| Research Article | ||
Open Vet. J.. 2024; 14(5): 1251-1258 Open Veterinary Journal, (2024), Vol. 14(5): 1251–1258 Research Article Cardiorespiratory effects of intramuscular alfaxalone combined with low-dose medetomidine and butorphanol in dogs anesthetized with sevofluraneKeiko Kato, Takaharu Itami*, Norihiko Oyama and Kazuto YamashitaDepartment of Companion Animal Clinical Sciences, School of Veterinary Medicine, Rakuno Gakuen University, Ebetsu, Japan *Corresponding Author: Takaharu Itami. Department of Companion Animal Clinical Sciences, School of Veterinary Medicine, Rakuno Gakuen University, Ebetsu, Japan. Email: t-itami [at] rakuno.ac.jp Submitted: 14/08/2023 Accepted: 27/04/2024 Published: 31/05/2024 © 2024 Open Veterinary Journal
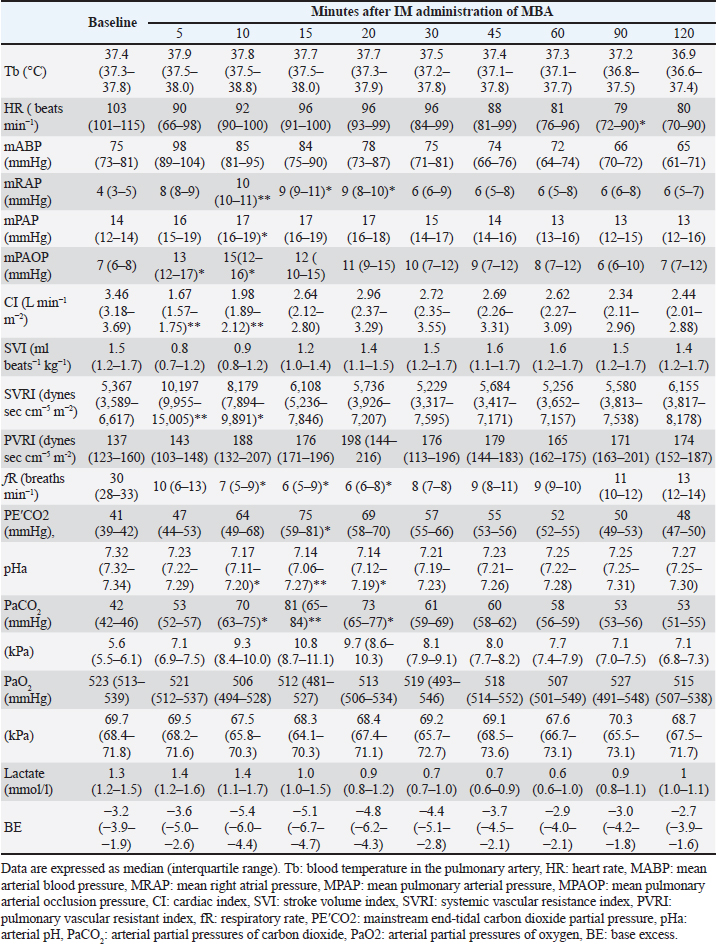
AbstractBackground: The intramuscular (IM) administration of 7.5–10 mg/kg of alfaxalone produces anesthetic effects that enable endotracheal intubation with mild cardiorespiratory depression in dogs. However, the effects of IM co-administration of medetomidine, butorphanol, and alfaxalone on cardiorespiratory function under inhalation anesthesia have not been studied. Aim: To assess the cardiorespiratory function following the IM co-administration of 5 μg/kg of medetomidine, 0.3 mg/kg of butorphanol, and 2.5 mg/kg of alfaxalone (MBA) in dogs anesthetized with sevoflurane. Methods: Seven intact healthy Beagles (three males and four females, aged 3–6 years old and weighing 10.0–18.1 kg) anesthetized with a predetermined minimum alveolar concentration (MAC) of sevoflurane were included in this study. The baseline cardiorespiratory variable values were recorded using the thermodilution method with a pulmonary artery catheter after stabilization for 15 minutes at 1.3 times their individual sevoflurane MAC. The cardiorespiratory variables were measured again following the IM administration of MBA. Data are expressed as median [interquartile range] and compared with the corresponding baseline values using the Friedman test and Sheff’s method. A p < 0.05 was considered statistically significant. Results: The intramuscular administration of MBA transiently decreased the cardiac index [baseline: 3.46 (3.18–3.69), 5 minutes: 1.67 (1.57–1.75) l/minute/m2 : p < 0.001], respiratory frequency, and arterial pH. In contrast, it increased the systemic vascular resistance index [baseline: 5,367 (3,589–6,617), 5 minutes:10,197 (9,955–15,005) dynes second/cm5/m2 : p =0.0092], mean pulmonary arterial pressure, and arterial partial pressure of carbon dioxide. Conclusion: The intramuscular administration of MBA in dogs anesthetized with sevoflurane transiently decreased cardiac output due to vasoconstriction. Although spontaneous breathing was maintained, MBA administration resulted in respiratory acidosis due to hypoventilation. Thus, it is important to administer MBA with caution to dogs with insufficient cardiovascular function. In addition, ventilatory support is recommended. Keywords: Alfaxalone, Medetomidine, Butorphanol, Dog, Cardiorespiratory effects. IntroductionAlfaxalone is a synthetic neuroactive steroid that causes neuro-depression and muscular relaxation associated with the gamma-aminobutyric acid A receptor in the central nervous system (Ferré et al., 2006). An alfaxalone formulation solubilized with 2-hydroxypropyl-beta-cyclodextrin has been approved for use as an intravenous (IV) anesthetic agent for dogs and cats in many countries owing to its smooth induction, rapid recovery, and minimal cardiorespiratory depression (Ferré et al., 2006; Muir et al., 2008; Keates and Whittem, 2012). Alfaxalone is also effective when administered intramuscularly (IM). Intramuscular administration of 7.5–10 mg/kg of alfaxalone produces anesthetic effects that enable endotracheal intubation with mild cardiorespiratory depression in dogs (Tamura et al., 2014). However, the approved product contains 10 mg/ml of alfaxalone and has a large IM dosage volume of 0.75–1.0 ml/kg (Tamura et al., 2014), making clinical applications with good animal welfare difficult (Diehl et al., 2001). In recent years, three prospective trials have reported that IM combinations of small doses of medetomidine, butorphanol, and alfaxalone provide anesthetic effects that can be clinically applied in dogs (Lee et al., 2016; Tamura et al., 2016; Kato et al., 2021). Lee et al. (2016) reported that the IM co-administration of 10 μg/kg of medetomidine, 0.1 mg/kg of butorphanol, and 1.5 mg/kg of alfaxalone induced anesthetic effects that enabled the maintenance of endotracheal intubation in dogs. Tamura et al. (2016) also reported that the IM co-administration of 2.5 μg/kg of medetomidine, 0.25 mg/kg of butorphanol, and 2.5 mg/kg of alfaxalone enabled endotracheal intubation without severe cardiorespiratory depression in dogs. Furthermore, we previously reported that an IM anesthetic protocol with 1–2.5 mg/kg of alfaxalone following premedication with 5 μg/kg of medetomidine and 0.3 mg/kg of butorphanol provided effective anesthesia without causing severe cardiorespiratory depression in dogs (Kato et al., 2021). Therefore, the IM co-administration of 2.5–5 μg/kg of medetomidine, 0.25–0.3 mg/kg of butorphanol, and 1–2.5 mg/kg of alfaxalone may provide clinically useful anesthetic effects without causing severe cardiorespiratory depression in dogs. These combinations are used as pre-anesthetic medications in clinical settings, and general anesthesia under inhalation anesthetics may be induced after the administration of these drugs. However, the effects of IM co-administration of medetomidine, butorphanol, and alfaxalone on cardiopulmonary function under inhalation anesthesia have not been studied in dogs. This study aimed to assess cardiorespiratory function following the IM co-administration of 5 μg/kg of medetomidine, 0.3 mg/kg of butorphanol, and 2.5 mg/kg of alfaxalone (MBA) in dogs anesthetized with sevoflurane because it is assumed that MBA is used with inhalant anesthesia and spontaneous breath in real clinical situation. An MBA will be one of premedication or sedative protocol that can be intramuscular administration in the primary clinic, e.g., of the aggressive patient. We hypothesized that the IM administration of MBA would cause significant cardiorespiratory depression in dogs anesthetized with sevoflurane. Materials and MethodsExperimental animalsSeven intact Beagle dogs (three males and four females, aged 3–6 years and weighing 10.0–18.1 kg, body conditioning score 4–6; using scare 1–9) were confirmed to be healthy based on the results of their complete blood count, biochemistry profile, and physical examination were included in this study. Determination of the minimum alveolar concentration (MAC)The MAC of sevoflurane of each dog was predetermined 2 weeks before the experiment. The MAC concentration was determined using an electrical stimulus (50 V, 50 Hz, 10 ms) (Electronic stimulator SEN-3301; Nihon Kohden Corporation, Tokyo, Japan) applied for 10 seconds (Yamashita et al., 2009). The electrical stimulus was applied using two 25-gauge, 1-inch needles (TOP injection needle; TOP Corporation, Tokyo, Japan) placed 5 cm apart on the right forelimb (Valverde et al., 2003). MAC was determined as the average of the highest end-tidal sevoflurane concentration (FE′Sev) at which purposeful movement occurred and the lowest concentration at which purposeful movement did not occur. FE′Sev was measured using a veterinary patient monitoring system (BP-608V; Omron Colin Co., Tokyo, Japan) that was calibrated using a standardized calibration gas (AG Calibration Gas and Adaptor Set; Omron Colin Co.). The MAC for each dog was determined in triplicate by the same researcher (K.K.). Study designThe cardiorespiratory variables of dogs anesthetized with 1.3-fold their individual sevoflurane MAC (1.3 MAC) were measured before and after the IM administration of MBA. The baseline cardiorespiratory values of the dogs were measured after stabilization for 15 minutes with 1.3 MAC sevoflurane. Subsequently, the dogs received an IM administration of the MBA mixture, and the cardiorespiratory values were recorded at 5, 10, 15, 20, 30, 45, 60, 90, and 120 minutes after IM administration. The MBA mixture was prepared by mixing 0.2 ml of 1 mg/ml of medetomidine (Medetomin injection Meiji; Meiji Seika Pharma Co. Ltd., Tokyo, Japan), 2.4 ml of 5 mg/ml of butorphanol (Vetorphale 5 mg; Meiji Seika Pharma Co. Ltd.), and 10 ml of 10 mg/ml of alfaxalone (Alfaxan; Meiji Seika Pharma Co. Ltd.). State how the quantity of each drug in the mixture of (0.2 ml) medetomidine + (2.4 ml) butorphanol + (10 ml) alfaxalone was arrived at. The MBA mixture was injected at a dose of 0.315 ml/kg into the right lumbar longissimus muscle to achieve an IM co-administration of 5 μg/kg of medetomidine, 0.3 mg/kg of butorphanol, and 2.5 mg/kg of alfaxalone. Anesthesia and instrumentationThe dogs were fasted for 12 hours but had access to water until 30 minutes before the commencement of the experiment. Anesthesia was induced by administering 5% sevoflurane (Sevoflo; Zoetis Japan Co. Ltd., Tokyo, Japan) in oxygen at 5 l/minute via a facemask using a circle rebreathing system and an anesthetic machine (Beaver 20; Kimura Medical Instrument Co., Tokyo, Japan) with an out-of-circuit vaporizer (Sevorex-200; Shin-ei Industries, Inc., Saitama, Japan). The trachea was intubated orally, and the endotracheal tube was connected to the anesthetic circuit. The dogs were placed in left lateral recumbency and allowed to breathe spontaneously, and anesthesia was maintained with 1.3 MAC of sevoflurane in > 95% oxygen (2 l/minute) in the left. FE′Sev was monitored using the same veterinary patient monitoring system used for MAC determination. A 22-gauge, 3.2-cm catheter (Terumo Surflo F&F; Terumo Co., Tokyo, Japan) was inserted into the left cephalic vein, and lactated Ringer’s solution was administered IV at a rate of 5 ml/kg/hour. Another 22-gauge catheter was inserted into the dorsal pedal artery to measure the arterial blood pressure and collect arterial blood samples. The right side of the neck was clipped and aseptically prepared, and approximately 0.5 ml of 2% lidocaine (Xylocaine injection 2%; Aspen Japan K. K., Tokyo, Japan) was administered subcutaneously at the catheter site. A 6-Fr catheter introducer (Catheter Introducer; Medikit Co. Ltd., Tokyo, Japan) was inserted percutaneously into the right jugular vein, and a 5-Fr, balloon-tipped, 4-lumen, 75-cm thermodilution output catheter (Swan-Ganz thermodilution catheter 5-Fr 132F5; Edwards Lifesciences Corporation, Irvine, CA) was inserted into the pulmonary artery through the introducer. The arterial catheter and proximal and distal ports of the thermodilution catheter were connected to pressure transducers (Meritrans DTXplus; MERIT MEDICAL, Tokyo, Japan) that were zeroed at the level of the sternal manubrium. Cardiorespiratory valuablesThe cardiorespiratory variables were measured in accordance with our previous reports on dogs (Yamashita et al., 2007; Itami et al., 2011). The cardiac output (CO) was determined using the thermodilution method. The systolic arterial blood pressure (SAP), mean arterial blood pressure (MAP), diastolic arterial blood pressure (DAP), mean right atrial pressure (mRAP), mean pulmonary arterial pressure (mPAP), and pulmonary arterial occlusion pressure (PAOP) were determined by connecting the catheters to the pressure transducers. The temperature of the blood in the pulmonary artery, heart rate (HR), respiratory frequency (fR), mainstream end-tidal carbon dioxide partial pressure (PE′CO2), electrocardiogram by lead II, SAP, MAP, DAP, mPAP, mRAP, PAOP, and CO were recorded using a multi-parameter anesthetic monitoring system (DS-7210; Fukuda Denshi Co. Ltd., Tokyo, Japan). The body surface area, cardiac index (CI), stroke volume index (SVI), systemic vascular resistance index (SVRI), and pulmonary vascular resistance index were calculated as described in a previous report (Haskins et al., 2005). Arterial blood samples (2 ml) were anaerobically collected using a 22-gauge catheter placed in the dorsal pedal artery and mixed with 30 units of heparin sodium per 1 ml of blood (heparin sodium 10,000 units/10 ml for injection; Mochida Pharmaceutical Co. Ltd., Tokyo, Japan). The samples were analyzed immediately using a blood gas analyzer (GEM Premier 3000; Instrumentation Laboratory Japan, Tokyo, Japan) to determine the arterial pH (pHa), arterial partial pressure of carbon dioxide (PaCO2) and oxygen (PaO2), lactate, base excess, and bicarbonate ion levels. Recovery from anesthesiaThe dogs were administered meloxicam (0.2 mg/kg subcutaneously; Metacam 0.2% injection; Boehringer Ingelheim Animal Health Japan Ltd, Tokyo, Japan), buprenorphine hydrochloride (0.01 mg/kg IM; Lepetan injection 0.2 mg; Otsuka Pharmaceutical Co., Ltd, Tokyo, Japan), and cefazolin sodium hydrate (25 mg/kg IV; Cefamezin α, LTL Pharma Co., Tokyo, Japan) at the end of the experiment and all instrumentation was removed. The dogs were then allowed to recover from anesthesia and were observed throughout the recovery period for adverse effects related to the experiment for at least 24 hours. Statistical analysisThe sample size of this study was determined by performing a power analysis based on our published data (Kato et al., 2021). It was determined that a sample size of seven dogs would enable the detection of a 19% difference in MAP with a standard deviation of 13 mmHg before and after the IM anesthetic protocol with MBA, with an alpha level of 0.05 and a power of 0.95. Subsequently, a Shapiro-Wilk Test was performed to assess the normality of the data, confirming a departure from a normal distribution. Data are expressed as median (interquartile range) and compared with their corresponding baseline values using the Friedman test and Scheffe method (Bell Curve for Excel; Social Survey Research Information Co., Ltd., Tokyo, Japan). A p-value < 0.05 was considered a statistically significant change. Ethical approvalAll procedures in this study were approved by the Animal Care and Use Committee of the Rakuno Gakuen University (approval no. VH18B12). ResultsEach individual sevoflurane MAC ranged between 1.6% and 2.1%, and the median (interquartile range) was 2.0% (1.9–2.05). The dogs were anesthetized with an individual concentration of FE′Sev (2.1%–2.7%; 1.3 times of sevoflurane MAC) during the measurement of cardiorespiratory variables, and the total median duration of anesthesia was 246 (220–256) minutes. Table 1 summarizes the changes in the cardiorespiratory variables resulting from the IM administration of MBA in dogs anesthetized with sevoflurane. Intramuscular administration of MBA resulted in statistically significant changes in CI, SVRI, mRAP, mPAP, PAOP, fR, PEĆO2, PaCO2, and pHa within the first 20 minutes of anesthesia. Regarding cardiovascular variables, significant decreases compared with the corresponding baseline values were detected in CI at 5 and 10 minutes (p < 0.001 and 0.0069, respectively) and HR at 90 minutes ( p =0.0372) after the IM administration of MBA. In contrast, significant increases were detected in SVRI at 5 and 10 minutes ( p =0.0092 and 0.0357); mRAP at 10, 15, and 20 minutes (p < 0.001, 0.0158, and 0.0355, respectively); mPAP at 10 minutes ( p =0.0138); and PAOP at 5 and 10 minutes (p =0.0389 and 0.0497, respectively) after the IM administration of MBA. Regarding respiratory variables, significant decreases were detected in fR at 10, 15, and 20 minutes (p =0.0263, 0.0202, and 0.0176, respectively), while significant increases were detected in PEĆO2 at 15 minutes ( p=0.0231) and PaCO2 at 10, 15, and 20 minutes (p =0.0469, < 0.001, and 0.0110, respectively) after the IM administration of MBA. In terms of acid-base balance, significant decreases were detected in pHa at 10, 15, and 20 minutes (p =0.0125, < 0.001, and 0.0108, respectively). No adverse effects were noted. Table 1. Changes in the cardiorespiratory variables caused by the IM co-administration of 5 μg/kg of medetomidine, 0.3 mg/kg of butorphanol, and 2.5 mg/kg of alfaxalone in dogs anesthetized with sevoflurane.
DiscussionIn this study, the IM administration of 5 μg/kg of medetomidine, 0.3 mg/kg of butorphanol, and 2.5 mg/kg of alfaxalone caused decreased CO and hypoventilation, in healthy dogs anesthetized with sevoflurane. Inhalational anesthetics exert dose-dependent respiratory and circulatory inhibitory effects (Mutoh et al., 1997). The MAC varies between individuals; therefore, the established concentration across individuals is likely to show a degree of variation in MAC determination studies (Magnusson et al., 2000; Yamashita et al., 2009). Therefore, we took steps to determine the MAC of each participant to evaluate the effect of MBA administration on cardiovascular function in more detail. The sevoflurane MAC of individual dogs was 1.6%–2.1% in this study, and the baseline values for HR, MAP, mRAP, mPAP, PAOP, CI, SVI, and SVRI were similar to those previously reported for dogs anesthetized with 1.3 MAC of sevoflurane (Itami et al., 2011; Endo et al., 2017a; Endo et al., 2017b). CI decreased significantly 5 and 10 minutes after MBA administration and then gradually improved. In this study, HR did not decrease significantly except for 90 minutes; however, a decrease in SVI was noted. Medetomidine is a highly selective α2-agonist, and it causes bradycardia by the baroreceptor reflex to vasoconstriction (Muir et al., 1999; Kuo and Keegan, 2004; Puighibet et al., 2015). Butorphanol causes decreased HR via its action on the opioid receptors (Greene et al., 1990; dos Santos et al., 2011). In contrast, alfaxalone has been reported to increase HR through the baroreceptor reflex of hypotension (Muir et al., 2008; Amengual et al., 2013; Zapata et al., 2018; Hampton et al., 2019). In addition, dogs anesthetized with 1.0–2.0 MAC sevoflurane showed an increase in HR to accommodate vasodilation (Mutoh et al., 1997). The decrease in HR due to medetomidine administration may have been alleviated by the vasodilatory effects of sevoflurane. SVI showed the lowest value 5 minutes after MBA administration; however, the difference was not significant, and it gradually recovered to the baseline value. Stroke volume is the opposite of systemic vascular resistance, which temporarily increases predominantly after MBA administration and then recovers to baseline. Medetomidine acts on the α2-adrenergic receptors in the peripheral vascular smooth muscle to increase arterial pressure by vasoconstriction (de Morais and Muir, 1995). Considering the absence of changes in HR and stroke volume, the transient decrease in CO observed after MBA administration is believed to be primarily attributed to the increase in systemic vascular resistance induced by medetomidine. CO increased 15 minutes after MBA administration, which is shorter than the duration of the effect of these drugs, which means an administration of medetomidine alone and butorphanol alone (de Morais and Muir, 1995; Pypendop and Verstege, 1998; Kuo and Keegan, 2004). Hypoventilation occurred for 10–20 minutes duration after MBA administration. A rapid increase in PaCO2 likely stimulates catecholamine secretion and enhances cardiovascular function (Walley et al., 1990; Itami et al., 2019, 2022). Although the catecholamine concentration was not measured in this study, the improvement in CO early after MBA administration is potentially due to hypercapnia. Furthermore, hypercapnia has vasodilatory effects (Walley et al., 1990, Itami et al., 2019, 2022). Decreased systemic vascular resistance is conceivably associated with increased stroke volume, which, in turn, is associated with increased CO. The effects of hypercapnia on cardiovascular function are possibly associated with changes in CO after MBA administration. In this study, CO did not return to the baseline levels during the experimental period. Sustained hypoventilation causes vasodilatory effects. In addition, respiratory acidosis suppresses cardiovascular function. Medetomidine causes bradycardia by suppressing the secretion of noradrenaline in the sympathetic nervous system. Although there was no significant difference, HR remained lower than the baseline value during the experimental period, and it was suggested that the decrease in HR which may have contributed to a decrease in CO. Elevated blood lactate levels suggest that oxygen consumption in peripheral tissues exceeds oxygen supply (Pang and Boysen, 2007). Although a decline in CO was observed, there was no significant change in the blood lactate concentration in this study. Unfortunately, it was not possible to calculate the mixed venous oxygen saturation and oxygen consumption, as pulmonary arterial blood samples were not collected; however, since there was no significant change in the blood lactate levels, unchanged lactate levels may indicate there was minimal effect on peripheral tissue oxygenation. Hypoventilation is caused by a decrease in minute ventilation, as defined by fR and tidal volume. Spontaneous breathing was maintained in this study, but the fR decreased significantly; however, the tidal volume could not be measured. Alfaxalone causes respiratory depression in a dose-dependent manner (Muir et al., 2008; Tamura et al., 2014). Apnea was also reported as an adverse event after the administration of 4, 6, and 10 mg/kg of alfaxalone (Muir et al., 2008; Hampton et al., 2019). Furthermore, medetomidine decreases fR by stimulating the α2-adrenergic receptors (Sinclair, 2003). Butorphanol is an opioid and opioids cause respiratory depression by decreasing the sensitivity of PaCO2 (Shook et al., 1990). Cremer and Riccó (2018) reported that the administration of alfaxalone with dexmedetomidine and butorphanol in cats decreased fR to less than the baseline value in 5 minutes and that fR did not return to the baseline value during the study period. This is similarly observed in the present study. Moreover, Mutoh et al. (1997) reported that sevoflurane decreased fR and ventilatory volume, resulting in increased PaCO2 and pHa and respiratory acidosis. PaCO2 is a measure of effective alveolar minute ventilation and normally ranges between 35 and 45 mmHg. The acceptable PaCO2 values may be higher in anesthetized animals. The PaCO2 levels were consistently high after MBA administration in this study. In particular, PaCO2 was ≥60 mmHg between 10 and 45 minutes after MBA administration. Acidemia (pH ≤7.2) was observed in the present study. Since there was no change in the base excess and lactate levels when evaluating metabolic factors, it was considered that PaCO2 was the cause of the decrease in pHa. The MBA administration transiently increased the PAOP, PAP, and RAP levels in this study. Kuo and Keegan (2004) reported that PAOP significantly increased 5 minutes after the IV administration of 20 µg/kg medetomidine. As PAOP is an indicator of left atrial pressure and left ventricular end-diastolic pressure, MBA should be administered with caution in patients with heart disease, such as mitral valve regurgitation. However, medetomidine has been reported to have no effect on PAP (Pypendop and Verstegen, 1998). The PAP has been reported to be elevated in a canine acute respiratory acidosis model, with a corresponding PaCO2 range of 90–110 mmHg (Itami et al., 2019). In conscious dogs, it has been reported that PAP increases due to pulmonary vasoconstriction at PaCO2 levels of 5%–8% (i.e., 38–61 mmHg) (Linde et al., 1963); thus, increased PAP may be associated with hypoventilation. Hypoventilation should be avoided in dogs with tricuspid valve regurgitation, as increased PAP may be associated with increased RAP. According to the results of our study, MBA may induce hypoventilation; thus, MBA should be administered with caution to dogs with tricuspid valve regurgitation. Medetomidine has an emetic effect (Colby et al., 1981; Sinclair, 2003). Lee et al. (2016) reported that nausea and vomiting were observed after the IM co-administration of 10 μg/kg of medetomidine, 0.1 mg/kg of butorphanol, and 1.5 mg/kg of alfaxalone in dogs. In contrast, Kato et al. (2021) reported that nausea and vomiting were not observed in conscious dogs following IM co-administration of 5 μg/kg of medetomidine, 0.3 mg/kg of butorphanol, and 2.5 mg/kg of alfaxalone. Nausea and vomiting were also not observed in the present study, possibly due to the administration of the drug under general anesthesia and the low dose of medetomidine. In addition, the antiemetic effect of butorphanol may have attributed to this finding (Hayashi et al., 1994). The low dose of medetomidine and high dose of butorphanol used in our study compared with Lee et al. (2016) may also be the reasons why these complications were not observed. Some reports have reported the incidence of tremors and ataxia during recovery from anesthesia in dogs receiving alfaxalone (Maddern et al., 2010; Maney et al., 2013; Maney, 2017; Tamura et al., 2014). In contrast, no adverse reactions were observed with the co-administration of medetomidine-butorphanol-alfaxalone (Lee et al., 2016; Kato et al., 2021). Medetomidine has a muscle-relaxant effect (Sinclair, 2003), and butorphanol has a sedative effect (Pfeffer et al., 1980; Troncy et al., 1996). Since these drugs were used in combination with alfaxalone, tremors, and ataxia were not observed in the present study. Also, time has passed is a possible reason why side effect was not observed. Our study has some limitations. First, clinically unacceptable hypercapnia was observed, which may have complicated the assessment of the effects of the drugs used in this study on cardiovascular function. It has been reported that in healthy conscious dogs, fR decreased at the same dose as in this study, but no hypoventilation was observed with a partial pressure of end-tidal carbon dioxide (Kato et al., 2021). The baseline value under 1.3 MAC of sevoflurane was normal for PaCO2 in the present study, suggesting that hypoventilation may be caused by the additive respiratory depression effect of MBA administration under sevoflurane anesthesia. PaCO2 >55 mmHg may be associated with excessive respiratory acidosis and is considered to represent sufficient hypoventilation to warrant positive pressure ventilation in small animals (Gaynor et al., 1999; Itami et al., 2019). Furthermore, in patients with spontaneous ventilation and significant hypoventilation, gas analyzer readings may not accurately reflect gas concentrations in the alveoli. Therefore, the utilization of mechanical ventilation is recommended for a more accurate determination of MAC and gas concentrations. Second, the drugs used in this study were administered under sevoflurane anesthesia to evaluate the effect of MBA administration on cardiorespiratory function. The results clarified that the effect on the circulatory system returned back to the baseline value early after administration. However, these drugs are usually used as pre-anesthetic medications for sedation. Therefore, when the MBA combination is used as a premedication, the subsequent effects on cardiorespiratory function under sevoflurane anesthesia may differ from those observed in this study. Intramuscular administration of MBA to dogs anesthetized with sevoflurane transiently decreased CO because of an increase in SVR. It is necessary to administer MBA with caution in dogs with insufficient cardiovascular function due to diseases because they cannot tolerate circulatory depression, unlike healthy dogs. Although spontaneous breathing was maintained, attention should be paid to the occurrence of respiratory acidosis secondary to respiratory depression. Ventilation support should be provided as needed. AcknowledgmentsThe authors thank Editage (www.editage.com) for English language editing. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis research received no specific grant. Authors’ contributionsKK: Study design, data collection, data interpretation, statistical analysis, and preparation of manuscript. TI: Study design, data collection, data interpretation, statistical analysis, and revision of manuscript. NO: Data collection and data interpretation. TS: Data collection and data interpretation. KY: Study design, data management, data interpretation, statistical analysis, and revision of manuscript. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesAmengual, M., Flaherty, D., Auckburally, A., Bell, A.M., Scott, E.M. and Pawson, P. 2013. An evaluation of anaesthetic induction in healthy dogs using rapid intravenous injection of propofol or alfaxalone. Vet. Anaesth. Analg. 40, 115–123. Colby, E.D., McCarthy, L.E. and Borison, H.L. 1981. Emetic action of xylazine on the chemoreceptor trigger zone for vomiting in cats. J. Vet. Pharmacol. Ther. 4, 93–96. Cremer, J. and Riccó, C.H. 2018. Cardiovascular, respiratory and sedative effects of intramuscular alfaxalone, butorphanol and dexmedetomidine compared with ketamine, butorphanol and dexmedetomidine in healthy cats. J. Feline Med. Surg. 20, 973–979. de Morais, H.S. and Muir, W.W. 3rd. 1995. The effects of medetomidine on cardiac contractility in autonomically blocked dogs. Vet. Surg. 24, 356–364. Diehl, K.H., Hull, R., Morton, D., Pfister, R., Rabemampianina, Y., Smith, D., Vidal, J.M. and van de Vorstenbosch, C. 2001. European Federation of Pharmaceutical Industries Association and European Centre for the Validation of Alternative Methods. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J. Appl. Toxicol. 21, 15–23. dos Santos, P.S., Nunes, N., de Souza, A.P., de Rezende, M.L., Nishimori, C.T., de Paula, D.P. and Ferro Lopes, P.C. 2011. Hemodynamic effects of butorphanol in desflurane-anesthetized dogs. Vet. Anaesth. Analg. 38, 467–474. Endo, Y., Kawase, K., Miyasho, T., Sano, T., Yamashita, K. and Muir, W.W. 2017a Plethysmography variability index for prediction of fluid responsiveness during graded haemorrhage and transfusion in sevoflurane-anaesthetized mechanically ventilated dogs. Vet. Anaesth. Analg. 44, 1303–1312. Endo, Y., Tamura, J., Ishizuka, T., Itami, T., Hanazono, K., Miyoshi, K., Sano, T., Yamashita, K. and Muir, W.W. 2017b. Stroke volume variation (SVV) and pulse pressure variation (PPV) as indicators of fluid responsiveness in sevoflurane anesthetized mechanically ventilated euvolemic dogs. J. Vet. Med. Sci. 79, 1437–1445. Ferré, P.J., Pasloske, K., Whittem, T., Ranasinghe, M.G., Li, Q. and Lefebvre, H.P. 2006. Plasma pharmacokinetics of alfaxalone in dogs after an intravenous bolus of Alfaxan-CD RTU. Vet. Anaesth. Analg. 33, 229–236. Gaynor, J.S., Dunlop, C.I., Wagner, A.E., Wertz, E.M., Golden, A.E. and Demme, W.C. 1999. Complications and mortality associated with anesthesia in dogs and cats. J. Am. Anim. Hosp. Assoc. 35, 13–17. Greene, S.A., Hartsfield, S.M. and Tyner, C.L. 1990. Cardiovascular effects of butorphanol in halothane-anesthetized dogs. Am. J. Vet. Res. 51, 1276–1279. Hampton, C.E., Riebold, T.W., LeBlanc, N.L., Scollan, K.F., Mandsager, R.E. and Sisson, D.D. 2019. Effects of intravenous administration of tiletamine-zolazepam, alfaxalone, ketamine-diazepam, and propofol for induction of anesthesia on cardiorespiratory and metabolic variables in healthy dogs before and during anesthesia maintained with isoflurane. Am. J. Vet. Res. 80, 33–44. Haskins, S., Pascoe, P.J., Ilkiw, J.E., Fudge, J., Hopper, K. and Aldrich, J. 2005. Reference cardiopulmonary values in normal dogs. Comp. Med. 55, 156–161. Hayashi, K., Nishimura, R., Yamaki, A., Kim, H., Matsunaga, S., Sasaki, N. and Takeuchi, A. 1994. Comparison of sedative effects induced by medetomidine, medetomidine-midazolam and medetomidine-butorphanol in dogs. J. Vet. Med. Sci. 56, 951–956. Itami, T., Hanazono, K., Makita, K. and Yamashita, K. 2022. Cardiovascular effects of intravenous pimobendan in dogs with acute respiratory acidosis. J. Vet. Emerg. Crit. Care (San Antonio). 32, 341–349. Itami, T., Hanazono, K., Oyama, N., Sano, T., Makita, K. and Yamashita, K. 2019. Cardiovascular effects of intravenous colforsin in normal and acute respiratory acidosis canine models: a dose-response study. PLoS One 14(7), e0213414. Itami, T., Tamaru, N., Kawase, K., Ishizuka, T., Tamura, J., Miyoshi, K., Umar, M.A., Inoue, H. and Yamashita, K. 2011. Cardiovascular effects of tramadol in dogs anesthetized with sevoflurane. J. Vet. Med. Sci. 73, 1603–1609. Kato, K., Itami, T., Nomoto, K., Endo, Y., Tamura, J., Oyama, N., Sano, T. and Yamashita, K. 2021. The anesthetic effects of intramuscular alfaxalone in dogs premedicated with low-dose medetomidine and/or butorphanol. J. Vet. Med. Sci. 83, 53–61. Keates, H. and Whittem, T. 2012. Effect of intravenous dose escalation with alfaxalone and propofol on occurrence of apnoea in the dog. Res. Vet. Sci. 93, 904–906. Kuo, W.C. and Keegan, R.D. 2004. Comparative cardiovascular, analgesic, and sedative effects of medetomidine, medetomidine-hydromorphone, and medetomidine-butorphanol in dogs. Am. J. Vet. Res. 65, 931–937. Lee, J., Suh, S., Choi, R. and Hyun, C. 2016. Cardiorespiratory and anesthetic effects produced by the combination of butorphanol, medetomidine and alfaxalone administered intramuscularly in Beagle dogs. J. Vet. Med. Sci. 77, 1677–1680. Linde, L.M., Simmons, D.H. and Lewis, N. 1963. Pulmonary hemodynamics in respiratory acidosis in dogs. Am. J. Physiol. 205, 1008–1012. Maddern, K., Adams, V.J., Hill, N.A. and Leece, E.A. 2010. Alfaxalone induction dose following administration of medetomidine and butorphanol in the dog. Vet. Anaesth. Analg. 37, 7–13. Magnusson, K.R., Scanga, C., Wagner, A.E. and Dunlop, C. 2000. Changes in anesthetic sensitivity and glutamate receptors in the aging canine brain. J. Gerontol. A. Biol. Sci. Med. Sci. 550, B448–B454. Maney, J.K., Shepard, M.K., Braun, C., Cremer, J. and Hofmeister, E.H. 2013. A comparison of cardiopulmonary and anesthetic effects of an induction dose of alfaxalone or propofol in dogs. Vet. Anaesth. Analg. 40(3), 237–244. Maney, J.K. 2017. Sedative and physiologic effects of low-dose intramuscular alfaxalone in dogs. Vet. Anaesth. Analg. 44, 1184–1188. Muir, W.W. 3rd, Ford, J.L., Karpa, G.E., Harrison, E.E. and Gadawski, J.E. 1999. Effects of intramuscular administration of low doses of medetomidine and medetomidine-butorphanol in middle-aged and old dogs. J. Am. Vet. Med. Assoc. 215, 1116–1120. Muir, W., Lerche, P., Wiese, A., Nelson, L., Pasloske, K. and Whittem, T. 2008. Cardiorespiratory and anesthetic effects of clinical and supraclinical doses of alfaxalone in dogs. Vet. Anaesth. Analg. 35, 451–462. Mutoh, T., Nishimura, R., Kim, H.Y., Matsunaga, S. and Sasaki, N. 1997. Cardiopulmonary effects of sevoflurane, compared with halothane, enflurane, and isoflurane, in dogs. Am. J. Vet. Res. 58, 885–890. Pang, D.S. and Boysen, S. 2007. Lactate in veterinary critical care: pathophysiology and management. J. Am. Anim. Hosp. Assoc. 43, 270–279. Pfeffer, M., Smyth, R.D., Pittman, K.A. and Nardella, P.A. 1980. Pharmacokinetics of subcutaneous and intramuscular butorphanol in dogs. J. Pharm. Sci. 69, 801–803. Puighibet, Z., Costa-Farré, C., Santos, L., Canfrán, S. and Gómez de Segura, I.A. 2015. The sedative effects of intramuscular low-dose medetomidine in combination with butorphanol or methadone in dogs. Vet. Anaesth. Analg. 42, 590–596. Pypendop, B.H. and Verstegen, J.P. 1998. Hemodynamic effects of medetomidine in the dog: a dose titration study. Vet. Surg. 27, 612–622. Shook, J.E., Watkins, W.D. and Camporesi, E.M. 1990. Differential roles of opioid receptors in respiration, respiratory disease, and opiate-induced respiratory depression. Am. Rev. Respir. Dis. 142, 895–909. Sinclair, M.D. 2003. A review of the physiological effects of alpha2-agonists related to the clinical use of medetomidine in small animal practice. Can. Vet. J. 44, 885–897. Tamura, J., Hatakeyama, N., Ishizuka, T., Itami, T., Fukui, S., Miyoshi, K., Sano, T., Pasloske, K. and Yamashita, K. 2016. The pharmacological effects of intramuscular administration of alfaxalone combined with medetomidine and butorphanol in dogs. J. Vet. Med. Sci. 78, 929–936. Tamura, J., Ishizuka, T., Fukui, S., Oyama, N., Kawase, K., Miyoshi, K., Sano, T., Pasloske, K. and Yamashita, K. 2014. The pharmacological effects of the anesthetic alfaxalone after intramuscular administration to dogs. J. Vet. Med. Sci. 77, 289–296. Troncy, E., Besner, J.G., Charbonneau, R., Cuvelliez, S.G. and Blais, D. 1996. Pharmacokinetics of epidural butorphanol in isoflurane-anaesthetized dogs. J. Vet. Pharmacol. Ther. 19, 268–273. Valverde, A., Morey, T.E., Hernández, J. and Davies, W. 2003. Validation of several types of noxious stimuli for use in determining the minimum alveolar concentration for inhalation anesthetics in dogs and rabbits. Am. J. Vet. Res. 64, 957–62. Walley, K.R., Lewis, T.H. and Wood, L.D. 1990. Acute respiratory acidosis decreases left ventricular contractility but increases cardiac output in dogs. Circ. Res. 67, 628–635. Yamashita, K., Iwasaki, Y., Umar, M.A. and Itami, T. 2009. Effect of age on minimum alveolar concentration (MAC) of sevoflurane in dogs. J. Vet. Med. Sci. 71, 1509–1512. Yamashita, K., Ueyama, Y., Miyoshi, K., Igarashi, R., Kushiro, T., Umar, M.A. and Muir, W.W. 2007. Minimally invasive determination of cardiac output by transthoracic bioimpedance, partial carbon dioxide rebreathing, and transesophageal Doppler echocardiography in beagle dogs. J. Vet. Med. Sci. 69, 43–47. Zapata, A., Laredo, F.G., Escobar, M., Agut, A., Soler, M. and Belda, E. 2018. Effects of midazolam before or after alfaxalone for co-induction of anaesthesia in healthy dogs. Vet. Anaesth. Analg. 45, 609–661. | ||
| How to Cite this Article |
| Pubmed Style Kato K, Itami T, Oyama N, Yamashita K. Cardiorespiratory effects of intramuscular alfaxalone combined with low-dose medetomidine and butorphanol in dogs anesthetized with sevoflurane. Open Vet. J.. 2024; 14(5): 1251-1258. doi:10.5455/OVJ.2024.v14.i5.20 Web Style Kato K, Itami T, Oyama N, Yamashita K. Cardiorespiratory effects of intramuscular alfaxalone combined with low-dose medetomidine and butorphanol in dogs anesthetized with sevoflurane. https://www.openveterinaryjournal.com/?mno=161876 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i5.20 AMA (American Medical Association) Style Kato K, Itami T, Oyama N, Yamashita K. Cardiorespiratory effects of intramuscular alfaxalone combined with low-dose medetomidine and butorphanol in dogs anesthetized with sevoflurane. Open Vet. J.. 2024; 14(5): 1251-1258. doi:10.5455/OVJ.2024.v14.i5.20 Vancouver/ICMJE Style Kato K, Itami T, Oyama N, Yamashita K. Cardiorespiratory effects of intramuscular alfaxalone combined with low-dose medetomidine and butorphanol in dogs anesthetized with sevoflurane. Open Vet. J.. (2024), [cited January 25, 2026]; 14(5): 1251-1258. doi:10.5455/OVJ.2024.v14.i5.20 Harvard Style Kato, K., Itami, . T., Oyama, . N. & Yamashita, . K. (2024) Cardiorespiratory effects of intramuscular alfaxalone combined with low-dose medetomidine and butorphanol in dogs anesthetized with sevoflurane. Open Vet. J., 14 (5), 1251-1258. doi:10.5455/OVJ.2024.v14.i5.20 Turabian Style Kato, Keiko, Takaharu Itami, Norihiko Oyama, and Kazuto Yamashita. 2024. Cardiorespiratory effects of intramuscular alfaxalone combined with low-dose medetomidine and butorphanol in dogs anesthetized with sevoflurane. Open Veterinary Journal, 14 (5), 1251-1258. doi:10.5455/OVJ.2024.v14.i5.20 Chicago Style Kato, Keiko, Takaharu Itami, Norihiko Oyama, and Kazuto Yamashita. "Cardiorespiratory effects of intramuscular alfaxalone combined with low-dose medetomidine and butorphanol in dogs anesthetized with sevoflurane." Open Veterinary Journal 14 (2024), 1251-1258. doi:10.5455/OVJ.2024.v14.i5.20 MLA (The Modern Language Association) Style Kato, Keiko, Takaharu Itami, Norihiko Oyama, and Kazuto Yamashita. "Cardiorespiratory effects of intramuscular alfaxalone combined with low-dose medetomidine and butorphanol in dogs anesthetized with sevoflurane." Open Veterinary Journal 14.5 (2024), 1251-1258. Print. doi:10.5455/OVJ.2024.v14.i5.20 APA (American Psychological Association) Style Kato, K., Itami, . T., Oyama, . N. & Yamashita, . K. (2024) Cardiorespiratory effects of intramuscular alfaxalone combined with low-dose medetomidine and butorphanol in dogs anesthetized with sevoflurane. Open Veterinary Journal, 14 (5), 1251-1258. doi:10.5455/OVJ.2024.v14.i5.20 |