| Research Article | ||
Open Vet. J.. 2023; 13(10): 1334-1345 Open Veterinary Journal, (2023), Vol. 13(10): 1334–1345 Original Research Protective effect of vitamin C against thiamethoxam-induced toxicity in male ratsIbrahim A. Hamed1, Refat M. Sherif1, El-Sayed A. El-Sheikh1, Ahmed M. Aldawek2* and Aly A. Shalaby11Department of Plant Protection, Faculty of Agriculture, Zagazig University, Zagazig, Sharkia, Egypt 2Department of Pathology and Clinical Pathology, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya *Corresponding Author: Ahmed M. Aldawek. Department of Pathology and Clinical Pathology, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya. Email: ahmad88876 [at] protonmail.com Submitted: 10/07/2023 Accepted: 22/09/2023 Published: 31/10/2023 © 2023 Open Veterinary Journal
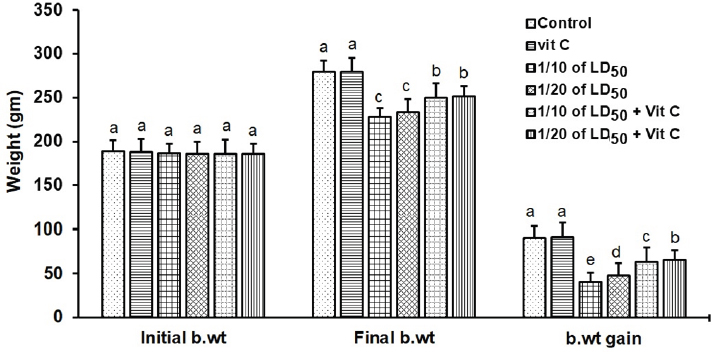
AbstractBackground: Thiamethoxam (THM) is a neonicotinoid insecticide used to control different insect pests on fruits, vegetables, and field crops. The misuse and continuous exposure to THM cause many harmful effects on health and the reproductive system. Aim: This work aims to investigate the efficiency of vitamin C (vit C) in reducing or eliminating the harmful effects of THM on the testes, liver, and kidney of male rats. Methods: Forty-eight sexually mature male Wister albino rats (weight: 170–190 g; age: 10–11 weeks) were randomly allocated into six groups (8 males/group). The control group was orally given distilled water, vit C group was orally treated with 200 mg/kg b.wt of vit C, group 1/10 of THM LD50 orally treated with 156.3 mg/kg b.wt of THM, group 1/20 of THM LD50 orally treated with 78.15 mg/kg b.wt of THM, group 1/10 of THM LD50 + vit C orally treated with 156.3 mg/kg b.wt of THM + 200 mg/kg b.wt of vit C, and group 1/20 of THM LD50 + vit C orally treated with 78.15 mg/kg b.wt of THM + 200 mg/kg b.wt of vit C. All groups were treated for five days per week for a whole period of 58 days. Blood samples were collected at the end of the experiment, and serum was extracted for liver and kidney functions and antioxidant measurements. Reproductive organs (testis, epididymis, and seminal vesicles) were collected and weighed at the end of the experiment. Results: The results showed that groups exposed to 1/10 and 1/20 of THM LD50 significantly (p < 0.05) decreased the body weight, the reproductive organ weights (testis, epididymis, and seminal vesicles), spermatid count, sperm (count and motility), and testosterone concentration with an increase in abnormalities. In addition, the groups exposed to THM showed a decrease in protein concentration, albumin, and globulin, and caused an increase in glucose concentration. The activities of alkaline phosphatase (ALP), alanine transaminase (ALT), aspartate transaminase (AST), creatinine, urea, and malondialdehyde (MDA) were increased while caused decrease in total antioxidant capacity (TAC) due to exposure to THM. The co-administration of vit C with HM modulated the harmful effects of the insecticide on testicular, liver, and kidney parameters, which confirmed in histopathological examination of testis. Groups orally treated with vit C showed a significant increase in spermatogenesis, spermatid numbers, and the weight of seminal vesicles. Conclusion: This study showed the importance of vit C in reducing toxic effects from exposure to THM. Accordingly, the intake of vit C by individuals who regularly handle this insecticide will be beneficial in reducing the adverse effects that may occur in the liver and kidney. Keywords: Thiamethoxam, Vitamin C, Oxidative stress, Antioxidant. IntroductionThe widespread use of pesticides to control agricultural pests has led to serious environmental contamination and health risks (Anaduaka et al., 2023). The increasing problems from the use of some groups of pesticides (i.e., organochlorines, organophosphates, and carbamates) led to the introduction of other groups that are characterized by being safer to achieve sustainable agriculture (Auwal et al., 2021). Neonicotinoids are a new class of pesticides that accounts for about 25% of the global insecticides market and is used in protecting domestic animals from fleas and crops from pest insects (Craddock et al., 2019). Thiamethoxam (THM) and imidacloprid (IMI) are the two major compounds of neonicotinoids that were utilized in large quantities, which might be problematic when considering the potential risks of occupational and environmental pollution (El-Hak et al., 2022). According to reports, neonicotinoids stimulate nicotinic acetylcholine receptors (nAChRs) (Moffat et al., 2016). Due to their specific binding affinity with nAChRs, they are considered highly poisonous to insects. Different studies and publications on the investigation of neonicotinoid-induced toxicity in vertebrate systems have been made that show potential effects on the biological system of mammals (Dhouib et al., 2017). Zhao et al. (2020) demonstrated that the metabolism of the neonicotinoid group involved the cytosolic aldehyde oxidase and the hepatic microsomal enzyme. Mammalian exposure to THM has shown that around 50% of the parent compound is metabolized with the existence of its metabolites in the brain tissues (Loser et al., 2021). The potential effects on mammals are extremely interesting as the generated metabolites take on a cationic character and are accordingly selective for the mammalian nAChRs (Costas-Ferreira and Faro, 2021). Pesticides and their metabolites act as free radicals that reduce the ability of antioxidants to protect the body from these substances and induce oxidative stress (Beslo et al., 2023). Antioxidant defense mechanisms defend against reactive oxygen species (ROS) damage (Irato and Santovito, 2021). Many ion channels, including calcium channels, have been demonstrated to be damaged by ROS (Kiselyov and Muallem, 2016). Vitamin C (also known as ascorbic acid; vit C) is a water-soluble vitamin sold as a dietary supplement and has been demonstrated to have preventive measures for xenobiotic intoxications (Zhong et al., 2017). In addition, it was reported that vit C has a powerful antioxidant that is widely known for shielding tissues from oxidative damage (Kurutas, 2016), decreasing oxidative cell death, and providing genome protection by quenching intracellular (Kaźmierczak-Barańska et al., 2020). Vit C plays a significant part in preventing the effects of free radicals on vital cells and protecting against pesticide toxicity, especially with regard to hepatic toxicity (Shati et al., 2021). It quickly removes reactive nitrogen species (RNS) as well as physiological ROS (Di Meo et al., 2016). According to reports, vit C reduces the hematological and biochemical changes that are caused by organophosphate pesticides in humans and animals (Saoudi et al., 2021). This chemical is an easily accessible, inexpensive, and comparatively non-toxic antioxidant that exhibits tremendous promise in the reduction of the toxic effects caused by the majority of xenobiotics (Ibrahim et al., 2019). Reproductive behavior is considered an effective way used in ecotoxicology that details the biochemical, physiological, and toxicological responses to a toxin that may affect reproduction (Ford et al., 2021). Therefore, this study aims to evaluate the effect of THM on different reproductive and biochemical parameters and the effect of vit C as an antioxidant in ameliorating the toxicity effects of the tested insecticide. Materials and MethodsChemicals and reagentsA commercial formulation of THM (Actara®, 25% WG) was purchased from the local distributor company of Syngenta crop protection Agrochemicals (Dokki, Egypt), which imported from Greenboro (Greenboro, NC, USA). L-ascorbic acid (100468) (vitamin C; vit C) was obtained from Sigma–Aldrich (St. Louis, MO). Kits used for biochemical determination were purchased from Bio-Diagnostic Company (Dokki, Egypt). Animals and experimental designForty-eight sexually mature male Wister albino rats (weight: 170–190 g; age: 10–11 weeks) were obtained from the Breeding Animal House, Faculty of Veterinary Medicine at Zagazig University, Egypt. Animals were housed in plastic cages at 23°C ± 2°C, 40%–60% relative humidity, and 12 hours light/dark cycle and kept under full hygienic conditions, and fed on rodent diet and water ad libitum throughout the experimental period (NRC, 1996). The rats were left for two weeks to get acclimatized to the experimental laboratory settings. The housing, care, and all experimental procedures were conducted in compliance with the guidelines of Zagazig University Care and Use of Laboratory Animals, under the permission number (ZU-IACUC/2/F/121/2022). The rats were weighed after the accommodation period and then randomly divided into six groups of eight males for each group. The groups were treated as follows: G1 (the control group) was orally administrated distilled water. G2 (vit C group) was orally administrated 200 mg/kg b.wt of vit C. G3 (1/10 of THM LD50 group) was orally administrated 156.3 mg/kg b.wt of THM. G4 (1/20 of THM LD50 group) was orally administrated 78.15 mg/kg b.wt of THM. G5 (1/10 of THM LD50 + vit C group) was orally administrated 156.3 mg/kg b.wt of THM + 200 mg/kg b.wt of vit C. G6 (1/20 of THM LD50 + vit C group) was orally administrated 78.15 mg/kg b.wt of THM + 200 mg/kg b.wt of vit C. Groups were orally given combination of THM and vit C were given vit C 30 minutes prior of THM. All groups were orally treated with 1/10 and 1/20 of THM LD50 and vit C for five days per week for 58 days. Doses of insecticide and vitamin CThe LD50 of THM is 1563 mg/kg b.wt (Maienfisch et al., 1999). In this study, 1/10 (156.3 mg/kg b.wt) and 1/20 (78.15 mg/kg b.wt) of THM LD50 were orally administrated to rats for 58 days. Insecticide was prepared in distilled water. A fresh daily, prepared aqueous solution of vit C was orally administrated to the treated rats throughout the experiment in a dose of 200 mg/kg b.wt (Hassan et al., 2021). Body and testis weightsThe animal body weights were recorded three times per week and at the end of the experiment. Clinical indications (posture and locomotor activities) were observed every day for mortality. The testicles were taken out after sacrifice, weighed, and microscopically examined to look for any enlargement, shrinkage, gaps caused by tissue loss, softening of the tissue, foreign coloring of the tissue, or altered content (US-EPA 2019). Reproductive organs (testis, epididymis, and seminal vesicles) were collected and weighed at the end of the experiment. Blood collectionThe rats were starved for an entire night on the last day of the experiment (the 58th day). They were weighed before sacrifice, and 2 ml of blood sample was drawn from the orbital venous retrograde plexus of the eye using a capillary tube, collected in a clean centrifuge glass tube and allowed to coagulate at laboratory temperature for 20 minutes, then centrifuged at 3,000 rpm for 10 minutes (Megafuge, Thermo Scientific, Germany). Serum samples were transferred and aliquots into Eppendorf tubes and stored at −20°C until used for biochemical analysis within two weeks. Biochemical analysisDetermination of liver and kidney functions Activities of alanine transaminase (ALT), aspartate transaminase (AST), and alkaline phosphatase (ALP) were determined colorimetrically in serum samples according to the method adopted by Reitman and Frankle (1957) for ALT and AST, and Belfield and Goldberg (1971) for ALP. Total protein and albumin concentrations were measured using the method of Grant et al. (1987) and Westgard and Poquette (1972), respectively. Serum globulin was calculated by deducting the value of albumin from total protein. The glucose level was determined according to Trinder (1969). Colorimetric determination of creatinine was carried out according to the method of Bartles et al. (1972). Urea was measured colorimetrically using the technique of Fawcett and Soctt (1960). All analyses were done using the microplate reader (Infinite M Nano, TECAN, Austria). Determination of MDA and TAC levels The colorimetric method for measuring malondialdehyde (MDA) as a sign of lipid peroxidation was carried out according to Ohkawa et al. (1979). Total antioxidant capacity (TAC) was measured in accordance with the guidelines provided in the BioDignostic kit’s instructions (Diagnostic Co., Dokki, Egypt). The method of Koracevic et al. (2001) was used to evaluate the rate of TAC. Analyses of MDA and TAC were done using the microplate reader (Infinite M Nano, TECAN, Austria). Estimation of serum testosterone hormone, luteinizing hormone, and serum follicle stimulating hormone levels Testosterone level in serum was predicted by the methods of Mukherjee et al. (2006) and Orcyzk et al. (1979). Serum follicle stimulating hormone (FSH) and luteinizing hormone (LH) levels were determined by ELISA as described by Levine et al. (1985), using a diagnostic kit (Immulite 1000 LH and FSH) supplied by Siemens Medical Solution Diagnostic Limited (USA). Features of semen The epididymis of every rat was removed to calculate the sperm count, according to (Amman et al. 1976). Analysis of sperm viability and motility was done using the techniques of Linder et al. (1990). Histological examination of testis Samples of the testicular specimens were collected from rats by the end of the experiment and stored in formalin. The samples were subjected to the automated tissue processing of dehydration and a two-step initial fixation procedure. Fixation involved immersing the tissues for 48 hours in 10% buffered formalin, followed by 30 minutes in distilled water to remove the fixative buffer. The tissues were then subjected to a graduated series of alcohol (70%, 90%, and 100%) to induce dehydration. The tissue was first subjected to 70% alcohol for 120 minutes, then to 90% alcohol for 90 minutes, and finally into two cycles of 100% alcohol, each lasting an hour. The samples were then cleared in numerous changes of xylene after dehydration. It involved immersing tissue for an hour in a combination of 50% alcohol and 50% xylene, then immersing in pure xylene for a further 1.5 hours. The samples were then embedded and blocked out after being impregnated with molten paraffin wax. Hematoxylin and eosin were used to stain paraffin slices (4–5 µm) (Suvarna et al. 2013). Staining allows looking for any pathological changes in the tissues, such as apoptosis, necrosis, degenerations, inflammation, and circulatory difficulties. Statistical analysisThe data were presented as mean ± SD. To ascertain statistical differences among groups, the data was examined using one-way analysis of variance (ANOVA) using the Statistical Package for the Social Sciences (SPSSs) 20.0 package program with Duncan’s Multiple Range Test at 0.05 significance value. Ethical approvalEthical clearance in this study was submitted and approved by the University of Zagazig with the following ethical clearance number: ZU-IACUC/2F/121/2022. ResultsEffect of THM on body weightNo death in rat groups was recorded during the whole experimental period due to exposure to THM, vit C, or their combination. Rats that were orally given both doses (1/10 and 1/20 of THM LD50) revealed a substantial reduction in final body weight and weight gain (p < 0.05) as compared to the control and other groups. Groups were given a combination of 1/10 or 1/20 of THM LD50 and vit C, which resulted in a significant increase in body weight or weight gain compared with groups that were treated only with 1/10 or 1/20 of THM LD50. In addition, no significant differences in the final body weight or weight gain were observed between the control and vit C groups (Fig. 1).
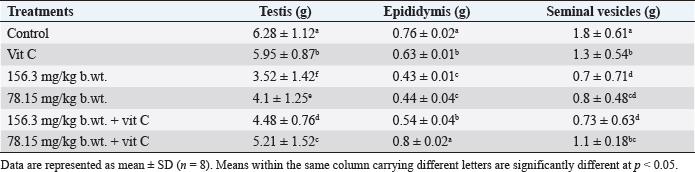
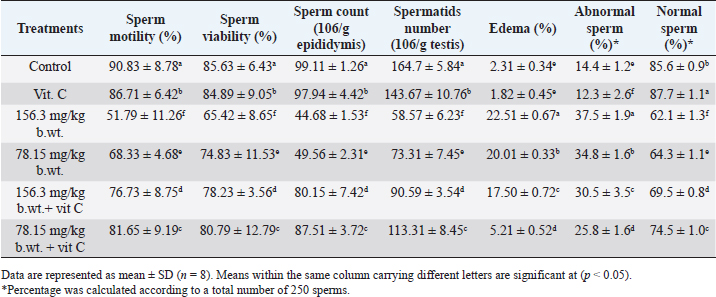
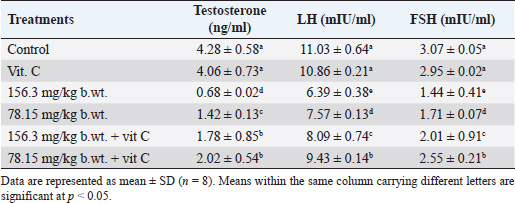
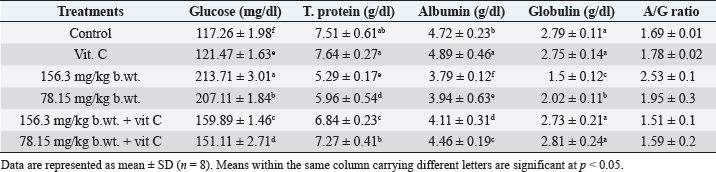
Fig. 1. Body weights (mean ± SD; n=8) of male rats treated with different doses of thiamethoxam, vitamin C, and their combination. Effect on reproductive organ weightsReproductive organ weights (testis, epididymis, and seminal vesicles) showed a significant decrease (p < 0.05) in groups that were orally given 1/10 or 1/20 of THM LD50 compared with the control group. While groups were orally given 1/10 or 1/20 of THM LD50 and co-administrated vit C, it resulted in a significant increase (p < 0.05) and enhancement in organ weights compared with groups treated with insecticide alone (Table 1). Effect on sperm characterizationResults in Table 2 showed a significant decrease (p < 0.05) in motility % and viability % of sperms in groups were orally given 1/10 and 1/20 of THM LD50 or in groups co-administrated vit C with insecticide when compared with control or vit C groups. Sperm count (106/g epididymis) and spermatids numbers (106/g testis) were increased significantly in groups treated with 1/10 or 1/20 of THM LD50 compared with control and vit C groups. Data showed an increase in sperm abnormalities in groups treated with 1/10 and 1/20 of THM LD50. Groups were orally given 1/10 and 1/20 of THM LD50 + vit C showed a significant decrease (p < 0.05) in sperm abnormalities compared with the control group. Effect on reproductive hormonesExposure of rats to both doses of THM (1/10 and 1/20 of LD50) caused a significant decline in serum sex hormone levels (Testosterone, LH, and FSH). While groups treated with both doses of THM + vit C induced significant increases in serum hormone levels compared with animals exposed to THM only. Groups orally vit C showed no significant changes in serum testosterone, LH, and FSH when compared with control (Table 3). Effect on glucose and total proteinSerum glucose was significantly decreased (p < 0.05) after 58 successive days of exposure to both doses of THM when compared either with control or other groups. Co-administration of vit C with THM showed a significant decrease (p < 0.05) when compared with THM groups (Table 4). Significant reductions in total protein, albumin, and globulin concentrations were reported in groups exposed to THM compared to other groups, including control (Table 4). Table 1. Effect of thiamethoxam, vitamin C, and their combination on reproductive organ weights.
Table 2. Effect of thiamethoxam, Vitamin C, and their combination on sperm count, motility, viability, spermatids number, edematous testicular tissue (% edema), and abnormality.
Table 3. Effect of thiamethoxam, vitamin C, and their combination on serum testosterone, LH and FSH levels.
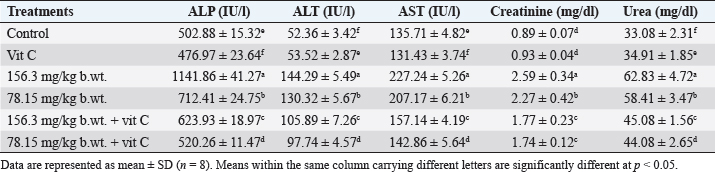
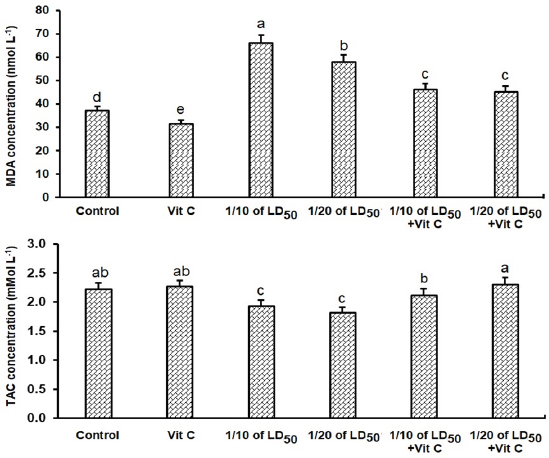
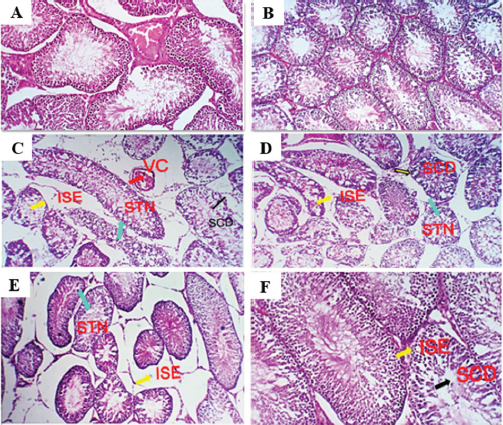
Effect on liver and kidney functionsGroups exposed to THM, vit C, and their combination showed a significant increase (p < 0.05) in activities of liver enzymes (ALP, ALT, and AST) in groups exposed to 1/10 and 1/20 of THM LD50 compared with the control group. The data also showed that co-administration of vit C with both THM doses exhibited some improvement, but still significantly different from the control. For kidney function parameters, creatinine and urea levels in the blood serum of animals exposed to THM were significantly increased compared to that of the control group. The data also showed that co-administration of vit C with both THM doses exhibited some protective effects but still significantly higher than what was recorded in the control group (Table 5). Effect on oxidative stress and antioxidant parametersFor oxidative stress parameters, the results showed that the level of MDA was significantly increased (p < 0.05) in rats administered 1/10 and 1/20 of THM LD50 compared with the control group. On the other hand, TAC level was significantly (p < 0.05) decreased as a result of exposure to the tested doses, whereas the co-administration of vit C with THM enhanced the decreased effects of insecticide compared with control (Fig. 2). Histological observation in testisExamined sections from the testis of control (Fig. 3A) and vit C (Fig. 3B) groups demonstrated healthy testicular architecture, including preserved seminiferous tubules that looked to be bordered by healthy spermatogonia, spermatocytes, spermatids, and sertoli cells. In their lumina, they had varying numbers of mature spermatozoa. Leydig cells, vascular structures, and interstitial tissue appeared to be normal. Dimensional scales represent the cellular contents of the tubules, including spermatozoa, at their full thickness and size of the primary spermatocytes, mature spermatozoa, and normal epithelium. Sections from testes of the group treated with 1/10 of THM LD50 (Fig. 3C) revealed moderate interstitial edema (ISE), vascular congestion (VC), germ cell degeneration (GCD), and spermatocytes degeneration and necrosis (STD and STN) associated with complete failure of spermatogenesis. Leydig cells moderately proliferated. Very few tubules were unaffected, with normal histological morphology of the seminiferous tubules and normal spermatogenesis (NSG). Dimensional scales represent the cellular contents of the tubules, including spermatozoa, at their full thickness and size of the primary spermatocytes. The percentages of edematous testicular interstitial tissue, partial filling of the epididymal tubules with mature spermatozoa, and the type of epithelium estimated focally degenerated and showed ISE. Sections from testes of the group treated with 1/20 of THM LD50 (Fig. 3D) revealed mild to moderate ISE, VC, multifocal STD and STN associated with partial failure of spermatogenesis. Leydig cells were unaffected. Fair number of seminiferous tubules showed preserved histological morphology and NSG. Examined sections from testes of the group treated with 1/10 and 1/20 of THM LD50 and co-treated with vit C (Fig. 3E and F, respectively) revealed healthy testicular architecture, including preserved seminiferous tubules that looked to be lined by normal spermatogonia, spermatocytes, spermatids, and sertoli cells. They also contained a range of mature spermatozoa in their Lumina. Leydig cells, vascular structures, and interstitial tissue appeared normal in most of the investigated sections; however, a few sections showed mild to moderate ISE, VC, and mild Leydig cell proliferation (LGP) (Fig. 3). Table 4. Effect of thiamethoxam, vitamin C, and their combinations on glucose, total protein, albumin, and globulin.
Table 5. Effect of thiamethoxam, vitamin C, and their combinations on some biochemical parameters related to liver and kidney functions in blood serum of adult male rats.
Fig. 2. Effects of thiamethoxam, vitamin C, and their combinations on the concentration of antioxidant and oxidative stress indicators in adult male rats.
Fig. 3. Photomicrograph of testes sections stained by H&E for histopathological changes: control (A), vit C (B) 1/10 of THM LD50 (C), 1/20 of THM LD50 (D), 1/10 of THM LD50 + vit C (E), and 1/20 of THM LD50 + vit C (F). DiscussionPesticides are widely used in agricultural production to protect crops from various pests with the aim of increasing production to meet consumer needs (El-Sheikh et al., 2023). Exposure to pesticides occurs either directly through exposure during the application or indirectly through eating food and drinking water contaminated with pesticide residues (Vasylieva et al., 2017; El-Sheikh and Ashour, 2022; El-Sheikh et al., 2022; Hassan et al., 2022; Shalaby et al., 2022). To identify the adverse effects of THM exposure, different biochemical and histological parameters of the reproductive system were determined in male rats. The weight of rats exposed to 1/10 or 1/20 of THM LD50 showed significant reduction compared with either control or vit C treated groups (Fig. 1). The changes in the body and organ weight are considered a sensitive sign of potential harmful effects (El-Okle et al., 2018) and connected to numerous structural and functional abnormalities (Keller and Banks, 2006). The co-administration of vit C with THM showed improvement in body weight reduction. The reduction in body weight was explained as a result of the toxic effect associated with exposure to THM on food intake and an increase in the breakdown of fat and protein (Mansour and Mossa, 2010). The co-administration of vit C with THM showed improvement in body weight and body weight gain, which is compatible with (Mosbah et al., 2018), who studied the administration of Nigella sativa oil as an antioxidant with acetamiprid on body weight and body weight gain, or vit C with abamectin (Khaldoun-Oularbi et al., 2013) improved the body weight gain. The weight of reproductive organs (testes, epididymis, and seminal vesicles) decreased in groups exposed to 1/10 and 1/20 of THM LD50, which ameliorated in groups co-treated with vit C and THM compared with groups exposed to insecticide only. This finding is in agreement with that of Bal et al. (2013), who studied the effect of IMI on organ weight. Similarly, Devan et al. (2015) found a reduction in the absolute weight of the testicles and an increase in the absolute weight of other organs. Londonkar et al. (2000) reported that groups treated with nicotine, either oral or intra-peritoneal routes, had weight reductions in the testicles, epididymis, seminal vesicles, and prostate. Results of groups treated with 1/10 and 1/20 of THM LD50 showed a marked decrease in effects on sperm characteristics with some improvement in aberrant sperm morphology, testicular spermatid number, epididymal sperm count, motility, and viability when co-administrated with vit C (Table 2). The obtained results were confirmed by Zhang et al. (2011) and Bal et al. (2012) when experimental animals were treated with either acetamiprid or IMI. Both studies identified a direct correlation between pesticide dose and sperm degradation level. Reports showed that LH stimulates the production of testosterone by targeting testicular Leydig cells (Oduwole et al., 2021), which confirms the lower level of testosterone in rats exposed to THM. In the same context, exposure to chlorpyrifos was shown to down-regulate genes necessary for steroidogenesis and the synthesis of gonadotropins, which may account for its impact on FSH and LSH levels (Gal et al., 2016). A significant decrease in total protein, albumin, and globulin was observed in male rats exposed to 1/10 and 1/20 of THM LD50 (Table 4), which is consistent with Abbassy et al. (2014), who reported that serum albumin, globulin, and total protein levels significantly decreased after exposure to several pesticides. Groups co-administrated vit C with THM showed to improve albumin, globulin, and total protein levels in rat serum, which may be attributed to the protective effect of vit C on liver tissues and appetite-stimulating properties. The harmful effects on liver function were reported in other study (Magdy et al., 2016) and the protective effects of vit C as an antioxidant for reducing oxidative stress and enhancing liver function was also investigated. It was reported that the increase in blood sugar may be caused by a disruption in the metabolism of liver glycogen. This may be mediated by an increase in the hormones adrenocorticotrophic and glycogen and/or a decrease in insulin activity (Raja et al., 1992), which confirms the increase in glucose level shown in the current study (Table 3). Groups treated with 1/10 and 1/20 of THM LD50 induced significant alterations in biomarkers linked to kidney and liver functions (ALT, AST, ALP, creatinine, and urea). Groups treated with vit C + THM showed some enhancement (Table 4). These findings are in agreement with Gul et al. (2020), as increasing GOT and GPT are common indications of liver damage since it is widely known that they represent changes in the permeability of the plasma membrane as a result of hepatic injury (Kaneko et al. 1997). Since an increase in these components over normal levels indicates that the kidneys are underactive or functioning abnormally, serum creatinine and urea were identified as markers of renal functions (El-Deeb et al., 2007). Accordingly, the increase in creatinine and urea levels can be linked to renal impairment, which reduces renal blood flow, glomerular filtration rate, and excretion of waste products (Romi et al., 2017). In the current study, the increase in the serum levels of AST and ALT may be caused by hepatotoxicity, which alters permeability and causes lysosomal enzymes to leak, increasing the release of enzymes (Choudhary et al., 2003). In support of our findings, abamectin administration to male rats increased the level of ALP (Nasr et al., 2016) due to insecticide-induced liver damage. When the body produces too many dangerous chemicals called free radicals for the tissue’s antioxidant defenses to handle, this is known as oxidative stress (Abdollahi et al., 2004; Mossa et al., 2015; Uchendu et al., 2012). Results of this study showed a notable increase in antioxidant capacity overall in groups exposed to 1/10 and 1/20 of THM LD50 and vit C. While, groups treated with 1/10 or 1/20 of THM LD50 showed a significant decrease compared with control groups (Fig. 2). This can be explained that vit C reduced the toxicity of THM. These findings are in agreement with those obtained by Jamil et al. (2020), who reported that THM significantly alters various physiologic and biochemical measurements in the treated animals. Magdy et al. (2016) reported that antioxidants (vit C and E) can improve the function of the liver and kidneys by reducing the oxidative stress caused by abamectin and reducing the harmful effects on histological alterations. The data on oxidative stress parameters confirm that THM insecticide caused tissue damage by producing free radicals and altering the antioxidant state, which is essential for oxidative metabolism (Chen et al., 2023). Hence, a compensatory mechanism that prevents the generation of pesticide-induced free radicals may be explained by the THM-induced elevation in the activities of antioxidant enzymes (Banerjee et al. 2001). According to several reports, acetamiprid, IMI, and nicotine showed to cause an imbalance in oxidative/antioxidative status by depleting antioxidant defense mechanisms and elevating MDA levels in the reproductive organs, which is consistent with our findings (Nagda and Bhatt, 2011; Mosbah et al., 2015). Groups exposed to THM experienced the destruction of testicular cells, represented by a significant decrease in seminiferous tubules and spermatogenic germ cells, irregular and undulating basement membranes, and luminal immature cell rashes. The side effects resulting from the current study agree with what was obtained by Elbetieha and Da’as (2003), who demonstrated that the reproductive system of male rats was damaged when exposed to abamectin. In addition, reports showed that testicular injury from THM exposure in rats included degenerative seminiferous tubules with altered cellular organization and a reduction in sperm production (Celik-Ozenci et al., 2011; Abd-Elhady and Abou-Elghar, 2013), which agree with our findings. The side effects showed in a histological profile of the testes due to exposure to THM were improved when rats co-administrated vit C. This indicates that the harmful effects of THM on the histological structure of rat testes are eliminated completely or partially by the administration of vit C as an antioxidant material. ConclusionThis study showed the importance of vit C in reducing toxic effects resulting from exposure to THM. Accordingly, the intake of vit C by individuals who regularly handle this insecticide will be beneficial in reducing the adverse effects that may occur on the liver and kidney function. It seems that THM could alter the reproductive function through its capacity to induce toxicity in testis, and vit C intake with THM could reverse partially or completely the reproductive effects induced by THM. AcknowledgmentsThanks to Prof. Dr. E.R. El-Attar for his help in performing histopathological examination. Authors contributionsIAH: Methodology, data curation, and writing the first draft. RMS: Supervision, concept development, methodology, writing, reviewing, and editing the manuscript. EAE: Supervision, methodology, writing, reviewing, and editing the manuscript. AMA: Writing and editing the manuscript. AAS: Supervision, writing, reviewing, and editing the manuscript. FundingPartial funding of the USC18-983 project by STDF is acknowledged. Data availabilityAll data of this study are included in this manuscript. Any other related data can be obtained through sending to the corresponding author. Conflict of interest The authors declare that there is no conflict of interest. ReferencesAbbassy, M.A., Marei, A.M., Al-Ashkar, M.A.M. and Mossa, A.T.H. 2014. Adverse biochemical effects of various pesticides on sprayers of cotton fields in El-Behira Governorate Egypt. Biomed. Aging Pathol. 4, 251–256. Abd-Elhady, H.K. and Abou-Elghar, G.E. 2013. Abamectin induced biochemical and histopathological changes in the albino rat, Rattus norvegicus. J. Plant Protect. Res. 53, 263–270. Abdollahi, M., Ranjbar, A., Shadnia, S., Nikfar, S. and Rezaie, A. 2004. Pesticides and oxidative stress: a review. Med. Sci. Monit. 10(6), 141–147. Amann, R., Johnson, L., Thompson, J. and Pickett, B. 1976. Daily spermatozoal production, epididymal spermatozoal reserves and transit time of spermatozoa through the epididymis of the rhesus monkey. Biol. Reprod. 15(5), 586–592. Anaduaka, E.G., Uchendu, N.O., Asomadu, R.O., Ezugwu, A.L., Okeke, E.S. and Chidike Ezeorba T.P. 2023. Widespread use of toxic agrochemicals and pesticides for agricultural products storage in Africa and developing countries: possible panacea for ecotoxicology and health implications. Heliyon 9(4), e15173. Auwal, M., Vinod, K., Salihu, S., Vinay, K. and Aryan, B. 2021. The effect of thiamethoxam and ameliorative property of quercetin on oxidative stress and antioxidant parameters in the liver of male rats. Science Forum J. Pur. Appl. Sci. 21, 10–19. Bal, R., Nazıroğlu, M., Türk, G., Yılmaz, O., Kuloğlu, T., Etem, E. and Baydaş, G. 2012. Insecticide imidacloprid induces morphological and DNA damage through oxidative toxicity on the reproductive organs of developing male rats. Cell Biochem. Funct. 30(6), 492–499. Bal, R., Türk, G., Tuzcu, M., Yılmaz, O., Kuloğlu, T., Baydaş, G., Naziroğlu, M., Yener, Z., Etem, E. and Tuzcu, Z. 2013. Effects of the neonicotinoid insecticide clothianidin on the reproductive organ system in adult male rats. Drug Chem. Toxicol. 36(4), 421–429. Banerjee, B., Seth, V. and Ahmed, R. 2001. Pesticide induced oxidative stress perspectives and trends. Rev. Environ. Health 16(1), 1–40. Bartels, H., Böhmer, M. and Heierli, C. 1972. Serum creatinine determination without protein precipitation. Clin. Chem. Acta. 37, 193–197. Belfield, A. and Goldberg, D.M. 1971. Colorimetric determination of alkaline phosphatase activity. Enzyme 12(5), 561–568. Bešlo, D., Golubić, N., Rastija, V., Agić, D., Karnaš, M., Šubarić, D. and Lučić, B. 2023. Antioxidant activity, metabolism, and bioavailability of polyphenols in the diet of animals. Antioxidants 12(6), 1141. Celik-Ozenci, C., Tasatargil, A., Tekcan, M., Sati, L., Gungor, E., Isbir, M. and Demir, R. 2011. Effects of abamectin exposure on male fertility in rats: Potential role of oxidative stress-mediated poly (ADP-ribose) polymerase (PARP) activation. Regul. Toxicol. Pharm. 61, 310–317. Chen, T., Chen, H., Wang, A., Yao, W., Xu, Z., Wang, B., Wang, J. and Wu, Y. 2023. Methyl parathion exposure induces development toxicity and cardiotoxicity in zebrafish embryos. Toxics 11(1), 84. Choudhary, T.V., Aksoylu, E. and Goodman, D.W. 2003. Nonoxidative activation of methane. Catal. Rev. 45(1), 151–203. Costas-Ferreira, C. and Faro, L.R.F. 2021. Neurotoxic effects of neonicotinoids on mammals: What is there beyond the activation of nicotinic acetylcholine receptors?-a systematic review. Int. J. Mol. Sci. 22(16), 8413. Craddock, H.A., Huang, D. and Turner, P.C. 2019. Trends in neonicotinoid pesticide residues in food and water in the United States, 1999–2015. Environ. Health 18, 7. Devan, K.S., Mishra, A., Prabu, P.C., Mandal, T.K. and Panchapakesan, S. 2015. Sub-chronic oral toxicity of acetamiprid in wistar rats. Toxicol. Environ. Chem. 97, 1236–1252. Dhouib, B., Annabi, A., Doghri R., Rejeb, I., Dallagi, Y., Bdiri, Y. and Gati, A. 2017. Neuroprotective effects of curcumin against acetamiprid-induced neurotoxicity and oxidative stress in the developing male rat cerebellum biochemical, histological, and behavioral changes. Environ. Sci. Poll. Res. 24(35), 27515–27524. Di Meo, S., Reed, T., Venditti, P. and Victor, V. 2016. Role of ROS and RNS sources in physiol and pathol conditions. Oxid. Med. Cell Longev. 2016, 1245049. Djurasevic, F., Cvijic, G., Djordjevic, J. and Davidovic, V. 2008. The influence of vitamin C supplementation on the oxidative status of rat interscapular brown adipose tissue. J. Thermal. Biol. 33(4), 238–243. E1-Deeb, A., Abd E1-Aleem, I. and Sherin, S. 2007. Harmful effect of some insecticides on vital parameters of albino rats. J. Egypt Soc. Toxicol. 36, 53–60. El Okle, O., El Euony, O., Khafaga, A. and Lebda, M. 2018. Thiamethoxam induced hepatotoxicity and pro-carcinogenicity in rabbits via motivation of oxidative stress, inflammation, and anti-apoptotic pathway. Environ. Sci. Pollut. Res. Int. 25(5), 4678–4689. Elbetieha, A. and Da’as, S.I. 2003. Assessment of antifertility activities of abamectin pesticide in male rats. Ecotoxicol. Environ. Safe 55, 307–313. EL-Hak, G., Al-Eisa, A. and Ryad, L. 2022. Mechanisms and histopathological impacts of acetamiprid and azoxystrobin in male rats. Environ. Sci. Pollut. Res. 29, 43114–43125. El-Sheikh, E.A. and Ashour, M.B. 2022. Diamide insecticides: efficacy, toxicity and analytical methods for residue monitoring in food samples, Egypt. J. Chem. 65(5), 165–177. El-Sheikh, E.A., Li, D., Hamed, I., Ashour, M.B. and Hammock, B.D. 2023. Residue analysis and risk exposure assessment of multiple pesticides in Tomato and Strawberry and their products from markets. Foods 12, 1936. El-Sheikh, E.A., Ramadan, M.M., El-Sobki, A.E., Shalaby, A.A., McCoy, M.R., Hamed, I., Ashour, M. and Hammock, B.D. 2022. Pesticide Residues in Vegetables and Fruits from Farmer Markets and Associated Dietary Risks. Molecules 27(22), 8072. Fawcett, J.K. and Soctt, J.E. 1960. A rapid and precise method for the determination of urea. J Clin Pathol. 13:156–159. Ford, A.T., Ågerstrand, M., Brooks, B.W., Allen, J., Bertram, M.G., Brodin, T., Dang, Z., Duquesne, S., Sahm, R., Hoffmann, F., Hollert, H., Jacob, S., Klüver, N., Lazorchak, J.M., Ledesma, M., Melvin, S.D., Mohr, S., Padilla, S., Pyle, G.G., Scholz, S., Saaristo, M., Smit, E., Steevens, J.A., van den Berg, S., Kloas, W., Wong, B.B.M., Ziegler, M. and Maack, G. 2021. The role of behavioral ecotoxicology in environmental protection. Environ. Sci. Technol. 55(9), 5620–5628. Gal, A., Lin, P., Cacioppo, J., Hannon, P., Mahoney, M., Wolfe, A., Fernandez-Valdivia, R., Lydon, J., Elias, C. and Ko, C. 2016. Loss of fertility in the absence of progesterone receptor expression in kisspeptin neurons of female mice. PLoS One 11(7), e0159534. Grant, G.H., Silverman, L.M. and Chistenson, R.H. 1987. Amino acids and protein In: Fundamental of Clinical Chem 3rd ed. Philadelphia: W. B. Saunders Company. Gul, S.T., Ahamd, I., Saleemi, M.K., Ahmad, M., Ahmad, L. and Khan, A. 2020. Toxicopathological effects of thiamethoxam on haemato-biochemical and productive performance of commercial laying hens. Pak. Vet. J. 40(4), 449–454. Hassan, A.A., Bel Hadj, S.K., Fahmy, E.M., Mansour, D.A., Mohamed, S.A.M., Abdallah, A.A., Ashkan, M.F., Majrashi, K.A., Melebary, S.J., El-Sheikh, E.A. and El-Shaer, N. 2022. Olive leaf extract attenuates chlorpyrifos-induced neuro- and reproductive toxicity in male albino rats. Life 12, 1500. Hassan, H., Toni, N. and Meligi, N. 2021. Toxicity induced by indoxacarb exposure in male albino rats and the possible protective effects of vitamin C and zinc. Egypt. Acad. J. Biol. Sci. C. Physiol. Mol. Biol. 13(2), 155–176. Ibrahim, R., ElKady, M. and Hassanein, A. 2019. Effect of some antioxidants on rats treated with Titanium dioxide nanoparticles. Egypt. J. Food Sci. 47(1), 91–103. Irato, P. and Santovito, G. 2021. Enzymatic and non-enzymatic molecules with antioxidant function. Antioxidants (Basel) 10(4), 579. Kaneko, J.J., Harvey, J.W. and Bruss, M.L. 1997. Clinical biochem of domestic animals 5th ed San Diego, CA: Academic Press. Kaźmierczak-Barańska, J., Boguszewska, K., Adamus-Grabicka, A. and Karwowski, B.T. 2020. Two faces of vitamin C-antioxidative and pro-oxidative agent. Nutrients 12(5), 1501. Keller, K.A. and Banks, C. 2006. Multidose general toxicology studies. In Toxicological testing handbook, principles, applications and data interpretation. 2nd edition. Eds., David Jacobson-kram and Kit A Keller Informa Healthcare USA Inc149. Khaldoun-Oularbi, H., Richeval, C., Djenas, N., Lhermitte, M., Humbert, L. and Bag, A. 2013. Effect of subacute exposure to abamectin (insecticide) on liver rat (Rattus norvegicus). Ann. Toxicol. Anal. 25(2), 63–70. Kiselyov, K. and Muallem, S. 2016. ROS and intracellular ion channels. Cell Calcium. 60(2), 108–114. Koracevic, D., Koracevic, G., Djordjevic, V., Andrejevic, S. and Cosic, V. 2001. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 54(5), 356–61. Kurutas, E. 2016. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr. J. 15(1), 71. Levine, J., Norman, R. and Oyama, T. 1985. In vivo gonadotropin releasing hormones measurements in ovarieactomized rats. Endocrinology 117, 711–721. Linder, R.E., Strader, L.F. and Rehnberg, G.L. 1990. Effect of acute exposure to boric acid on the male reproductive system of the rat. J. Toxicol. Environ. Health 31, 133–146. Londonkar, R.L., Sonar, A., Patil, S. and Patil, S.B. 2000. Nicotine delays puberty in male rat. Pharm. Biol. 38(4), 291–297. Loser, D., Grillberger, K., Hinojosa, M.G., Blum, J., Haufe, Y., Danker, T., Johansson, Y., Möller, C., Nicke, A., Bennekou, S.H., Gardner, I., Bauch, C., Walker, P., Forsby, A., Ecker, G.F., Kraushaar, U. Leist, M. 2021. Acute effects of the imidacloprid metabolite desnitro-imidacloprid on human nACh receptors relevant for neuronal signaling. Arch. Toxicol. 95(12), 3695–3716. Magdy, B., Mohamed, F., Amin, A. and Rana, S. 2016. Ameliorative effect of antioxidants (vitamins C and E) against abamectin toxicity in liver, kidney and testis of male albino rats. J. Basic Appl. Zool. 77(C), 69–82. Maienfisch, P., Brandl, F., Kobel, W., Rindlisbacher, A. and Senn, R. 1999. CGA 293343: a novel, broad-spectrum neonicotinoid insecticide. Eds., Yamamoto, I., Casida, J.E. Nicotinoid insecticides and the nicotinic acetylcholine receptor. Tokyo: Springer, Verlag, pp: 177–209. Mansour, S.A. and Mossa, A.H. 2010. Oxidative damage, biochemical and histopathological alterations in rats exposed to chlorpyrifos and the antioxidant role of zinc. Pest. Biochem. Physiol. 96(1), 14–23. Moffat, C., Buckland S.T., Samson, A.J., McArthur, R., Chamosa Pino, V., Bollan, K.A., Huang, J.T. and Connolly C.N. 2016. Neonicotinoids target distinct nicotinic acetylcholine receptors and neurons, leading to differential risks to bumblebees. Sci. Rep. 28(6), 24764. Mosbah, R., Djerrou, Z. and Mantovani, A. 2018. Protective effect of Nigella sativa oil against acetamiprid induced reproductive toxicity in male rats. Drug Chem. Toxicol. 41(2), 206–212. Mosbah, R., Yousef, M. and Mantovani, A. 2015. Nicotine induced reproductive toxicity, oxidative damage, histological changes and haematotoxicity in male rats: the protective effects of green tea extract. Exp. Toxicol. Pathol. 67, 253–259. Mossa, A.T.H., Heikal, T.M. and Belaiba, M. 2015. Antioxidant activity and hepatoprotective potential of Cedrelopsis grevei on cypermethrin induced oxidative stress and liver damage in male mice. BMC Complement Altern. Med. 15, 251. Mukherjee, D., Mukherjee, D., Sen, U., Paul, S. and Bhattacharyaya, S. 2006. In vitro effects of insulin-like growth factors and insulin on oocyte maturation and maturation inducing steroid production in ovarian follicles of common carp, Cyprinus carpio Comp. Biochem. Physiol. Part A. 144, 63–77. Nagda, G. and Bhatt, D.K. 2011. Alleviation of lindane induced toxicity in testis of Swiss mice (Musmusculus) by combined treatment with vitamin C, vitamin E and alpha-lipoic acid. Indian J. Exper. Biol. 49, 191–199. Nasr, S., Collins, A., Alexander, M., Schraith, D., Herrera, H.L., Fidler, M., Sethi, S., Leung, N., Fervenza, F. and Cornell, L. 2016. The clinicopathologic characteristics and outcome of atypical anti-glomerular basement membrane nephritis. Kidney Int. 89(4), 897–908. NRC 1996. Guide for the care and use of laboratory animals National Research Council, Washington DC: Academic Press. Oduwole, O.O., Huhtaniemi, I.T. and Misrahi, M. 2021. The roles of luteinizing hormone, follicle-stimulating hormone and testosterone in spermatogenesis and folliculogenesis revisited. Int. J. Mol. Sci. 22(23), 12735. Ohkawa, H., Ohishi, N. and Yagi, K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95(2), 351–358. Orcyzk, G., Hichens, M., Arth, G. and Behrman, H. 1979. In methods of hormone radio immunoassay. Eds., Jaffe, B.M., Jaffe, B.M., Behrman, H.R.. New York: Academic Press, pp: 170. Raja, M., Al-Fatah, A., Ali, M., Hassan, R. A., Menon, M. and Dhami, M. S. 1992. Modification of liver and serum enzymes by parquet treatment in rabbits. Drug Metab. Drug Int. 10, 279–291. Reitman, S. and Frankle, S. 1957. Coloremetric method for determination of serum transaminase activity. Am. J. Clin. Pathol. 28, 56–68. Romi, M.M., Arfian, N. and Tranggono, U. 2017. Uric acid causes kidney injury through inducing fibroblast expansion, Endothelin-1expression, and inflammation. BMC Nephrol. 18, 326. Saoudi, M., Badraoui, R., Rahmouni, F., Jamoussi, K. and El Feki, A. 2021. Antioxidant and protective effects of Artemisia campestris essential oil against chlorpyrifos-induced kidney and liver injuries in rats. Front. Physiol. 12, 618582. Shalaby, A., El-Sheikh, E., Refaat, A. and Ragheb, D. 2022. Residue analysis and associated risk assessment of hexythiazox and spinosad applied on strawberry plants. Egypt. J. Chem. 65(11), 489–498. Shati, A.A., Zaki, M.S.A., Alqahtani, Y.A., Haidara, M.A., Al-Shraim, M., Dawood, A.F. and Eid, R.A. 2021. Potential protective effect of vitamin C on qunalphos-induced cardiac toxicity: histological and tissue biomarker assay. Biomed. 10(1), 39. Suvarna, K.S., Christopher, L. and Bancroft, J.D. 2013. Bancroft’s theory and practice of histological techniques, 7th Edition. Trinder, P. 1969. Enzymatic determination of glucose in blood serum. Ann. Clin. Biochem. 6(1), 24–27. U.S., E.P.A. 2019. Guidelines for reproductive toxicity risk assessment proposed guidelines. Proposed guidelines for female reproductive risk and proposed guidelines for male reproductive risk report No: EPA/630/R-96/009. Uchendu, C., Ambali, F. and Ayo, O. 2012. The organophosphate, chlorpyrifos, oxidative stress and the role of some antioxidants: a review. Afr. J. Agric. Res. 7(18), 2720–2728. Vasylieva, N., Barnych, B., Rand, A., Inceoglu, B., Gee, S.J. and Hammock, B.D. 2017. Sensitive immunoassay for detection and quantification of the neurotoxin, tetramethylene disulfotetramine. Anal. Chem. 89(10), 5612–5619. Westgard, J.O. and Poquette, M.A. 1972. Determination of serum albumin with the “SMA 12-60” by a bromcresol green dye-binding method. Clin. Chem. 18(7): 647-53. Wise and Loveys Information Services Ltd. 2005. Zhang, J., Wang, Y., Xiang, H., Meng-xue, L.I., Wen-hao, L., Kai-ge, M., Wang, X. and Zhang, J. 2011. Oxidative stress: role in acetamiprid-induced impairment of the male mice reproductive system. Agric. Sci. China 10(5), 786–796. Zhao, G.P., Yang, F.W., Li, J.W., Xing, H.Z., Ren, F.Z., Pang, G.F. and Li, Y.X. 2020. Toxicities of neonicotinoid-containing pesticide mixtures on nontarget organisms. Environ. Toxicol. Chem. 39, 1884–1893. Zhong, X., Zeng, M., Bian, H., Zhong, C. and Xiao, F. 2017. An evaluation of the protective role of vitamin C in reactive oxygen species-induced hepatotoxicity due to hexavalent chromium in vitro and in vivo. J. Occup. Med. Toxicol. 15, 12–15. | ||
| How to Cite this Article |
| Pubmed Style Hamed IA, Sherif RM, El-sheikh EA, Aldawek AM, Shalaby AA. Protective effect of vitamin C against thiamethoxam-induced toxicity in male rats. Open Vet. J.. 2023; 13(10): 1334-1345. doi:10.5455/OVJ.2023.v13.i10.13 Web Style Hamed IA, Sherif RM, El-sheikh EA, Aldawek AM, Shalaby AA. Protective effect of vitamin C against thiamethoxam-induced toxicity in male rats. https://www.openveterinaryjournal.com/?mno=158931 [Access: January 24, 2026]. doi:10.5455/OVJ.2023.v13.i10.13 AMA (American Medical Association) Style Hamed IA, Sherif RM, El-sheikh EA, Aldawek AM, Shalaby AA. Protective effect of vitamin C against thiamethoxam-induced toxicity in male rats. Open Vet. J.. 2023; 13(10): 1334-1345. doi:10.5455/OVJ.2023.v13.i10.13 Vancouver/ICMJE Style Hamed IA, Sherif RM, El-sheikh EA, Aldawek AM, Shalaby AA. Protective effect of vitamin C against thiamethoxam-induced toxicity in male rats. Open Vet. J.. (2023), [cited January 24, 2026]; 13(10): 1334-1345. doi:10.5455/OVJ.2023.v13.i10.13 Harvard Style Hamed, I. A., Sherif, . R. M., El-sheikh, . E. A., Aldawek, . A. M. & Shalaby, . A. A. (2023) Protective effect of vitamin C against thiamethoxam-induced toxicity in male rats. Open Vet. J., 13 (10), 1334-1345. doi:10.5455/OVJ.2023.v13.i10.13 Turabian Style Hamed, Ibrahim A., Refat M. Sherif, El-sayed A. El-sheikh, Ahmed M. Aldawek, and Aly A. Shalaby. 2023. Protective effect of vitamin C against thiamethoxam-induced toxicity in male rats. Open Veterinary Journal, 13 (10), 1334-1345. doi:10.5455/OVJ.2023.v13.i10.13 Chicago Style Hamed, Ibrahim A., Refat M. Sherif, El-sayed A. El-sheikh, Ahmed M. Aldawek, and Aly A. Shalaby. "Protective effect of vitamin C against thiamethoxam-induced toxicity in male rats." Open Veterinary Journal 13 (2023), 1334-1345. doi:10.5455/OVJ.2023.v13.i10.13 MLA (The Modern Language Association) Style Hamed, Ibrahim A., Refat M. Sherif, El-sayed A. El-sheikh, Ahmed M. Aldawek, and Aly A. Shalaby. "Protective effect of vitamin C against thiamethoxam-induced toxicity in male rats." Open Veterinary Journal 13.10 (2023), 1334-1345. Print. doi:10.5455/OVJ.2023.v13.i10.13 APA (American Psychological Association) Style Hamed, I. A., Sherif, . R. M., El-sheikh, . E. A., Aldawek, . A. M. & Shalaby, . A. A. (2023) Protective effect of vitamin C against thiamethoxam-induced toxicity in male rats. Open Veterinary Journal, 13 (10), 1334-1345. doi:10.5455/OVJ.2023.v13.i10.13 |