| Research Article | ||
Open Vet. J.. 2024; 14(8): 1761-1770 Open Veterinary Journal, (2024), Vol. 14(8): 1761–1770 Research Article Bovine umbilical mesenchymal stem cell-conditioned medium increased expression of GLUT-4 in pancreas of diabetic rats induced by nicotinamide-streptozotocinHerawati Herawati1, Hevi Wihadmadyatami2, Muhammad Ali Zulfikar3, Ricadonna Raissa4, Made Bagus Auriva Mataram5, Siti Kurniawati6 and Dyah Ayu O.A. Pratama7*1Laboratory of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Brawijaya, Malang, Indonesia 2Department of Anatomy, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia 3Department of Chemistry, Faculty of Mathematics and Science, Institute Teknologi Bandung, Bandung, Indonesia 4Laboratory of Veterinary Pharmacology, Faculty of Veterinary Medicine, Universitas Brawijaya, Malang, Indonesia 5Laboratory of Veterinary Clinical Pathology, Faculty of Veterinary Medicine, Universitas Brawijaya, Malang, Indonesia 6Laboratory of Veterinary Microbiology, Faculty of Veterinary Medicine, Universitas Brawijaya, Malang, Indonesia 7Laboratory of Veterinary Pathology, Faculty of Veterinary Medicine, Universitas Brawijaya, Malang, Indonesia *Corresponding Author: Dyah Ayu Oktavianie Ardhiana Pratama. Laboratory of Veterinary Pathology, Faculty of Veterinary Medicine, Universitas Brawijaya, Malang, Indonesia. Email: dyah_ayu [at] ub.ac.id Submitted: 08/12/2023 Accepted: 11/07/2024 Published: 31/08/2024 © 2024 Open Veterinary Journal
ABSTRACTBackground: Diabetes is a degenerative disease associated with metabolic disorders. The majority of people have type 2 diabetes mellitus (DM) insulin resistance due to an unhealthy lifestyle. The development of DM treatment is also growing, one of which is using conditioned medium. Aim: This study aims to determine the effect of Bovine umbilical mesenchymal stem cell-conditioned medium (BUMSC-CM) on nicotinamide (NA) and streptozotocin (STZ) induced rats as an animal model of DM. Methods: The study began with the in vitro docking of Cholecalciferol with aldolase reductase and glucokinase. In the in vivo study, animal models were divided into five groups: group A (negative control), group B (diabetic rats), group C (NA+STZ+Metformin), group D (NA+STZ+ BUMSC-CM 0.2 ml/kg BW), and group E (NA+STZ+ BUMSC-CM 0.5 ml/kg BW). Blood sugar levels were checked, and BUMSC-CM was administered by intramuscular injection at four-day intervals for a duration of 16 days. Blood sugar levels were also sampled, and GLUT4 histochemical and immunohistochemical staining was performed. Results: The results showed that Cholecalciferol can bind to aldolase reductase ASP43 and TYR48 and bind to glucokinase at TYR214 with hydrogen bonds. BUMSC-CM administration was able to reduce blood sugar well. In addition, BUMSC-CM also helped repair the tissue structure of the pancreas damaged by inflammation from STZ administration. Conclusion: This study can be concluded that the administration of BUMSC-CM can be an alternative cell-free therapy for patients with DM. Keywords: Diabetes melitus, BUMSC-CM, Cholecalciferol, GLUT-4. IntroductionDiabetes mellitus (DM) is a chronic metabolic disorder with multiple etiologies characterized by high blood sugar levels and impaired carbohydrate, lipid, and protein metabolism due to impaired insulin secretion and/or insulin resistance (American Diabetes Association, 2009; Galicia-Garcia et al., 2020). Diabetes is one of the biggest global public health problems, imposing a heavy global burden on public health and socioeconomic development. Approximately 422 million people worldwide have diabetes, the majority living in low- and middle-income countries and 1.5 million deaths are directly attributable to diabetes each year (Lin et al., 2020). By 2019, an estimated 463 million people are expected to be living with diabetes, representing 9.3% of the global adult population (20–79 years). This number is expected to increase to 578 million (10.2%) by 2030 and 700 million (10.9%) by 2045 (Saeedi et al., 2019). DM is a chronic metabolic disorder in which the pancreas does not produce enough insulin, or the body cannot use the insulin produced effectively (Sone, 2017; Dilworth et al., 2021). Insulin is a hormone that regulates the balance of blood sugar levels. Insulin resistance develops in the early stages of T2DM and is defined as a loss of insulin sensitivity, resulting in impaired glucose uptake from the blood. Glucose transporter type 4 (GLUT4) is an exocytotic vesicle that regulates insulin in glucose uptake in fat and muscle cells. Reduced gene expression of this transporter is directly related to the development of insulin resistance (Leguisamo et al., 2012; Leto and Saltiel, 2012). Inflammatory cytokines produced by adipose tissue, such as TNF-α (tumor necrosis factor-α) and interleukin-6 (IL-6) have been associated with reduced GLUT4 expression (Lainampetch et al., 2019). With the development of technology and knowledge in the field of health, treatment using stem cells provides hope for people with diabetes. However, this treatment still gives concerns about stem cell rejection of infections that occur due to a decrease in the immune system (Hoang et al., 2022). Some studies reveal that stem cells are able to repair tissues because they have the ability to secrete growth factors that have an impact on tissue repair (Pawitan, 2014; Ozkan et al., 2022). Growth factors secreted in stem cells can be found in a culture medium, called conditioned medium. The use of CM has several advantages over the use of stem cells as it can be produced, frozen, packaged and transported more easily. In addition, since it does not contain cells, there is no need to match donors and recipients to avoid rejection issues (Pawitan, 2014; Chouaib et al., 2023). Previous studies reported various benefits from CM such as increasing skeletal tissue regeneration, stimulating vascular repair mechanisms, improving acute lung injury, and other benefits in the medical field (Timmers et al., 2011; Ionescu et al., 2012; Ando et al., 2014). Therefore, stem cell-derived CMs have promising prospects to be produced as drugs for regenerative medicine and will develop rapidly shortly. We also hypothesized that CM may also exert beneficial effects on diabetes. The objective of this study was to explore the effect of bovine umbilical mesenchymal stem cell-conditioned medium (BUMSC-CM) on rats induced nicotinamide (NA) and streptozotocin (STZ) to mimic DM. Materials and MethodsIn silico molecular dockingBUMSC-CM metabolite compounds were analyzed using liquid chromatography-mass spectrometry (LC-MS). The screening results can be seen in Larasati et al. (2021). BUMSC-CM metabolite compounds were downloaded from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). The target proteins namely (PDB ID: 3G5E and glucokinase (PDB ID: 4IXC) were downloaded from the RCSB PDB database database (https://www.rcsb.org/ligand/). The bioactive compound of BUMSC-CM and target proteins (aldolase reductase and glucokinase) were prepared sequentially with the PyRX 0.8 and discovery studio ver 19.0.0 program (https://www.3ds.com/products-services/biovia/) (Widiyananda et al., 2021). Then, the proteins and bioactive compounds were interacted using AutoDock Vina software and visualized with Discovery Studio Visualizer ver.19.0.0 (https://www.3ds.com/products -service/biovia/) (Widyananda et al., 2023). BUMSC-CM preparationIsolation of endothelial cells from the umbilical cord vein was obtained by dissociating endothelial cells by introducing collagenase in the umbilical vein. The initial stage is umbilical sterilization with sterile DPBS and antibiotics, and then the umbilical cord is cut into 15 cm. One end of the umbilical cord is closed so that the collagenase solution in HBBS can be deposited in the lumen of the umbilical vein; close the other end with arterial clamps, then incubate for 30 minutes. After incubation, the umbilical is massaged so that the endothelial cells are released and can be collected in a conical tube. The collagenase solution mixed with endothelial cells was centrifuged at 2,400 rpm for 5 minutes at 24◦C. The supernatant was discarded, and the pellet was collected to be grown on a DMEM growth medium supplemented with 10% FBS, 2% Penicillin/Streptomycin, and 0.5% amphotericin). Cells were cultured in T25 flasks with 7 ml of medium. After 80%, the medium is collected and filtered, while the endothelial cells will be subcultured or cryopreserved if not used. Experimental designTwenty-five healthy male Sprague Dawley (SD) rats aged 7–9 weeks weighing 150–180 g were used in this study. Rats were obtained from the Integrated Research and Testing Laboratory (LPPT) of Gadjah Mada University and maintained at the Laboratory of Animal Experiments, Faculty of Veterinary Medicine, Brawijaya University. Animals were adapted 7 days before the experiment started. Animals were kept in group cages with covers and well-conditioned in a controlled room with standard temperature (22°C), and humidity (55%). Mice were provided with standard feed and water i. A 12-hour alternating light-dark cycle was maintained. After acclimatization, male SD rats were randomly divided into five groups,
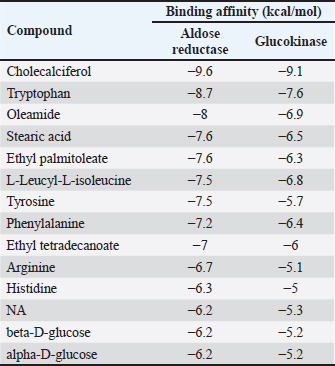
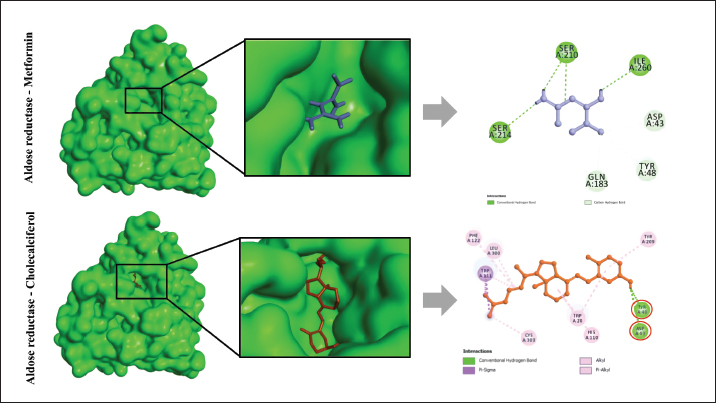
Experimental procedure and induction of type 2 diabetes animal modelDiabetes induction refers to the method of Furman et al. (2021) with several modifications (Furman et al., 2021). NA (Merck KGaA, Darmstadt, Germany) dose 110 mg/kg BW dissolved in 0.9% NaCl was administered intraperitoneally. STZ (Nucalai Tesque, Kyoto, Japan) dose of 65 mg/kg BW dissolved in citrate buffer was administered intraperitoneally given 15 minutes after NA administration. NA + STZ was used in this study to induce type 2 diabetes. Seven days post NA + STZ induction, blood sugar was checked using a glucometer (EasyTouch-GCU, Miaoli, Taiwan). The animals with blood glucose concentrations >200 mg/dl will be used for the study. Blood sugar checks were performed on days 0, 7, 12, 17, 22, and 27. Rats were sacrificed on day 28 using high dose ketamine 10% (Holland, Metaalweg, Netherland) 100 mg/kg BW and xylazine 2% 10 mg/kg BW were collected for hepatic organ samples. Histological investigation by hematoxylin-eosin stainingLiver samples were fixed in 10% formalin. After fixation, dehydration using graded alcohol and clearing using xylene were performed before immersion in paraffin wax. Block preparations were cut to a 5-μm thickness using a microtome. For histological examination, staining using hematoxylin and eosin (H&E) was performed. Observations were made with a microscope and Optilab (Cardiff et al., 2014). GLUT4 immunohistochemical stainingGLUT 4 immunohistochemistry was carried out according to the method of Feitosa et al. (2014) with some necessary modifications (Feitosa et al., 2018). Immunohistochemical staining with StreptAvidin biotin complex (SABC) technique was performed using an IHC Kit (Biocare Medical, Prinsessegracht, The Netherland) to determine GLUT 4 in the preparations. The IHC step begins with deparaffinization and rehydration, then washing with phosphate buffer saline (PBS) for 5 minutes. Drop biocore background sniper and incubate for 15 minutes at room temperature, followed by administration of the primary antibody, rabbit anti-GLUT-4 (Invitrogen, USA), dilution ratio antibody and diluent was 1:800 for 2 hours at room temperature; slides were washed again with PBS for 5 minutes then drop Trek Universal Link and incubate for 20 minutes at room temperature. wash slides using PBS 5 minutes before administration of Trek Avidin HRP. The Avidin HRP tracks are incubated for 15 minutes. Wash with PBS and drop diaminobenzidine (DAB) in the dark condition. Images were observed under a microscope. Immune-rectified cells were scored by five observers. -: no intensity; +: weak intensity; ++: moderate intensity; +++: strong intensity, ++++: very strong intensity. Ethical approvalEthical standards were followed and all protocols were authorized by the ethical clearance committee of Brawijaya University (No. 16-KEP-UB-2023). ResultsMolecular docking of cholecalciferol with aldose reductase and glucokinaseInteraction between aldose reductase and BUMSC-CM compounds. The docking result shows that from 14 compounds had a more negative binding affinity than Metformin as the native ligand when interacting with aldose reductase. In the active compounds of BUMSC-CM the most negative binding affinity value was cholecalciferol, which was −9.6 kcal/mol (Table 1). Both compounds bind to the active site of the aldose reductase. Cholecalciferol interacts at the same site as the native ligand therefore it has potential as a competitive inhibitor of aldose reductase. Cholecalciferol interacted with aldose reductase by forming two hydrogen bonds in the active site, the amino acid residues are ASP43 and TYR48 (Fig. 1). Table 1. Binding affinity value of the interaction between BUMSC-CM metabolite compounds and Metformin with the proteins.
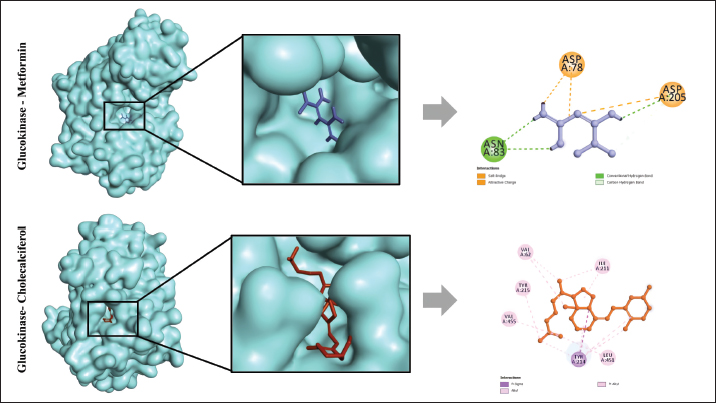
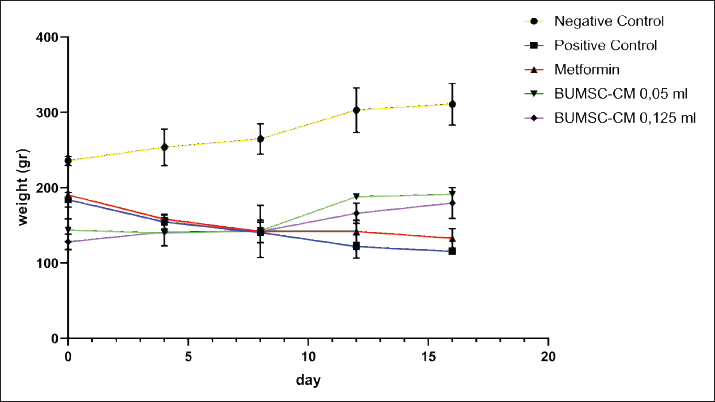
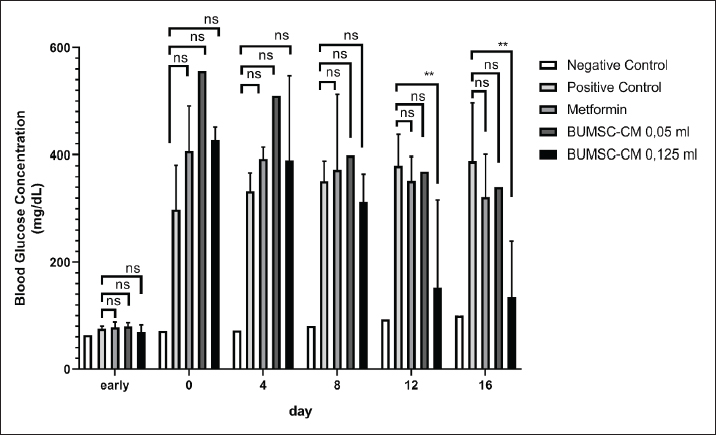
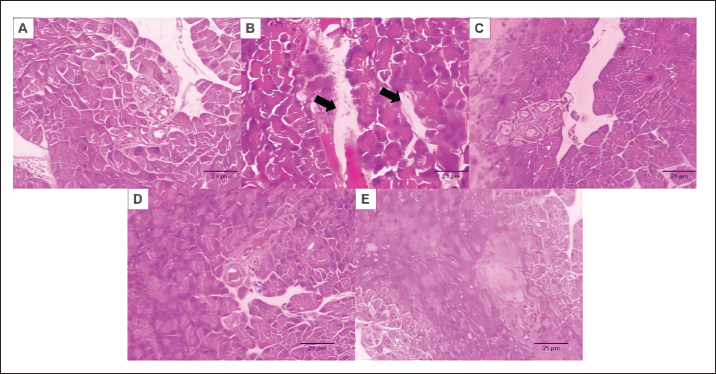
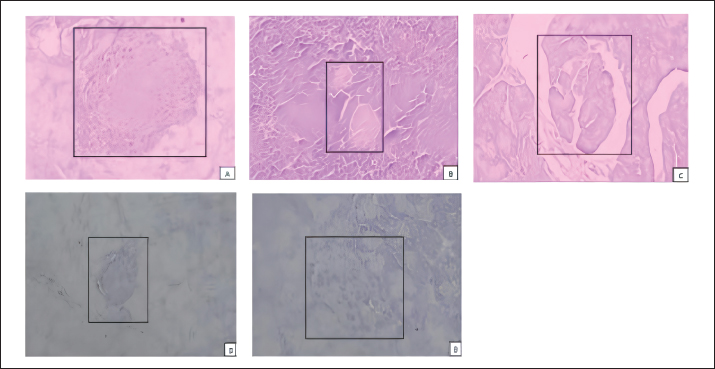
Fig. 1. The interaction between aldose reductase with ligands. (A) Ligands binds in the aldolase reductase active site. (B) The visualization of the interaction between aldose reductase and ligands. The ligands are metformin and cholecalciferol Interaction between glucokinase and BUMSC-CM compounds. The docking result shows that 14 compounds had a more negative binding affinity than Metformin as the native ligand when interacting with glucokinase. In the active compounds of BUMSC-CM the most negative binding affinity value was cholecalciferol, which was −9.1 kcal/mol (Table 1). Both compounds bind to the active site of the aldose reductase. Cholecalciferol interacts at the same site as the native ligand; therefore, it has potential as an inductor of glucokinase. Cholecalciferol interacts with glucokinase by forming phi sigma bonds in the active site, the amino acid residue is TYR214 (Fig. 2). The effect of BUMSC-CM body weights in T2DM ratsOn the sixteenth day after treatment, groups of BUMSC-CM treatment do not show reduced body weight as the metformin treatment and negative control do (Fig. 3). Between the BUMSC-CM at 0.05 and 0.125 ml, the dose of 0.05 ml shows better body weight increases. The effect of BUMSC-CM on fasting blood glucose levels in T2DM ratsOn day seven after STZ induction, groups 2, 3, 4, and 5 experienced an increase in fasting blood sugar levels to > 200 mg/dl (Fig. 4). Group 2 experienced an increased fasting blood sugar until the 27th day of treatment (Fig. 4). After treatment for 20 days, there was a decrease in fasting blood sugar in groups 3, 4, and 5. The decrease in fasting blood sugar in groups 3 and 4 was not significant compared to group 2, whereas in group 5 on days 22 and 27, after administering 0.125 ml BUMSC, there was a significant decrease in fasting blood sugar compared to group 2 (p < 0.05, Fig. 4). Histopathological change of pancreasHistopathology of the pancreas showed that the pancreas of control rats (A) showed no structural changes. The islets of Langerhans could be distinguished from the surrounding acinar cells. Significant changes were seen in the pancreas of the positive control (diabetic rats), the acinar cells showed small vacuoles indicating swelling. The difference between islets of Langerhans and acinar cells was less clearly observed and almost disappeared. In rats given metformin (C), there was also swelling of acinar cells, thickened connective tissue, and congestion. In the treatment groups (D) and (E), it was seen that the acinar cells and Langerhans islets could be distinguished, although there was still congestion. Immunoreactivity of GLUT-4 in rat’s pancreas tissueBased on the results of immunoreactivity, in the pancreas, using GLUT-4 in IHC staining, no brown color was detected, indicating a small amount of GLUT-4 expression. The analysis by visualized is included in Table 2. DiscussionType 2 DM is one of the most common metabolic problems in the world, and its development is primarily caused by a combination of two main factors: inadequate insulin secretion by pancreatic cells and the failure of insulin-sensitive tissues to respond to insulin (Vaiserman, 2017). Insulin release and action must precisely meet metabolic demand; thus, the molecular mechanisms involved in insulin synthesis and release and insulin response in tissues must be closely controlled (Galicia-Garcia et al., 2020). Diabetes-related complications are exacerbated by treatment issues. Treatment issues also support current problems related to diabetes. Recently, therapy using endothelial cells has been considered, one of which is BUMSC-CM. In this study, we explore BUMSC Type 2 DM in Mouse (Rattus norvegicus).
Fig. 2. The interaction between glucokinase with ligands. (A) Ligands binds in the glucokinase active site. (B) The visualization of the interaction between glucokinase and ligands. The ligands are metformin and cholecalciferol.
Fig. 3. The effect of BUMSC on the body weight of rats after 16 days of treatment.
Fig. 4. The effect of BUMSC on the fasting blood sugar levels of rats after 20 days of treatment. Table 2. The GLUT-4 expression in pancreas.
Based on the in silico modeling, we have to explore the aldose reductase, which is present in all targeted tissues for diabetes. In hyperglycemic conditions, aldose leads to further complications in diabetes due to the polyol pathway (Thakur et al., 2021). The polyol pathway operates parallel with glycolysis (a minor route of glucose metabolism). An aldose reductase inhibitor can subtract the secondary diabetic complication (Singh et al., 2021). Different from aldose reductase, glucokinase has an important role in blood glucose homeostasis (Ren et al., 2022). Glucokinase recognizes levels of blood glucose like a sensor, so this is an important key role. In pancreatic beta-cells, it ensures that insulin secretion is matched to the circulating blood glucose level, in the liver it facilitates glycogen storage and the post-prandial clearance of glucose from the bloodstream, and in certain neurons and neuroendocrine cells, it mediates glucose sensing. Its critical importance for insulin secretion is convincingly demonstrated by inactivating mutations in the glucokinase gene that cause diabetes, whereas activating mutations result in congenital hyperinsulinism. Another component is Cholecalciferol, which binds to aldolase and glucokinase proteins at their active sites. Its binding affinity value is also lower than Metformin as a drug standard, indicating that cholecalciferol can act as a good competitor for aldose glucokinase and an inductor for glucokinase. Cholecalciferol, also known as vitamin D3, is a secosteroid that can be found in dairy products and eggs. Cholecalciferol has a role in maintaining calcium and phosphate levels (Borojević et al., 2022). Cholecalciferol also has an important role in improving blood glucose control and cholesterol profiles in vitamin D3-deficient individuals who have type 2 diabetes. In another study, the indication for cholecalciferol is one of the dietary supplementations for hypothyroidism, osteoporosis prevention, and vitamin D deficiency/insufficiency in healthy patients or patients with chronic kidney disease (Lips et al., 2017).
Fig. 5. Histopathological Micrograph of Rat’s Pancreas Tissue induced by NA-STZ treated by BUMSC-CM. (A) Negative control group, (B) diabetic rats group (NA+STZ), (C) diabetic rats + metformin, (d) diabetic rats BUMSC-CM 0.2 ml/kgBB, and (E) diabetic rats BUMSC-CM 0.5 ml/kgBB. There are no structural changes in the control group, acinar cells and langerhans islets cannot be distinguished, there is congestion and hemorrhagic. The treatment group with BUMSC-CM showed an improvement in structure, and acinar cells and islets of langerhans began to be distinguished. Scale bar 50 µm.
Fig. 6. Immunohistochemical staining of Rattus norvegicus pancreatic tissue (100x magnification) (A) Negative control group, (B) diabetic rats group (NA+STZ), (C) diabetic rats + metformin, (D) diabetic rats BUMSC-CM 0.2 ml/kgBB, (E) diabetic rats BUMSC-CM 0.5 ml/kgBB., no detected brown color on the of Langerhans. In this study, also observed in histopathology. The data were also obtained in this investigation. The findings revealed that there were substantial disparities between the groups, specifically between group E (STZ+BUMSC 0.5 ml/kg) and the other diabetic groups. Bovine umbilical mesenchymal stem cells (BUMSCs) can also influence and regulate angiogenesis, as well as play a function in immunomodulation and angiogenesis (Cajero-Jua Rez et al., 2023). In contrast, in the pancreas, using GLUT-4 immunohistochemical staining, no brown color was found, indicating the absence of GLUT-4 expression. GLUT-4 can be found in muscle and fat. Meanwhile, in the pancreas, fat tissue is also found. The results of this study are slightly different from previous studies which reported that CM increased insulin resistance in C2C12 cells with increased GLUT4 expression (Kim et al., 2019). Based on this, it shows that things can be concluded: first, the pancreas did not experience fatty degeneration, second, the pancreas did not experience fat infiltration, and third, GLUT4 expression in the pancreas was not found (da Silva Rosa et al., 2020; Yin et al., 2022). In another study, in contrast, glucose transporter GLUT4 is widely known for facilitating blood glucose transfer into insulin-sensitive muscle and adipose tissue. GLUT4 is also found in the endocrine pancreas of mice, rats, and humans (Bähr et al., 2012). Furthermore, high glucose lowered and insulin increased GLUT4 expression in pancreatic cells. In contrast, high glucose levels boosted GLUT4 expression, whilst insulin decreased GLUT4 expression in pancreatic cells. In vivo, tests revealed that GLUT4 expression is much higher in the pancreatic tissue of type 2 diabetic rats and patients than in the nondiabetic control group. ConclusionThe findings of our study elucidated that Cholecalciferol exhibited notable interactions, forming hydrogen bonds with aldose reductase at amino acid residues ASP43 and TYR48, and with glucokinase at TYR214. Moreover, administration of BUMSC-CM demonstrated efficacy in effectively lowering blood sugar levels. Additionally, BUMSC-CM exhibited promising capabilities in restoring the structural integrity of pancreatic tissue damaged by inflammation induced by STZ administration. Additional research involving a broader spectrum of diabetes-related target proteins is essential to substantiate and corroborate the promising antidiabetic efficacy of BUMSC-CM. AcknowledgmentThe authors would like to thank all veterinarians who helped with the sample collection. FundingThis Research was Supported by Indonesia Collaboration Research, Lembaga Penelitian dan Pengabdian Masyarakat (LPPM) Universitas Brawijaya with contract number no: 801.13/UN10.C10/TU/2023. Author contributionsConceptualization, HH, DAOP; Methodology, HH, DAOP, MAL, RR, MBAM; Validation, HH, RR, MBAM, SK; Formal analysis, HH, DAOP, RR; Investigation, HH, RR, MBAM, SK; Resources, HH, DAOP, MAL; Data curation, HH, DAOP, RR, MBAM, SK; Writing-original draft preparation, HH, DAOP, RR, MBAM, SK; Writing-review editing, HH, DAOP, RR, MBAM, SK; Visualization, RR, SK; Supervision, HH, DAOP; Project administration, HH; Funding acquisition, HH. Conflict of interestAll authors declared that there is no conflict of interest. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesAmerican Diabetes Association. 2009. Diagnosis and classification of diabetes mellitus. Diabetes Care. Available via https://doi.org/10.2337/dc09-S062 Ando, Y., Matsubara, K., Ishikawa, J., Fujio, M., Shohara, R., Hibi, H., Ueda, M. and Yamamoto, A. 2014. Stem cell-conditioned medium accelerates distraction osteogenesis through multiple regenerative mechanisms. Bone 61, 82–90. Bähr, I., Bazwinsky-Wutschke, I., Wolgast, S., Hofmann, K., Streck, S., Mühlbauer, E., Wedekind, D. and Peschke, E. 2012. GLUT4 in the endocrine pancreas—indicating an impact in pancreatic islet cell physiology? Horm. Metab. Res. 44, 442–450. Borojević, A., Jauković, A., Kukolj, T., Mojsilović, S., Obradović, H., Trivanović, D., Živanović, M., Zečević, Ž., Simić, M., Gobeljić, B., Vujić, D. and Bugarski, D. 2022. Vitamin D3 stimulates proliferation capacity, expression of pluripotency markers, and osteogenesis of human bone marrow mesenchymal stromal/stem cells, partly through SIRT1 signaling. Biomolecules 12, 323. Cajero-Jua Rez, M., Avila, B., Ochoa, A., Garrido-Guerrero, E., Varela-Echavarrìa, A., Martìnez De La Escalera, G. and Clapp, C. 2002. Immortalization of Bovine Umbilical Mesenchymal Stem Cells: a model for the study of vascular endothelium. Eur. J. Cell. Biol. 81(1), 1–8. Cardiff, R.D., Miller, C.H. and Munn, R.J. 2014. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb. Protoc. 2014(6), 655–658. Chouaib, B., Haack-Sørensen, M., Chaubron, F., Cuisinier, F. and Collart-Dutilleul, P.Y. 2023. Towards the standardization of mesenchymal stem cell secretome-derived product manufacturing for tissue regeneration. Int. J. Mol. Sci. 24(16), 12594. da Silva Rosa, S.C., Nayak, N., Caymo, A.M. and Gordon, J.W. 2020. Mechanisms of muscle insulin resistance and the cross-talk with liver and adipose tissue. Physiol. Rep. 8, 314607. Dilworth, L., Facey, A. and Omoruyi, F. 2021. Diabetes mellitus and its metabolic complications: the role of adipose tissues. Int. J. Mol. Sci. 22(14), 7644. Feitosa, S.G., Viana, K.F., Luna, E.C.M., Costa, F.W.G., Cavalcante, R.B., Chaves, F.N. and Pereira, K.M.A. 2018. Immunohistochemical evaluation of GLUT-3 and GLUT-4 in oral epithelial dysplasia and oral squamous cell carcinoma. Asian Pac. J. of Cancer Prev. 19(7), 1779–1783. Furman, B.L. 2021. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. 1(4), e78. Galicia-Garcia, U., Benito-Vicente, A., Jebari, S., Larrea-Sebal, A., Siddiqi, H., Uribe, K.B., Ostolaza, H. and Martín, C. 2020. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 21(17), 6275. Hoang, D.M., Pham, P.T., Bach, T.Q., Ngo, A.T.L., Nguyen, Q.T., Phan, T.T.K., Nguyen, G.H., Le, P.T.T., Hoang, V.T., Forsyth, N.R., Heke, M. and Nguyen, L.T. 2022. Stem cell-based therapy for human diseases. Signal Transduct. Target Ther. 7, 272. Ionescu, L., Byrne, R.N., van Haaften, T., Vadivel, A., Alphonse, R.S., Rey-Parra, G.J. and Thébaud, B. 2012. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am. J. of Physiol. Lung Cell. Mol. Physiol. 303(11), 967–977. Kim, K.S., Choi, Y.K., Kim, M.J., Hwang, J.W., Min, K., Jung, S.Y. et al. 2021. Umbilical cord-mesenchymal stem cell-conditioned medium improves insulin resistance in C2C12 cell. Diabetes Metab. J. 45(2), 260. Lainampetch, J., Panprathip, P., Phosat, C., Chumpathat, N., Prangthip, P., Soonthornworasiri, N., Puduang, S., Wechjakwen, N. and Kwanbunjan, K. 2019. Association of tumor necrosis factor alpha, interleukin 6, and C-reactive protein with the risk of developing type 2 diabetes: a retrospective cohort study of Rural Thais. J. Diabetes Res. 2019, 9051929. Larasati, V.A., Lembang, G.V., Tjahjono, Y., Winarsih, S., Ana, I.D., Wihadmadyatami, H. and Kusindarta, D.L. 2022. In Vitro neuroprotective effect of the bovine umbilical vein endothelial cell conditioned medium mediated by downregulation of IL-1β, caspase-3, and caspase-9 expression. Vet. Sci. 9, 48. Leguisamo, N.M., Lehnen, A.M., Machado, U.F., Okamoto, M.M., Markoski, M.M., Pinto, G.H. and Schaan, B.D. 2012. GLUT4 content decreases along with insulin resistance and high levels of inflammatory markers in rats with metabolic syndrome. Cardiovasc. Diabetol. 11(100), 1–10. Leto, D. and Saltiel, A.R. 2012. Regulation of glucose transport by insulin: Traffic control of GLUT4. Nat. Rev. Mol. Cell Biol. 13, 383–396. Lin, X., Xu, Y., Pan, X., Xu, J., Ding, Y., Sun, X., Song, X., Ren, Y. and Shan, P.F. 2020. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci. Rep. 10(1), 1–11. Lips, P., Goldsmith, D. and de Jongh, R. 2017. Vitamin D and osteoporosis in chronic kidney disease. J. Nephrol. 30, 671–675. Ozkan, S., Isildar, B., Ercin, M., Gezginci-Oktayoglu, S., Konukoglu, D., Neşetoğlu, N., Oncul, M. and Koyuturk, M. 2022. Therapeutic potential of conditioned medium obtained from deferoxamine preconditioned umbilical cord mesenchymal stem cells on diabetic nephropathy model. Stem Cell Res. Ther. 13(1), 438. Pawitan, J.A. 2014. Prospect of stem cell conditioned medium in regenerative medicine. Biomed. Res. Int. 2014, 965849. Ren, Y., Li, L., Wan, L., Huang, Y. and Cao, S. 2022. Glucokinase as an emerging anti-diabetes target and recent progress in the development of its agonists. J. Enzyme Inhib. Med. Chem. 37(1), 606–615. Saeedi, P., Petersohn, I., Salpea, P., Malanda, B., Karuranga, S., Unwin, N., Colagiuri, S., Guariguata, L., Motala, A.A., Ogurtsova, K., Shaw, J.E., Bright, D. and Williams, R. 2019. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 157, 107843. Singh, M., Kapoor, A. and Bhatnagar, A. 2021. Physiological and pathological roles of aldose reductase. Metabolites. 11(10), 655. Sone, H. 2017. Diabetes Mellitus. In: Encyclopedia of cardiovascular research and medicine. Amsterdam, The Netherlands: Elsevier, pp: 9–16. Thakur, S., Gupta, S.K., Ali, V., Singh, P. and Verma, M. 2021. Aldose Reductase: a cause and a potential target for the treatment of diabetic complications. Arch. Pharm. Res. 44, 655–667. Timmers, L., Lim, S.K., Hoefer, I.E., Arslan, F., Lai, R.C., van Oorschot, A.A., Goumans, M.J., Chaylendra, S., Sze, S.K., Choo, A., Piek, J.J., Doevendans, P.A., Pasterkamp, G. and de Kleijn, D.P.V. 2011. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res. 6(3), 206–214. Vaiserman, A.M. 2017. Early-life nutritional programming of type 2 diabetes: experimental and quasi-experimental evidence. Nutrients. 9(3), 236. Widyananda, M.H., Fatchiyah, F., Muflikhah, L., Ulfa, S.M. and Widodo, N. 2023. Computational examination to reveal Kaempferol as the most potent active compound from Euphorbia hirta against breast cancer by targeting AKT1 and ERα. Egypt. J. Basic Appl. Sci. 10(1), 753–767. Widyananda, M.H., Pratama, S.K., Samoedra, R.S., Sari, F.N., Kharisma, V.D., Ansori, A.N.M., and Antonius, Y. 2021. Molecular docking study of sea urchin (Arbacia lixula) peptides as multi-target inhibitor for non-small cell lung cancer (NSCLC) associated proteins. J. Pharm. Pharmacogn. Res. 9(4), 484–496. Yin, X., Chen, Y., Ruze, R., Xu, R., Song, J., Wang, C. and Xu, Q. 2022. The evolving view of thermogenic fat and its implications in cancer and metabolic diseases. Signal Transduct. Target Ther. 7(1), 324. | ||
| How to Cite this Article |
| Pubmed Style Herawati H, Wihadmadyatami H, Zulfikar MA, Raissa R, Mataram MBA, Kurniawati S, Pratama DAO. Bovine umbilical mesenchymal stem cell-conditioned medium increased expression of GLUT-4 in pancreas of diabetic rats induced by nicotinamide-streptozotocin. Open Vet. J.. 2024; 14(8): 1761-1770. doi:10.5455/OVJ.2024.v14.i8.3 Web Style Herawati H, Wihadmadyatami H, Zulfikar MA, Raissa R, Mataram MBA, Kurniawati S, Pratama DAO. Bovine umbilical mesenchymal stem cell-conditioned medium increased expression of GLUT-4 in pancreas of diabetic rats induced by nicotinamide-streptozotocin. https://www.openveterinaryjournal.com/?mno=179840 [Access: January 24, 2026]. doi:10.5455/OVJ.2024.v14.i8.3 AMA (American Medical Association) Style Herawati H, Wihadmadyatami H, Zulfikar MA, Raissa R, Mataram MBA, Kurniawati S, Pratama DAO. Bovine umbilical mesenchymal stem cell-conditioned medium increased expression of GLUT-4 in pancreas of diabetic rats induced by nicotinamide-streptozotocin. Open Vet. J.. 2024; 14(8): 1761-1770. doi:10.5455/OVJ.2024.v14.i8.3 Vancouver/ICMJE Style Herawati H, Wihadmadyatami H, Zulfikar MA, Raissa R, Mataram MBA, Kurniawati S, Pratama DAO. Bovine umbilical mesenchymal stem cell-conditioned medium increased expression of GLUT-4 in pancreas of diabetic rats induced by nicotinamide-streptozotocin. Open Vet. J.. (2024), [cited January 24, 2026]; 14(8): 1761-1770. doi:10.5455/OVJ.2024.v14.i8.3 Harvard Style Herawati, H., Wihadmadyatami, . H., Zulfikar, . M. A., Raissa, . R., Mataram, . M. B. A., Kurniawati, . S. & Pratama, . D. A. O. (2024) Bovine umbilical mesenchymal stem cell-conditioned medium increased expression of GLUT-4 in pancreas of diabetic rats induced by nicotinamide-streptozotocin. Open Vet. J., 14 (8), 1761-1770. doi:10.5455/OVJ.2024.v14.i8.3 Turabian Style Herawati, Herawati, Hevi Wihadmadyatami, Muhammad Ali Zulfikar, Ricadonna Raissa, Made Bagus Auriva Mataram, Siti Kurniawati, and Dyah Ayu O.a. Pratama. 2024. Bovine umbilical mesenchymal stem cell-conditioned medium increased expression of GLUT-4 in pancreas of diabetic rats induced by nicotinamide-streptozotocin. Open Veterinary Journal, 14 (8), 1761-1770. doi:10.5455/OVJ.2024.v14.i8.3 Chicago Style Herawati, Herawati, Hevi Wihadmadyatami, Muhammad Ali Zulfikar, Ricadonna Raissa, Made Bagus Auriva Mataram, Siti Kurniawati, and Dyah Ayu O.a. Pratama. "Bovine umbilical mesenchymal stem cell-conditioned medium increased expression of GLUT-4 in pancreas of diabetic rats induced by nicotinamide-streptozotocin." Open Veterinary Journal 14 (2024), 1761-1770. doi:10.5455/OVJ.2024.v14.i8.3 MLA (The Modern Language Association) Style Herawati, Herawati, Hevi Wihadmadyatami, Muhammad Ali Zulfikar, Ricadonna Raissa, Made Bagus Auriva Mataram, Siti Kurniawati, and Dyah Ayu O.a. Pratama. "Bovine umbilical mesenchymal stem cell-conditioned medium increased expression of GLUT-4 in pancreas of diabetic rats induced by nicotinamide-streptozotocin." Open Veterinary Journal 14.8 (2024), 1761-1770. Print. doi:10.5455/OVJ.2024.v14.i8.3 APA (American Psychological Association) Style Herawati, H., Wihadmadyatami, . H., Zulfikar, . M. A., Raissa, . R., Mataram, . M. B. A., Kurniawati, . S. & Pratama, . D. A. O. (2024) Bovine umbilical mesenchymal stem cell-conditioned medium increased expression of GLUT-4 in pancreas of diabetic rats induced by nicotinamide-streptozotocin. Open Veterinary Journal, 14 (8), 1761-1770. doi:10.5455/OVJ.2024.v14.i8.3 |