| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 136-143 Original Research Immunological effects of ractopamine in rabbits receiving the viral inactivated rabbit hemorrhagic disease vaccineGamal A. Shams1, Hosny A. Ibrahim1, Heba M. Hassan2, Nashwa S. Semary3* and Abeer F. I. Hassan41Department of Pharmacology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 2Animal Health Research Institute (AHRI), Agriculture Research Center (ARC), Giza, Egypt 3Animal Health Research Institute (AHRI), Agriculture Research Center (ARC), Zagazig, Egypt 4Educational Veterinary Hospital, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt *Corresponding Author: Nashwa S. Semary. Animal Health Research Institute (AHRI), Agriculture Research Center (ARC), Zagazig, Egypt. Email: Nashwasemary [at] ahri.gov.eg Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/01/2024 © 2024 Open Veterinary Journal
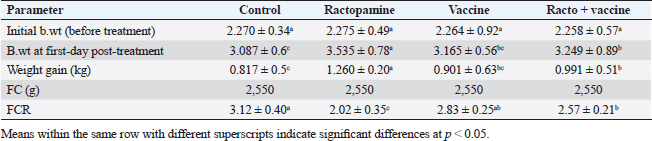
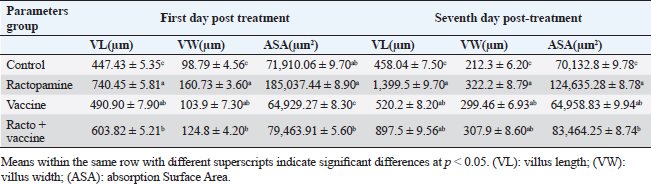
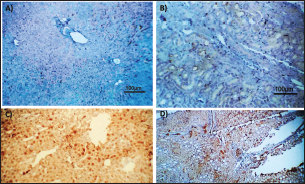
AbstractBackground: There is an obvious lack of information about the effects of ractopamine, a ß-adrenergic agonist, on the growth performance and immune responses of rabbits, particularly in those receiving the viral rabbit hemorrhagic disease (RHD) vaccine. Aim: The current study was undertaken to study the effects of ractopamine on growth performances and immunological parameters in rabbits inoculated with the viral RHD vaccine. Methods: Experimental rabbits were grouped into four groups, the first acted as a control and received distilled water, the second received ractopamine, the third received inactivated RHD vaccine, and the fourth received both ractopamine, and inactivated RHD vaccine. Then, blood analysis, histopathological, histomorphometric, and immunohistochemistry (IHC) examinations were followed. Results: The obtained results demonstrated that ractopamine induced significant increases in body weight gain, neutrophils, monocytes, nitric oxide, lysosome, and improved feed conversion rate. A significant decrease in lymphocytes with insignificant decreases in eosinophils, phagocytic % and index, serum total protein, α, β, and γ globulin were observed. Vaccinated rabbits only showed a marked rise in WBCs, neutrophils, monocytes, eosinophils, basophils, phagocytic index and activity, nitric oxide, lysosome activity, total protein, albumin, γ globulin, and a decrease in lymphocytes. Rabbits that received ractopamine and then vaccinated had insignificant increases in body weight, weight gain, WBCs, neutrophils, monocyte, eosinophils, basophils, phagocytic activity, and index, globulins besides a significant decrease in lymphocytes. Pathologically, rabbits that received ractopamine alone, with a vaccine or vaccinated only showed an increase in villus length, villus width, and absorption surface area. IHC of rabbits’ liver and kidneys of the control and vaccinated group showed negative expression for caspase-3, but rabbits received ractopamine only or rabbits vaccinated and received ractopamine showed diffuse positive moderate expression for caspase-3. Conclusion: Ractopamine induced several adverse effects on the immune responses of the rabbits inoculated with the viral HRD vaccine. Keywords: Rabbits, Ractopamine, Rabbit hemorrhagic virus vaccine, Immunity, Viral diseases. IntroductionAnimals and birds use their immune systems as one of their defense mechanisms to maintain the integrity of their bodies (Fabio et al., 2021). The immunity that is either naturally occurring or acquired does not have antigen specificity or memory, and it only receives linear reinforcement. On the other hand, acquired immunity is influenced by antigens and has an immune memory (Gergely, 2006). The first case of rabbit hemorrhagic disease (RHD) was discovered in China in 1984. Since then, it has been discovered in more than 40 countries across Asia, Africa, the Americas, and Europe (Mcintosh et al., 2007). It affects domestic and wild rabbits older than 2 months and is extremely contagious. It is brought on by the calicivirus, a nonenveloped tiny round RNA virus with just one main capsid protein (Abd El-Moaty et al., 2014). In both human and veterinary medicine, ractopamine is primarily utilized as a mucolytic and bronchodilator drug. It belongs to the phenethylamine category of ß-adrenergic agonists (Watkins et al., 1990). Beta-adrenergic agonists are categorized based on the binding type of beta-adrenergic receptors. Its exogenous component modifies animal metabolism and nutrient distribution in a way that favors muscular position (Moody et al., 2000). This drug is gaining popularity in the intensive production of pigs, chickens, and cattle (Brambilla et al., 2000; Davis and Belk, 2018). Beta-agonists effectively stimulate cattle growth (Kootstra et al., 2005). It is used to enhance physical performance (Ronald et al., 2019). According to Mirhendi et al. (2014), beta-adrenergic agonists enhance the growth performance of chicken. Their physiological effect is similar to that of phenethanolamines as upon ligand binding to receptors, it causes various physiological reactions (Mersmann, 1998). However, there is an obvious lack of information about the effects of ractopamine on the growth performance and immune responses of rabbits, particularly in those receiving the viral RHD vaccine. In sight of the previous facts, this study was done to study the effects of ractopamine on growth performances and immune responses in rabbits inoculated with the viral inactivated RHD vaccine. Materials and MethodsChemicals and vaccineThe analytical standard of ractopamine hydrochloride Vetranal™, purity 95.0%, was purchased from Sigma-Aldrich in St. Louis, MO. A dose of 0.5 ml of the inactivated RHD virus vaccine was administered subcutaneously to each rabbit after being acquired from the Veterinary Serum and Vaccine Research Institute, Abbassia, Cairo, Egypt (Mostafa et al., 2009). Animals and experimental designFor this investigation, 60 male white New Zealand rabbits weighing between 2.258 and 2.275 kg and 2 months old were used. Wire cages with perfect hygiene were utilized to house the rabbits. The rabbits were provided free access to water and fed on a balanced diet devoid of any drugs. The rabbits were acclimated for a week before the trial. Four equal groups of rabbits were separated (15 rabbits in each). The first group of rabbits received 0.2 ml of DW orally as a control. The second group of rabbits received ractopamine at 20 mg/kg dry matter orally for 21 consecutive days. The third group of rabbits received an inactivated RHD virus vaccine at 0.5 ml/rabbit subcutaneously once at the end of the experiment (on the 21st day). The fourth group of rabbits received both ractopamine at 20 mg (on the 21st day) and an inactivated RHD virus vaccine. To calculate weight increase and the feed conversion rate (FCR), each rabbit was weighed separately at the start of the experiment and on the first day after treatment. SamplingFive rabbits from each group had blood drawn from the ear vein on the first and second post-vaccination days, which was centrifuged to provide clear serum for lysozyme and nitric oxide estimate (Schltz, 1987; Rajaraman et al., 1998; Ramadan and Attia, 2003). Three blood samples were taken on the third day after vaccination; the first sample was centrifuged to estimate lysozyme and nitric oxide, and the second sample was collected in a tube containing heparin to gauge phagocytic activity and percentage (Wilkinson, 1977; Lucy and Larry, 1982). For the purpose of estimating the total and differential leukocytic count, the third sample was taken in a tube containing EDTA (Feldman et al., 2000). Blood samples were taken on days 7, 14, and 21 following immunization and centrifuged to generate clear sera for measurement of serum total protein and serum protein fractions using cellulose acetate electrophoresis (Henry et al., 1974; Doumas et al., 1981). Histopathological examinationEach group’s collected intestinal samples from the first and seventh post-treatment days were processed using the paraffin embedding procedure, fixed in 10% formalin, and stained with hematoxylin and eosin (Survarna et al., 2013). HistomorphometricDifferent small intestine segment parameters were carried out using the prior (H&E) staining technique. All observations and morphological measurements were performed on five villus crypt units that had intact lamina propria (Ashraf et al., 2013) using photographs taken at various magnifications (OMAX 40X-2000X), which were then analyzed using Image J software. Villus length (VL), width (VW), and absorptive surface area (ASA) were the parameters examined for intestinal segments (De Los Santos et al., 2007). Immunohistochemistry (IHC)Additional liver and kidney slices were employed for the IHC procedure with ABs designating the target parts on positively charged coated slides. Slides treated with the anti-SMA antibody (eBioscience, Tokyo, Japan). Using caspase-3 antibodies, the IHC staining for caspase-3 apoptosis was localized. The avidin-biotin-peroxidase technique was used to incubate a rabbit monoclonal caspase-3 antibody with paraffin sections that were 4 m thick (Krajewska et al., 1997). Areas with a higher concentration of positive cells (referred to as “hot spots” areas) on each slide were chosen for the investigation of protein expression associated with apoptosis (caspase 3). Protein labeling was not always visible diffusely in the samples under study; therefore, this methodology was employed. The slides were stained with peroxidase stain with a counter-stain of hematoxylin, mounted, and examined microscopically to gauge the staining intensity. Standard procedures were used for each slide at the same time. As external controls, known positive and negative examples were used. The brown peroxidase stain color was used to determine the positive expression for each positive marker. Strong (+++), moderate (++), and mild (+) color intensities were utilized in a semi-quantitative way. Statistical analysisThe automated SPSS program version 16 was used to examine the data that were obtained (Tambane and Dunlop, 2000). Ethical approvalThe committee for animal welfare and research ethics guidelines (Protocol number: ZU-IACUC/3/F/173/2021) were followed while using any experimental rabbits. ResultsThe present investigation declared that rabbits that received ractopamine only or in combination with the vaccine showed a marked increase in body weight, weight gain, and a reduction in the FCR, while rabbits with the vaccine alone did not show significant alterations in such the tested parameters (Table 1). The obtained data in Table 2 revealed that rabbits received ractopamine at the recommended dose orally for 21 successive days induced an insignificant increase in WBCs besides a significant increase in neutrophils, monocytes, and basophils associated with a decrease in lymphocytes, eosinophils, phagocytic activity, and index. It was noticed that rabbits that received ractopamine in the tested dose displayed a noninsignificant increase in nitric oxide and lysosome at the first, second, and third days post-treatment (Table 3). Ractopamine reduced serum total protein, α, β, γ, and total globulin, but increased the albumin all over the experimental period in comparison with control rabbits (Table 4). IHC analysis of the rabbit liver and kidney (Figs. 1 and 2) of the control and vaccinated groups only showed a negative expression for caspase-3, while rabbits that received ractopamine alone or in a combination with the vaccine showed a diffuse positive moderate expression for caspase-3 in the same organs, livers, and kidneys. Intestinal examination revealed that rabbits with ractopamine alone or with the vaccine and rabbits that received the vaccine only showed an increase in the VL, VW, and absorption surface area (Table 5, Figs. 3 and 4). DiscussionIn the present study, ractopamine caused significant enhancement in body weight, body gain, and FCR. Such results were agreeable with those reported by Mirhendi et al. (2014) who stated that β-adrenergic agonists could enhance the weight gain and FCR in poultry. Ractopamine is also used to favor the FCR in the livestock industry (Hales et al. 2016). Besides, Ronald et al. (2019) reported that ractopamine could induce a significant rise in weight gain and improve FCR. Likely, Abd-elfattah et al. (2019) stated that vaccination with the RHD virus vaccine did not significantly affect body weight and FCR. Moreover, Boimpoundi and Hao (2021) stated that ractopamine improved body weight gain coupled with a decrease in FCR. Table 1. The effect of ractopamine on body performance in rabbits vaccinated with RHD virus vaccine (mean ± SE) (n=5).
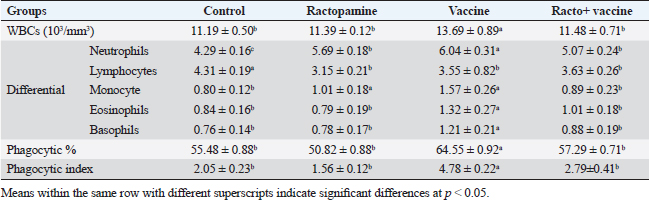
Table 2. The effect of ractopamine on total and differential count, phagocytic%, and phagocytic index in rabbits vaccinated with RHD virus vaccine (mean ± SE) (n=5).
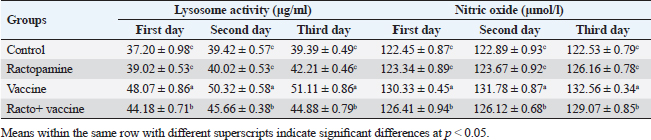
Table 3. The effect of ractopamine on nitric oxide and lysosome activity in rabbits vaccinated with RHD virus vaccine (mean ± SE) (n=5).
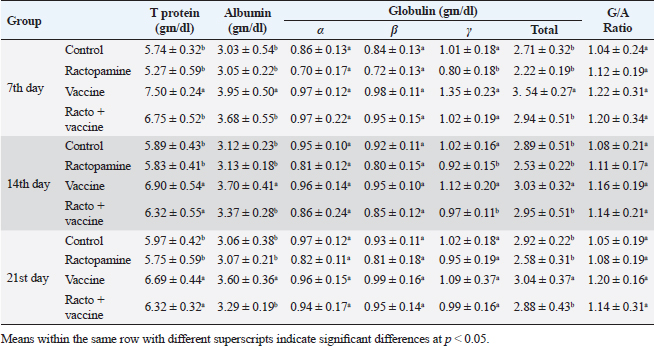
Table 4. The effect of ractopamine on protein picture in rabbits vaccinated with RHD virus vaccine (mean ± SE) (n=5).
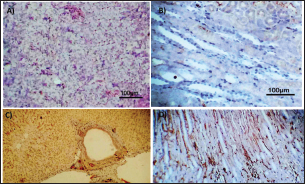
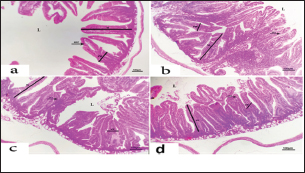
Table 5. The effect of ractopamine on intestinal characters in rabbits vaccinated with RHD virus vaccine (mean ± SE) (n=5).
Ractopamine administration caused a clear alteration in the blood picture of the examined rabbits. This result agreed with Spencer and Oliver (1996) who reported that administration of clenbuterol, another ß-adrenergic agonist, to rats did not alter WBCs besides insignificant neutrophilia and monocytosis associated with a decrease in lymphocytes and eosinophils (Ken et al., 2007). The same observation was reported previously by Ken et al. (2013) who mentioned clenbuterol caused a clear reduction in phagocytic activity and index.
Fig. 1. Photomicrograph of peroxidase-stained rabbit A) control liver, B) control kidney group showing negative expression for caspase-3 (scale bar=100 µm), C) livers of the ractopamine-treated group, and D) kidneys of the ractopamine-treated group showing focal positive moderate expression for caspase-3 (scale bar=100 µm).
Fig. 2. Photomicrograph of peroxidase stained rabbit A) liver and B) kidney of group 3 showing negative expression for caspase-3 (scale bar=100 µm), C) liver of group 4 showing diffuse positive moderate expression for caspase-3, and D) kidney of group 4 showing focal interstitial moderate expression for caspase-3 (scale bar=100 µm).
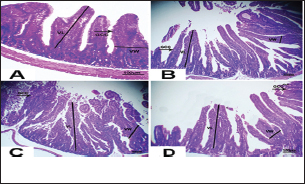
Fig. 3. Photomicrograph of intestine portions of different groups at first scarification showing mucosal demonstration for VL; VW and Goblet cells (GCC) for groups a–d consequently (H&E X bar=100 µm).
Fig. 4. Photomicrograph of intestine portions of different groups at second scarification showing mucosal demonstration for VL; VW and GCC for groups A–D consequently (H&E X bar=100 µm). The rabbits vaccinated with the RHD vaccine only or received ractopamine in the tested dose and then vaccinated with the vaccine resulted in a marked rise in WBCs, neutrophils, monocytes, eosinophils, basophils, phagocytic activity, and phagocytic index beside a significant decrease in lymphocytes. Likely, Chenggang et al. (2001) stated that the RHD virus vaccine induced an increase in WBCs, neutrophils, monocytes, eosinophils, basophils, phagocytic activity, and index due to improving the immunological status post vaccination by stimulation of the lymphoid tissue T-cell system, increasing the production of lymphocyte active substances and increasing phagocytosis of macrophage. Likely, Abd El Lateef (2006) demonstrated that the RHD virus vaccine increased the phagocytic activity and phagocytic index, besides improving the total and differential leukocytic count. Furthermore, Abd El-Wahab (2015) recorded that the RHD virus vaccine stimulates the phagocytic activity and index, WBCs, neutrophils, monocytes, eosinophils, and basophils. Similarly, Said et al. (2019) demonstrated that vaccinated rabbits had a significant rise in the leukocytic count, neutrophils, monocytes, eosinophils, basophils, phagocytic percent, and phagocytic index. Ractopamine caused marked changes in nitric oxide and lysosome. Such changes corresponded well with Yates et al. (1997) who mentioned that short- and long-acting inhaled beta2-agonists induced insignificant increases in serum nitric oxide and lysosome. Masao et al. (1999) reported that celiprolol and nebivolol, other beta-agonists caused a dose-dependent insignificant increase in nitric oxide and lysosome release. Besides, Liu et al. (2012) found that rats that received another beta-agonist named isoproterenol showed an insignificant increase in serum nitric oxide and lysosome. Niaolin et al. (2012) found that bopindolol, another beta-agonist could increase nitric oxide and lysosome production. Vaccinated rabbits in the present study either alone or in combination with ractopamine had a significant elevation in serum nitric oxide and lysosome. The same phenomenon was recorded in several reports before (Abd El-Wahab, 2015; Halaby, 2015; Ghandour, 2017; Said et al., 2019). Ractopamine caused clear changes in the protein picture of the tested rabbits throughout the experimental period in comparison with control rabbits. Likely, ractopamine decreased protein synthesis (Liu et al., 1994; Mersmann, 1998; Vandenberg and Moccia, 1998). Similarly, Spencer and Oliver (1996) found that clenbuterol suppressed the humoral immunological function. The observed higher serum albumin levels agreed with that recorded in the supplemented diet containing beta-adrenergic agonists (terbutaline and metaproterenol), and this might be due to the enhanced protein synthesis (Abadi et al., 2010). Likely, Kheiri et al. (2011) showed that broilers that received ractopamine had a marked rise in serum albumin. While total protein and globulin decreased in male Japanese quails that received 7.5 ppm ractopamine (Sayed et al., 2014). Vaccinated rabbits showed an elevation in total protein, albumin, and globulins . On similar grounds, Zheng et al. (1990) stated that γ globulin was increased in rabbits vaccinated against rabbits’ hemorrhagic disease virus. The same results were recorded before as α, β, and γ globulins were increased in the vaccinated rabbits, such increases were due to the increase in antibody-producing cells in the lymphoid organs (Villares, 1991; Perrin, 1996; Boga et al., 1997; Elkady et al., 2016; Said et al., 2019). IHC examination of the internal organs of the rabbits (liver and kidney) showed a negative expression for caspase-3 in the control and vaccinated groups only. Rabbits that received ractopamine alone or in combination with the vaccine showed a positive expression for caspase-3 in the same organs. Intestinal examination revealed an increase in the VL, VW, and absorption surface area in rabbits that were treated with ractopamine alone or with the vaccine. Likely, similar IHC observation was reported before as exposure to natural or chemical compounds (drug) induced liver injury and positive expression for caspase-3 (Fisher et al., 2015). The same change in caspase-3 was observed by Eid et al. (2015) in albino rats vaccinated with the swine flu (H1N1) vaccine. Moreover, Li et al. (2020) stated that the Newcastle disease vaccine caused a negative expression for caspase 3. ConclusionIn conclusion, administration of ractopamine could lead to several adverse effects on the immune responses in rabbits. Interestingly, using the hemorrhagic disease virus vaccine either alone or with the ractopamine could improve the immune responses in rabbits. AcknowledgmentThe authors would like to thank all staff members of the Department of Pharmacology, Faculty of Veterinary Medicine, Zagazig University, and Animal Health Research Institute, Agriculture Research Center, Egypt. Conflict of interestThe authors declare that there is no conflict of interest. Data availabilityAll data are provided in the manuscript. Any extra data needed can be provided by the corresponding author upon reasonable request. ReferencesAbadi, S., Mahboobi, N. and Riazi, G. 2010. Effects of supplemental dietary L-carnitine and ractopamine on the performance of juvenile rainbow trout, Oncorhynchus mykiss. Aqua. Res. 41, 1582–1591. Abd-elfattah, H., Marwa, M., Hanaa, S. and Mohamed, A. 2019. Effect of colibacillosis on the immune response to a rabbit viral hemorrhagic disease vaccine. Vet. Res. 43(1), 12–18. Abd El Lateff, A. 2006. Clinicopathological studies on rabbit hemorrhagic disease vaccines. Ph.D. Thesis, Cairo University, Giza, Egypt. Abd El-Moaty, A., El-Bagoury, G.F., El-Zeedy, A. and Salman, G. 2014. Egyptian hemagglutinating isolates of rabbit hemorrhagic disease virus can change to variable ha profile. Benha. Vet. Med. J. 26(2), 71–83. Abd El-Wahab, A. 2015. Influence of cephalexin on immuno status of vaccinated rabbits. Master Thesis, Bird and rabbit diseases, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. Ashraf, S., Zaneb, H. and Yousaf, M. 2013. Effect of dietary supplementation of prebiotics and probiotics on intestinal microarchitecture in broilers reared under cyclic heat stress. J. Anim. Physiol. Anim. Nutr. 97, 68–73. Boga, J., Martín, J., Casais, R. and Parra, F. 1997. A single dose immunization with rabbit hemorrhagic disease virus major capsid protein produced in Saccharomyces induces protection. J. General. Virol. 78(9), 563–572. Boimpoundi, E. and Hao, W. 2021. Beta-agonist drugs modulate the proliferation and differentiation of skeletal muscle cells in vitro. Bioch. Biophys. Rep. 18(2), 26–39. Brambilla, G., Cenc, T. and Francni, F. 2000. Pharmacological profile in clenbuterol epidemic poisoning of contaminated beef meat in Italy. Tox. Lett. 114(3), 47–53. Chenggang, T., Qiang, W. and Guolian, B. 2001. The relationship between maternal antibody against RHDV and infection. Chin. J. Rabbit. Farm. 3, 7–8. Davis, H. and Belk, E. 2018. Managing meat exports considering production technology challenges. Anim. Front. 8(3), 23–29. De Los Santos, F., Donoghue, A. and Farnell, M. 2007. Gastrointestinal maturation is accelerated in Turkey supplemented with mannanoligosaccharide yeast extract (Alphamune). Poul. Sci. 86, 21–30. Doumas, B., Baysa, D., Peler, T. and Schaffer, R. 1981. A candidate reference method for determination of total protein in serum. I development and validation. Clin. Chem. 27, 1642–1650. Eid, F., Mohamed, A., Aly, A. and Ibrahim, N. 2015. Effects of swine flu (H1N1) vaccine on albino rats. J. Biosci. Appl. Res. 1(3), 113–126. Elkady, A., Emad, M. and Amani, A. 2016. Development of fluorescein-labelled ant-isera for detection of rabbit haemorrhagic disease virus. Alex. J. Vet. Sci. 51(2), 46–51. Fabio, A., Carolina, M. and Maria, C. 2021. A potential atypical case of rabbit haemorrhagic disease in a Dwarf rabbit. Animals (Basel). 11(1), 40–47. Feldman, B., Zinki, J. and Jain, V. 2000. Schalms veterinary hematology, 5th ed. Philadelphia, PA: Lippincott Williams and Wilkins. Fisher, K., Vuppalanchi, R. and Saxena, R. 2015. Drug-induced liver injury. Arch. Pathol. Lab. Med. 139(7), 876–887. Gergely, J. 2006. Introduction to the immunology. In Immuno-biology (in Hungarian). Eds., Gergely, J. and Erdei, A. Budapest, Hungary: Medicina Konyvkiado Rt., pp: 15–17. Ghandour, M. 2017. Some pharmacological studies on rafoxanide. Master Thesis, Faculty of Veterinary Medicine (Pharmacology), Zagazig University, Zagazig, Egypt. Halaby, R. 2015. Role of lysosomes in cancer therapy. Res. Rep. Bio. 6, 47–55. Hales, K., Shackelford, S., Wells, J., King, D., Pyatt, N., Freetly, H. and Wheeler, T. 2016. Effects of dietary protein concentration and ractopamine hydrochloride on performance and carcass characteristics of finishing beef steers. J. Anim. Sci. 94, 2097–2102. Henry, R., Cannon, D. and Winkelman, J. 1974. Clinical chemistry: principals and techniques. Hagerstown, MD: Harper and Row. Ken, S., Jun, T. and Kazuhiko, I. 2007. Beta2-agonist clenbuterol induced changes in the distribution of white blood cells in rats. J. Pharm. Sci. 104(2), 146–152. Ken, S., Shogo, S., Yoko, H. and Kazuhiko, I. 2013. β2-agonist clenbuterol suppresses bacterial phagocytosis of splenic macrophages expressing high levels of macrophage receptor with collagen-ous structure. Biol. Pharm. Bull. 36(3), 475–480. Kheiri, F., Pourreza, J. and Abadi, S. 2011. Effect of supplemental ractopamine and L—carnitine on growth performance, blood biochemical parameters and carcass traits of male broiler chicks. Afr. J. Biotechnol. 68, 15450–15455. Kootstra, P., Wus, D. and Stephany, R. 2005. Analysis of beta-agonists in bovine muscle using molecular imprinted polymers with ion trap LCM screening. Anal. Chem. Acta. 529, 75–81. Krajewska, M., Wang, H.G. and Krajewski, S. 1997. Immunohistochemical analysis of in vivo patterns of expression of CPP32 (caspase-3), a cell death protease. Cancer. Res. 57, 1605–1613. Li, S., Liu, Z., Yuan, J., Yi, Y., Tian, J., Wu, L. and Wen, Z. 2020. Effects of induced stress from the live LaSota Newcastle disease vaccination on the growth performance and immune function in broiler chickens. Poult. Sci. 99(1), 1896–1905. Liu, C., Grant, A., Ji, S. and Anderson, D. 1994. Limitations of ractopamine to affect adipose tissue metabolism in swine. J. Anim. Sci. 72(1), 62–67. Liu, H., Zhang, L. and Lu, S., 2012. Evaluation of antioxidant and immunity activities of quercetin in isoproterenol-treated rats. Molecules 17(4), 4281–4291. Lucy, F. and Larry, D. 1982. Ontogeny and line differences as in mitogenic responses of chicken lymphocytes. Poul. Sci. 62, 579–594. Masao, K., Etsu, S. and Masao, O. 1999. Effects of vasodilatory β-adrenoceptor antagonists on endothelium-derived nitric oxide release in rat kidney. Hypertension 33, 467–471. Mcintosh, M., Behan, S., Mohamed, F. and Metwally, S. 2007. A pandemic strain of calicivirus threatens rabbit industries in the Americas. Virol. J. 4, 96. Mersmann, H. 1998. Overview of the effects of beta-adrenergic receptor agonists on animal growth including mechanisms of action. J. Anim. Sci. 76, 160–172. Mirhendi, S., Jalali, S. and Zare A. 2014. Effects of dietary ractopamine on growth performance and blood biochemical parameters in male Japanese quail (Coturnix japonica). Res. Opin. Anim. Vet. Sci. 4, 442–445. Moody, D., Hanck, D. and Anderon, D. 2000. Phenethanolamine repartitioning agents. In Farm animal metabolism and nutrition. Ed., D’Mello, F. New York, NY: CABI Publications, pp: 65–96. Mostafa, H.A., Sayed, E.A. and Salman, O. 2009. Effect of some antibiotics and antiparasitic on the immune response of rabbit vaccinated with rabbit hemorrhagic disease vaccine. In 6th International Science Conference, 2009, vol. 62, pp 93–95. Niaolin, X., Karen, M. and Djahida, K. 2012. Cardioprotective effect of beta-3 adrenergic receptor agonism: role of neuronal nitric oxide synthase. J. Am. Coll. Cardiol. 60(5), 481. Perrin, A. 1996. Techniques for the preparation of rabies conjugates. Lab. Tech. Rabies. 4, 433–444. Rajaraman, V., Nonnecke, B. and Horst, R. 1998. Effect of vitamins E on nitric oxide production by blood mononuclear leukocytes from neonatal calves fed milk replacer. J. Dairy. Sci. 81, 378–385. Ramadan, A. and Attia, R. Natural killing molecules in cervical mucus of buffaloes during the estrous cycle. 7th Sci Congress Egyptian Society for Cattle Diseases, Assuit, Egypt, 2003. Ronald, J.T., Kasey, R.M.C. and Kendall, C.S. 2019. Effects of ractopamine hydrochloride supplementation on feeding behavior, growth performance, and carcass characteristics of finishing steers. Trans. Anim. Sci. 3(4), 1143–1152. Said, A., Nagah, E., Omar, M. and Lotfy, A., 2019. Immunomodulating effects of isoprinosine in vaccinated rabbit. SCVMJ 1, 123–129. Sayed, R., Sayed, M. and Ahmad, Z. 2014. Effects of dietary ractopamine on growth performance and blood biochemical parameters in male Japanese quail (Coturnix japonica). Res. Opin. Anim. Vet. Sci. 4(8), 442–445. Schltz, L. 1987. Methods in clinical chemistry. St. Louis, MO: C. V. Mosby, pp: 42–46. Spencer, G. and Oliver, M. 1996, Suppression of immune response in lambs during treatment by beta-adrenergic agonist clunbuterol. J. Anim. Sci. 74, 151–153. Survarna, K., Lyton, C. and Bancroft, J., 2013, Bancroft’s theory and practice of histological techniques, 7th ed. Oxford, UK: Churchill Livingston, Elsevier, pp: 654. Tambane, A.C. and Dunlop, D. 2000. Statistics and data analysis from elementary to intermediate. Hoboken, NJ: Prentic Hall. Vandenberg, G. and Moccia, R. 1998. Performance and carcass composition of rainbow trout, Oncorhynchus mykiss (walbaum), fed the agonist ractopamine. Aqua. Res. 29, 69–79. Villares, J.L.A. 1991. Viral hemorrhagic disease in rabbits: vaccination and immune response. Rev. Sci. Tech. 10, 71–80. Watkins, D., Jones, D., Mowrey, D.H., Anderson, D.B. and Veenhuizen, L. 1990. The effect of various levels of ractopamine hydrochloride on performance and carcass characteristics of finishing swine. J. Anim. Sci. 68, 35–35. Wilkinson, P. 1977. Techniques in clinical immunology. In Ed., Thompson, R. Oxford, UK: Blackwell Scientific Publications. Yates, D., Kharitonov, A. and Barnes, P. 1997. Effect of short- and long-acting inhaled beta2-agonists on nitric oxide in asthmatic patients. Eur. Respir. J. 10(7), 83–88. Zheng, X., Bao, E. and Bao, J. 1990. Histopathological studies on the immune system of RHDV infected rabbits (in Chinese). Chin. J. Vet. Sci. Technol. 20(6), 3–4. | ||
| How to Cite this Article |
| Pubmed Style Shams GA, Ibrahim HA, Hassan HM, Semary NS, Hassan AF. Immunological effects of ractopamine in rabbits receiving the viral inactivated rabbit hemorrhagic disease vaccine. Open Vet. J.. 2024; 14((1) (Zagazig Veterinary Conference)): 136 -143 . doi:10.5455/OVJ.2024.v14.i1.12 Web Style Shams GA, Ibrahim HA, Hassan HM, Semary NS, Hassan AF. Immunological effects of ractopamine in rabbits receiving the viral inactivated rabbit hemorrhagic disease vaccine. https://www.openveterinaryjournal.com/?mno=174699 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i1.12 AMA (American Medical Association) Style Shams GA, Ibrahim HA, Hassan HM, Semary NS, Hassan AF. Immunological effects of ractopamine in rabbits receiving the viral inactivated rabbit hemorrhagic disease vaccine. Open Vet. J.. 2024; 14((1) (Zagazig Veterinary Conference)): 136 -143 . doi:10.5455/OVJ.2024.v14.i1.12 Vancouver/ICMJE Style Shams GA, Ibrahim HA, Hassan HM, Semary NS, Hassan AF. Immunological effects of ractopamine in rabbits receiving the viral inactivated rabbit hemorrhagic disease vaccine. Open Vet. J.. (2024), [cited January 25, 2026]; 14((1) (Zagazig Veterinary Conference)): 136 -143 . doi:10.5455/OVJ.2024.v14.i1.12 Harvard Style Shams, G. A., Ibrahim, . H. A., Hassan, . H. M., Semary, . N. S. & Hassan, . A. F. (2024) Immunological effects of ractopamine in rabbits receiving the viral inactivated rabbit hemorrhagic disease vaccine. Open Vet. J., 14 ((1) (Zagazig Veterinary Conference)), 136 -143 . doi:10.5455/OVJ.2024.v14.i1.12 Turabian Style Shams, Gamal A., Hosny A. Ibrahim, Heba M. Hassan, Nashwa S. Semary, and Abeer F.i. Hassan. 2024. Immunological effects of ractopamine in rabbits receiving the viral inactivated rabbit hemorrhagic disease vaccine. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 136 -143 . doi:10.5455/OVJ.2024.v14.i1.12 Chicago Style Shams, Gamal A., Hosny A. Ibrahim, Heba M. Hassan, Nashwa S. Semary, and Abeer F.i. Hassan. "Immunological effects of ractopamine in rabbits receiving the viral inactivated rabbit hemorrhagic disease vaccine." Open Veterinary Journal 14 (2024), 136 -143 . doi:10.5455/OVJ.2024.v14.i1.12 MLA (The Modern Language Association) Style Shams, Gamal A., Hosny A. Ibrahim, Heba M. Hassan, Nashwa S. Semary, and Abeer F.i. Hassan. "Immunological effects of ractopamine in rabbits receiving the viral inactivated rabbit hemorrhagic disease vaccine." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 136 -143 . Print. doi:10.5455/OVJ.2024.v14.i1.12 APA (American Psychological Association) Style Shams, G. A., Ibrahim, . H. A., Hassan, . H. M., Semary, . N. S. & Hassan, . A. F. (2024) Immunological effects of ractopamine in rabbits receiving the viral inactivated rabbit hemorrhagic disease vaccine. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 136 -143 . doi:10.5455/OVJ.2024.v14.i1.12 |