferi in experimentally infected dogs after antibiotic treatment. J. Clin. Microbio. 35(1), 111–116. Sýkora, J., Pokorný, P., Bukovjan, K. and Radek, J. 1990. Occurrence of Borrelia antibodies in field hares (Lepus europaeus). Ceskoslovenska. Epidemiol. Mikrobiol. Imunol. 39(2), 120–125. Uesaka, K., Maezawa, M. and Inokuma, H. 2016. Serological survey of Borrelia infection of dogs in Sapporo, Japan, where Borrelia garinii infection was previously detected. J. Vet. Med. Sci. 78(3), 463–465. Verbsky, M.P. 1997. A cross sectional pilot study of lyme disease in case associated dogs to determine endemic areas in Ohio. Doctoral Dissertation, The Ohio State University, Columbus, OH. Wagner, B., Freer, H., Rollins, A., Garcia-Tapia, D., Erb, H.N., Earnhart, C. and Meeus, P. 2012. Antibodies to Borrelia burgdorferi OspA, OspC, OspF, and C6 antigens as markers for early and late infection in dogs. mSphere 19(4), 527–535. Wang, J., Kelly, P., Zhang, J., Shi, Z., Song, C., Zheng, X. and Wang, C. 2018. Detection of Dirofilariaimmitis antigen and antibodies against Anaplasma phagocytophilum, Borrelia burgdorferi and Ehrlichia canis in dogs from ten provinces of China. Acta. Parasitol. 63(2), 412–415. | ||
| How to Cite this Article |
| Pubmed Style Ajaj EA, Al-jumaa ZM. Molecular detection of Spirochetes and Borrelia burgdorferi in stray dogs of Nineveh province, Iraq. Open Vet. J.. 2023; 13(10): 1318-1325. doi:10.5455/OVJ.2023.v13.i10.11 Web Style Ajaj EA, Al-jumaa ZM. Molecular detection of Spirochetes and Borrelia burgdorferi in stray dogs of Nineveh province, Iraq. https://www.openveterinaryjournal.com/?mno=157240 [Access: January 18, 2026]. doi:10.5455/OVJ.2023.v13.i10.11 AMA (American Medical Association) Style Ajaj EA, Al-jumaa ZM. Molecular detection of Spirochetes and Borrelia burgdorferi in stray dogs of Nineveh province, Iraq. Open Vet. J.. 2023; 13(10): 1318-1325. doi:10.5455/OVJ.2023.v13.i10.11 Vancouver/ICMJE Style Ajaj EA, Al-jumaa ZM. Molecular detection of Spirochetes and Borrelia burgdorferi in stray dogs of Nineveh province, Iraq. Open Vet. J.. (2023), [cited January 18, 2026]; 13(10): 1318-1325. doi:10.5455/OVJ.2023.v13.i10.11 Harvard Style Ajaj, E. A. & Al-jumaa, . Z. M. (2023) Molecular detection of Spirochetes and Borrelia burgdorferi in stray dogs of Nineveh province, Iraq. Open Vet. J., 13 (10), 1318-1325. doi:10.5455/OVJ.2023.v13.i10.11 Turabian Style Ajaj, Eva Ayser, and Zahraa Mustafa Al-jumaa. 2023. Molecular detection of Spirochetes and Borrelia burgdorferi in stray dogs of Nineveh province, Iraq. Open Veterinary Journal, 13 (10), 1318-1325. doi:10.5455/OVJ.2023.v13.i10.11 Chicago Style Ajaj, Eva Ayser, and Zahraa Mustafa Al-jumaa. "Molecular detection of Spirochetes and Borrelia burgdorferi in stray dogs of Nineveh province, Iraq." Open Veterinary Journal 13 (2023), 1318-1325. doi:10.5455/OVJ.2023.v13.i10.11 MLA (The Modern Language Association) Style Ajaj, Eva Ayser, and Zahraa Mustafa Al-jumaa. "Molecular detection of Spirochetes and Borrelia burgdorferi in stray dogs of Nineveh province, Iraq." Open Veterinary Journal 13.10 (2023), 1318-1325. Print. doi:10.5455/OVJ.2023.v13.i10.11 APA (American Psychological Association) Style Ajaj, E. A. & Al-jumaa, . Z. M. (2023) Molecular detection of Spirochetes and Borrelia burgdorferi in stray dogs of Nineveh province, Iraq. Open Veterinary Journal, 13 (10), 1318-1325. doi:10.5455/OVJ.2023.v13.i10.11 |