| Research Article | ||
Open Vet. J.. 2024; 14(5): 1259-1268 Open Veterinary Journal, (2024), Vol. 14(5): 1259–1268 Research Article Anti-tumour effects of lapatinib on HER2-positive canine prostatic carcinoma cell linesKenjiro Kaji, Tomoki Motegi, Tomohiro Yonezawa, Yasuyuki Momoi and Shingo Maeda*Department of Veterinary Clinical Pathobiology, Graduate School of Agricultural and Life Sciences, The University of Tokyo, Tokyo, Japan *Corresponding Author: Shingo Maeda. Department of Veterinary Clinical Pathobiology, Graduate School of Agricultural and Life Sciences, The University of Tokyo, Tokyo, Japan. Email: amaeda [at] g.ecc.u-tokyo.ac.jp Submitted: 07/12/2023 Accepted: 22/04/2024 Published: 31/05/2024 © 2024 Open Veterinary Journal
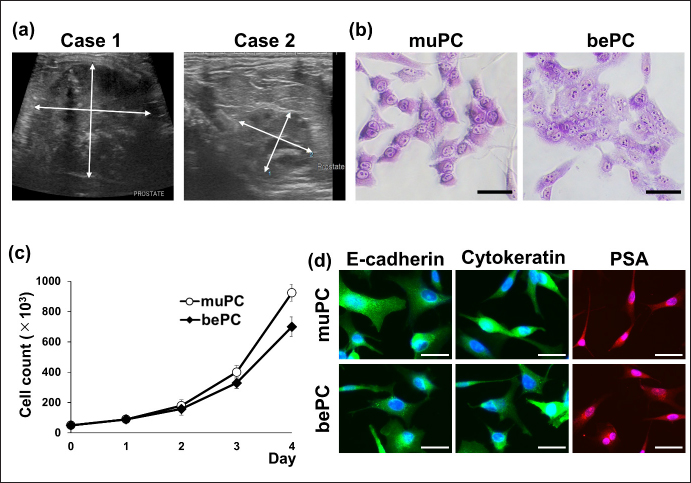
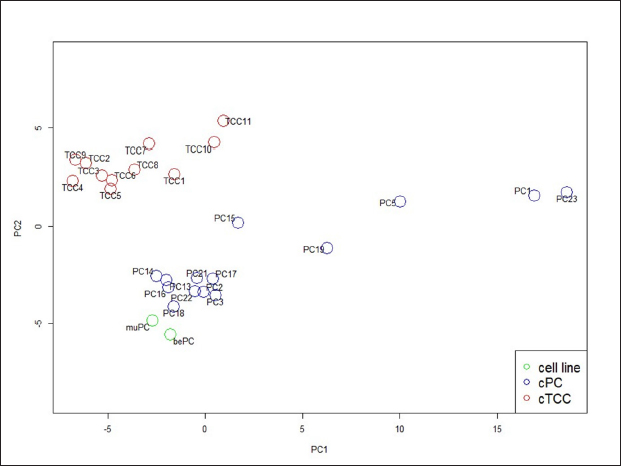
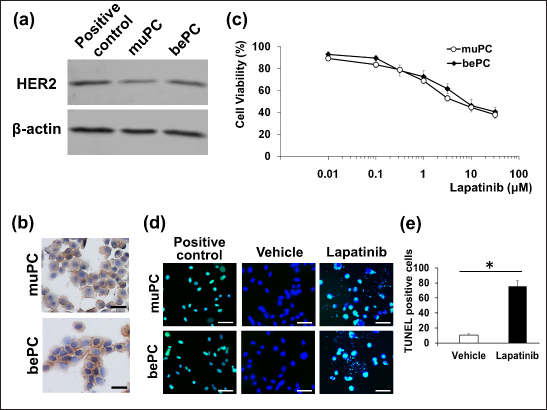
AbstractBackground: Canine prostatic carcinoma (cPC) is a urogenital tumour with a poor prognosis, for which no effective treatment has been established. Recently, it has been shown that human epidermal growth factor receptor type 2 (HER2) is overexpressed in cPC cells; however, the efficacy of HER2-targeted therapy remains unclear. Aim: Investigate the anti-tumour effect of lapatinib on HER2-positive cPC cell lines. Methods: Two cell lines (muPC and bePC) were established from two dogs with cPC and the effects of lapatinib treatment on cell proliferation, apoptosis, and HER2 downstream signalling were investigated. Furthermore, muPC was used to generate tumour-bearing mice, and the anti-tumour effects of lapatinib were examined in vivo. Results: Lapatinib treatment inhibited the proliferation and phosphorylation of Erk1/2 and Akt, which are downstream signals of HER2. Furthermore, the TUNEL assay showed that lapatinib induced apoptosis in both cell lines. The muPC-engrafted nude mouse model showed that lapatinib significantly inhibited tumour growth and increased the area of necrotic tumour tissue compared to the vehicle-treated groups. Conclusion: Lapatinib exerts anti-tumour effects on cPC cells by inhibiting HER-2 signalling. Keyword: Dogs, Human epidermal growth factor receptor type 2, Target therapy, Prostate cancer. IntroductionCanine prostatic carcinoma (cPC) is a malignant tumour that occurs primarily in the prostate glands of older dogs. In human males, prostate cancer is the second most common malignancy after lung cancer, accounting for about 7% of all malignancies (Rawla, 2019). On the other hand, the incidence of cPC is reported to be lower than that of humans, generally less than 1%, and the actual incidence may be even lower when differential diagnosis with canine transitional cell carcinoma (cTCC) is taken into account (Weaver, 1981; Cornell et al., 2000; Schrank and Romagnoli, 2020). In the early stages of the disease, cPC presents with non-specific symptoms. As the disease progresses, an enlarged prostate can lead to hematuria and difficulty urinating and defecating. Owing to these subtle initial symptoms, diagnosis is often delayed. In addition, because cPC is highly metastatic, the tumour may have already progressed or metastasized at the time of detection in many patients (LeRoy et al., 2009). Surgical treatment, radiotherapy, and chemotherapy are used to treat cPC. However, due to the frequently advanced state of the disease at diagnosis, the effectiveness of surgery and radiotherapy is limited (Axiak et al., 2012). Moreover, there is no universally established chemotherapy protocol for cPC, contributing to the poor prognosis of cPC cases (Cornell et al., 2000). Therefore, the development of effective medical options for cPC is desirable. Human epidermal growth factor receptor 2 (HER2) is a receptor tyrosine kinase belonging to the HER family that plays a role in the survival and proliferation of normal cells(Gutierrez and Schiff, 2011). HER2 overexpression resulting from gene copy number variations has been reported in various human cancers, such as prostate, breast, and bladder cancers, and is considered to be a poor prognostic factor (Cooke et al., 2001; Hernes et al., 2004; Dai et al., 2008; Lei et al., 2017; Soria et al., 2017). Therefore, in recent years, molecularly targeted drugs that target this HER2, such as lapatinib and trastuzumab, have been used for cancer therapy. Lapatinib blocks the intracellular tyrosine kinase regions of human epidermal growth factor receptor 1 (EGFR) and HER2, and inhibits MAPK and PI3K/Akt pathways to inhibit cancer cell growth and induce apoptosis (D’Amato et al., 2015). It has been reported that lapatinib and trastuzumab improve the prognosis of human patients with HER2-overexpressing breast cancer (Piccart-Gebhart et al., 2005; Burstein et al., 2008; Xu et al., 2017; Yuan et al., 2022). Similarly, HER2 overexpression has been reported in various canine cancers such as mammary gland carcinoma, lung cancer, intestinal carcinoma, cTCC, and cPC (Peña et al., 2014; Tsuboi et al., 2019; Yoshimoto et al., 2020; Brunetti et al., 2021; Sakai et al., 2021; Muscatello et al., 2022). Our previous study showed that the HER2 inhibitor lapatinib improves the prognosis of dogs with cTCC (Maeda et al., 2022). As HER2 overexpression is also observed in cPC, lapatinib may also be effective for therapy. In this study, we established novel cPC cell lines expressing HER2 and evaluated the anti-tumour effects of lapatinib in these cell lines. Materials and MethodsReagents and antibodiesLapatinib used for both in vitro and in vivo experiments was acquired from Selleck Chemicals (Texas, USA) and LC Laboratories (Massachusetts, USA), respectively. It was then dissolved in dimethyl sulfoxide (FUJIFILM Wako, Tokyo, Japan). Recombinant human epidermal growth factor (EGF) was purchased from RandD Systems (Minneapolis, MN, USA) and dissolved in phosphate-buffered saline (PBS; FUJIFILM Wako). Anti-HER2 (polyclonal, Dako, California, USA), anti-E-cadherin (4A2C7, Invitrogen, Massachusetts, USA), anti-pan-cytokeratin (AE1/AE3, Dako), and anti-prostate-specific antigen (PSA; polyclonal, Agilent, California, USA) antibodies were used for immunostaining of cPC cells. The secondary antibodies Alexa Fluor™ 488 and Alexa Fluor™ 568 were obtained from Thermo Fisher Scientific (Massachusetts, USA). Western blotting utilized anti-HER2 (19G5, NanoTools, Teningen, Germany), anti-Erk 1/2 (137F5, Cell Signaling Technology, Massachusetts, USA), anti-phospho-Tyr 202/Tyr 204-Erk 1/2 (D13.14.4E, Cell Signaling Technology), anti-Akt (C67E7, Cell Signaling Technology), anti-phospho-Ser 473-Akt (D9E, Cell Signaling Technology), and anti-β-actin (4967S, Cell Signaling Technology) antibodies. Secondary antibodies, IRDye680RD and IRDye800CW, were purchased from MandS TechnoSystems (Osaka, Japan). All the antibodies used for immunostaining and western blotting were diluted according to the manufacturer's recommendations. Establishment and cell culture of cPC cell lines, muPC, and bePCTumour samples were collected from two animals (case 1: Miniature Dachshund, 10 years old, castrated, and case 2: Toy Poodle, 14 years old, castrated) with primary prostate cancer using a urethral catheter and diagnosed by cytology. The samples were treated with collagenase (0.35%; Worthington, California, USA) at 37°C for 2 hours. Dissociated cells were cultured in RPMI1640 medium (Sigma-Aldrich, Missouri, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, California, USA) at 37°C under 5% CO2. Following single-cell cloning, the cell lines exhibiting monoclonal proliferation after 30 passages were designated as muPC (case 1) and bePC (case 2). Classifying prostate cancer and transitional cell carcinoma based on expressed genesTo develop a classifier for cPC and cTCC, we analyzed differential expression genes (DEGs) between prostate and bladder tissues. This analysis utilized previously published mRNA expression data in the DDBJ Sequenced Read Archive repository under accession numbers DRA011773 and DRA005844 (Maeda et al., 2018; Maeda et al., 2022). We identified DEGs between normal and tumour tissues for both organ types by applying a well-established method. We isolated 458 tumour-specific DEGs from this dataset, excluding those expressed in normal tissues, to define a gene expression profile for each tumour type's classifier. Our established cell lines were classified by extracting these DEGs using Transcripts Per Million (TPM) data, calculated from transcript abundance via RSEM V.1.3.3, and Principal Component Analysis (PCA) using R version 4.2.1 (R Core Team, Vienna, Australia). ImmunocytochemistryMuPC and bePC cells were seeded onto cover glasses at 2 x 105 cells/well. After 1 day, the samples were fixed in acetone for 10 minutes and washed three times with Tris-buffered saline (TBS; FUJIFILM Wako). The membranes were blocked with 5% skim milk in TBS for 1 hour. They were then treated with HER2 antibody (1:100) for 30 minutes at room temperature or with E-cadherin antibody (1:500), cytokeratin antibody (1:500), or PSA antibody (1:500) at 4°C overnight. After washing, samples treated with HER2 antibody were incubated with EnVision polymer reagent (Dako) for 10 minutes at 37°C, visualized with diaminobenzidine (Dako), and contrast stained with Mayer's hematoxylin (FUJIFILM Wako). Samples treated with E-cadherin, cytokeratin, and PSA were treated with secondary antibodies Alexa Fluor™ 488 and Alexa Fluor™ (1:1000 each) for 20 minutes at room temperature. Nuclear staining was performed with 4′,6-diamidino-2-phenylindole (DAPI). Doubling time assayThe muPC and bePC were seeded at 50 × 103 in 12-well plates and incubated in 10% FBS-RPMI 1640 for 3 days at 37°C in 5% CO2. Cells were detached with trypsin (Thermo Fisher Scientific) every 24 hours from the start of the culture, and cell counts were calculated using a blood cell calculator. The doubling time (Td) of each cell was calculated using the following formula: Td=t × log2 / [logNt—logN0], where t is the incubation time, N0 is the number of cells at inoculation, and Nt is the number of cells after t hours of incubation. Cell proliferation assayCell proliferation was assessed using Cell Counting Kit-8 (Dojindo, Kumamoto, Japan) and MTT assays. MuPC and bePC cells were seeded in a 96–well plate at 5 x 103 cells/well and incubated at 37°C in 5% CO2 for 1 day. Various concentrations of lapatinib (0.01–30µM) were then added, followed by incubation for 24 hours. Viability was determined based on the absorbance measured at 450 nm using an iMark Microplate Reader (Bio-Rad, Hercules, California, USA). IC50 was calculated simply using the following formula: IC50=10^[log(A / B) × (50—C) / (D—C) + logB], where A is the Lapatinib concentration at which cell viability is less than 50%, B is the Lapatinib concentration at which cell viability is greater than 50%, C is the inhibition rate at B, and D is the inhibition rate at A Western blottingmuPC and bePC cells, seeded in 12–well plates and cultured, were washed with Hanks’ Balanced Salt Solution (HBSS; Sigma-Aldrich) and incubated for an hour in serum-free media with varying concentrations of lapatinib (0.1, 1, 10µM). The cells were treated with 10 ng/ml EGF for 30 minutes. After washing with HBSS, proteins were extracted using RIPA buffer (Cell Signaling Technology) containing protease and phosphatase inhibitor cocktails (Roche Diagnostics, Mannheim, Germany). The extracted samples were subjected to electrophoresis on a 10% sodium dodecyl sulfate-polyacrylamide gel and transferred onto a nitrocellulose membrane. The membrane was blocked for an hour at room temperature in Tris-buffered saline (TBS; FUJIFILM Wako) containing 5% skim milk. Primary antibodies were incubated overnight at 4°C (HER2 1:200, p-ERK 1:300, ERK 1:300, p-Akt 1:300, Akt 1:300, β-actin 1:1,000). After washing the membrane in TBS containing 0.1% Tween 20 (Sigma-Aldrich), it was incubated for an hour at room temperature with the appropriate secondary antibodies. Protein detection was performed using an ODYSSEY CLx (MandS TechnoSystems Inc.). The canine transitional cell carcinoma cell line TCCUB, which has been confirmed to express HER2 in previous studies, was used as a positive control (Tsuboi et al., 2019). Apoptosis evaluationMuPC and bePC cells were seeded onto cover glasses at 2 x 105 cells/well. After 1 day, cells were treated with 10 μM lapatinib or vehicle for 24 hours. After washing with HBSS, the cells were fixed in 4% paraformaldehyde (FUJIFILM Wako) at 4°C for 25 minutes. Terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick-end labelling (TUNEL) staining was performed using the DeadEnd Fluorometric TUNEL System (Promega, Madison, WI, USA) according to the manufacturer's protocol. The positive control consisted of cells treated with 1U DNase I (Promega). The percentage of TUNEL-positive cells was quantified by counting 100 cells from a random microscopic field. Xenograft mouse modelFemale BALB/cSlc nude mice (5 weeks old) were purchased from Japan SLC Inc. (Shizuoka, Japan) and acclimated to our facility 1 week prior to the experiments. To generate tumour-bearing mice, muPC cells were subcutaneously injected into the left flank of each mouse at 2 x 106 cells. Tumour dimensions were measured every 2 days using calipers, and tumour volume was calculated using the following formula: tumour volume=(length) × (width) 2 × 0.52. Starting on the day of muPC transplantation, mice received lapatinib (100 mg/kg, n=4) or vehicle control (saline containing 10% DMSO and 10% Tween 20, n=4) daily for 14 days via intraperitoneal administration. At the end of the experiment, the mice were euthanized, and tumour samples were collected and fixed in 10% neutral-buffered formalin and embedded in paraffin. HistopathologyFormalin-fixed, paraffin-embedded tumour tissues from the lapatinib and vehicle groups were sectioned at 3 μm and stained with hematoxylin and eosin (HandE). Tumour tissues were captured at 2 × objective and 10 × ocular magnification. The ratio of necrotic area to the total tumour tissue area was calculated using ImageJ software (U.S. National Institutes of Health, Maryland, USA). ImmunohistochemistryThe 4-μm sections were deparaffinized with xylene and hydrated with ethanol; antigen removal was performed in 10 mM citrate buffer (pH 6.0) for 10 minutes at 121°C. The slides were treated with a peroxidase blocking solution (Dako) at room temperature for 10 minutes and washed three times with TBS containing 0.1% Tween 20 (TBS-T). After blocking with TBS-T containing 5% skim milk (FUJIFILM Wako) for 1 hour, the sections were incubated with anti-HER2 antibody (1:100) and incubated at 37°C for 1 hour. The sections were then incubated with the secondary antibody at 37°C for 40 minutes. Peroxidase reactions were performed with 3,3′-diaminobenzidine tetrahydrochloride (Dako) for 10 minutes. The reaction was stopped by washing twice with distilled water and contrast staining was performed using Mayer's hematoxylin. The sections were incubated with an antibody diluent as a negative control. Statistical analysisStudent's t-test was used for comparisons between the two groups. Two-way ANOVA was performed for tumour diameter comparisons in tumour-bearing mice. Prism software version 5.0.1 (Graph Pad Software, California, USA) was used for statistical analysis. Statistical significance was defined as p < 0.05. Doubling time assays and cell proliferation assays were performed in triplicates. All quantified data are presented as mean ± standard error. ResultsCytological features of muPC and bePCFigure 1a shows the echographic images of the two cases that were sampled in this study. The prostate was greatly enlarged to 36.9 mm × 41.0 mm in case 1; and to 13.8 mm × 17.5 mm in case 2. The muPC cells isolated from case 1 were polygonal with one to several nuclei, distinct nucleoli, and granular cytoplasm. The bePC cells isolated from case 2 also had morphological characteristics similar to those of muPC, but the cell-to-cell attachment surfaces were more indistinct than those of muPC (Fig. 1b). Proliferation curves were generated for muPC and bePC, and cell doubling times were calculated to be 31.9 hours for muPC and 35.2 hours for bePC, respectively (Fig. 1c). Immunostaining with epithelial markers E-cadherin, cytokeratin, and the prostate-specific marker PSA was performed, and these markers were positive in both muPC and bePC cells (Fig. 1d). Principal component analysis of RNA-Seq dataWe employed PCA to navigate the complexity of multidimensional gene expression datasets and discern the similarity among our cell lines based on tumour-specific DEGs. The PCA result in Figure 2 highlights variance distribution across the dataset: the first principal component (PC1) accounted for 54% of the variance, while the second principal component (PC2) explained an additional 14%. A clear segregation of cell lines from cTCC tissues was observed, and our established cell lines indicated gene expression patterns closer to those of the cPCs. muPC and bePC express HER2 and exhibit inhibited cell proliferation by lapatinibWestern blot analysis revealed a single HER2 band in both muPC and bePC, which was similar to that in the positive control (Fig. 3a). Immunocytochemistry was also performed and showed HER2 localization at the plasma membrane in both muPC and bePC (Fig. 3b). The anti-tumour effects of lapatinib were examined using a cell proliferation assay. Lapatinib treatment inhibited the proliferation of both muPCs and bePCs (Fig. 3c) in a dose-dependent manner. The 50% inhibitory concentration (IC50) of lapatinib was 4.61 µM and 7.47 µM for muPC and bePC, respectively. Lapatinib induces apoptosis in muPC and bePCTo investigate the effect of lapatinib on apoptosis in cPC cell lines, TUNEL staining was performed. After lapatinib treatment for 24 hours, a substantial number of DNA-fragmented TUNEL-positive cells were observed for both muPC and bePC cells (Fig. 3d and e).
Fig. 1. (a) Ultrasound images of prostate masses in cPC cases 1 and 2. The arrows represent the diameter of the masses. (b) Wright-Giemsa stained images of muPC and bePC. Bars=50μm. (c) Cell proliferation curves for muPC and bePC. Cell counts were calculated using a blood cell calculator for each day for 4 days. Data are presented as mean ± SE. Each experiment was performed three times. (d) Cellular immunostaining images of E-cadherin, cytokeratin, and prostate-specific antigen (PSA) in muPC and bePC; E-cadherin and cytokeratin are indicated by green. PSA is indicated by red. Blue fluorescence indicates DAPI. Bars=50μm. DAPI; 4′,6-diamidino-2-phenylindole.
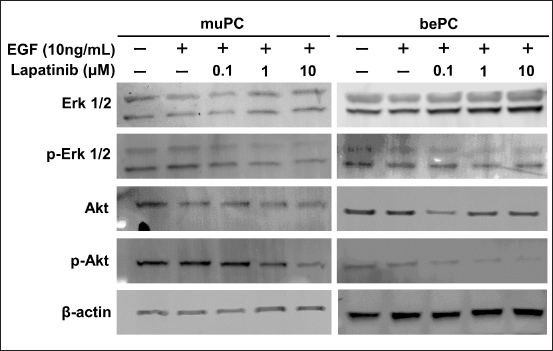
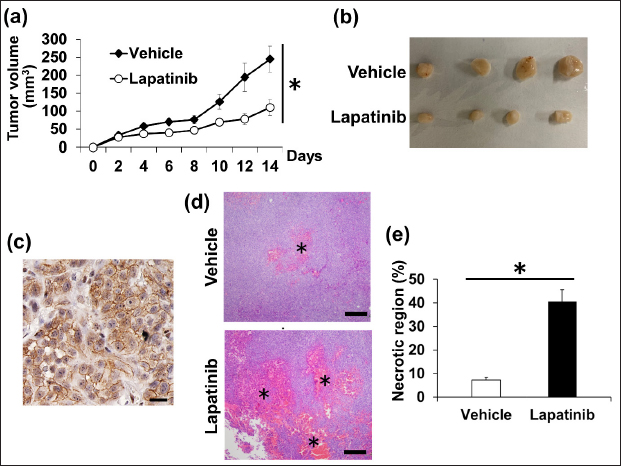
Fig. 2. PCA of the RNA-seq data for 11 cTCCs, 14 cPCs, and 2 of our established cell lines. The cell lines are shown in green color circles, cPCs are blue, and cTCCs are red. Lapatinib suppresses phosphorylation in the HER2 signalling pathway of muPC and bePCThe effect of lapatinib on the phosphorylation of downstream signals of HER2, Erk1/2, and Akt, was examined by western blotting. In muPCs and bePCs, phosphorylation of Erk1/2 and Akt was observed without EGF stimulation. In addition, phosphorylation was not enhanced by EGF stimulation. Lapatinib inhibited the phosphorylation of Erk1/2 and Akt in both muPC and bePC in a dose-dependent manner (Fig. 4). Lapatinib inhibits tumour growth in muPC xenograft miceTo investigate the tumour growth-inhibitory effect of lapatinib in vivo, muPC cells were subcutaneously transplanted to create tumour-bearing mice. Up to day 8 post-transplantation, muPC exhibited slow tumour growth; however, from day 9 onward, tumour growth accelerated. Similarly, the inhibitory effect of lapatinib on tumour growth was minimal up to day 8 but became significantly evident from day 10 (Fig. 5a). In contrast, subcutaneous transplantation of bePC did not result in tumour formation in either the vehicle or lapatinib group after 14 days (data not shown). The gross images of the sampled tumours are shown in Figure 5b. Immunohistochemistry was performed on the muPC-transplanted tissue, and HER2 localization was observed in the cell membranes of the tumour cells (Fig. 5c). Histopathological analysis of the tumours stained with HandE revealed that the lapatinib-treated group had a significantly larger tumour necrotic area than that of the vehicle group (Fig. 5d and e). DiscussionWe have previously demonstrated that HER2 is overexpressed, and that the HER2 inhibitor lapatinib suppresses tumour progression, not only in experimental models but also in clinical cases (Sakai et al., 2018; Maeda et al., 2022). Similarly, we reported the expression of HER2 in cPC (Sakai et al., 2021), suggesting the possibility of a HER2-targeted therapy for cPC, similar to that for cTCC. However, little is known about the efficacy of HER2-targeted therapies for cPC. One reason for the lack of research on cPC is that very few HER2-positive cPC cell lines are available. In this study, we established two new HER2-positive cPC cell lines and evaluated the anti-tumour effects of lapatinib on these lines. Lapatinib exerted growth-inhibitory effects on both muPC and bePC. Furthermore, lapatinib strongly induces apoptosis in these cells. We believe that inhibition of the phosphorylation of Erk and Akt, which are downstream signals of HER2, is an important molecular mechanism that leads to growth inhibition and apoptosis by lapatinib. It has been reported that activation of overexpressed HER2 regulates cell proliferation and apoptosis via the phosphorylation of Akt and mTOR in various cancers, including human breast cancer (Xia et al., 2002; Rusnak and Gilmer, 2011; Uy et al., 2022; Liu et al., 2024). HER2 activation contributes to similar tumour progression mechanisms via phosphorylation of MEK and ERK (Chi et al., 2016; Matkar et al., 2017). In the present study, lapatinib suppressed Erk and Akt phosphorylation in muPC and bePC, suggesting that the anti-tumour effect of lapatinib may be due to the suppression of HER2 downstream signalling. In a previous study, lapatinib induced minimal apoptosis in canine cTCC cell lines, which led us to consider that lapatinib mainly worked by inhibiting cell proliferation in cTCC (Sakai et al., 2018). However, the induction of apoptosis and inhibition of cell proliferation were observed in cPC cell lines. Therefore, lapatinib may exert a stronger anti-tumour effect on cPC. The fact that lapatinib treatment inhibited tumour growth and expansion of necrotic areas in muPC tumour-bearing mice further supports this hypothesis. It is now suggested that Lapatinib may exert more potent therapeutic effects in human prostate cancer when used in combination with not only chemotherapeutic agents but also androgen receptor antagonists and σ2 receptor agonists (Li et al., 2011; Shiota et al., 2015; Garcia et al., 2022). Since this study demonstrated that lapatinib is effective in the treatment of cPC, it is possible that in the future, as in humans, more potent therapies may be established in combination with multiple drugs.
Fig. 3. (a) Protein expression of human epidermal growth factor receptor 2 (HER2) in muPC and bePC. TCCUB, a cTCC cell line, was used as a positive control. (b) Immunocytochemical testing for HER2 in muPC and bePC. Brown-stained areas indicate HER2 positivity. (c) Cell viability of muPC and bePC after lapatinib treatment. muPC and bePC were treated with 0.01–30 μM lapatinib. After 24 hours, cell viability was determined using the methylthiazolyl tetrazolium assay. Data are presented as mean ± SE. Each experiment was performed three times. (d) Detection of apoptosis in muPC and bePC. Cells were treated with 10 μM lapatinib for 24 hours. Apoptotic cells were stained by the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) method. Cells treated with DNase were used as positive controls. Green fluorescence indicates TUNEL signal and blue fluorescence indicates DAPI signal. Bars=50μm. dUTP; deoxyuridine triphosphate. DAPI; 4′,6-diamidino-2-phenylindole. (e) Comparison of TUNEL-positive cell counts in vehicle and lapatinib groups. Data are shown as mean ± SE. * p < 0.05. In this study, PSA immunostaining was performed to confirm whether the two cPC cell lines were derived from clinical cases. However, the suitability of PSA as a marker for cPC remains controversial. Previous studies have reported significantly elevated serum PSA levels in dogs with benign prostatic hyperplasia (Golchin-Rad et al., 2019). Elevated serum PSA levels in benign prostatic hyperplasia are thought to be due to leakage from prostate destruction, and it is unclear whether malignant transformation of cPC increases PSA production. histopathologic studies of cPC cases have shown few PSA positive cases, and if positive, the expression was very weak Although there may be case differences in PSA expression, there are no reports evaluating PSA expression in canine prostate cancer at the protein level. Also, in this experiment, only immunostaining for PSA was performed due to antibody problems, which limits the use of PSA alone as a marker for cPC. Furthermore, the cases included in this study have not undergone surgical treatment and have not been adequately histologically studied. Currently, there is no method to histologically differentiate cTCC from PC, relying primarily on the location of the primary site. In this study, we examined whether cTCC and cPC could be differentiated by differences in gene expression patterns using DEGs analysis. The results showed the possibility of distinguishing between cTCC and cPC based on the characteristics of gene expression, but the data are not yet sufficient, so we plan to increase the number of cases in the future. Therefore, more detailed histological and genetic studies are needed to determine whether the cells established in this study are actually derived from canine PC.
Fig. 4. Human epidermal growth factor receptor 2 (HER2) downstream signal changes in muPC and bePC after lapatinib treatment. Cells were incubated with each concentration of lapatinib (0.1, 1, and 10 μM) for an hour. Cells were then treated with 10 ng/ml EGF for 30 minutes. Erk 1/2; extracellular regulatory kinase 1/2. p-Erk 1/2; Tyr 202/Tyr 204-phosphorylated Erk 1/2. Akt; protein kinase B. p-Akt; Ser 473-phosphorylated Akt. In our in vivo study, muPCs were subcutaneously transplanted into nude mice. The tumour microenvironment, including blood vessels, fibroblasts, immune cells, and the extracellular matrix surrounding the tumour cells, plays an important role in cancer progression (Knapp et al., 2013; Fulkerson et al., 2015; Maeda et al., 2018). Therefore, the transplantation of cancer cells at a site different from the original site may have affected the relationship between lapatinib and cPC. Previous reports have shown that subcutaneous transplantation of human prostate cancer cells resulted in low viability and low histological differentiation levels. In fact, in the present study, bePCs were not viable by subcutaneous transplantation. In recent years, subrenal transplantation has been reported to be a good model for transplantation of prostate cancer cells, and orthotopic transplantation into the prostate under the actual microscope has also been attempted. In view of these facts, it is possible that more clear data will be obtained in the future by selecting the appropriate method for prostate cancer transplantation using the established cells. cPC has a very poor prognosis as it is often advanced at the time of detection; however, effective medical therapy is not yet available. In this study, we found that lapatinib has tumour-suppressive effects on cPC cell lines via inhibition of HER2 downstream signalling in in vitro and in vivo models. Therefore, it is expected to be an effective therapy for cPC with HER2 overexpression.
Fig. 5. (a) Tumour growth in muPC transplanted mice. Data are shown as mean ± SE. * p < 0.05. (b) Image of a tumour tissues removed from muPC transplanted mice 14 days later. (c) Immunohistochemistry for human epidermal growth factor receptor 2 (HER2) in tumour tissues derived from muPC transplanted mice. Brown-stained areas indicate HER2 expression. Bars=50μm. (d) Histological characteristics of tumours derived from muPC transplanted mice in groups vehicle and lapatinib (HandE). Asterisks indicate necrotic areas. Bars=100μm. (e) Necrotic areas were measured and calculated using image analysis software. Data are shown as mean ± SE. * p < 0.05. AcknowledgmentsThe authors would like to thank Editage (www.editage.jp) for English language editing. Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionsShingo Maeda (SM) conceived the idea for the study, and Kenjiro Kaji (KK) established the cells and conducted the experiments. Tomoki Motegi (TM) analyzed the RNA-seq data. Yonezawa (TY) and Momoi (YM) reported improvements in their experiments. KK wrote the manuscript, and SM, TY, and YM commented on and revised it. FundingThis study was supported by JSPS KAKENHI and a Grant-in-Aid for Science Research (Grant Numbers19H00968, 23H00357, and 23K14078). Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesAxiak, S.M. and Bigio, A. 2012. Canine prostatic carcinoma. Compend. Contin. Educ. Vet. 34, E1–5. Brunetti, B., Bacci, B., Sarli, G., Pancioni, E. and Muscatello, L.V. 2021. Immunohistochemical screening of HER2 in canine carcinomas: a preliminary study. Animals 11, 1006. Burstein, H.J., Storniolo, A.M., Franco, S., Forster, J., Stein, S., Rubin, S., Salazar, V.M. and Blackwell, K.L. 2008. A phase II study of lapatinib monotherapy in chemotherapy-refractory HER2-positive and HER2-negative advanced or metastatic breast cancer. Annal. Oncol. 19, 1068–1074. Chi, F., Wu, R., Jin, X., Jiang, M. and Zhu, X. 2016. HER2 induces cell proliferation and invasion of non-small-cell lung cancer by upregulating COX-2 expression via MEK/ERK signaling pathway. Onco. Targets Ther. 9, 2709–2716. Cooke, T., Reeves, J., Lanigan, A. and Stanton, P. 2001. HER2 as a prognostic and predictive marker for breast cancer. Annal. Oncol. 12, S23–S28. Cornell, K.K., Bostwick, D.G., Cooley, D.M., Hall, G., Harvey, H.J., Hendrick, M.J., Pauli, B.U., Render, J.A., Stoica, G., Sweet, D.C. and Waters, D.J. 2000. Clinical and pathologic aspects of spontaneous canine prostate carcinoma: a retrospective analysis of 76 cases. The Prostate 45, 173–183. Dai, B., Kong, Y.Y., Ye, D.W., Ma, C.G., Zhou, X.Y. and Yao, X.D. 2008. Human epidermal growth factor receptor type 2 protein expression in Chinese metastatic prostate cancer patients correlates with cancer specific survival and increases after exposure to hormonal therapy. Asian J. Androl. 10, 701–709. D’Amato, V., Raimondo, L., Formisano, L., Giuliano, M., De Placido, S., Rosa, R. and Bianco, R. 2015. Mechanisms of lapatinib resistance in HER2-driven breast cancer. Cancer Treat. Rev. 41, 877–883. Fulkerson, C.M. and Knapp, D.W. 2015. Management of transitional cell carcinoma of the urinary bladder in dogs: a review. Vet. J. 205, 217–225. Garcia, E.A., Bhatti, I., Henson, E.S. and Gibson, S.B. 2022. Prostate Cancer cells are sensitive to lysosomotropic agent siramesine through generation reactive oxygen species and in combination with tyrosine Kinase inhibitors. Cancers 14, 5478. Golchin-Rad, K., Mogheiseh, A., Nazifi, S., Ahrari Khafi, M.S., Derakhshandeh, N. and Abbaszadeh-Hasiri, M. 2019. Changes in specific serum biomarkers during the induction of prostatic hyperplasia in dogs. BMC Vet. Res. 15, 440. Gutierrez, C. and Schiff, R. 2011. HER 2: biology, detection, and clinical implications. Arch. Pathol. Lab. Med. 135, 55–62. Hernes, E., Fosså, S.D., Berner, A., Otnes, B. and Nesland, J.M. 2004. Expression of the epidermal growth factor receptor family in prostate carcinoma before and during androgen-independence. Br. J. Cancer 90, 449–454. Knapp, D.W. and Mcmillan, S.K. 2013. Withrow and MacEwen’s small animal clinical oncology. Philadelphia, PA: W.B. Saunders. Lei, Y., Huang, J., Zhao, Q., Jiang, N., Xu, H., Wang, Z., Li, H., Zhang, S. and Sun, Z. 2017. The clinicopathological parameters and prognostic significance of HER2 expression in gastric cancer patients: a meta-analysis of literature. World J. Surg. Oncol. 15, 68. LeRoy, B.E. and Northrup, N. 2009. Prostate cancer in dogs: comparative and clinical aspects. Vet. J. 180, 149–162. Li, F., Danquah, M., Singh, S., Wu, H. and Mahato, R.I. 2011. Paclitaxel- and lapatinib-loaded lipopolymer micelles overcome multidrug resistance in prostate cancer. Drug Deliv. Transl. Res. 1, 420–428. Liu, H., Zhou, D., Liu, D., Xu, X., Zhang, K., Hu, R., Xiong, P., Wang, C., Zeng, X., Wang, L. and Zhang, S. 2024. Synergistic antitumor activity between HER2 antibody-drug conjugate and chemotherapy for treating advanced colorectal cancer. Cell Death Dis. 15, 1–13. Maeda, S., Sakai, K., Kaji, K., Iio, A., Nakazawa, M., Motegi, T., Yonezawa, T. and Momoi, Y. 2022. Lapatinib as first-line treatment for muscle-invasive urothelial carcinoma in dogs. Sci. Rep. 12, 4. Maeda S., Motegi T., Iio A., Kaji K., Goto-Koshino Y., Eto S., Ikeda N., Nakagawa T., Nishimura R., Yonezawa T., Momoi Y. 2022. Anti-CCR4 treatment depletes regulatory T cells and leads to clinical activity in a canine model of advanced prostate cancer. J. Immunother. Cancer. 10, e003731. Maeda, S., Tomiyasu, H., Tsuboi, M., Inoue, A., Ishihara, G., Uchikai, T., Chambers, J. K., Uchida, K., Yonezawa, T. and Matsuki, N. 2018. Comprehensive gene expression analysis of canine invasive urothelial bladder carcinoma by RNA-Seq. BMC Cancer 18, 472. Matkar, S., An, C. and Hua, X. 2017. Kinase inhibitors of HER2/AKT pathway induce ERK phosphorylation via a FOXO-dependent feedback loop. Am. J. Cancer Res. 7, 1476–1485. McEntee, M., Isaacs, W. and Smith, C. 1987. Adenocarcinoma of the canine prostate: immunohistochemical examination for secretory antigens. The Prostate 11, 163–170. Muscatello, L. V., Gobbo, F., Di Oto, E., Sarli, G., De Maria, R., De Leo, A., Tallini, G. and Brunetti, B. 2022. HER2 Overexpression and cytogenetical patterns in canine mammary carcinomas. Vet. Sci. 9, 583. Peña, L., Gama, A., Goldschmidt, M. H., Abadie, J., Benazzi, C., Castagnaro, M., Díez, L., Gärtner, F., Hellmén, E., Kiupel, M., Millán, Y., Miller, M. A., Nguyen, F., Poli, A., Sarli, G., Zappulli, V. and Mulas, J. M. de las 2014. Canine mammary tumors: a review and consensus of standard guidelines on epithelial and myoepithelial phenotype markers, HER2, and hormone receptor assessment using immunohistochemistry. Vet. Pathol. 51, 127–145. Piccart-Gebhart, M.J., Procter, M., Leyland-Jones, B., Goldhirsch, A., Untch, M., Smith, I., Gianni, L., Baselga, J., Bell, R., Jackisch, C., Cameron, D., Dowsett, M., Barrios, C.H., Steger, G., Huang, C.S., Andersson, M., Inbar, M., Lichinitser, M., Láng, I., Nitz, U., Iwata, H., Thomssen, C., Lohrisch, C., Suter, T. M., Rüschoff, J., Sütő, T., Greatorex, V., Ward, C., Straehle, C., McFadden, E., Dolci, M.S. and Gelber, R.D. 2005. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. New Engl. J. Med. 353, 1659–1672. Rawla, P. 2019. Epidemiology of prostate cancer. World J. Oncol. 10, 63–89. Rusnak, D. and Gilmer, T. M. 2011. The discovery of lapatinib (GW572016). Mol. Cancer Therapeut. 10, 2019. Rusnak, D.W., Affleck, K., Cockerill, S.G., Stubberfield, C., Harris, R., Page, M., Smith, K.J., Guntrip, S.B., Carter, M.C., Shaw, R.J., Jowett, A., Stables, J., Topley, P., Wood, E.R., Brignola, P.S., Kadwell, S.H., Reep, B.R., Mullin, R.J., Alligood, K.J., Keith, B.R., Crosby, R.M., Murray, D.M., Knight, W.B., Gilmer, T.M. and Lackey, K. 2001. The characterization of novel, dual ErbB-2/EGFR, tyrosine kinase inhibitors: potential therapy for cancer. Cancer Res. 61, 7196–7203. Sakai, K., Maeda, S., Saeki, K., Nakagawa, T., Murakami, M., Endo, Y., Yonezawa, T., Kadosawa, T., Mori, T., Nishimura, R. and Matsuki, N. 2018. Anti-tumour effect of lapatinib in canine transitional cell carcinoma cell lines. Vet. Comp. Oncol. 16, 642–649. Sakai, K., Shinohara, Y., Kaji, K., Yonezawa, T., Momoi, Y. and Maeda, S. 2021. Human epidermal growth factor receptor 2 is overexpressed in canine prostate carcinoma. Translat. Regulat. Sci. 3, 1–8. Schrank, M. and Romagnoli, S. 2020. Prostatic neoplasia in the intact and castrated dog: how dangerous is castration? Animals 10, 85. Shiota, M., Bishop, J.L., Takeuchi, A., Nip, K.M., Cordonnier, T., Beraldi, E., Kuruma, H., Gleave, M.E. and Zoubeidi, A. 2015. Inhibition of the HER2-YB1-AR axis with Lapatinib synergistically enhances Enzalutamide anti-tumor efficacy in castration resistant prostate cancer. Oncotarget 6, 9086–9098. Soria, F., Moschini, M., Haitel, A., Wirth, G.J., Karam, J. A., Wood, C.G., Rouprêt, M., Margulis, V., Karakiewicz, P.I., Briganti, A., Raman, J.D., Kammerer-Jacquet, S.F., Mathieu, R., Bensalah, K., Lotan, Y., Özsoy, M., Remzi, M., Gust, K.M. and Shariat, S.F. 2017. HER2 overexpression is associated with worse outcomes in patients with upper tract urothelial carcinoma (UTUC). World J. Urol. 35, 251–259. Tsuboi, M., Sakai, K., Maeda, S., Chambers, J.K., Yonezawa, T., Matsuki, N., Uchida, K. and Nakayama, H. 2019. Assessment of HER2 Expression in Canine urothelial carcinoma of the urinary bladder. Vet. Pathol. 56, 369–376. Uy, N. F., Merkhofer, C. M. and Baik, C. S. 2022. HER2 in non-small cell lung cancer: a review of emerging therapies. Cancers (Basel) 14, 4155. Weaver, A.D. 1981. Fifteen cases of prostatic carcinoma in the dog. Vet. Rec. 109, 71–75. Xia, W., Mullin, R.J., Keith, B.R., Liu, L.H., Ma, H., Rusnak, D.W., Owens, G., Alligood, K.J. and Spector, N.L. 2002. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene 21, 6255–6263. Xu, Z., Zhang, Y., Li, N., Liu, P., Gao, L., Gao, X. and Tie, X. 2017. Efficacy and safety of lapatinib and trastuzumab for HER2-positive breast cancer: a systematic review and meta-analysis of randomised controlled trials. BMJ Open 7, e013053. Yoshimoto, S., Kato, D., Kamoto, S., Yamamoto, K., Tsuboi, M., Shinada, M., Ikeda, N., Tanaka, Y., Yoshimoto, R., Eto, S., Saeki, K., Chambers, J., Hashimoto, Y., Uchida, K., Nishimura, R. and Nakagawa, T. 2020. Overexpression of human epidermal growth factor receptor 2 in canine primary lung cancer. J. Vet. Med. Sci. 82, 804–808. Yuan, Y., Liu, X., Cai, Y. and Li, W. 2022. Lapatinib and lapatinib plus trastuzumab therapy versus trastuzumab therapy for HER2 positive breast cancer patients: an updated systematic review and meta-analysis. Syst. Rev. 11, 264. | ||
| How to Cite this Article |
| Pubmed Style Kaji K, Yonezawa T, Yonezawa T, Momoi Y, Maeda S. Anti-tumor effects of lapatinib on HER2-positive canine prostatic carcinoma cell lines. Open Vet. J.. 2024; 14(5): 1259-1268. doi:10.5455/OVJ.2024.v14.i5.21 Web Style Kaji K, Yonezawa T, Yonezawa T, Momoi Y, Maeda S. Anti-tumor effects of lapatinib on HER2-positive canine prostatic carcinoma cell lines. https://www.openveterinaryjournal.com/?mno=180194 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i5.21 AMA (American Medical Association) Style Kaji K, Yonezawa T, Yonezawa T, Momoi Y, Maeda S. Anti-tumor effects of lapatinib on HER2-positive canine prostatic carcinoma cell lines. Open Vet. J.. 2024; 14(5): 1259-1268. doi:10.5455/OVJ.2024.v14.i5.21 Vancouver/ICMJE Style Kaji K, Yonezawa T, Yonezawa T, Momoi Y, Maeda S. Anti-tumor effects of lapatinib on HER2-positive canine prostatic carcinoma cell lines. Open Vet. J.. (2024), [cited January 25, 2026]; 14(5): 1259-1268. doi:10.5455/OVJ.2024.v14.i5.21 Harvard Style Kaji, K., Yonezawa, . T., Yonezawa, . T., Momoi, . Y. & Maeda, . S. (2024) Anti-tumor effects of lapatinib on HER2-positive canine prostatic carcinoma cell lines. Open Vet. J., 14 (5), 1259-1268. doi:10.5455/OVJ.2024.v14.i5.21 Turabian Style Kaji, Kenjiro, Tomohiro Yonezawa, Tomohiro Yonezawa, Yasuyuki Momoi, and Shingo Maeda. 2024. Anti-tumor effects of lapatinib on HER2-positive canine prostatic carcinoma cell lines. Open Veterinary Journal, 14 (5), 1259-1268. doi:10.5455/OVJ.2024.v14.i5.21 Chicago Style Kaji, Kenjiro, Tomohiro Yonezawa, Tomohiro Yonezawa, Yasuyuki Momoi, and Shingo Maeda. "Anti-tumor effects of lapatinib on HER2-positive canine prostatic carcinoma cell lines." Open Veterinary Journal 14 (2024), 1259-1268. doi:10.5455/OVJ.2024.v14.i5.21 MLA (The Modern Language Association) Style Kaji, Kenjiro, Tomohiro Yonezawa, Tomohiro Yonezawa, Yasuyuki Momoi, and Shingo Maeda. "Anti-tumor effects of lapatinib on HER2-positive canine prostatic carcinoma cell lines." Open Veterinary Journal 14.5 (2024), 1259-1268. Print. doi:10.5455/OVJ.2024.v14.i5.21 APA (American Psychological Association) Style Kaji, K., Yonezawa, . T., Yonezawa, . T., Momoi, . Y. & Maeda, . S. (2024) Anti-tumor effects of lapatinib on HER2-positive canine prostatic carcinoma cell lines. Open Veterinary Journal, 14 (5), 1259-1268. doi:10.5455/OVJ.2024.v14.i5.21 |