| Research Article | ||
Open Vet J. 2023; 13(11): 1409-1415 Open Veterinary Journal, (2023), Vol. 13(11): 1409–1415 Original Research Hematology and biochemistry parameters of the Central American bushmaster (Lachesis stenophrys) under human care in Costa RicaRandall Arguedas1,2*, Esteban Castro1 and Lizbeth Ovares11VetLab, Curridabat, P.O 11801, San José, Costa Rica 2Sede Atenas, Universidad Técnica Nacional, P.O 7-4013, Alajuela, Costa Rica *Corresponding Author: Randall Arguedas. VetLab, San José, Costa Rica Email: ranarg [at] gmail.com Submitted: 17/08/2023 Accepted: 04/10/2023 Published: 30/11/2023 © 2023 Open Veterinary Journal
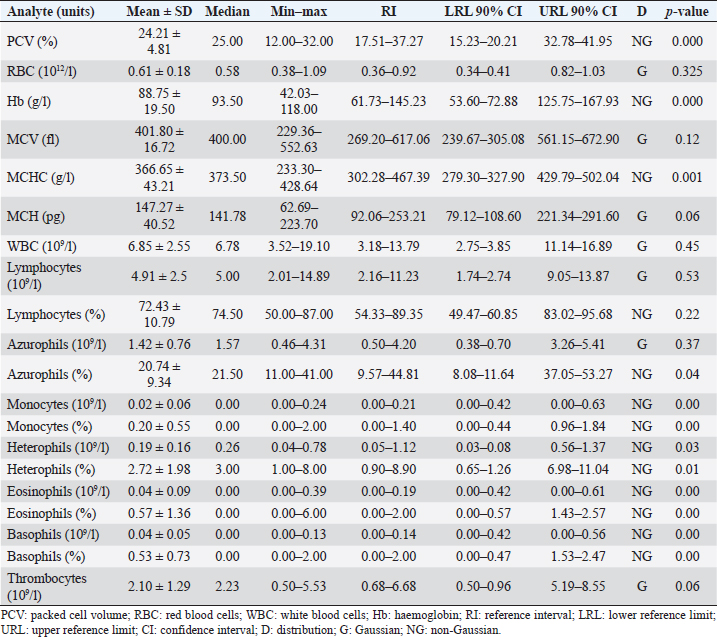
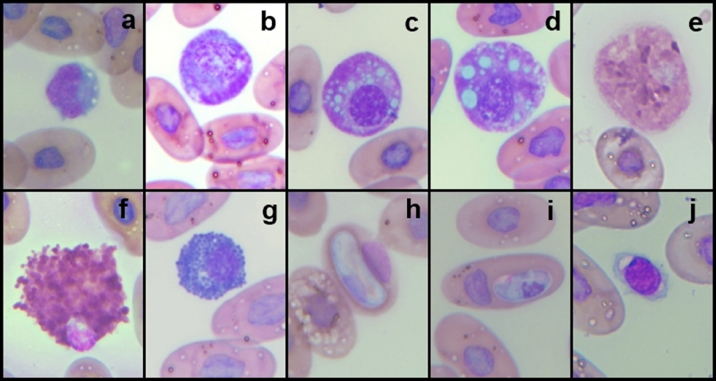
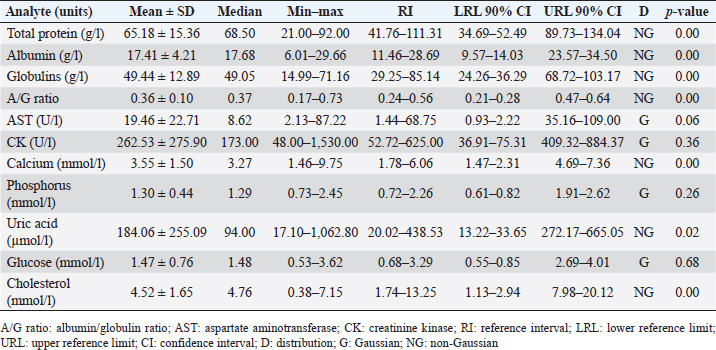
AbstractBackground: The Central American bushmaster (Lachesis stenophrys) is one of the largest pitvipers in the Americas, with relatively low abundance, suspected population declines, and continuing loss, fragmentation, and habitat degradation. Aim: Conservation actions, both in the wild and in captivity, bear the need for health parameters that allow managers and veterinarians to have a better understanding of health, especially when there are relatively few individuals in captivity to obtain robust information since there is no published information on the genus. To have hematological and biochemical reference ranges on the genus Lachesis. Methods: Blood samples were collected from 32 individuals (18 females and 14 males) under human care from 7 zoological institutions from August 2022 to January 2023 and performed hematological and biochemical analyses. Results: Reference intervals of hematological analytes included packed cell volume (17.51%–37.27%), total red blood cell count (0.36–0.92 × 1012/l), hemoglobin (61.73–145.23 g/l), white blood cell count (3.18–13.79 × 109/l), lymphocytes (2.16%–11.23%), azurophils (0.50%–4.20%), monocytes (0.00%–0.21%), heterophils (0.05%–1.12%), eosinophils (0.00%–0.19%), basophils (0.00%–2.00%), and total thrombocyte count (0.68–6.68 × 109/l), and biochemistry reference intervals included total protein (41.76–111.31 g/l), albumin (11.46–28.69 g/l), globulins (29.25–85.14 g/l), aspartate aminotransferase (1.44–68.75 U/l), creatinine kinase (52.72–625.00 U/l), uric acid (20.02–438.53 µmol/l), glucose (0.68–3.29 mml/l), cholesterol (41.74–13.25 mmol/l), calcium (1.78–6.06 mmol/l), and phosphorus (0.72–2.26 mmol/l). Conclusion: This is the first report on the genus Lachesis reporting hematological and biochemical reference ranges. Keywords: Biochemistry, Bushmaster, Costa Rica, Hematology, Lachesis stenophrys. IntroductionThe genus Lachesis has four species, which are the largest pitvipers in the Americas and the only pitvipers that lay eggs (Savage, 2002; Leenders, 2019). This is likely one of the factors that limit their distribution to hot and humid tropical forests since incubation requires a high ambient temperature and a high relative humidity (Leenders, 2019). The Central American bushmaster (Lachesis stenophrys) is one of the longest vipers, with males being slightly longer than females, averaging 2–2.10 and 1.9–2.05 m, respectively (Solórzano, 2004), and frequently over 4 kg in weight (Corrales et al., 2014). It is distributed along the Caribbean versant of Nicaragua to western and central Panama (Campbell and Lamar, 2004), and in Costa Rica, it is found in tropical and subtropical rainforests on the Caribbean versant (Leenders, 2019). The breeding season occurs from February to March. The species is cataloged as Near Threatened (A2c criterion), given the species’ relatively low abundance, suspected population declines, and continuing loss, fragmentation, and habitat degradation, since this species lives in close association with unaltered humid forests (Solórzano, 2004). The rate of decline is likely less than 30% over 10 years or three generations (Acosta-Chaves et al., 2021). On the other hand, this species has public health importance since it may be implicated in ophidic accidents (e.g., snake bites) throughout its distribution, even though other viper species, such as Bothrops asper, are the most frequently involved in accidents (Sasa and Cano, 2020). The size and venom composition make this species very dangerous (Lomonte et al., 2014). This is one of the reasons why it is kept and bred in captivity in institutions in Costa Rica, both for venom extraction and antivenom production. At the same time, it is maintained in some other captive institutions for breeding and conservation programs and exhibitions in Costa Rica and worldwide. Because this is an uncommon species, the captive population is limited to a small number of individuals in the country. Many of these individuals came from the wild since many people in rural areas see the snake as a menace to their family or community, but they prefer to call the local authorities or emergency services before killing a snake (Quesada-Acuña, 2019); therefore, because some individuals cannot be relocated near or have serious injuries, they have to be taken to captive institutions. Other individuals were rescued from illegal traffic since this is a very expensive specimen on the wild animal black market. For reptile collections, blood analyses are necessary for health assessment, entry or exit of collection, baseline determination, presurgical or therapeutic assessment, and diagnosing clinical suspicion of inflammation or organ dysfunction (Heatley and Russel, 2019). Hematology is commonly used to evaluate conditions such as anemia, inflammatory diseases, or parasitemia, while blood chemistry profiles are used to reflect the physiological function and activity of certain organs and physiological status (Nardini et al., 2013; Campbell, 2015). However, reference intervals for total erythrocyte count, hemoglobin, hematocrit, total leukocyte count, and leukocyte differential are difficult to establish for reptiles, mainly due to physiological adaptations that can occur in response to a large number of intrinsic and extrinsic factors. Likewise, studies designed to clarify the relationship between the function of certain organs and changes in chemical values or hematology in reptiles, compared to domestic mammals, are lacking (Campbell, 2015), and most hematology information available for reptiles does not conform to the current American Society for Veterinary Clinical Pathology (ASVCP) guidelines for reference range creation (Heatley and Russel, 2019). For neotropical viperids, there is a lack of information regarding hematology and biochemistry values, both for free-living species and those in captivity (Grego et al., 2006). Conservation actions of the Central American bushmaster both in the wild and in captivity bear the need for health parameters that allow managers and veterinarians to have a better understanding of health, especially when there are relatively few individuals in captivity to obtain robust information. Materials and MethodsStudy population and inclusion criteriaWe looked for all the zoological institutions that kept bushmasters in their facilities and then offered to do complete blood work as part of their annual veterinary checkup in our laboratory, since all zoological facilities in Costa Rican legislation are required to provide health assessments to all of their collection. We found eight authorized centers with L. stenophrys in their collection, but only seven wanted to be part of the study. The total population of the species in captivity according to the information given to us was 47 individuals. The institution that did not want to participate in the study held 14 adult individuals. We found 33 individuals distributed in the following ways in the captive centers who agreed to participate in the study: 16, 8, 4, 2, 1, 1, and 1. Inclusion criteriaWe included clinically healthy animals (no visible lesions, eating regularly, no ectoparasites, signs of dysecdysis), more than 2 years of being in captivity, and more than 1 m of total length. We did not include sex or origin (if it was wild-caught or born in captivity). Only one individual from a facility was excluded because it was less than a meter long and had 1 year of being in captivity. Thus, we ultimately selected 32 individuals (18 females and 14 males). Captive conditions were different between facilities in terms of enclosure size and enrichment, although all facilities maintained controlled temperature and humidity and fed on mice or rats. Most of the enclosures have adequate size but differ in vegetation, furniture, and substrate. Each individual was kept alone in all the institutions. Sampling methodsWe sampled from August 2022 to January 2023, but in Costa Rica, there was no important change in temperature throughout the year, and they were not in the breeding season. All the animals were sampled in the zoological institution, so we did not transport them to avoid stress. Before venipuncture, each snake was scrutinized for a routine physical examination to rule out any obvious lesion, external parasites, dysecdysis, internal palpable abnormalities, or abnormal secretions, as well as body condition. Each snake was manually restrained within an acrylic tube, while 2.5–3 ml of blood was obtained from the ventral coccygeal vein with a 23-gauge needle attached to a 3.0 ml syringe. Blood films were made immediately on clean glass microscope slides, and after that, the samples were placed on 3 ml VACUETTE® LH lithium heparin tubes, taken to the laboratory, and processed the same day. After blood collection, each individual was measured for total body length and snout-vent length (SVL), weighed, and sexed using stainless steel probes. Total red blood cell count (RBC), total white blood cell count (WBC), and total thrombocyte count (TBC) were performed using standard methods with a Natt and Herrick solution (1/200) Neubauer improved chamber. To calculate RBCs, we used the following formula: RBC=[(# erythrocytes counted in 5 squares) × 10]. To calculate WBC and TBC, we used the following formula: WBC/TBC=[(# cells in 9 large squares) × 1.1 × 0.2]. Hematocrit was determined using high-speed centrifugation (15,000 rpm) of blood-filled Vitrex® nonheparinized microhematocrit tubes for 5 minutes. Hemoglobin was measured with a HemoCue® 201+ System, which uses an azide methemoglobin reaction with dual wavelengths (570 and 880 nm). Differential WBCs were made by examining a peripheral smear stained with Wright stain at 100× with a Delphi-X ObserverTM, Euromex®, with ocular eyepieces 10 × 25 mm. Total proteins were determined utilizing a clinical refractometer using plasma from the microhematocrit tube. Biochemistry parameters were determined by spectrophotometry in a Beckman Coulter AU480® analyzer following the manufacturer’s specifications. All diagnostic laboratory analyses were performed by the same person to minimize result bias. Statistical analysisThe mean, standard deviation, median, minimum and maximum values, reference intervals, 90% confidence intervals around each reference limit, distribution, and p-values for all blood parameters were calculated. Differences in weight, biochemistry, and hematological values between the sexes were examined using the Mann–Whitney U test with a standard α level of 0.05. Tests of normality were performed using Shapiro‒Wilks with an α level of 0.05. Logarithmic transformation was used, and reference intervals were calculated with the Robust method with 1,000 iterations, according to the ASVCP guidelines (Gunn‐Christie et al., 2012) using Med-Calc®v22.009 software. The other statistical analyses were performed using IBM SPSS®v24 software. Ethical approvalIn Costa Rica, it is only legal to have native species in captive institutions authorized by the government, specifically by the Sistema Nacional de Áreas de Conservación (SINAC) (which means that people cannot have them as pets or in private collections). Here, we provide the permit number given by SINAC for each institution: SINAC-ACC-OH-RES-575-2021, SINAC-ACC-OA-VS-039-2020, ACAT-D-VS-006-2019, ACLA-P-D-219-2019, ACLAC-DRFVS-002-2021, SINAC-ACC-OSJ-re428-2021, and SINAC-ACC-OH-R-1515-2020. ResultsWe sampled a total of 32 individuals ranging from 2 to 8 years of being in captivity. All snakes appeared to be active and healthy. Weight ranged from 470 to 5,130 g (median 2,560 g, range 4,660 g). There were 18 females, with weights ranging from 470 to 5,130 g (median 3,110, range 4,660), and 14 males, with weights ranging from 1,210 to 4,330 g (median 1,370, range 3,120). The SVL ranged from 980 to 1,910 cm (median 1.56 cm, range 0.93 cm), and the total length ranged from 1,070 to 2,003 cm (median 1.73 cm, range 0.96 cm). No evidence of lesions was detected during physical examinations. No ectoparasites or dysecdysis were observed, and none of the females had palpable eggs. The general body condition of all individuals was between 3 and 4/5 (3/5 (n=30) and 4/5 (n=2). No significant differences between sexes in any of the hematological or biochemical parameters were found. Hematological values are presented in Table 1. The morphologies of lymphocytes (Fig. 1a), azurophils (Fig. 1b–d), heterophils (Fig. 1e), eosinophils (Fig. 1f), and basophils (Fig. 1g) are shown. Azurophils were the leukocytes with more morphologic variation ranging from a blue to reddish cytoplasm with no vacuoles to different degrees of vacuolization (Fig. 1b–d). Both the shape and appearance of erythrocytes and thrombocytes (Fig. 1j) were similar to those reported for other reptiles. Erythrocytes were ellipsoid with a central positioned oval nucleus that contained dense purple chromatin with irregular margins. The cytoplasm is stained orange or pale pink. Thrombocytes are rounded with the nucleus located in a central position, containing dense chromatin that is stained purple and with colorless cytoplasm. Intraerythrocytic parasites were found in 3 of the 32 individuals (9.37% of the total sample) (Fig. 1h and i). Biochemistry values are included in Table 2. DiscussionOur results provide a first view of the hematological and biochemical parameters of L. stenophrys based on the values of almost all captive individuals in the country. Although samples were taken from several captive populations, all the animals that were used in this research were clinically healthy, even though there were two individuals who we considered to be in a high corporal condition. One important aspect to be said is that all of them were at similar temperature and humidity ranges, and all of them ate adult rats (from a frozen stock kept by each institution). This is a very important aspect to consider in ectotherms, as extrinsic factors such as nutrition, temperature, and relative humidity may cause variations in biochemical and hematological values (Campell, 2015). Most of the information on hematology and blood biochemistry in neotropical vipers comes from studies carried out in Brazil, Argentina, Peru, and Costa Rica (Troiano et al., 1997, 1999, 2000, 2001; Grego et al., 2006; Rameh-de-Albuquerque et al., 2007; Silva et al., 2010; Ghilardi and Zacariotti, 2013; Glaser et al., 2013; Gómez et al., 2016; Trujillo et al., 2016; Pereira et al., 2022). These investigations were made for the genera Crotalus, Bothrops, Bothropoides, and Caudisona, obtaining ranges of values. Studies with free-living animals have been carried out by Grego et al. (2006) and Gómez et al. (2016) in Bothrops leucurus and Crotalus simus, respectively. Grego et al. (2006) worked only with hematology and reported several hemoparasites. There are no data for the genus Lachesis. In general, most of the hematological parameters do not differ significantly from those of other tropical vipers, and the leukocyte distribution is similar to that of Crotalus durissus, B. leucurus, Bothrops jararaca, and Bothoriopoides jararacussu, where lymphocytes are the most prevalent cell population, followed by azurophils. However, for example, Bothrops ammodytes have lymphocytes as the first cell but eosinophils and then heterophils as the second and third cells, leaving the azurophil as the fourth cell population, which is different from what Pereira et al. (2022) found for Bothrops atrox, where heterophils were the first line, followed by lymphocytes. Although Lachesis is a genus confined to tropical areas, it is more closely related phylogenetically to the genera Ophryacus (a genus endemic to Mexico) and Agkistrodon (Castoe and Parkinson, 2006; Alencar et al., 2016; Pyron, 2017); therefore, it is probably more accurate to compare its parameters with those of copperheads. An article published by Cerreta et al. (2020), with Agkistrodon contortix, has a very similar leukocyte population percentage to our study, where lymphocytes are the first line, and azurophils and heterophils are in third place. Table 1. Hematological values (n=32) of the Central America bushmaster (L. stenophrys) under human care in Costa Rica.
Biochemistry parameters are also similar when compared with some of the investigations mentioned above (Troiano et al., 1999; Grego et al., 2006; Rameh-de-Albuquerque et al., 2007; Silva et al., 2010; Ghilardi and Zacariotti, 2013; Glaser et al., 2013; Gómez et al, 2016; Trujillo et al., 2016; Pereira et al., 2022); however, comparing different biochemistry results done with different equipment may be handled with care, since there can be an important degree of heterogeneity, especially from in-house analyzers (Campbell, 2015). Thus, every clinician must take into consideration the specific methodology because these differences would influence the interpretation of test results and may affect patient management (Campbell, 2015).
Fig. 1. Photographs taken from blood smears of selected Central America bushmaster (L. stenophrys) under human care in Costa Rica. Wright stains at 100×. (a) Lymphocytes; (b–d) azurophils; (e) heterophil; (f) eosinophil; (g) basophil; (h and i) hemoparasites; and (j) thrombocyte. Table 2. Biochemical values (n=32) of the Central America bushmaster (L. stenophrys) under human care in Costa Rica.
We did not find differences between sexes in any of the values. Some studies in snakes and lizards have found differences between sexes in some parameters; for example, in Natrix natrix, there were differences in the red blood count (hematocrit, RBC, and Hb), and in Sistrurus catenatus, there were lower blood glucose, packed cell volumes, absolute azurophil counts, and higher plasma calcium and phosphorus concentrations than in males (Allender et al., 2006). Even so, our data should be taken cautiously, since we have a small sample and had to use nonparametric analyses. Hemoparasites were also found in three individuals. The presence of hemoparasites in wild reptiles is common (Telford, 2009), and hemoparasites are usually considered nonpathogenic (Stacy et al., 2011) and are usually transmitted by an intermediate host, such as ticks or mites (Telford, 2009; Campbell, 2015). We did not find any individuals with ectoparasites, and since asexual reproduction can occur in the reptilian host, they were already present in animals that came from the wild, which we could verify on their records. We are aware of limitations in our study that include a small sample of individuals, undetected underlying causes of disease, and, as with other reptiles, any environmental factor that could possibly affect an individual at the sampling time. This is the first study on the clinical pathology values in L. stenophrys, an uncommon threatened viper species. We consider that the generated data can be very valuable and can be used as baseline data for clinicians, researchers, and wildlife managers for both captive and wild individuals or population health assessments of bushmasters. However, more studies must be performed on these viper species to build robust information. AcknowledgmentsThe authors want to thank all the collaborators from the different institutions, Luis Morales, Sergio Arguedas, Esteban Arrieta, Loyal Clarke, Florian Wollinger, Aaron Gómez, Greivin Corrales, Natalia Montero, Josimar Estrella, Pompilio Campos C, Pompilio Campos B, and Roberto Peña. Conflict of interestThe authors declare that there is no conflict of interest. Author contributionRandall Arguedas: Main idea, field work, and sampling, laboratory analyses, statistical analyses, and manuscript writing. Esteban Castro: Field work and sampling, laboratory analyses, and manuscript writing. Lizbeth Ovares: Field work and sampling, laboratory analyses, and manuscript writing. FundingOwn funds and VetLab Laboratory. Data availabilityData are available from the authors upon reasonable request. ReferencesAcosta-Chaves, V., Batista, A., García Rodríguez, A., Vargas Álvarez, J. and Dwyer Q. 2021. Lachesis stenophrys. The IUCN red list of threatened species 2021: e.T203669A2769592. Available from: https://dx.doi.org/10.2305/IUCN.UK.20213.RLTS.T203669A2769592.en Alencar, L.R., Quental, T.B., Grazziotin, F.G., Alfaro, M.L., Martins, M., Venzon, M. and Zaher H. 2016. Diversification in vipers: phylogenetic relationships, time of divergence and shifts in speciation rates. Mol. Phylogenet. Evol. 105, 50–62. Allender, M.C., Mitchell, M.A., Phillips, C.A., Gruszynski, K. and Beasley V.R. 2006. Hematology, plasma biochemistry, and antibodies to select viruses in wild-caught eastern massasauga rattlesnakes (Sistrurus catenatus catenatus) from Illinois. J. Wildl. Dis. 42, 107–114. Campbell, J.A. and Lamar, W.W. 2004. The venomous reptiles of the western hemisphere. Ithaca, NY; London, UK: Cornell University Press. Campbell, T.W. 2015. Exotic animal hematology and cytology. 4th ed. Oxford, IA: Iowa State University Press, John Wiley & Sons. Castoe, T.A. and Parkinson, C.L. 2006. Bayesian mixed models and the phylogeny of pitvipers (Viperidae: Serpentes). Mol. Phylogenet. Evol. 39(1), 91–110. Cerreta, A.J., Cannizzo, S.A., Smith, D.C. and Minter, L.J. 2020. Venous hematology, biochemistry, and blood gas analysis of free-ranging Eastern Copperheads (Agkistrodon contortrix) and Eastern Ratsnakes (Pantherophis alleghaniensis). PLoS One 15(2), e0229102. Corrales, G., Meidinger, R., Rodríguez, S., Chacón, D. and Gómez, A. 2014. Reproduction in captivity of the Central American bushmaster (Lachesis stenophrys, Serpentes: Viperidae), in Costa Rica. Cuad. Herpetol. 28, 137–139. Ghilardi, R. and Zacariotti, R.L. 2013. Estudo Da Hematologia E Bioquímica Sérica De Serpentes Do Gênero Bothrops insularis (Jararaca-Ilhoa). Caderno Enic 1, 1. Glaser, V., Boni, A.P., da Silva Pitz, H., de Albuquerque, C.A.C. and Zeni A.L.B. 2013. Parâmetros hematológicos e bioquímicos de Bothropoides jararaca e Bothrops jararacussu (Ophidia-Viperidae) mantidas em cativeiro. Arch. Vet. Sci, 18, 3. Gómez, A., Arroyo, C., Astorga, W., Chacón, D., Rodríguez, S. and Jiménez, M. 2016. Hematological and biochemical reference intervals for Bothrops asper and Crotalus simus (Serpentes: Viperidae), maintained in captivity for venom extraction. Comp. Clin. Path. 25, 615–623. Grego, K.F., Alves, J.A.S., Albuquerque, L.C. and Fernandes W. 2006. Referências hematológicas para a jararaca de rabo branco (Bothrops leucurus) recém capturadas da natureza. Arq. Bras. Med. Vet. Zootec. 58, 1240–1243. Gunn-Christie, R.G., Flatland, B., Friedrichs, K.R., Szladovits, B., Harr, K.E., Ruotsalo, K., Knoll, J.S., Wamsley, H.L. and Freeman K.P. 2012. ASVCP quality assurance guidelines: control of preanalytical, analytical, and postanalytical factors for urinalysis, cytology, and clinical chemistry in veterinary laboratories. Vet. Clin. Path. 41, 18–26. Heatley, J.J. and Russel, K.E. 2019. Hematology. In Eds., Divers, S.J. and Stahl, S.J. St Louis, MO: Elsevier Health Sciences. Leenders, T. 2019. Reptiles of Costa Rica: a field guide. New York, NY: Cornell University Press. Lomonte, B., Fernández, J., Sanz, L., Angulo, Y., Sasa, M., Gutiérrez, J.M. and Calvete J.J. 2014. Venomous snakes of Costa Rica: biological and medical implications of their venom proteomic profiles analyzed through the strategy of snake venomics. J. Proteomics 105, 323–339. Nardini, G., Leopardi, S. and Bielli., M. 2013. Clinical hematology in reptilian species. Vet. Clin. N. Am. Exot. Anim. Pract. 16, 1–30. Pereira, H.C., Telles, L.P.J.D., Hirano, L.Q.L, Rios, M.P. and Santos, A.L.Q. 2022. Hematologic parameters of captive Bothrops atrox (Squamata: Viperidae). J. Biosci. 38(E38089), 1981–3163. Pyron, R.A. 2017. Novel approaches for phylogenetic inference from morphological data and total-evidence dating in squamate reptiles (lizards, snakes, and amphisbaenians). Syst. Biol. 66, 38–56. Quesada-Acuña, S.G. 2019. Percepción y conocimiento sobre serpientes en funcionarios de una universidad pública costarricense. UNED Res. J. 11, 369–377. Rameh-de-Albuquerque, L.C., Almeida, C.D., Sano-Martins, I.S. and Catão-Dias J.L. 2007. Características morfológicas e citoquímicas de espécies selecionadas dos gêneros Bothrops e Crotalus (Ophidia: viperidae), mantidos em cativeiro no Instituto Butantan. Resumos. São Paulo, Brazil: Faculdade de Medicina Veterinária e Zootecnia, Universidade de São Paulo. Sasa, M. and Cano S.E.S. 2020. New insights into snakebite epidemiology in Costa Rica: a retrospective evaluation of medical records. Toxicon X. 7, 100055. Savage, J.M. 2002. The amphibians and reptiles of Costa Rica. A herpetofauna between two continents, and two seas. Chicago, IL: The University of Chicago Press. Silva, W.B., Soares, R.M., Machado, C., Freire, I.M., Silva, L.C., Moreira, S.B., Goldberg, D.W. and Almosny N.R. 2010. Bioquímica plasmática de cascavéis (Caudisona durissa LINNAEUS, 1758) em cativeiro. Ciência Rural. 40, 2510–2514. Solórzano, A. 2004. Serpientes de Costa Rica: distribución, taxonomía e historia natural. San José, Costa Rica: Instituto Nacional de Biodiversidad, Editorial INBio. Stacy, N.I., Alleman, A.R. and Sayler K.A. 2011. Diagnostic hematology of reptiles. Clin. Lab. Med. 31, 87–108. Telford Jr, S.R. 2009. Hemoparasites of the reptilia: color atlas and text. New York, NY: CRC Press. Troiano, J.C., Gould, E.G., Althaus, R., Malinskas, G., Gould, J.A., Heker, J., Vidal, J.C., Amantini, E. and Simoncini C. 2001. Blood biochemical profile of the South American rattlesnake (Crotalus durissus terrificus) in captivity. J. Venom. Anim. Toxins. 7, 183–189. Troiano, J.C., Vidal, J.C., Gould, E.F., Heker, J., Gould, J., Vogt, A.U., Simoncini, C., Amantini, E. and De Roodt A. 2000. Hematological values of some Bothrops species (Ophidia-Crotalidae) in captivity. J. Venom. Anim. Toxins 6, 194–204. Troiano, J.C., Vidal, J.C., Gould, E.F., Malinskas, G., Gould, J., Scaglione, M., Scaglione, L., Heker, J.J., Simoncini, C. and Dinápoli, H. 1999. Haematological and blood chemical values from Bothrops ammodytoides (Ophidia-Crotalidae) in captivity. Comp. Haematol. Int. 9, 31–35. Troiano, J.C., Vidal, J.C., Gould, J. and Gould E. 1997. Haematological reference intervals of the south american rattlesnake ( Crotalus durissus terrificus, Laurenti, 1768) in captivity. Comp. Haematol. Int. 7, 109–112. Trujillo, E., Elias, R., Silva, W. and Montes D. 2016. Valores hematológicos de Bothrops atrox mantenidos en cautiverio en la ciudad de Lima. Salud Tecnol. Vet. 4(2), 44. | ||
| How to Cite this Article |
| Pubmed Style Arguedas R, Castro E, Ovares L. Hematology and biochemistry parameters of the Central American bushmaster (Lachesis stenophrys) under human care in Costa Rica. Open Vet J. 2023; 13(11): 1409-1415. doi:10.5455/OVJ.2023.v13.i11.3 Web Style Arguedas R, Castro E, Ovares L. Hematology and biochemistry parameters of the Central American bushmaster (Lachesis stenophrys) under human care in Costa Rica. https://www.openveterinaryjournal.com/?mno=165688 [Access: August 17, 2025]. doi:10.5455/OVJ.2023.v13.i11.3 AMA (American Medical Association) Style Arguedas R, Castro E, Ovares L. Hematology and biochemistry parameters of the Central American bushmaster (Lachesis stenophrys) under human care in Costa Rica. Open Vet J. 2023; 13(11): 1409-1415. doi:10.5455/OVJ.2023.v13.i11.3 Vancouver/ICMJE Style Arguedas R, Castro E, Ovares L. Hematology and biochemistry parameters of the Central American bushmaster (Lachesis stenophrys) under human care in Costa Rica. Open Vet J. (2023), [cited August 17, 2025]; 13(11): 1409-1415. doi:10.5455/OVJ.2023.v13.i11.3 Harvard Style Arguedas, R., Castro, . E. & Ovares, . L. (2023) Hematology and biochemistry parameters of the Central American bushmaster (Lachesis stenophrys) under human care in Costa Rica. Open Vet J, 13 (11), 1409-1415. doi:10.5455/OVJ.2023.v13.i11.3 Turabian Style Arguedas, Randall, Esteban Castro, and Lizbeth Ovares. 2023. Hematology and biochemistry parameters of the Central American bushmaster (Lachesis stenophrys) under human care in Costa Rica. Open Veterinary Journal, 13 (11), 1409-1415. doi:10.5455/OVJ.2023.v13.i11.3 Chicago Style Arguedas, Randall, Esteban Castro, and Lizbeth Ovares. "Hematology and biochemistry parameters of the Central American bushmaster (Lachesis stenophrys) under human care in Costa Rica." Open Veterinary Journal 13 (2023), 1409-1415. doi:10.5455/OVJ.2023.v13.i11.3 MLA (The Modern Language Association) Style Arguedas, Randall, Esteban Castro, and Lizbeth Ovares. "Hematology and biochemistry parameters of the Central American bushmaster (Lachesis stenophrys) under human care in Costa Rica." Open Veterinary Journal 13.11 (2023), 1409-1415. Print. doi:10.5455/OVJ.2023.v13.i11.3 APA (American Psychological Association) Style Arguedas, R., Castro, . E. & Ovares, . L. (2023) Hematology and biochemistry parameters of the Central American bushmaster (Lachesis stenophrys) under human care in Costa Rica. Open Veterinary Journal, 13 (11), 1409-1415. doi:10.5455/OVJ.2023.v13.i11.3 |