| Research Article | ||
Open Vet. J.. 2024; 14(5): 1167-1171 Open Veterinary Journal, (2024), Vol. 14(5): 1167–1171 Research Article Transcranial photobiomodulation therapy improves cognitive test scores in dogs with presumptive canine cognitive dysfunction: A case series of five dogsCurtis Wells Dewey1,2*, Mark Rishniw3, Matthew Warren Brunke1,4, Joyce Gerardi5 and Kasie Sakovitch11Elemental Pet Vets, PLLC, Freeville, New York, USA 2Department of Veterinary Clinical Sciences, Long Island University College of Veterinary Medicine, New York, USA 3Department of Clinical Sciences, College of Veterinary Medicine, Cornell University, Ithaca, New York, USA 4Veterinary Referral Associates, Gaithersburg, Maryland, USA 5Synergy Integrative Veterinary Clinic, New Bern, North Carolina, USA *Corresponding Author: Curtis W. Dewey. Elemental Pet Vets, PLLC, 1610 Dryden Road, Freeville, NY 13068, USA. Email: elementalpetvets [at] outlook.com Submitted: 30/01/2024 Accepted: 22/04/2024 Published: 31/05/2024 © 2024 Open Veterinary Journal
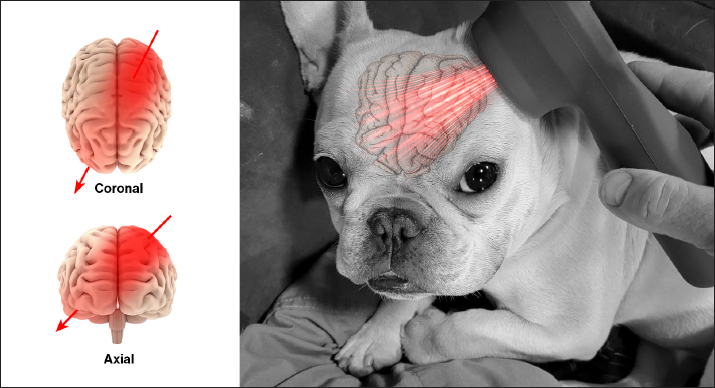
AbstractBackground: Canine cognitive dysfunction (CCD) is considered the canine version of human Alzheimer’s disease (AD). As with AD, CCD is a multifactorial and progressive neurodegenerative disorder for which effective treatment options are continuously being sought. Transcranial photobiomodulation (tPBMT) or transcranial laser therapy has shown promise as a treatment for cognitive impairment in rodent AD investigations and several human AD clinical trials. Aim: The purpose of this prospective case series was to evaluate the effect of tPBMT on cognitive scores when applied to senior dogs with CCD over a 60-day period. Methods: Five senior (>9-year-old) dogs with moderate (16–33) to severe (>33) cognitive scores were enrolled. Owners were instructed on the use of a Class IM laser device and administered a specific dose of laser energy transcranially to both sides of the patient’s head, three times per week for one month and two times per week for a second month. No additional therapeutic measures aimed at enhancing cognitive ability were permitted during the 60-day evaluation time. Baseline cognitive scores were compared with scores obtained at 30- and 60-days post-treatment. Results: Cognitive scores showed improvement in 4/5 dogs at 30 days (27.6% reduction) and all dogs at 60 days (43.4% reduction). There were no adverse effects attributable to tPBMT. Conclusion: Results of our small case series suggest that tPBMT may improve cognitive scores in dogs with moderate to severe CCD by 30 days of application and the improvement is sustained at 60 days. Further studies are needed to ascertain optimal tPBMT protocols for CCD. Keywords: Canine, Cognitive, Alzheimer’s, Transcranial, Photobiomodulation, Laser. IntroductionCanine cognitive dysfunction (CCD) is a progressive neurodegenerative disorder of senior dogs that is considered a naturally occurring model of human Alzheimer’s disease (AD) (Landsberg et al., 2012; Chapagain et al., 2018; Dewey et al., 2019). Numerous pathophysiologic processes have been implicated in the onset and progression of AD/CCD. These include damage to brain vasculature, neuronal mitochondrial dysfunction, toxic β-amyloid protein accumulation around neurons and blood vessels, and oxidative/inflammatory brain injury. Progressive loss of dendrites, synapses, and neurons (especially in the cerebral cortex and hippocampus) ensues over time because of these damaging events, ultimately culminating in cognitive decline (Landsberg et al., 2012; Chapagain et al., 2018; Dewey et al., 2019; Dewey et al., 2020; Ailioaie et al., 2023). Photobiomodulation (PBMT) or laser therapy entails the delivery of photons of light energy in the red to near-infrared wavelength range (approximately 600 to 1,200 nm) to tissues, to induce a positive biological response. PBMT is commonly implemented in veterinary practice, often used to decrease inflammation, accelerate healing, and attenuate pain (Anders et al., 2017; Riegel and Godbold, 2017; Stephens, 2019). Transcranial PBMT (tPBMT) therapy, also known as transcranial laser therapy, involves the delivery of photons of near-infrared to infrared light from the skin surface of the scalp to the underlying brain. Several potentially beneficial molecular mechanisms of action for tPBMT have been discovered that may be beneficial for AD treatment. These mechanisms include stimulation of neuronal mitochondrial energy production (ATP synthesis), enhancement of brain blood flow, diminution of β-amyloid production and accumulation around neurons and blood vessels, mitigation of dendrite and neuronal loss, reduced inflammatory and oxidative neuronal damage, and enhanced production of neurotrophic factors (Lapchak, 2012; Barrett and Gonzalez-Lima, 2013; Gonzalez-Lima et al., 2014; Hamblin, 2016; Hennessy and Hamblin, 2017; Saltmarche et al., 2017 Hamblin, 2019; Enengl et al., 2020; Dewey et al., 2022; Ailioaie et al., 2023). There is evidence in rodent AD models and human clinical trials that tPBMT improves cognitive function (Barrett and Gonzalez-Lima, 2013; Naeser et al., 2014; Saltmarche et al., 2017; Salehpour et al., 2017; Chan et al., 2018). There is one canine study, in which single wavelength (808 nm) tPBMT was shown to improve cerebral energy production in normal laboratory beagles, as measured via magnetic resonance spectroscopy (Mintzopoulos et al., 2017). To the best of the authors’ knowledge, tPBMT has not been investigated as a therapeutic tool for CCD. The purpose of this open-label prospective case series was to investigate a specific tPBMT protocol for the treatment of cognitive impairment in aging dogs. Materials and MethodsWe conducted a prospective, open-label, prospective case series investigation. Senior dogs (≥ 9 years old) with a clinical diagnosis of CCD were recruited. The diagnosis of CCD was based upon signalment, history, the nature and duration of clinical signs of dysfunction, in addition to a neurologic examination. Case inclusion was limited to dogs weighing 10 kg or less, to standardize tPBMT dose administration. Other inclusion criteria included the absence of any metabolic cause for cognitive dysfunction based on recent (within 3 months) bloodwork (CBC, serum biochemistry). Clients were required to submit an informed consent form before case enrolment. Each client filled out a previously published cognitive dysfunction questionnaire (Pan, et al., 2018) (Table 1) initially (baseline) and 30- and 60-days after starting tPBMT. Dog owners were instructed on the use of a Class IM laser (My Pet 2 laser, Multi Radiance Medical). Using a specific program on the laser device (Program 3), a total of 64 joules were delivered to each side of the head, as illustrated in Figure 1. This dose was approximated based on doses published for rodents and people (Gonzalez-Lima and Barrett, 2014; Salehpour et al., 2017; Enengl et al., 2020). The laser device (4.0 cm2 aperture) was placed just rostral to the external ear (caudal aspect of the temporal fossa) and aimed toward the opposite orbit. This trajectory was chosen to target both cerebral cortical and hippocampal tissue. Wavelengths of light administered during each treatment included 660, 850, and 905 nm, at a frequency range of 1,000 to 3,000 Hz. Other tPBMT treatment parameters included the following: total power=0.3564 W; power density=0.0891 W/cm2; energy density=16.04 J/cm2. Each treatment was completed in 6 minutes (3 minutes per side). Owners were instructed to administer treatments 3 times per week (Monday, Wednesday and Friday) for the first 30 days, then 2 times per week (Monday and Friday) for the following 30 days. No additional therapies aimed at improving cognitive status were permitted during the study period. Table 1. DISHAA scoring system used to assess cognitive ability in dogs. Each subcategory (bullet point) is scored as: 0=none, 1=mild, 2=moderate, and 3=severe. Total scores are assessed as: 4–15=mild impairment, 16–33=moderate impairment, and >33=severe impairment.
To examine the change in disorientation; interactions-social; sleep/wake cycle; house soiling, learning, and memory; activity; and anxiety (DISHAA) scores, we performed a Friedman’s test with post-hoc pairwise comparisons using the Dunn-Conover method.
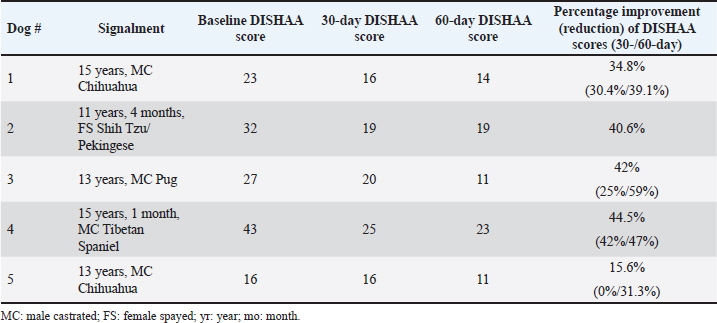
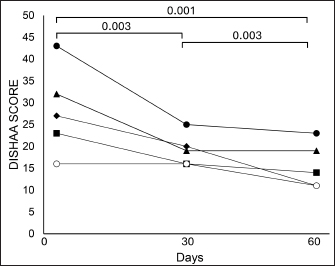
Fig. 1. Illustration depicting tPBMT delivery to a dog’s brain. Ethical approvalNot needed for this study. ResultsWe recruited five dogs for this case series: two Chihuahuas, a Tibetan Spaniel, a Pug mix, and a Shih Tzu/Pekingese mix. Ages ranged from 11 to 15 years (median=13 years); three were castrated males and two were spayed females. Routine hematology and biochemistry revealed no evidence of concurrent metabolic disease. DISHAA scores decreased at both 30-day and 60-day follow-up periods (Table 2, Fig. 2, p < 0.005). We observed no adverse effects attributed to tPBMT. DiscussionTranscranial PBMT has the potential to improve cognition in dogs afflicted by CCD via several mechanisms, including improving neuronal mitochondrial function, reducing toxic brain amyloid load, mitigating inflammatory/oxidative brain damage, and stimulating production of neurotrophic factors (Lapchak, 2012; Barrett and Gonzalez-Lima, 2013; Gonzalez-Lima and Barrett, 2014; Hamblin, 2016; Saltmarche et al., 2017; Hennessy and Hamblin, 2017; Hamblin, 2019; Enengl et al., 2020; Dewey et al., 2022; Ailioaie et al., 2023). Our small, open-label, uncontrolled case series showed an improvement in cognitive scores for dogs with CCD treated with tPBMT. There are several limitations to this study, including a small sample size, lack of a control (placebo) group, and lack of more rigorous methods of cognitive assessment. Because the cognitive scoring method used in our investigation engenders some level of owner subjectivity, it is possible that the perceived positive effects of tPBMT on cognition were exaggerated by owner bias. However, considering that all dogs experienced an improvement in cognitive scores, combined with the ease of home tPBMT application and lack of adverse effects, we believe our results warrant further investigation into this therapeutic modality for CCD. Anecdotally, 2 of our patients experienced documented worsening of cognitive scores within one month of discontinuing treatment; tPBMT was reinstituted in one of these dogs, resulting in a return to approximately the level of cognitive improvement previously attained within one month. Further investigation into the effectiveness of tPBMT for CCD would likely benefit from the use of a control group (e.g., sham laser device) and more objective and non-biased methods of measuring cognitive function/dysfunction. Such objective methods include delayed nonmatching to positioning memory tasks, attention tasks, food-searching tasks, and problem-solving tasks (Landsberg et al., 2012; Gonzalez-Martinez et al., 2013; Chapagain et al., 2018). Considering the progressive nature of CCD, future investigation into tPBMT for this disorder should ideally involve longer follow-up evaluation periods than presented in this study. Table 2. Summary of pre-and post-tPBMT DISHAA scores in five dogs.
Fig. 2. DISHAA scores in five dogs treated with tPBMT. Scores at 30 and 60 days differed from day 0; scores at 60 days differed from scores at 30 days. The ideal tPBMT treatment protocol for CCD-if one exists-remains unknown. We based our protocol on limited information from rodent AD models and human AD clinical trials (Gonzalez-Lima and Barrett, 2014; Salehpour et al., 2017; Enengl et al., 2020). Although we suspect that twice-a-week treatment may be sufficient, there may be more effective dosing regimens than what we used for our small clinical trial. Since PBMT is considered to have cumulative biological effects (Riegel and Godbold, 2017), it is possible that the initial month of therapy (three treatments per week) served as an induction phase, allowing for twice-a-week therapy in the second month to remain effective. Hopefully, further clinical research into various tPBMT protocols for CCD will help to better establish the most effective protocols for dogs affected by CCD. AcknowledgmentsThe authors wish to thank Multi Radiance Medical for providing the lasers for the study. Conflict of interestThere are no conflicts of interest associated with this investigation. FundingThis study was partially funded by Global Pet Project, INC, a 501c3 non-profit foundation. Authors’ contributionsDr. Dewey was responsible for the study’s inception, case recruitment, study design, and manuscript preparation. Dr. Rishniw conducted data analysis and edited the manuscript. Dr. Gerardi and Dr. Brunke were involved in case recruitment and manuscript editing. Ms. Sakovitch was responsible for client communication, data collection, and manuscript editing. Data availabilityAll data from this investigation are available upon request. ReferencesAilioaie, L.M., Ailioaie, C. and Litscher, G. 2023. Photobiomodulation in Alzheimer’s disease-a complementary method to state-of-the-art pharmaceutical formulations and nanomedicine? Pharmaceutics 15(3), 916. Anders, J.J., Ketz, A.K. and Wu, X. 2017. Basic principles of photobiomodulation and its effects at the cellular, tissue, and system levels. In Laser therapy in veterinary medicine: photobiomodulation. Eds., Reigel, R.J. and Godbold, J.C., Ames, IA: Wiley, pp: 36–51. Barrett, D.W. and Gonzalez-Lima, F. 2013. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience 230, 13–23. Chan, A.S., Lee, T.K., Yeung, M.K. and Hamblin, M.R. 2019. Photobiomodulation improves the frontal cognitive function of older adults. Int. J. Geriatr. Psychiatry. 34, 369–377. Chapagain, D., Range, F., Huber, L. and Virányi, Z. 2018. Cognitive aging in dogs. Gerontology 64(2), 165–171. Dewey, C.W., Davies, E.S., Xie, H. and Wakshlag, J.J. 2019. Canine cognitive dysfunction: pathophysiology, diagnosis, and treatment. Vet. Clin. Small Anim. Pract. 49, 477–499. Dewey, C.W., Rishniw, M., Johnson, P.J., Platt, S., Robinson, K., Sackman, J. and O’Donnell, M.O. 2020. Canine cognitive dysfunction patients have reduced total hippocampal volume compared with aging control dogs: a comparative magnetic resonance imaging study. Open Vet. J. 10, 438–442. Dewey, C.W., Brunke, M.W. and Sakovitch, K. 2022. Transcranial photobiomodulation (laser) therapy for cognitive impairment: a review of molecular mechanisms and potential application to canine cognitive dysfunction (CCD). Open Vet. J. 12, 256–263. Enengl, J., Hamblin, M.R. and Dungel, P. 2020. Photobiomodulation for Alzheimer’s disease: translating basic research to clinical application. J. Alzheim. Dis. 75, 1073–1082. Gonzalez-Lima, F. and Barrett, D.W. 2014. Augmentation of cognitive brain functions with transcranial lasers. Front. Syst. Neurosci. 8, 36. Gonzalez-Martinez, A., Rosado, B., Pesini, P., Garcia-Belenguer, S., Palacio, J., Villegas, A., Suarez, M.L., Santamarina, G. and Sarasa, M. 2013. Effect of age and severity of cognitive dysfunction on two simple tasks in pet dogs. Vet. J. 198(1), 176–181. Hamblin, M.R. 2016. Shining light on the head: photobiomodulation for brain disorders. BBA Clin. 6, 113–124. Hamblin, M.R. 2019. Photobiomodulation for Alzheimer’s disease: Has the light dawned? Photonics 6(3), 77. Hennessy, M. and Hamblin, M.R. 2017. Photobiomodulation and the brain: a new paradigm. J. Opt. 19(1), 013003. Landsberg, G.M., Nichol, J. and Araujo, J.A. 2012. Cognitive dysfunction syndrome: a disease of canine and feline brain aging. Vet. Clin. Small Anim. 42, 749–768. Lapchak, P.A. 2012. Transcranial near-infrared laser therapy applied to promote clinical recovery in acute and chronic neurodegenerative diseases. Expert. Rev. Med. Devices. 9, 71–83. Mintzopoulos, D., Gillis, T.E., Tedford, C.E. and Kaufman, M.J. 2017. Effects of near-infrared light on cerebral bioenergetics measured with phosphorous magnetic resonance spectroscopy. Photomed. Laser Surg. 35, 395–400. Naeser, M.A., Zafonte, R., Krengel, M.H., Martin, P.I., Frazier, J., Hamblin, M.R., Knight, J.A., Meehan, W.P. and Baker, E.H. 2014. Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: open-protocol study. J. Neurotrauma. 31, 1008–1017. Pan, Y., Landsberg, G., Mougeot, I., Kelly, S., Xu, H., Bhatnagar, S., Gardner, C.L. and Milgram, N.W. 2018. Efficacy of a therapeutic diet on dogs with signs of cognitive dysfunction syndrome (CDS): a prospective double blinded placebo controlled clinical study. Front. Nutr. 12(5), 1–10. Riegel, R.J. and Godbold, J.C. 2017. Fundamental information. In Laser therapy in veterinary medicine: photobiomodulation. Eds., Reigel, R.J. and Godbold, J.C., Ames, IA: Wiley, pp: 9–18. Salehpour, F., Ahmadian, N., Rasta, S.H., Farhoudi, M., Karimi, P. and Sadigh-Eteghad, S. 2017. Transcranial low-level laser therapy improves brain mitochondrial function and cognitive impairment in D-galactose-induced aging mice. Neurobiol. Aging. 58, 140–150. Saltmarche, A.E., Naeser, M.A., Ho, K.F., Hamblin, M.R. and Lim, L. 2017. Significant improvement in cognition in mild to moderately severe dementia cases treated with transcranial plus intranasal photobiomodulation: case series report. Photomed. Laser Surg. 35, 432–441. Stephens, B.J. 2019. Light is just the catalyst. In Veterinary laser therapy in small animal practice. Eds., Redondo, M.S. and Stephens, B.J. Sheffield, UK: 5M Publishing, pp: 3–9. | ||
| How to Cite this Article |
| Pubmed Style Dewey CW, Rishniw M, Brunke MW, Gerardi J, Sakovitch K. Transcranial photobiomodulation therapy improves cognitive test scores in dogs with presumptive canine cognitive dysfunction (CCD): A case series of five dogs. Open Vet. J.. 2024; 14(5): 1167-1171. doi:10.5455/OVJ.2024.v14.i5.11 Web Style Dewey CW, Rishniw M, Brunke MW, Gerardi J, Sakovitch K. Transcranial photobiomodulation therapy improves cognitive test scores in dogs with presumptive canine cognitive dysfunction (CCD): A case series of five dogs. https://www.openveterinaryjournal.com/?mno=179510 [Access: January 12, 2026]. doi:10.5455/OVJ.2024.v14.i5.11 AMA (American Medical Association) Style Dewey CW, Rishniw M, Brunke MW, Gerardi J, Sakovitch K. Transcranial photobiomodulation therapy improves cognitive test scores in dogs with presumptive canine cognitive dysfunction (CCD): A case series of five dogs. Open Vet. J.. 2024; 14(5): 1167-1171. doi:10.5455/OVJ.2024.v14.i5.11 Vancouver/ICMJE Style Dewey CW, Rishniw M, Brunke MW, Gerardi J, Sakovitch K. Transcranial photobiomodulation therapy improves cognitive test scores in dogs with presumptive canine cognitive dysfunction (CCD): A case series of five dogs. Open Vet. J.. (2024), [cited January 12, 2026]; 14(5): 1167-1171. doi:10.5455/OVJ.2024.v14.i5.11 Harvard Style Dewey, C. W., Rishniw, . M., Brunke, . M. W., Gerardi, . J. & Sakovitch, . K. (2024) Transcranial photobiomodulation therapy improves cognitive test scores in dogs with presumptive canine cognitive dysfunction (CCD): A case series of five dogs. Open Vet. J., 14 (5), 1167-1171. doi:10.5455/OVJ.2024.v14.i5.11 Turabian Style Dewey, Curtis Wells, Mark Rishniw, Matthew Warren Brunke, Joyce Gerardi, and Kasie Sakovitch. 2024. Transcranial photobiomodulation therapy improves cognitive test scores in dogs with presumptive canine cognitive dysfunction (CCD): A case series of five dogs. Open Veterinary Journal, 14 (5), 1167-1171. doi:10.5455/OVJ.2024.v14.i5.11 Chicago Style Dewey, Curtis Wells, Mark Rishniw, Matthew Warren Brunke, Joyce Gerardi, and Kasie Sakovitch. "Transcranial photobiomodulation therapy improves cognitive test scores in dogs with presumptive canine cognitive dysfunction (CCD): A case series of five dogs." Open Veterinary Journal 14 (2024), 1167-1171. doi:10.5455/OVJ.2024.v14.i5.11 MLA (The Modern Language Association) Style Dewey, Curtis Wells, Mark Rishniw, Matthew Warren Brunke, Joyce Gerardi, and Kasie Sakovitch. "Transcranial photobiomodulation therapy improves cognitive test scores in dogs with presumptive canine cognitive dysfunction (CCD): A case series of five dogs." Open Veterinary Journal 14.5 (2024), 1167-1171. Print. doi:10.5455/OVJ.2024.v14.i5.11 APA (American Psychological Association) Style Dewey, C. W., Rishniw, . M., Brunke, . M. W., Gerardi, . J. & Sakovitch, . K. (2024) Transcranial photobiomodulation therapy improves cognitive test scores in dogs with presumptive canine cognitive dysfunction (CCD): A case series of five dogs. Open Veterinary Journal, 14 (5), 1167-1171. doi:10.5455/OVJ.2024.v14.i5.11 |