| Research Article | ||
Open Vet. J.. 2024; 14(8): 1771-1778 Open Veterinary Journal, (2024), Vol. 14(8): 1771–1778 Research Article Metazoan ectoparasites of Lithognathus mormyrus (Linnaeus, 1758) from the western coast of LibyaMalak T. Altikbali1, Mohamed. L. Showehdi2, Sarah A. Benzeglam3, Aisha A. Seif-Alnaser3 and Esmail A. Shakman1*1Zoology Department, Faculty of Sciences, University of Tripoli, Tripoli, Libya 2Poultry and Fish Diseases Department, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya 3Biology Department, Faculty of Sciences, University of Tripoli, Tripoli, Libya *Corresponding Author: Esmail A. Shakman. Zoology Department, Faculty of Sciences, University of Tripoli, Tripoli, Libya. Email: Shugmanism [at] yahoo.com Submitted: 20/01/2024 Accepted: 12/07/2024 Published: 31/08/2024 © 2024 Open Veterinary Journal
ABSTRACTBackground: Lithognathus mormyrus fish is one of the fish of high economic importance in the countries of the world in general and the Mediterranean countries in specific, including the Libyan Sea waters, for this it is necessary to know everything related to this type of fish, including its infection with parasites. Parasites are considered one of the factors threatening the life of fish, being one of the food chains and the ecological composition of life. Aim: The current study aims to isolate and identify the parasites that infect the L. mormyrus. Methods: A total of 368 specimens of L. mormyrus were collected by fishermen, from the western coast of Libya. The study was focused on metazoan parasites. When fish were fully examined for the presence of ectoparasites under a dissecting microscope with incident light, the software camera connected with a microscope and semichon’ acetocarmine technique for identification of parasites. Results: Two species of Monogenea (Encotyllabe valley, Pagellicotyle mormyri, and Lamellodiscus spp.), Isopoda (Gnathia), Copepoda (Lernaeolophus sultanus), and Annelidae (Trachelobdella lubrica) has been isolated from this species of fish. The highest prevalence of infection was Gnathia parasites (8.47%). Conclusion: There were differences in the parasite species that infect L. mormyrus from one country to another, and also from city to other cities in the same country, as in the Tunisian waters. Keywords: Lithognathus mormyrus, Encotyllabe valley, Lernaeolophus sultanus, Trachelobdella lubrica, Libya. IntroductionStriped sea bream (Lithognathus mormyrus) is a valuable economic resource in the Mediterranean Sea, including Libya. This demersal marine fish, which is a member of the Sparidae family, has over 100 species worldwide. The Northeast Atlantic and Mediterranean Seas have the most diversity, with 24 described species from 11 genera (Kallianiotis et al., 2005). In Libya, these species of fish live in groups near the sandy bottoms and sometimes on Posidonia beds at a depth of 30 m. These species of fish are very mobile and depend on the sea bottom to get their food: crustaceans, worms, and molluscs. They reproduce in spring and summer; hermaphroditic protandrous, where juveniles are male, after 14 cm total length; however, the female character is dominant, and it reaches maturity at 2 years (about 14 cm) (Bauchot and Hureau, 1990; Aydın, 2018; Karadurmus and Aydin, 2022). Geographically distributed throughout the Mediterranean and Atlantic, from the Bay of Biscay to the Cape of Good Hope; the Canaries, Cape Verde, the Black Sea, and elsewhere the Red Sea and the south-western Indian Ocean (Smith and Smith, 1986; Bauchot and Hureau, 1986; Bauchot and Hureau, 1990; Wirtz et al., 2008; Russell et al., 2014). Different species of L. mormyrus’s parasites have been recorded in many previous studies from different geographical locations around the world: the Western Mediterranean Sea (Euzet, 1984; Bartoli et al., 1989, 1993; Bartoli and Bray, 1996; Jousson et al., 1999, 2000; Sasal et al., 1999; Benmansour et al., 2001; Jovelin and Justine, 2001; Desdevises et al., 2002; Bartoli et al., 2005; Ramdane et al., 2007, 2009; Gargouri Ben Abdallah and Maamouri, 2008; Boudaya et al., 2009; Boualleg et al., 2010, 2011; Gargouri Ben Abdallah et al., 2011, 2015; Poisot et al., 2011; Derbel et al., 2012; Samn et al., 2014; Antar et al., 2015); the Eastern Mediterranean Sea (Saad Fares and Maillard, 1990; Saad Fares and Combes, 1992; Bruce et al., 1994; Akmırza, 2010; Cafer et al., 2015; Demirkale et al., 2015) and from Adriatic (Radujkovic and Raibaut, 1989; Radujkovic and Euzet, 1989; Orecchia et al., 1988; Radujkovic et al., 1989; Bray and Bartoli, 1996), and from the North-Eastern Atlantic Ocean (Gijon-Botella and Lopez-Roman, 1989). Also, from the South-Eastern Atlantic Ocean (Gaevskaya and Aleshkina, 1988), the Aegean Sea (Akmirza, 2013; Çinar, 2014), and the Red Sea (Bray and Cribb, 1989). Some of these parasites may affect the fisheries economy significantly, by damaging and killing these fish. Also, heavy infestation by different types of parasites causes significant loss of fish condition, which may result in reduced growth rates and delayed reproductive effort, and thus, affects the health status, which may lead to mortality (Mehanna, 2020). If there are no visible significant effects on wildlife, it will accidentally influence the human population as a consumer of marine seafood, and also, in the fish aquacultures (Koyuncu et al., 2015). Although parasites cause severe damage to marine organisms, especially fish, they can be used as an indicator of fish health, as well as a biological and ecological indicator for the environment surrounding fish, through which fish migration can be tracked and the presence of pollutants can be determined (Derbel et al., 2012). In Libya, there are not many studies on fish parasites, especially in Striped sea bream fish, for which no parasites have been recorded that may infect them. For this reason, this study aimed to detect and identify metazoan ectoparasites that can infect this species of fish. Materials and MethodsA total of 368 specimens of L. mormyrus (Fig. 1) were collected from fishermen and immediately transferred to the laboratory of the Marine Biology Department of Zoology, Faculty of Science at the University of Tripoli. These specimens were collected from the western coast of Libya, during the period from September 2020 to October 2021. Morphometric measurements have been taken, and the total weight of each individual has been measured in grams (Dwivedi and Menezes, 1974). The study was focused on metazoan parasites, where fish were fully examined for the presence of ectoparasites under a dissecting microscope with incident light. The parasite examination was carried out according to Euzet and Trilles (1960). Identification of parasites, and staining by using the software camera connected with a microscope and semichon’ acetocarmine technique. Parasitic indicates adopted in the calculations by Bush et al. (1997). Ethical approvalNot needed for this study. ResultsOne hundred and fifty-two different genera of ectoparasites were isolated from three hundred sixty-eight individuals of L. mormyrus. The percentage of infection was 28.3% of the total examined fish (intensity 1.46 and abundance 0.41). Parasite prevalence was significantly higher in the Bychowskicotyla mormyri at 13.23% (intensity 1.14 and abundance 0.13) and the lowest infection rate was in Encotyllabe spari (0.27%) and Lamellodiscus sp. (0.27%) as shown in Table 1.
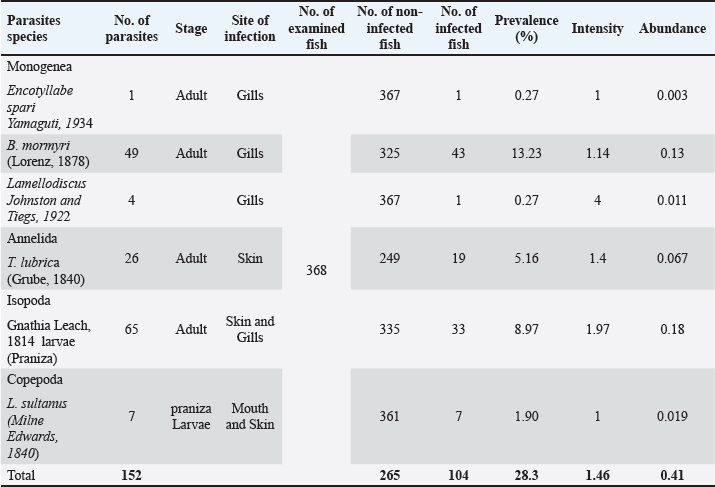
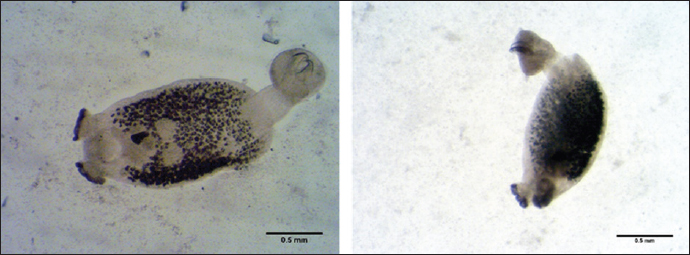
Fig. 1. Lithognathus mormyrus (Linnaeus, 1758). Encotyllabe spari Yamaguti, 1934Family: Capsalidae Genus: Encotyllabe Species: Encotyllabe spari Yamaguti, 1934 Sites: Gills Description: A total of one Encotyllabe spari belongs to the Capsalidae family was collected. The main characteristics of this species depend on the Body ellipsoidal, sub cylindrical long sides of the body to folded ventrally tegument smooth as in (Fig. 2), total length is 1.818 mm long and 0.243 mm wide; anterior suckers 0.086 long. Mouth bordered by digitiform projections on the anterior border two pairs of eye spots are present. The pharynx is muscular, 0.75) 0.226, Two pairs of eyespots are at the level of the pharynx. The haptor is bell-shaped (0.122× 0.112; marginal membrane 0.015 wide; one pair of large anchors, one pair of small anchors and 14 marginal hooks. Large anchors (0.077× 0.027). Testes are two juxtaposed, pre-equatorial, differ in size; left, right part (0.190 ×0.192)— left part (0.190 × 0.212). Ovary noticed pretesticular, oval, (0.135 ×0.149). The Vitelline reservoir is pre-ovarian, (0.114 × 0.166). Bychowskicotyla mormyri (Lorenz, 1878)Family: Microcotylidae Genus: Bychowskicotyla Unnithan, 1971 Species: B. mormyri (Lorenz, 1878) Synonyms: Microcotyle mormyri Lorenz, 1878.Atrispinum mormyri (Lorenz, 1878) , Orecchia and Paggi, 1983. Pagellicotyle mormyri (Lorenz, 1878) Mamev, 1984. Sites: Gill Description: The elongated body’s total length is about 7 to 8 mm while the width is approximately 0.6 mm; the haptor, symmetrical, is triangular in shape as in (Fig. 3), which has clamps 90–100 on each side. The mouth, ventral sub terminal, is located at the anterior end. The pharynx is median. The esophagus, initially straight, bifurcates at the level of the genital atrium. The two intestinal branches descend laterally along the body, forming numerous axial and lateral caeca The ventral genital opening is located at the anterior end. The atrium size is 150 µm which includes in its anterior part ten large slender spines, arranged in an arc of a circle their size 75 µm, and in its posterior two lateral groups of hooked small spines. These two groups, symmetrical with respect to the mid-sagittal plane, each have about twenty spines their size is about 35 µm. Table 1. Parasites have been found in the L. mormyrus.
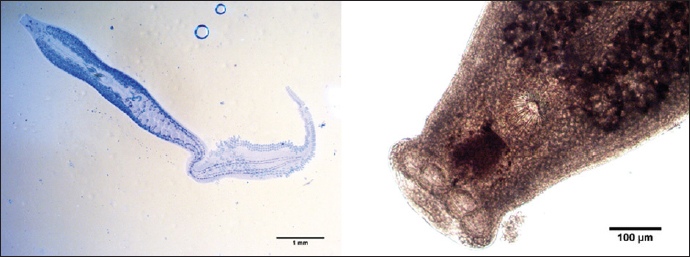
Fig. 2. Encotyllabe spari Yamaguti, 1934 (left: whole worm; right: lateral view). Lamellodiscus Johnston and Tiegs, 1922Family: Diplectanidae Genus: Lamellodiscus Species: Lamellodiscus Johnston and Tiegs, 1922. Sites: Gills Description: In this study, the gills of 300 Lithognathus mormy were examined, which revealed a parasite of the genus Lamellodiscinae Oliver, 1969. It haptor is characterized by three transverse bars (two dorsal and one ventral medial) as in (Fig. 4); Two pairs of handles (each consisting of a dorsal grip and a ventral grip), and a dorsal grip at the lateral end of each dorsal rod. The lamellidian discus consists of 10 pairs of plates, the former closed, almost heart-shaped, and the latter in a circular arc. Fourteen uncinuli. Three pairs of cephalic glandular organs. Eye spots are present (two pairs) or absent, left vas deferens. The male copulatory apparatus generally consists of a genital part and a more or less complex accessory part. The ovary surrounds the right intestinal branch. The opening of the vagina, lateral, is in the left half of the body (exceptionally on the right). Eggs opposite, tetrahedral, with long filaments at one of the apexes opposite the operculum. Trachelobdella lubrica (Grube, 1840)Class: Clitellata Genus: Trachelobdella Species: T. lubrica Orig. name Pontobdella lubrica Grube, 1840 Synonyms: P. lubrica Grube, 1840 Trachelobdella kolleri (Diesing, 1850)Trachelobdella luederitzi (Augener, 1936)Trachelobdella muelleri (Diesing, 1850) Sites: Skin Description: Form body slender, sub-fusiform, it is symmetrical bilateral. The colour of living specimens is yellow-orange. The body has obvious paired pulsatile vesicles. The head region dilated into a circular sucker (Fig. 5), distinct from the body and has one pair of eyes, its body has two ellipses that represent suckers, trapeziums situated between them, and the body structure is an anterior sucker. Trachelosome, which is divided into many trapeziums, is located in the anterior part of the body. Another structure is located in the posterior part of their body, which is called the urosome and posterior sucker which is very small.
Fig. 3. Bychowskicotyla mormyri (Lorenz, 1878) Unnithan, 1971 (left: whole worm; right: genital atrium).
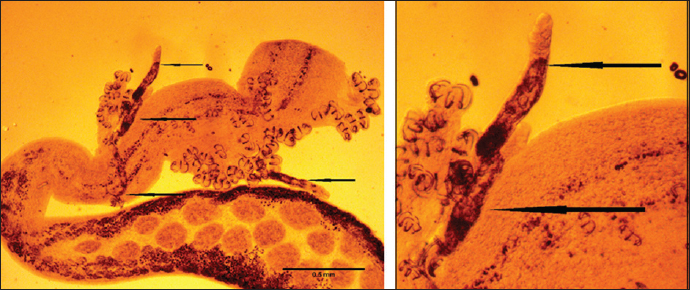
Fig. 4. Left: Lamellodiscus Johnston and Tiegs, 1922 attached with haptor of B. mormyri Scale-bars: 0.5 mm. Right: magnification to lamellodiscus. Gnathia Leach, 1814 larvae (Praniza) Family: Gnathiidae Genus: Gnathia Sites: Skin and Gills Description: A total of 65 Gnathia larvae (Praniza) were collected through this study. Its body is described as divided into the cephalosome, the peroen with five pairs of pereopods, the pleon consists of five pleonites each with a pair of pleopods, and the telson with one pair of uropods. The cephalon is narrowing anteriorly with a truncate frontal margin, the antennae are slightly longer than the antennule extended pereonite 1, the posterior margin of cephalon being is much narrower than the anterior margin of pereonite 1, the compound eyes are large, oval, and located on the lateral margin as in (Fig. 6). Lernaeolophus sultanus (Milne Edwards, 1840) Family: Pennellidae Burmeister, 1835 Genus: Lernaeolophus Heller, 1865 Species: L. sultanus (Milne Edwards, 1840) Original name: Pennella sultana Milne-Edwards, 1840 Synonymised names: Lernaea hemirhamphi Krøyer, 1863 · Lernaea sieboldi Koch, 1860 · Lernaeolophus hemirhamphi (Krøyer, 1863) · Lernaeolophus recurvus Wilson C.B., 1913 · Pennella sultana Milne-Edwards, 1840· Sites: Mouth and Skin Description: Mouth region, the most highly modified elongated body was deeply embedded into the host’s tissue. the globular cephalosome is attached with three cephalic horns as in (Fig. 7), the globular head had a width of 2.19 mm, while the sigmoid trunk had 2.09 mm. Three pairs of lobes in the circular oral region have an anterior lobe size of 0.32 mm, middle lobe of 0.47, and posterior lope of 0.74 mm, continuously after the neck there were found the cylindrical truck measured 6.68 mm. The abdomen was shorter than the trunk with a length of 2.45mm. branched posterior processes giving it a brush-like appearance with spirally coiled egg sacs. DiscussionToday, the world is looking for knowledge of the biodiversity of living organisms, especially the marine biodiversity, which plays a major role in the marine environmental balance in addition to its food importance as it follows this path with research studies that study the risks that can threaten these marine organisms, including worms parasitizing on fish and other such organisms. Moreover, these parasites are considered one of the marine biodiversity. For this reason, it is necessary to study and identify them, as this study was conducted in which they isolated the Monogenea (Encotyllabe valley, Pagellicotyle mormyri, and Lamellodiscus). Isopoda (Gnathia), Copepoda (L. sultanus), and Annelidae (Leeches). By searching for previous studies in particular, especially in the waters of the sea of the countries adjacent to the marine water of the Libyan state, such as Tunisia on the western side and Egypt on the eastern side. a difference has been found in the isolated parasites in the same fish species (L. mormyrus). Alaa et al. (2014) have isolated Nerocila bivittata from L. mormyrus of Abu Qir Bay, Alexandria, showed higher digenean diversity (Derogenes latus, Allopodocotyle pedicellate, Diphterostomum brusinae, Holorchis pycnoporus, Lepocreadium album, Lepocreadium pegorchis, Macvicaria maillard, Macvicaria mormyri, Proctoeces maculatus, and Pycnadenoides senegalensis) in the L. mormyrus was catch from Bizerte Lagoon-Tunisia (Gargouri Ben Abdallah et al., 2011). In addition, mature spermatozoon of Holorchis pycnoporus from the digestive tract of the Striped seabream from off the Gulf of Gabès at La Chebba (Tunisia) were isolated, and Ramdane et al. (2007) have isolated Anilocra frontalis and Solea vulgaris from L. mormyrus in the gulf of Béjaïa and gulf of Jijel from Algerian fauna.
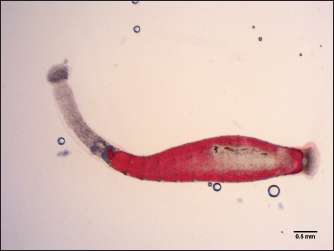
Fig. 5. Trachelobdella lubrica (Grube, 1840).
Fig. 6. Left and right: Praniza larvae.
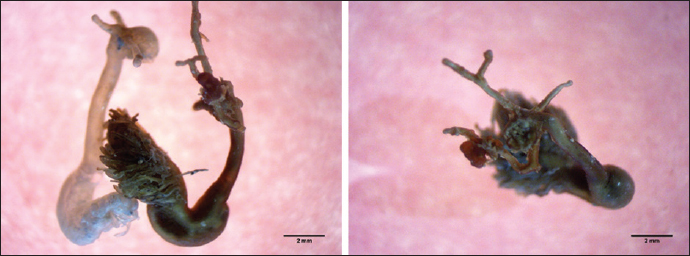
Fig. 7. Lernaeolophus sultanus (Milne Edwards, 1840) (left: whole body; right: the head). Distomum mormyri was redescribed from the intestine of L. mormyrus L. in the western Mediterranean Scandola Nature Reserve, Corsica, France (Bartoli et al., 1993). Differences have been noted in the type of parasites that infect spindle fish from one country to another, and even within the same country, the types that have been isolated from this type of fish also vary from one city to another. AcknowledgmentThe authors would like to express their deepest gratitude to Dr. Fawzia Al-Wafi for her support and contributions that have been instrumental in the completion of this research. Authors’ contributionsAll authors contributed equally to this research. FundingNone. Conflict of interestThe authors declare that there is no conflict of interest. Data availabilityAll data are provided in the manuscript. ReferencesAkmirza, A. 2010. Investigation of the monogenean trematods and crustacean parasites of the cultured and wild marine fishes near Salih Island. J. Fac. Vet. Med. Kafkas Univ. 16, 353–360. Akmirza, A. 2013. Digenean trematodes of fish in the waters off Gökçeada, the Aegean Sea, Turkey. J. Black Sea/Mediterranean Environ. 19, 283–298. Alaa, A.M., Samn, K.M., Metwally, F., Zeina, H.M. and Khalaf, A. 2014. First occurrence of Nerocila bivittata: parasitic Isopods (skin shedders) on Lithognathus mormyrus (Osteichthyes, Sparidae) from Abu Qir Bay, Alexandria, Egypt. J. Am. Sci. 10(7), 171–179. Antar, R., Georgieva, S., Ben Abdallah, L. and Kostadinova, A. 2015. Molecular evidence for the existence of species complexes within Macvicaria Gibson and Bray, 1982 (Digenea: Opecoelidae) in the western Mediterranean, with descriptions of two new species. Syst. Parasitol. 91, 211–229. Aydın, M. 2018. The new maximum length of the striped sea bream ( Lithognathus mormyrus L., 1758) in the black sea region. Aquat. Sci. Engin. 33(2), 50–52. Bartoli, P. and Bray, R.A. 1996. Description of three species of Holorchis Stossich, 1901 (Digenea: Lepocreadiidae) from marine fishes off Corsica. Syst. Parasitol. 35, 133–143. Bartoli, P., Bray, R.A. and Gibson, D.I., 1989. The opecoelidae (Digena) of sparid fishes of the western Mediterranean. II. Pycnadenoides Yamaguti, 1938 and Pseudopycnadena Saad Fares and Maillard, 1986. Syst. Parasitol. 13, 35–51. Bartoli, P., Gibson, D.I. and Bray R.A. 1993. The opecoelidae (Digenea) of sparid fishes of the western Mediterranean. VI. A redescription of Macvicaria mormyri (Stossich, 1885) n. comb. and a key to the opecoelids from western Mediterranean sparids. Syst. Parasitol. 26, 59–67. Bartoli, P., Gibson, D.I. and Bray, R.A. 2005. Digenean species diversity in teleost fish from a nature reserve off Corsica, France (Western Mediterranean), and a comparison with other Mediterranean regions. J. Nat. History 39, 47–70. Bauchot, M.-L. and J.-C. Hureau, 1986. Sparidae. In Fishes of the north-eastern Atlantic and the Mediterranean. Eds., Whitehead, P.J.P., Bauchot, M.-L., Hureau, J.-C., Nielsen, J. and E. Tortonese. Volume 2. Paris, France: UNESCO, pp: 883–907. Bauchot, M.-L. and J.-C. Hureau, 1990. Sparidae. In Check-list of the fishes of the eastern tropical Atlantic (CLOFETA). Eds., Quero, J.C., Hureau, J.C., Karrer, C., Post, A. and L. Saldanha, A. JNICT, Lisbon; SEI, Paris; and UNESCO, Paris. 2, 790–812. Benmansour, B., Ben Hassine, O.K., Diebakate, D. and Raibaut, A., 2001. Sur deux espèces de Copépodes Lernaeopodidae (Siphonostomatoida) parasites du marbré Lithognathus mormyrus (Linnaeus, 1758) (Pisces, Sparidae). Zoosystema 23, 695–703. Boualleg, C., Kaouachi, N., Seridi, M., Ternango, S. and Bensouilah, M.A., 2011. Copepod parasites of gills of 14 teleost fish species caught in the gulf of Annaba (Algeria). Afri. J. Microbiol. Res. 5, 4253–4259. Boualleg, C., Seridi, M., Kaouachi, N., Quiliquini, Y. and Bensouillah, M., 2010. Les Copépodes parasites des poissons téléostéens du littoral Est-algérien. Bulletin de l’Institut Scientifique, Rabat, section Sciences de la Vie 32, 65–72. Boudaya, L., Neifar, L. and Euzet, L. 2009. Diplectanid parasites of Lithognathus mormyrus (L.) (Teleostei: Sparidae) from the Mediterranean Sea, with the description of Lamellodiscus flagellatus n. sp. (Monogenea: Diplectanidae). Syst. Parasitol. 74, 149–159. Bray, R.A. and Bartoli, P. 1996. A redescription of Lepidauchen stenostoma Nicoll, 1913 (Digenea), and a reassessment of the status of the genus Lepidauchen Nicoll, 1913. Syst. Parasitol. 33, 167–176. Bray, R.A. and Cribb, T.H. 1989. Digeneans of the family Opecoelidae Ozaki, 1925 from the southern Great Barrier Reef, including a new genus and three new species. J. Nat. History 23, 429–473. Bruce, N.L., Adlard, R.D. and Cannon, L.R.G. 1994. Synoptic checklist of ascaridoid parasites (Nematoda) from fish hosts. Invertebrate Taxonomy 8, 583–674. Bush, A.O., Lafferty, K.D., Lotz, J.M. and Shostak, A.W. 1997. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 83(4), 575–583. Cafer, E.K., Raul, C.R. and Ercument, G. 2015. Clavellotis briani (Copepoda, Lernaeopodidae) Infestation on Striped Seabream, Lithognathus mormyrus (Sparidae) from the Northeast Mediterranean Sea, Turkey. J. Agricult. Sci. 21, 152–157. Çinar, M.E. 2014. Checklist of the phyla platyhelminthes, xenacoelomorpha, nematoda, acanthocephala, myxozoa, tardigrada, cephalorhyncha, nemertea, echiura, brachiopoda, phoronida, chaetognatha and chordata (tunicata, cephalochordata, and hemichordata) from the coasts of Turkey. Turkish J. Zool. 38, 698–722. Demirkale, I., Ozak, A.A. and Boxshall, G.A. 2015. The discovery of the male of Caligus ligusticus Brian, 1906 (Copepoda: Caligidae) parasitic on the sand steenbras Lithognathus mormyrus (L.) in the eastern Mediterranean. Syst. Parasitol. 91, 81–90. Derbel, H., Chaari, M. and Neifar, L. 2012. Digenean species diversity in teleost fishes from the Gulf of Gabes, Tunisia (Western Mediterranean). Parasite 19, 129–135. Desdevises, Y., Morand, S. and Legendre P. 2002. Evolution and determinants of host specificity in the genus Lamellodiscus (Monogenea). Biol. J. Linnean Soc. 77, 431–443. Dwivedi, S.N. and Menezes, Maria, R. 1974. A note on the morphometry and ecology of Brachiurus orientalis (Bloch and Scheneder) in the estuaries of Goa. Geobios. 1(4), 80–83. Euzet, L. 1984. Diplectanidae (Monogenea) parasites de poissons des Iles Kerkenneh (Tunisie). Archives de l’Institute Pasteur de Tunis 61, 463–474. Gaevskaya, A.V. and Aleshkina, L.D., 1988. Fauna of monogenean of the South-East Atlantic, its ecological and geographical analysis. Zoologicheskii Zhurnal 67, 325–330. Gargouri Ben Abdallah, L. and Maamouri, F. 2008. Digenean fauna diversity in sparid fish from Tunisian coasts. Bull. Euro. Assoc. Fish Pathol. 28, 129–136. Gargouri Ben Abdallah, L., Antar, R. and Maamouri, F. 2011. Diversity of the digenean fauna in sparid fishes from the Lagoon of Bizerte in Tunisia. Acta Parasitol. 56, 34–39. Gargouri Ben Abdallah, L., Antar, R., Zarrouk, F. and Maamouri, F. 2015. The occurrence of acanthocephalans in teleost fish from the Bizerte lagoon, Tunisia. J. Helminthol. 90, 96–101. Gijon-Botella, H. and Lopez-Roman, R. 1989. Aportacion al catalogo de Digenea de peces marinos del Archipelago de Canarias. Revista Iberica de Parasitologia 49, 137–138. Jousson, O., Bartoli, P. and Pawlowski J. 1999. Molecular identification of developmental stages in Opecoelidae (Digenea). Inter. J. Parasitol. 29, 1853–1858. Jousson, O., Bartoli, P. and Pawlowski J., 2000. Cryptic speciation among intestinal parasites (Trematoda: Digenea) infecting sympatric host fishes (Sparidae). J. Evol. Biol. 13, 778–785. Jovelin, R. and Justine, J.L. 2001. Phylogenetic relationships within the polyopisthocotylean monogeneans (Platyhelminthes) were inferred from partial 28S rDNA sequences. Inter. J. Parasitol. 31, 393–401. Kallianiotis, A., Torre, M. and Argyri, A. 2005. Age, growth, mortality, reproduction and feeding habits of the striped seabream, Lithognathus mormyrus (Pisces: Sparidae) in the coastal waters of the Thracian Sea, Greece. Sci. Mar. 69(3), 391–404. Karadurmus, U. and Aydin, M. 2022. Morphological characterization of Lithognathus mormyrus (Linnaeus, 1758) populations in the southern Black Sea (Turkey). Aquat. Sci. Eng. 37(1), 38–45. Koyuncu, C.E, Romerob, R.C. and Genc, E. 2015. Clavellotis briani (Copepoda, Lernaeopodidae) Infestation on Striped Seabream, Lithognathus mormyrus (Sparidae) from the Northeast Mediterranean Sea, Turkey. J. Agricult. Sci. 21, 152–155. Mehanna, S.F. 2020. Isopod parasites in the Egyptian fisheries and its impact on fish production: Lake Qarun as a case study. Egyptian J. Aquat. Biol. Fisheries. 24(3), 181–191. Orecchia, P., Paggi, L. and Radujkovic, B.M. 1988. Digeneans of fishes from the Adriatic Sea with a description of Lecithaster atherinae n. sp. from Atherina (Hepsetia) boyeri. Parassitologia, 30, 225–229. Poisot, T., Verneau, O. and Desdevises, Y. 2011. Morphological and molecular evolution are not linked in Lamellodiscus (Platyhelminthes, Monogenea). PLoS One 6, e26252. Radujkovic, B.M. and Euzet, L. 1989. Parasites des poissons marins du Montenegro: Monogenes. Acta Adriatica 30, 51–135. Radujkovic, B.M. and Raibaut, A. 1989. Parasites des poissons marins du Monténégro: liste des espèces de poissons avec leurs parasites. Acta Adriatica 30, 307–320. Radujkovic, B.M., Orecchia, P. and Paggi, L. 1989. Parasites des poissons marins du Montenegro: Digenes. Acta Adriatica 30, 137–187. Ramdane, Z., Bensouilah, M. and Trilles J.P. 2009. Étude comparative des crustacés isopodes et copépodes ectoparasites de poissons marins algériens et marocains. Cybium 33, 123–131. Ramdane, Z., Bensouilah, M.A. and Trilles, J.P. 2007. The Cymothoidae (Crustacea, Isopoda), are parasites on marine fishes, from the Algerian fauna. Belg. J. Zool. 137, 67–74. Russell, B., Carpenter, K.E., Pollard, D., Mann, B.Q. and Buxton, C.D. 2014. Lithognathus mormyrus. The IUCN Red List of Threatened Species 2014, e.T170160A1284573. Saad Fares, A. and Maillard, C. 1990. Digenetic trematodes of lebanese coast fishes: the species complexes lepocreadium album (Stossich, 1890) and lepocreadium pegorchis (Stossich, 1900) (Lepocreadiidae). Syst. Parasitol. 17, 87–95. Saad Fares, A. and Combes, C. 1992. Comparative allometry growth of some marine fish digenetic tremaodes. Memorias do Instituto Oswaldo Cruz 1, 233–237. Samn, A.A., Metwally, K.M., Zeina, A.F. and Khalaf Allah, H.M. 2014. The first occurrence of Nerocila bivittata: parasitic Isopods (skin shedders) on Lithognathus mormyrus (Osteichthyes, Sparidae) from Abu Qir Bay, Alexandria, Egypt. J. Am. Sci. 10, 171–197. Sasal, P., Niquil, N. and Bartoli, P. 1999. Community structure of digenean parasites of sparid and labrid fishes of the Mediterranean Sea: a new approach. Parasitol. 119, 635–648. Smith, J.L.B. and Smith, M.M. 1986. Sparidae. In Smiths’ sea fishes. Eds., M.M. Smith and P.C. Heemstra. Berlin, Germany: Springer-Verlag, pp: 580–594. Wirtz, P., Fricke, R. and Biscoito, M.J. 2008. The coastal fishes of Madeira Island—new records and an annotated checklist. Zootaxa 1715, 1–26. | ||
| How to Cite this Article |
| Pubmed Style Altikbali MT, Showehdi ML, Benzeglam SA, Seif-alnaser AA, Shakman EA. Metazoan ectoparasites of Lithognathus mormyrus (Linnaeus, 1758) from the western coast of Libya. Open Vet. J.. 2024; 14(8): 1771-1778. doi:10.5455/OVJ.2024.v14.i8.4 Web Style Altikbali MT, Showehdi ML, Benzeglam SA, Seif-alnaser AA, Shakman EA. Metazoan ectoparasites of Lithognathus mormyrus (Linnaeus, 1758) from the western coast of Libya. https://www.openveterinaryjournal.com/?mno=180416 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i8.4 AMA (American Medical Association) Style Altikbali MT, Showehdi ML, Benzeglam SA, Seif-alnaser AA, Shakman EA. Metazoan ectoparasites of Lithognathus mormyrus (Linnaeus, 1758) from the western coast of Libya. Open Vet. J.. 2024; 14(8): 1771-1778. doi:10.5455/OVJ.2024.v14.i8.4 Vancouver/ICMJE Style Altikbali MT, Showehdi ML, Benzeglam SA, Seif-alnaser AA, Shakman EA. Metazoan ectoparasites of Lithognathus mormyrus (Linnaeus, 1758) from the western coast of Libya. Open Vet. J.. (2024), [cited January 25, 2026]; 14(8): 1771-1778. doi:10.5455/OVJ.2024.v14.i8.4 Harvard Style Altikbali, M. T., Showehdi, . M. L., Benzeglam, . S. A., Seif-alnaser, . A. A. & Shakman, . E. A. (2024) Metazoan ectoparasites of Lithognathus mormyrus (Linnaeus, 1758) from the western coast of Libya. Open Vet. J., 14 (8), 1771-1778. doi:10.5455/OVJ.2024.v14.i8.4 Turabian Style Altikbali, Malak. T., Mohamed. L. Showehdi, Sarah A. Benzeglam, Aisha A. Seif-alnaser, and Esmail A. Shakman. 2024. Metazoan ectoparasites of Lithognathus mormyrus (Linnaeus, 1758) from the western coast of Libya. Open Veterinary Journal, 14 (8), 1771-1778. doi:10.5455/OVJ.2024.v14.i8.4 Chicago Style Altikbali, Malak. T., Mohamed. L. Showehdi, Sarah A. Benzeglam, Aisha A. Seif-alnaser, and Esmail A. Shakman. "Metazoan ectoparasites of Lithognathus mormyrus (Linnaeus, 1758) from the western coast of Libya." Open Veterinary Journal 14 (2024), 1771-1778. doi:10.5455/OVJ.2024.v14.i8.4 MLA (The Modern Language Association) Style Altikbali, Malak. T., Mohamed. L. Showehdi, Sarah A. Benzeglam, Aisha A. Seif-alnaser, and Esmail A. Shakman. "Metazoan ectoparasites of Lithognathus mormyrus (Linnaeus, 1758) from the western coast of Libya." Open Veterinary Journal 14.8 (2024), 1771-1778. Print. doi:10.5455/OVJ.2024.v14.i8.4 APA (American Psychological Association) Style Altikbali, M. T., Showehdi, . M. L., Benzeglam, . S. A., Seif-alnaser, . A. A. & Shakman, . E. A. (2024) Metazoan ectoparasites of Lithognathus mormyrus (Linnaeus, 1758) from the western coast of Libya. Open Veterinary Journal, 14 (8), 1771-1778. doi:10.5455/OVJ.2024.v14.i8.4 |