| Research Article | ||
Open Vet. J.. 2024; 14(2): 730-737 Open Veterinary Journal, (2024), Vol. 14(2): 730-737 Original Research Clinacanthus nutans leaf extract reduces pancreatic β-cell apoptosis by inhibiting JNK activation and modulating oxidative stress and inflammation in streptozotocin-induced diabetic ratsNurlaili Susanti1,2, Arifa Mustika3* and Junaidi Khotib41Doctoral Program of Medical Science, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia 2Faculty of Medicine and Health Science, Maulana Malik Ibrahim State Islamic University, Malang, Indonesia 3Department of Anatomy, Histology, and Pharmacology, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia 4Department of Pharmacy Practice, Faculty of Pharmacy, Universitas Airlangga, Surabaya, Indonesia *Corresponding Author: Arifa Mustika. Department of Anatomy, Histology, and Pharmacology, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: arifa-m [at] fk.unair.ac.id Submitted: 29/10/2023 Accepted: 29/01/2024 Published: 29/02/2024 © 2024 Open Veterinary Journal
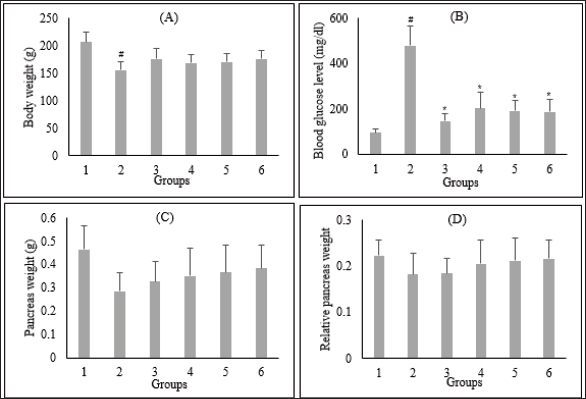
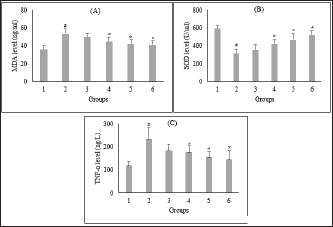
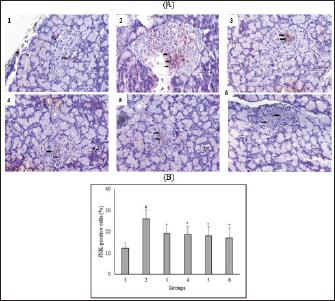
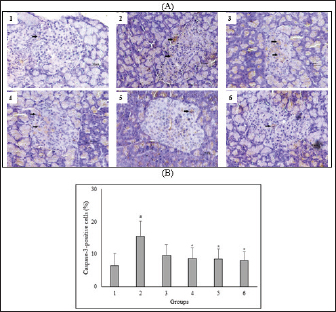
AbstractBackground: Controlling apoptosis induced by oxidative stress in pancreatic β-cells provides promising strategies for preventing and treating diabetes. Clinacanthus nutans leaves possess bioactive constituents with potential antioxidant and anti-diabetic properties. Aim: This study aimed to investigate the molecular mechanisms by which C. nutans extract protects pancreatic β-cells from apoptotic damage in streptozotocin (STZ)-induced diabetic rats. Methods: Diabetes was induced in male Wistar rats by intraperitoneal injection of 45 mg/kg STZ, followed by 28 days of treatment with C. nutans leaf extract and Glibenclamide as the standard drug. At the end of the study, blood samples were collected to measure glucose levels, oxidative stress markers, and inflammation. Pancreatic tissue was stained immunohistochemically to detect c-Jun N-terminal kinase (JNK) and Caspase-3 expression. Results: The administration of C. nutans leaf extract to diabetic rats significantly reduced fasting blood glucose, malondialdehyde, and tumor necrosis factor-α levels, while concurrently enhancing the activity of superoxide dismutase. The immunohistochemical studies revealed a decrease in the expression of JNK and caspase-3 in the pancreatic islets of diabetic rats. Conclusion: Clinacanthus nutans exhibits the potential to protect pancreatic β-cells from apoptosis by suppressing oxidative stress and inflammation. Keywords: Apoptosis, Clinacanthus nutans, Diabetes, Inflammation, Oxidative stress. IntroductionThe prevalence of diabetes mellitus (DM) has reached 10.5% of the worldwide population by 2021, which makes it a significant global health problem (Sun et al., 2022). DM is distinguished by elevated levels of glucose in the bloodstream, which arise from chronic progressive metabolic abnormalities related to insufficient production and/or utilization of insulin by the body (Banday et al., 2020; ADA, 2021) The persistence of chronically elevated blood glucose levels has detrimental effects on several tissues, including the pancreatic β-cells. This condition is responsible for the advancement of DM and the development of multiple complications affecting both macrovascular and microvascular systems (Harding et al., 2019; Goyal and Jialal, 2020). Alternative pathways for glucose metabolism are activated when oxidative phosphorylation becomes saturated due to hyperglycemia. As a consequence, the production of reactive oxygen species (ROS) is upregulated (Costes et al., 2021). Conversely, it has been observed that hyperglycemia may suppress the synthesis of endogenous antioxidants, such as superoxide dismutase (SOD) (Gerber and Rutter, 2017). ROS production that exceeds the body’s antioxidant defense capacity can result in a state of oxidative stress (Wang and Wang, 2017). ROS can oxidize lipid components inside cellular structures, generating malondialdehyde (MDA). Therefore, quantifying MDA levels becomes a marker for assessing cellular damage caused by oxidative stress (Ohiagu et al., 2021). On the other hand, the production of proinflammatory cytokines, mainly tumor necrosis factor-alpha (TNF-α), is enhanced in the presence of high quantities of ROS, exacerbating oxidative stress. Both conditions, oxidative stress, and inflammation, have been identified as the primary mechanisms underlying hyperglycemia-induced dysfunction and death of pancreatic β-cell (Ortega-Camarillo, 2019). One of the molecular pathways contributing to pancreatic β-cell damage by oxidative stress and inflammation is the involvement of c-Jun N-terminal kinase (JNK) (Kaneto et al., 2005). It has been reported that multiple stress signals, such as cytokines and glucolipotoxicity, can activate the JNK pathway. Previous research has demonstrated that the levels of JNK protein expression are elevated in cultures of pancreatic β-cell and pancreas of diabetic rats exposed to STZ (Tang et al., 2018; Liu et al., 2019). The JNK pathway modulates apoptotic signaling in pancreatic β-cell by modulating the caspase cascade (Dhanasekaran and Reddy, 2017). Caspase-3 is recognized as one of the key apoptotic executors, and its elevated activity indicates the presence of cell apoptosis (Palai and Mishra, 2015). Regarding the potential detrimental effect of higher ROS generation on pancreatic β-cell, modulating the capacity for antioxidants could provide advantageous outcomes. Therefore, controlling cellular stress-induced apoptosis in pancreatic β-cell suggests a potential therapeutic strategy for diabetes (Salazar-García and Corona, 2021). Several studies have demonstrated that natural compounds with antioxidant capability can reduce oxidative stress and proinflammatory cytokines, protecting β-cell from damage caused by ROS (Tabatabaie and Yazdanparast, 2017). Hanchang et al., 2019). Clinacanthus nutans is a widely utilized herbal plant that has been investigated for its properties in treating diabetes (Chia et al., 2022) C. nutans has demonstrated anti-diabetic properties by inhibiting α-glycosidase in vitro (Alam et al., 2017). This plant also showed the ability to decrease blood glucose levels and oxidative stress markers while enhancing markers of antioxidant status in diabetic rats (Sarega et al., 2016a; Umar-Imam et al., 2019). However, the exact mechanisms of how C. nutans exhibits its beneficial effect on pancreatic β-cells in diabetes remain unknown. This study examines the molecular mechanisms by which C. nutans extract preserves pancreatic β-cells from apoptotic damage in diabetic rats. Specifically, the study intends to determine how the extract modulates the JNK pathway through the antioxidant and anti-inflammatory defense systems. Material and MethodsChemicalsStreptozotocin (STZ) was acquired from Santa Cruz Biotechnology USA. The ELISA kit used to detect TNF-α was acquired from Bioassay Technology Laboratory China (No. E0764Ra). The Total SOD Activity (No. E-BC-K019-S) and MDA Colorimetric (No. E-BC-K025-S) Assay Kit were obtained from Elabscience Biotechnology China. The polyclonal antibodies against JNK (No. bs-2900R) and Caspase3 (No. bs-0081R) were acquired from Bioss USA. Plant extractionExtraction of C. nutans leaves was according to the method described in our previous study (Susanti et al., 2023). The leaves of C. nutans were acquired from the UPT Herbal Laboratory of Materia Medica Batu, Indonesia, with determination number 074/042/102.7-A/2022. The leaves undergo the process of drying in an oven set at a temperature of 50oC, followed by subsequent grinding and sieving procedures. One hundred grams of C. nutans powder was dissolved in 1,000 ml of 70% ethanol as solvent (ratio 1:10). The extraction process was carried out using the sonication method with the SONICA 2400EP S3 instrument. Solvents were evaporated under reduced pressure before drying in an oven. The resulting dry extract was subsequently stored in a glass container at a temperature of 4oC for subsequent experimental procedures. AnimalsThis study used male Wistar rats weighing 190.63 ± 19.00 g and aged approximately three months. Rats were obtained from animal farms in Malang, Indonesia, and were certified healthy by a veterinarian from the Department of Agriculture of Malang City No. 524.3/088/35.73.309/2022. All rats were kept in cages at a regulated temperature with alternate light cycles for 12 hours and ad libitum access to standard feed and water. The animals were housed according to the Guidelines for the Care and Use of Laboratory Animals of the US National Institutes of Health and the EU recommendations (Directive 2010/63/EU) for animal experiments. Diabetes inductionFollowing a period of acclimatization lasting seven days, rats that had undergone overnight fasting were induced to develop diabetes with the intraperitoneal injection of a single dose of Streptozotocin (STZ). Before injection, STZ was freshly prepared in a 0.1 M citrate buffer pH 4.5. The dosage administered was 45 mg/kg of body weight. The rats were given a 10% sucrose solution for 48 hours following injection to prevent the risk of sudden death caused by hypoglycemia. Seven days after the injection of STZ, fasting blood glucose levels were measured by a glucometer (Accu-Check Active, Roche Diagnostics). Rats with fasting blood glucose levels of more than 200 mg/dl were identified as diabetic and used in this experiment. The increase in blood sugar belongs to the intermediate level category (151–250 mg/dl) (Fajarwati et al., 2023). Experimental designThe rats were divided into six groups using a random allocation method. Each group included six rats. Group 1 consisted of rats not injected with STZ (normal control). Group 2 consisted of STZ-injected rats (diabetes control). Group 3 consisted of rats injected with STZ and administered 0.45 mg/kg BW Glibenclamide. Rats in Groups 4, 5, and 6 were injected with STZ and administered C. nutans leaf extract at 100, 200, and 400 mg/kg BW, respectively. The administration of C. nutans leaf extract and Glibenclamide were given using an intragastric tube for 28 days. The dosage was adjusted based on weekly variations in body weight to ensure a consistent dosage per kilogram of body weight in rats. Measurement of biomarkers for oxidative stress and inflammationOn the 29th day of the experiment, after an overnight fasting period, the rats were sacrificed following anesthetization with Ketamine (100 mg/kg) and Xylazine (10 mg/kg) injection. Blood samples were obtained by cardiac puncture and deposited in a vacutainer without ethylenediaminetetraacetic acid (EDTA). The blood samples were left at room temperature for approximately two h and then centrifuged at 4000 g, 4°C for 15 minutes to obtain serum. The assessment of oxidative stress markers involved the quantification of MDA and measuring SOD, a key antioxidant enzyme. In addition, inflammatory activity was determined by measuring TNF-α levels. Serum levels of TNF-α were measured with a rat TNF-α ELISA kit, while serum SOD activity was assessed using the SOD Assay kit following the instructions provided by the manufacturer. In addition, MDA concentrations were measured by the thiobarbituric acid (TBA) reactive substances (TBARSs) method (Đorđević et al., 2019). The reaction mixture of 0.1 ml serum, 0.2 ml sodium dodecyl sulfate 8.1%, 1.5 ml acetic acid 20% (pH 3.5), and 1.5 ml TBA 0.8% was heated for 60 minutes at 95°C. Following the cooling step, the mixture was centrifuged at 4,000 rpm for 10 minutes at a temperature of 4°C. Subsequently, the supernatant was quantified its absorbance at 532 nm. The preparation of calibration curves was conducted using MDA standards. The serum MDA levels were calculated using the equation based on the calibration curve. Immunohistochemical staining of pancreasThe histological examination was conducted on pancreatic tissue samples obtained from all experimental groups. The samples were washed using a normal saline solution to remove any remains of blood stains. After that, the samples were weighed and fixed in a solution of 10% formalin. Relative organ weight is determined by the percentage ratio of organ to body weight. The tissues were dehydrated using increasing concentrations of ethanol solutions, followed by embedding in paraffin blocks. Tissues embedded in paraffin were sliced into sections of 5 µm thickness. For immunohistochemical staining, tissue sections were incubated overnight with anti-JNK and Caspase 3 primary antibodies, followed by secondary antibodies conjugated with horseradish peroxidase. The peroxidase reaction used Diaminobenzidine as the substrate, whereas hematoxylin was used for counterstaining. Images were visualized by a microscope with a digital camera at 400× magnification and ten fields of view, displaying different Langerhans islets. The analysis was conducted with the ImageJ software program. The percentage of immunopositive cells was determined by dividing the number of brown-stained cells by the total number of cells in the islets of Langerhans. Statistical analysisAll data were presented as the mean ± standard deviation. The statistical analysis was conducted utilizing SPSS 26 software. One-way analysis of variance (ANOVA) followed by a Tukey post hoc test was used to analyze the differences between groups. A p-value less than 0.05 indicated a statistically significant difference. Ethical approvalAnimals were maintained following institutional guidelines for animal care. All efforts were taken to minimize any discomfort or pain. Experiment procedures were approved by the Health Research Ethics Committee of the State Islamic University of Maulana Malik Ibrahim, Indonesia (083/EC/KEPK-FKIK/2022). ResultsEffect of C. nutans extract on body weight, blood glucose, and pancreas and relative pancreas weight in diabetic ratsAfter treatment, body weight, blood glucose level, pancreas weight, and relative pancreas weight were analyzed, and the data are displayed in Figure 1. There was a reduction (P<0.05) in the body weight of diabetic rats that were not treated in comparison to the body weight of normal rats. Treatment with C. nutans extract at 400, 200, and 100 mg/kg BW doses have increased body weight by 13%, 10%, and 8%, respectively. Diabetic rats also displayed increased blood glucose levels in comparison with normal rats, whereas rats that received C. nutans displayed a hypoglycemic effect (p < 0.05). There was no significant difference between pancreas and relative pancreatic weight in all groups (p > 0.05). Effect of C. nutans extract on oxidative stress and inflammation markers in diabetic ratsAs illustrated in Figure 2, induction of diabetes led to an increase in MDA and TNF- α levels, together with a decrease in SOD levels (p < 0.05). This effect was reversed after all doses of C. nutans extract were given for four weeks. There was a decrease in MDA and TNF-α levels and an increase in SOD levels (p < 0.05). The study’s findings indicated that C. nutans extracts decreased markers associated with oxidative stress and inflammation in rats with diabetes. Effect of C. nutans extract on the expression of JNK and Caspase-3 in the pancreas of diabetic ratsThe expression of JNK dan Caspase-3 was determined by immunohistochemical staining of the pancreas, which gives a brown appearance on immunopositive islet cells. Only a small brown-stained islet of Langerhans was found in the pancreas of the normal rats. In contrast, the number of brown-stained islets of Langerhans increased markedly in the diabetes rats. Administration of C. nutans extracts decreased the distribution area of the brown-stained islets (Figs. 3A and 4A). JNK and Caspase-3 immunopositive cells are shown in Figures 3B and 4B. The percentage of JNK immunopositive cells in the diabetes rats was higher than in the normal rats (p < 0.05). Treatment with C. nutans extract decreased the percentage of JNK and Caspase-3 immunopositive cells compared to untreated diabetic rats (p < 0.05). The data suggest that the administration of C. nutans extract has the potential to attenuate apoptotic signaling pathways and markers in rats with diabetes. DiscussionPreserving functional β-cell mass offers a promising therapeutic approach to managing diabetes (Zhong and Jiang, 2019; Marrano et al., 2020). Oxidative stress has been considered a promising therapeutic target for decelerating β-cell loss due to its detrimental effects (Puddu et al., 2013). Some evidence indicates that antioxidants in plant extract formulations may efficiently reduce oxidative stress (Adwas et al., 2019; Hrelia and Angeloni, 2020). Clinacanthus nutans is widely consumed as a traditional medicinal plant in Southeast Asia because of its anti-diabetic properties (Yeo et al., 2018). Recent studies have shown that C. nutans can potentially reduce blood glucose levels and enhance insulin production in rats with diabetes (Sarega et al., 2016b; Azemi et al., 2020). Nevertheless, the effect of this plant on alleviating the damage in rat pancreatic β-cells by STZ remains uncertain. Elevated blood glucose levels and decreased body weight are common signs of diabetes (Erukainure et al., 2020). This condition was also observed in rats with diabetes in our research study. The cellular catabolic processes ultimately result in the breakdown of fat and muscle tissue, which causes weight loss. The formation of ROS caused by STZ induces β-cells death, thus reducing insulin release and elevating blood glucose levels (Šoltésová and Herichová, 2011; Eleazu et al., 2013). Nevertheless, the administration of C. nutans extracts relieved these symptoms, indicating its potential as an anti-diabetic agent. Hyperglycemia induces an overproduction of ROS and triggers oxidative stress (Yaribeygi et al., 2020). Based on phytochemical analyses, the active compounds of C. nutans extract contain flavonoids such as vitexin, isovitexin, orientin, isoorientin, apigenin, and shaftoside, which contribute potent antioxidant and anti-inflammatory activities (Khoo et al., 2018; Susanti et al., 2023). Previous studies demonstrated that flavonoids can prevent oxidative stress in rats with diabetes by scavenging free radicals and improving the endogenous antioxidant capacity of the body (Ghorbani et al., 2019; Wickramasinghe et al., 2021). In these results, MDA levels were decreased, and SOD activity was significantly increased after four weeks of treatment with C. nutans extract. This study corresponds with previous studies demonstrating that C. nutans extracts in rats induced by a high-fat and high-cholesterol diet can decrease MDA levels and increase SOD activity in the liver (Sarega et al., 2016b).
Fig. 1. Effect of C. nutans extract on body weight (A), blood glucose levels (B), pancreas weight (C), and relative pancreas weight (D). Data represented mean ± SD. *p < 0.05 significantly different from group 2; #p < 0.05 significantly different from group 1. Group 1 normal control; Group 2 diabetes control; Group 3 diabetic rats were administered Glibenclamide 0.45 mg/kg BW; Group 4 diabetic rats were administered C. nutans leaf extract at 100 mg/kg BW; Group 5 diabetic rats were administered C. nutans leaf extract at 200 mg/kg BW; Group 6 diabetic rats were administered C. nutans leaf extract at 400 mg/kg BW.
Fig. 2. Effect of C. nutans extract on serum levels of MDA (A), SOD (B), and TNF-α (C). Data represented mean ± SD. *p < 0.05 significantly different from group 2; #p < 0.05 significantly different from group 1. Group 1 normal control; Group 2 diabetes control; Group 3 diabetic rats were administered Glibenclamide 0.45 mg/kg BW; Group 4 diabetic rats were administered C. nutans leaf extract at 100 mg/kg BW; Group 5 diabetic rats were administered C. nutans leaf extract at 200 mg/kg BW; Group 6 diabetic rats were administered C. nutans leaf extract at 400 mg/kg BW.
Fig. 3. Effect of C. nutans on Immunohistochemical features of JNK expression from the pancreas of diabetic rats (A) and the percentage of JNK-positive cells of Langerhans islet (B). Magnification 400×, scale 50 μm, black arrows indicate the representative immunopositive cells. Data represented mean ± SD. *p < 0.05 significantly different from group 2; #p < 0.05 significantly different from group 1. Group 1 normal control; Group 2 diabetes control; Group 3 diabetic rats were administered Glibenclamide 0.45 mg/ kg BW; Group 4 diabetic rats were administered C. nutans leaf extract at 100 mg/kg BW; Group 5 diabetic rats were administered C. nutans leaf extract at 200 mg/kg BW; Group 6 diabetic rats were administered C. nutans leaf extract at 400 mg/kg BW.
Fig. 4. Effect of C. nutans on Immunohistochemical features of Caspase-3 expression from the pancreas of diabetic rats (A) and the percentage of Caspase-3-positive cells of Langerhans islet (B). Magnification 400×, scale 50 μm, black arrows indicate the representative immunopositive cells. Data represented mean ± SD. *p < 0.05 significantly different from group 2; #p < 0.05 significantly different from group 1. Group 1 normal control; Group 2 diabetes control; Group 3 diabetic rats were administered Glibenclamide 0.45 mg/kg BW; Group 4 diabetic rats were administered C. nutans leaf extract at 100 mg/kg BW; Group 5 diabetic rats were administered C. nutans leaf extract at 200 mg/kg BW; Group 6 diabetic rats were administered C. nutans leaf extract at 400 mg/kg BW. Hyperglycemia was also reported to increase proinflammatory cytokines released via NF-κB activation mediated by oxidative stress (Boarescu et al., 2022). These cytokines enhance the generation of ROS by activating NADPH oxidase, thereby exacerbating oxidative stress and ultimately damaging pancreatic β-cell. Furthermore, proinflammatory cytokines play a significant role not only in the process of inflammation but also in the initiation of pancreatic β-cell death. Prior studies showed increased proinflammatory cytokine marker TNF-α concentrations in animals with diabetes (Yapislar et al., 2022). The study findings suggest that C. nutans extract can decrease TNF-α levels in diabetic rats, thus confirming its anti-inflammatory properties. Oxidative stress induced by prolonged hyperglycemia can adversely affect pancreatic β-cells. Much evidence points to the involvement of the JNK pathway in oxidative stress-induced pancreatic β-cell damage (Dhanasekaran and Reddy, 2017; Baumel-Alterzon and Scott, 2022). ß-cell dysfunction was observed in rats infused with glucose, but not in JNK-1-null rats treated with JNK inhibition using SP600125 (Tang et al., 2018). On the other hand, STZ-induced rats lead to JNK activation, negatively impacting pancreatic β-cells survival (Liu et al., 2019; Sadek et al., 2017). This condition supports the evidence that the JNK pathway plays a role in the impairment of β-cell function mediated by glucose. In this study, we found that C. nutans extract could decrease JNK expression in the pancreas of diabetic rats. The findings presented in this study correspond with prior research, which has demonstrated that plants possessing antioxidant properties can protect against diabetes-induced pancreatic β-cell damage by attenuating oxidative stress and inhibiting the JNK pathway (Tabatabaie and Yazdanparast, 2017; JNK-mediated signaling pathways regulate apoptosis through extrinsic and intrinsic pathways (Šrámek et al., 2016). The extrinsic pathway is activated following the ligation of the cell surface death receptor, triggering downstream effector mechanisms and activating caspase-3. The intrinsic pathway is triggered by the translocation of the pro-apoptotic protein Bcl to mitochondria. It causes the Cytochrome-C release to form the apoptosome complex and thus activates caspase-3 (Tomita, 2016). Caspase-3 facilitates the release of caspase-activated endonucleases from their inhibitory states, thereby leading to DNA fragmentation (Kitazumi and Tsukahara, 2011). These findings indicate that C. nutans extract can reduce caspase-3 expression in the pancreas of diabetic rats, strengthening the protective effect of this plant against diabetic-induced pancreatic β-cell apoptosis. This anti-apoptotic effect has also been proven in previous studies where C. nutans extract exerts a neuroprotective effect by reducing neuronal apoptosis and repairing ischemic brain injury (Wu et al., 2018). ConclusionThis study provides scientific evidence that C. nutans may possess a protective effect against pancreatic β-cell apoptosis in diabetic rats. The observed effect is associated with its ability to regulate oxidative stress and inflammatory response, stimulate antioxidant enzymes, suppress the JNK pathway, and inhibit caspase-3 activation. Further research is necessary to validate the molecular pathways underlying the anti-diabetic properties of C. nutans before clinical application. AcknowledgmentsThe authors thank the Project Management Unit, Universitas Islam Negeri Maulana Malik Ibrahim Malang for the scholarship under the scheme The Development of Maulana Malik Ibrahim State Islamic University of Malang Phase II, East Java Project supported by the Indonesian Ministry of Religion and the Saudi Fund for Development. FundingSupported by the Indonesian Ministry of Religion and the Saudi Fund for Development. Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionsNS: conceptualized the study, conducted the experiment and laboratory examination, analyzed the data, and wrote and revised the manuscript; AM: supervised the study, wrote and reviewed the manuscripts; JK: supervised the study, wrote and reviewed the manuscripts. Data availabilityAll data are provided in the manuscript. ReferencesAdwas, A.A., Elsayed, A.S.I., Azab, A.E. and Quwaydir, F.A. 2019. Oxidative stress and antioxidant mechanisms in human body. J. Appl. Biotechnol. Bioeng. 6(1), 43–47. Alam, M.A., Zaidul, I.S.M., Ghafoor, K., Sahena, F., Hakim, M., Rafii, M.Y., Abir, H.M., Bostanudin, M.F., Perumal, V. and Khatib, A. 2017. In vitro antioxidant and, α-glucosidase inhibitory activities and comprehensive metabolite profiling of methanol extract and its fractions from Clinacanthus nutans. BMC Complement. Alternat. Med. 17(181), 1–10. American Diabetes Assosiation. 2021. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2021. Diabetes Care, 44(1), S15–S33. Azemi, A.K., Mokhtar, S.S. and Rasool, A.H.G. 2020. Clinacanthus nutans leaves extract reverts endothelial dysfunction in type 2 diabetes rats by improving protein expression of eNOS. Oxidative Med. Cell. Longevity, 2020, 1–10. Banday, M., Sameer, A. and Nissar, S. 2020. Pathophysiology of diabetes : an overview. Avicenna J. Med. 10, 174–188. Baumel-Alterzon, S. and Scott, D.K. 2022. Regulation of Pdx1 by oxidative stress and Nrf2 in pancreatic beta-cells. Front. Endocrinol. 13, 1–9. Boarescu, P.M., Boarescu, I., Pop, R.M., Roşan, Ş.H., Bocșan, I.C., Rus, V., Mada, R.O., Popa, I.D., Neagu, N., Bulboacă, A.E., Buzoianu, A.D. and Bolboacă, S.D. 2022. Evaluation of oxidative stress biomarkers, pro-inflammatory cytokines, and histological changes in experimental hypertension, dyslipidemia, and type 1 diabetes mellitus. Int. J. Mol. Sci. 23(3), 1–17. Chia, T.Y., Gan, C.Y., Murugaiyah, V., Hashmi, S.F., Fatima, T., Ibrahim, L., Abdulla, M.H., Alswailmi, F.K., Johns, E.J. and Ahmad, A. 2022. A narrative review on the phytochemistry, pharmacology and therapeutic potentials of Clinacanthus nutans (Burm. f.) lindau leaves as an alternative source of future medicine. Molecules, 27(139), 1–29. Costes, S., Bertrand, G. and Ravier, M.A. 2021. Mechanisms of beta-cell apoptosis in type 2 diabetes-prone situations and potential protection by GLP-1-based therapies. Int. J. Mol. Sci. 22(5303), 1–23. Dhanasekaran, D.N. and Reddy, E.P. 2017. JNK-signaling : a multiplexing hub in programmed cell death. Genes Cancer, 8(9–10), 682–694. Đorđević, M., Grdović, N., Mihailović, M., Arambašić Jovanović, J., Uskoković, A., Rajić, J., Sinadinović, M., Tolić, A., Mišić, D., Šiler, B., Poznanović, G., Vidaković, M. and Dinić, S. 2019. Centaurium erythraea extract improves survival and functionality of pancreatic beta-cells in diabetes through multiple routes of action. J. Ethnopharmacol. 242, 112043. Eleazu, C.O., Eleazu, K.C., Chukwuma, S. and Essien, U.N. 2013. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J. Diabetes Metab. Disord. 12(60), 1–7. Erukainure, O.L., Ijomone, O.M., Chukwuma, C.I., Xiao, X., Salau, V.F. and Islam, M.S. 2020. Dacryodes edulis (G. Don) H.J. Lam modulates glucose metabolism, cholinergic activities and Nrf2 expression, while suppressing oxidative stress and dyslipidemia in diabetic rats. J. Ethnopharmacol. 255(112744), 1–15. Fajarwati, I., Solihin, D.D., Wresdiyati, T. and Batubara, I. 2023. Self-recovery in diabetic Sprague Dawley rats induced by intraperitoneal alloxan and streptozotocin. Heliyon 9(5), 1–7. Gerber, P.A. and Rutter, G.A. 2017. The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxid. Redox Signal. 26(10), 501–518. Ghorbani, A., Rashidi, R. and Shafiee-Nick, R. 2019. Flavonoids for preserving pancreatic beta cell survival and function: a mechanistic review. Biomed. Pharmacother. 111, 947–957. Goyal, R. and Jialal, I. 2020. Diabetes mellitus type 2. In NCBI Bookshelf. St. Petersburg, FL: StatPearls Publishing LLC, pp: 1–9. Hanchang, W., Khamchan, A., Wongmanee, N. and Seedadee, C. 2019. Hesperidin ameliorates pancreatic β-cell dysfunction and apoptosis in STZ streptozotocin-induced diabetic rat model. Life Sci. 235(2019), 1–9. Harding, J.L., Pavkov, M.E., Magliano, D.J., Shaw, J.E. and Gregg, E.W. 2019. Global trends in diabetes complications: a review of current evidence. Diabetologia 62, 3–16. Hrelia, S. and Angeloni, C. 2020. New mechanisms of action of natural antioxidants in health and disease. Antioxidants, 9(4), 1–5. Ju, L., Wen, X., Wang, C., Wei, Y., Peng, Y., Ding, Y., Feng, L. and Shu, L. 2017. Salidroside, a natural antioxidant, improves β-cell survival and function via activating AMPK pathway. Front. Pharmacol. 8, 1–12. Kaneto, H., Matsuoka, T.A., Nakatani, Y., Kawamori, D., Miyatsuka, T., Matsuhisa, M. and Yamasaki, Y. 2005. Oxidative stress, ER stress, and the JNK pathway in type 2 diabetes. J. Mol. Med. 83(6), 429–439. Khoo, L.W., Audrey Kow, S., Lee, M.T., Tan, C.P., Shaari, K., Tham, C.L. and Abas, F. 2018. A comprehensive review on phytochemistry and pharmacological activities of Clinacanthus nutans (Burm.f.) Lindau. Evidence-Based Complement. Altern. Med. 2018, 1–39. Kitazumi, I. and Tsukahara, M. 2011. Regulation of DNA fragmentation: the role of caspases and phosphorylation. FEBS J. 278(3), 427–441. Liu, Y., Han, J., Zhou, Z. and Li, D. 2019. Paeoniflorin protects pancreatic β cells from STZ-induced damage through inhibition of the p38 MAPK and JNK signaling pathways. Euro. J. Pharmacol. 853, 18–24. Marrano, N., Biondi, G., Cignarelli, A., Perrini, S., Laviola, L., Giorgino, F. and Natalicchio, A. 2020. Functional loss of pancreatic islets in type 2 diabetes: How can we halt it? Metab. Clin. Exper. 110, 154304. Ohiagu, F.O., Chikezie, P.C. and Chikezie, C.M. 2021. Pathophysiology of diabetes mellitus complications: metabolic events and control. Biomed. Res. Ther. 8(3), 4243–4257. Ortega-Camarillo, C. 2019. Dysfunction and death of pancreatic beta cells in type 2 diabetes. In The Diabetes Textbook, pp: 169–184. Palai, T.K. and Mishra, S.R. 2015. Caspases : an apoptosis mediator. J. Adv. Vet. Anim. Res. 2(1), 18–22. Puddu, A., Sanguineti, R., Mach, F., Dallegri, F., Viviani, G.L. and Montecucco, F. 2013. Update on the protective molecular pathways improving pancreatic beta-cell dysfunction. Mediat. Inflamm. 2013, 1–14. Sadek, K.M., Lebda, M.A., Nasr, S.M. and Shoukry, M. 2017. Spirulina platensis prevents hyperglycemia in rats by modulating gluconeogenesis and apoptosis via modification of oxidative stress and MAPK-pathways. Biomed. Pharmacother. 92, 1085–1094. Salazar-García, M. and Corona, J.C. 2021. The use of natural compounds as a strategy to counteract oxidative stress in animal models of diabetes mellitus. Int. J. Mol. Sci. 22(13), 1–13. Sarega, N., Imam, M.U., Esa, N.M., Zawawi, N. and Ismail, M. 2016a. Effects of phenolic-rich extracts of Clinacanthus nutans on high fat and high cholesterol diet-induced insulin resistance. BMC Complement. Alternat. Med. 16(88), 1–11. Sarega, N., Imam, M.U., Ooi, D.J., Chan, K.W., Esa, N.M., Zawawi, N. and Ismail, M. 2016b. Phenolic rich extract from Clinacanthus nutans attenuates hyperlipidemia-associated oxidative stress in rats. Oxidative Med. Cell. Longev. 2016, 1–16. Šoltésová, D. and Herichová, I. 2011. On the mechanisms of diabetogenic effects of alloxan and streptozotocin. Diabetol. Metab. Endokrinol. Vyziva 14(3), 130–138. Šrámek, J., Němcová-Fürstová, V. and Kovář, J. 2016. Kinase signaling in apoptosis induced by saturated fatty acids in pancreatic β-cells. Int. J. Mol. Sci. 17(9), 1–15. Sun, H., Saeedi, P., Karuranga, S., Pinkepank, M., Ogurtsova, K., Duncan, B.B., Stein, C., Basit, A., Chan, J.C.N., Claude, J., Pavkov, M.E., Ramachandaran, A., Wild, S.H., James, S., Herman, W.H., Zhang, P., Bommer, C., Kuo, S., Boyko, E.J. and Magliano, D.J. 2022. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 183, 1–13. Susanti, N., Mustika, A., Khotib, J., Muti’ah, R. and Rochmanti, M. 2023. Phytochemical, metabolite compound, and antioxidant activity of Clinacanthus nutans leaf extract from Indonesia. Sci. Technol. Indonesia 8(1), 38–44. Tabatabaie, P.S. and Yazdanparast, R. 2017. Teucrium polium extract reverses symptoms of streptozotocin-induced diabetes in rats via rebalancing the Pdx1 and FoxO1 expressions. Biomed. Pharmacother. 93, 1033–1039. Tang, C., Yeung, L.S.N., Koulajian, K., Zhang, L., Tai, K., Volchuk, A. and Giacca, A. 2018. Glucose-induced B-cell dysfunction in vivo: Evidence for a causal role of C-jun N-terminal kinase pathway. Endocrinology 159(11), 3643–3654. Tomita, T. 2016. Apoptosis in pancreatic β-islet cells in Type 2 diabetes. Bosnian J. Basic Med. Sci. 16(3), 162–179. Umar Imam, M., Ismail, M., George, A., Chinnappan, S.M. and Yusof, A. 2019. Aqueous leaf extract of Clinacanthus nutans improved metabolic indices and sorbitol-related complications in type II diabetic rats (T2D). Food Sci. Nutr. 7(4), 1482–1493. Wang, J. and Wang, H. 2017. Oxidative stress in pancreatic beta cell regeneration. Oxidative Med. Cell. Longevity 2017, 1–9. Wickramasinghe, A.S.D., Kalansuriya, P. and Attanayake, A.P. 2021. Herbal medicines targeting the improved β-cell functions and β-cell regeneration for the management of diabetes mellitus. Evidence-Based Complement. Alternat. Med. 2021, 1–32. Wu, J.S., Kao, M.H., Tsai, H.D., Cheung, W.M., Chen, J.J., Ong, W.Y., Sun, G.Y. and Lin, T.N. 2018. Clinacanthus nutans mitigates neuronal apoptosis and ischemic brain damage through augmenting the C/EBPβ-Driven PPAR-γ transcription. Mol. Neurobiol. 55(7), 5425–5438. Yapislar, H., Haciosmanoglu, E., Sarioglu, T., Degirmencioglu, S., Sogut, I., Poteser, M. and Ekmekcioglu, C. 2022. Anti-inflammatory effects of melatonin in rats with induced type 2 diabetes mellitus. Life 12(4), 1–18. Yaribeygi, H., Sathyapalan, T., Atkin, S.L. and Sahebkar, A. 2020. Molecular mechanisms linking oxidative stress and diabetes mellitus. Oxidative Med. Cell. Longevity 2020, 1–13. Yeo, B.S., Yap, Y.J., Koh, R.Y., Ng, K.Y. and Chye, S.M. 2018. Medicinal properties of Clinacanthus nutans: a review. Trop. J. Pharma. Res. 17(2), 375–382. Zhong, F. and Jiang, Y. 2019. Endogenous pancreatic β cell regeneration: a potential strategy for the recovery of β cell deficiency in diabetes. Front. Endocrinol. 10(101), 1–14. | ||
| How to Cite this Article |
| Pubmed Style Susanti N, Mustika A, Khotib J. Clinacanthus nutans leaf extract reduces pancreatic β-cell apoptosis by inhibiting JNK activation and modulating oxidative stress and inflammation in Streptozotocin-induced diabetic rats. Open Vet. J.. 2024; 14(2): 730-737. doi:10.5455/OVJ.2024.v14.i2.13 Web Style Susanti N, Mustika A, Khotib J. Clinacanthus nutans leaf extract reduces pancreatic β-cell apoptosis by inhibiting JNK activation and modulating oxidative stress and inflammation in Streptozotocin-induced diabetic rats. https://www.openveterinaryjournal.com/?mno=174651 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i2.13 AMA (American Medical Association) Style Susanti N, Mustika A, Khotib J. Clinacanthus nutans leaf extract reduces pancreatic β-cell apoptosis by inhibiting JNK activation and modulating oxidative stress and inflammation in Streptozotocin-induced diabetic rats. Open Vet. J.. 2024; 14(2): 730-737. doi:10.5455/OVJ.2024.v14.i2.13 Vancouver/ICMJE Style Susanti N, Mustika A, Khotib J. Clinacanthus nutans leaf extract reduces pancreatic β-cell apoptosis by inhibiting JNK activation and modulating oxidative stress and inflammation in Streptozotocin-induced diabetic rats. Open Vet. J.. (2024), [cited January 25, 2026]; 14(2): 730-737. doi:10.5455/OVJ.2024.v14.i2.13 Harvard Style Susanti, N., Mustika, . A. & Khotib, . J. (2024) Clinacanthus nutans leaf extract reduces pancreatic β-cell apoptosis by inhibiting JNK activation and modulating oxidative stress and inflammation in Streptozotocin-induced diabetic rats. Open Vet. J., 14 (2), 730-737. doi:10.5455/OVJ.2024.v14.i2.13 Turabian Style Susanti, Nurlaili, Arifa Mustika, and Junaidi Khotib. 2024. Clinacanthus nutans leaf extract reduces pancreatic β-cell apoptosis by inhibiting JNK activation and modulating oxidative stress and inflammation in Streptozotocin-induced diabetic rats. Open Veterinary Journal, 14 (2), 730-737. doi:10.5455/OVJ.2024.v14.i2.13 Chicago Style Susanti, Nurlaili, Arifa Mustika, and Junaidi Khotib. "Clinacanthus nutans leaf extract reduces pancreatic β-cell apoptosis by inhibiting JNK activation and modulating oxidative stress and inflammation in Streptozotocin-induced diabetic rats." Open Veterinary Journal 14 (2024), 730-737. doi:10.5455/OVJ.2024.v14.i2.13 MLA (The Modern Language Association) Style Susanti, Nurlaili, Arifa Mustika, and Junaidi Khotib. "Clinacanthus nutans leaf extract reduces pancreatic β-cell apoptosis by inhibiting JNK activation and modulating oxidative stress and inflammation in Streptozotocin-induced diabetic rats." Open Veterinary Journal 14.2 (2024), 730-737. Print. doi:10.5455/OVJ.2024.v14.i2.13 APA (American Psychological Association) Style Susanti, N., Mustika, . A. & Khotib, . J. (2024) Clinacanthus nutans leaf extract reduces pancreatic β-cell apoptosis by inhibiting JNK activation and modulating oxidative stress and inflammation in Streptozotocin-induced diabetic rats. Open Veterinary Journal, 14 (2), 730-737. doi:10.5455/OVJ.2024.v14.i2.13 |