| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 564-570 Original Research Heavy metals load in chicken meat and its reduction by probiotic strainsHeba M. Kamouh1*, Ghada A. Kirrella2, Saleh Shafik1 and Nader Y. Mostafa21Food Hygiene Department, Agricultural Research Center (ARC), Animal Health Research Institute (AHRI), Giza, Egypt 2Food Control Department, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafr El-Sheikh, Egypt *Corresponding Author: Heba M. Kamouh. Department of Food Hygiene, Animal Health Research Institute, Giza, Egypt. Email: heba.mostafa2011 [at] gmail.com Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/01/2024 © 2024 Open Veterinary Journal
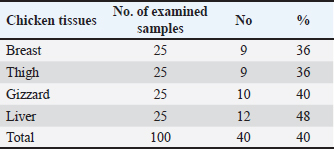
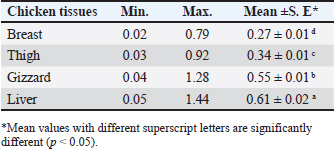
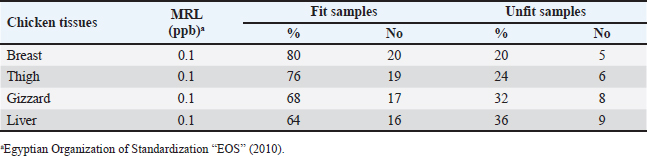
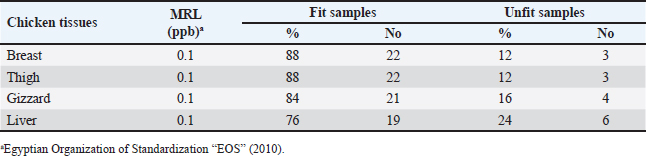
AbstractBackground: Around the world, poultry meat is a staple of everyday meals. However, chicken flesh is not always as healthy as it looks since it becomes contaminated by pollutants from the environment, especially heavy metals, which can bioaccumulate and magnify, and endanger human health. Aim: The current investigation set out to evaluate the degree of contamination in a subset of chicken samples and the potential risks to human health associated with the ingestion of chicken meat. Methods: Using atomic absorption spectroscopy, the heavy metal (lead, cadmium, and copper) contents of chicken samples were evaluated. To assess the residual content, 100 samples of chicken meat, 25 of each from the breast, thigh, gizzard, and liver were randomly selected from chicken butchers in Kafr El-Sheikh and El-Gharbia governorate in Egypt. Results: Lead had mean values of 0.27 ± 0.01, 0.34 ± 0.01, 0.55 ± 0.01, and 0.61 ± 0.02 (mg/kg ww), in the examined breast, thigh, gizzards, and liver, respectively. Cadmium, on the other hand, had mean levels (mg/kg ww) of 0.09 ± 0.01, 0.12 ± 0.01, 0.18 ± 0.01, and 0.25 ± 0.01 in the same samples, respectively. The copper residues had mean values (mg/kg ww) of 1.53 ± 0.14, 1.69 ± 0.16, 2.05 ± 0.17, and 2.71 ± 0.22, in the same samples, respectively. Conclusion: Further government efforts are required to reduce environmental pollution and enhance the quality of the El-Gharbia governorate to prevent the accumulation of heavy metals, even though the majority of the samples that were examined fell within the acceptable limits established by the Egyptian Organization for Standardization and Quality Control. Keywords: Heavy metals, Chicken flesh, Health risks, Reduction. IntroductionThe transfer of heavy metals from polluted sources to the food chain has posed a severe concern to human health. This is also a result of the world's population growing at an accelerated rate, which has created a lot of waste from residential, commercial, industrial, and agricultural sources, negatively affecting the ecosystem. The chicken industry is a major contributor to human diets in many parts of the world (Alturiqi and Albedair, 2012). Because of their significance from the perspectives of necessity and toxicity, heavy metals have been thoroughly investigated for their toxic effects (Tao et al., 2012; Ahmed et al., 2015). In addition to being necessary for human nutrition, several micronutrients, such as copper (Cu), may also be toxic in excessive concentrations (Rahman et al., 2014). Certain metals, such as lead (Pb) and cadmium (Cd), may inadvertently find their way into the food chain and endanger both people and animals (Rahman et al., 2014). According to Lei et al. (2010), the US Environmental Protection Agency has concluded that the most dangerous substances found in the environment are heavy metals. If ingested over an extended period of time, hazardous compounds can be extremely deadly even in tiny amounts (Forti et al., 2011). Lead has been connected to abnormal changes to the central nervous system and organs, which lower children's intelligence quotients. Cadmium is dangerous for the bones, kidneys, and circulatory system even though it is an inorganic substance. According to Ahmed et al. (2015), this is the most dangerous type of arsenic and causes cancer in humans. In underdeveloped countries, excessive amounts of heavy metal residue have been connected to municipal wastes. In the absence of appropriate control methods, heavy metal contamination is likely to reach dangerous levels when anthropogenic activities increase beyond normal. Growing numbers of hazardous, open disposal sites for home, municipal, industrial, and medical waste are regular occurrences (Solomon et al., 2014). At dump sites, heavy metals from human activity have accumulated in the soil. It has been stated that these metals pose a risk to crops, livestock, and people. Furthermore, concentrations of heavy metals can spread through the food chain, building up in the food web, and eventually reaching humans (Adewuyi and Opasina, 2010). The type of food and the path the food took to be exposed to the hazard element determine the concentrations of hazard elements in food. These substances can infiltrate food through a variety of environmental channels, including tainted water, untreated industrial and municipal waste, and contaminated air. The primary route via which humans are exposed to potentially hazardous components is thought to be food consumption; other pathways include inhalation and skin contact with polluted water (Arslan et al., 2016). Because heavy metals are harmful, even in very low quantities, the risk of heavy metal contamination in meat is extremely concerning for the safety of animal and human health (Akan et al., 2010). Heavy metals can build up in food after years of exposure and have long-term effects. To stop the accumulation of heavy metals in the food chain, it is crucial to monitor the amounts of these metals in food (Al-Hwaiti and Al-Khashman, 2015). Heavy metals come from a variety of sources, including batteries, metal melting, metal products manufacturing, car emissions, cable coating businesses, diesel generators, and re-suspended road dust. All these resources have the potential to be major contributors to food contamination. Another source of heavy metals in the field is irrigation water tainted by industrial waste (Hariri et al., 2015; Adejumo et al., 2016). One of the many experiments that have been carried out to control these factors and their main issue is probiotics. According to Veglio et al. (1997), their method of action in reducing these hazardous risks may be ascribed to the metal ions' ability to attach to extracellular polysaccharides and cell walls of both active and inactive cells through a variety of processes including adsorption, ion exchange, complexation, chelation, and microprecipitation. Thus, the goals of this research are to determine the levels of Cu, Cd, and Pb in frequently consumed meat products in Egypt, as well as to assess the health hazards associated with the consumption of such meat types. Besides, a trial to reduce the metal load in the contaminated samples using probiotics was tested. Materials and MethodsSample collectionA total of 100 randomly selected samples of chicken, consisting of 25 pieces of each of the gizzard, liver, breast, and thigh, were gathered from several chicken slaughterhouses situated in the governorates of Kafr El-Sheikh and El-Gharbia, Egypt. Each sample was wrapped and then transported and cooled to the laboratory for the determination of their heavy metal residues (lead, cadmium, and copper). Method of digestionOne gram of each sample was macerated and digested in 10 ml of a mixture (60 ml of 65% nitric acid and 40 ml of 70% perchloric acid) in a screw-capped tube (Tsoumbaris and Papadopoulou, 1994; AOAC, 2006). Heavy metals measurementLead, Cd, and Cu residues were recorded using a Flame Atomic Absorption Spectrophotometer. Lead, Cd, and Cu absorbencies were taken straight from the digital AAS scale, and their concentrations were computed using the formula, C=R × (D/W), where R is the reading of the AAS digital scale and C is the lead concentration (mg/kg) wet weight. D stands for prepared sample dilution and W for sample weight. Impact of Lactobacillus rhamnosus on experimentally inoculated chicken fillets'The probiotic L. rhamnosus strain utilized in this investigation was acquired from the Graduate School of Medicine, Department of Microbiology and Immunology, Osaka University, Osaka, Japan. To create the diluted solutions used for binding studies, Fluka (Sigma-Aldrich) provided a standard solution of cadmium and lead (1,000 mg/l) in HNO3 (2%, w/w). To create an overnight culture, a strain of L. rhamnosus was cultured for 24 hours at 37°C in brain heart infusion (BHI) broth (Sigma-Aldrich, Chemie GmbH). Sterile peptone water (0.1%, w/v) was used to decimally dilute 1 ml of the grown bacterial suspension (Merck, Darmstadt, Germany). Thus, using the plate count method (a volume of the culture broth corresponding to about 1 × 107) and BHI Agar (Sigma-Aldrich Chemie GmbH), the viable count of L. rhamnosus strain was performed (Halttunen et al., 2008). Assay for bindingOne kilogram of chicken fillets was mixed in the bacterial strain to achieve a final concentration of either 1 × 107 bacteria and 20 mg/kg ionic lead standard solution, 1 × 107 bacteria plus 20 mg/kg ionic cadmium standard solution. The chicken fillets were incubated for 24 hours on a fine Mixer SH2000 Orbital Shaker (FINEPCR, Seoul, Korea) with gentle agitation after being treated with bacterial stain and metal solution. As a result, the metal-contaminated chicken fillets were used as the control sample. On the other hand, the treated groups received L. rhamnosus, while the test groups consisted of heavy metal-contaminated beef fillets. Ultrapure HNO3 was used to acidify the samples, which were then measured for each free metal using a flame atomic absorption spectrophotometer at 0, 8, 16, and 24-hour intervals (Halttunen et al., 2008). Statistical analysisIn accordance with Feldman et al. (2003), the ANOVA was carried out for the statistical evaluation of the acquired results, where p values of 0.05 or less were considered to be significant. Ethical approvalNot needed for this study. ResultsLeadThe data gathered are presented in Table 1, which indicates that Pb contamination was detected in 36%, 36%, 40%, and 48% of the raw chicken meat (liver, thigh, gizzards, and breast, in that order). The Pb concentrations in the examined samples of chicken flesh (breast, thigh, gizzard, and liver) had a mean value of 0.27 ± 0.01 µg/g (breast), 0.34 ± 0.01 µg/g (thigh), 0.55 ± 0.01 µg/g (gizzards), and 0.61 ± 0.02 µg/g (liver), according to the results in Table 2. For Pb residues in meat and offal, the Egyptian Organization for Standardization and Quality Control (EOS, 2010) established a permissible level of 0.1 mg/kg for meat and 0.5 mg/kg for edible offal. According to Table 3, the proportions of undesirable chicken breast, thigh, gizzard, and liver were, respectively, 20%, 24%, 32%, and 36%. Table 1. Occurrence of Pb residues in the examined samples.
Table 2. Concentrations of Pb (mg/kg) in the examined samples.
Table 3. Fitness of the examined samples of chicken meat and offal according to their Pb residues.
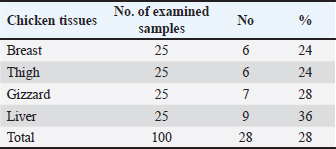
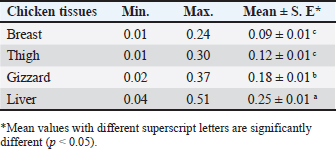
CadmiumTable 4 presented the percentages of Cd residues found in the liver, gizzard, thigh, and chicken breast samples that were analyzed (24%, 24%, 28%, and 36%, respectively). The amounts of Cd in samples of chicken liver, gizzard, thighs, and breast are shown in Table 5. The mean Cd contents in the subsequent samples were 0.09 ± 0.01, 0.12 ± 0.01, 0.18 ± 0.01, and 0.25 ± 0.01 µg/g, in that order. For Cd residues in meat and offal, a permissible limit of 0.05 µg/g for meat and 0.1 µg/g for edible offal has been established (EOS, 2010). Table 6 shows that the percentages of chicken breast, thigh, gizzard, and liver that were deemed undesirable were 12%, 12%, 16%, and 24%, respectively. Table 4. Occurrence of Cd residues in the examined samples.
Table 5. Concentrations of Cd (mg/kg) in the examined samples.
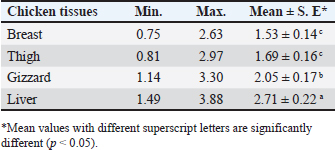
CopperAll examined samples had Cu residues. The average concentrations (µg/g ww) of Cu in the examined poultry meat samples, breast, thigh, gizzard, and liver were 1.53 ± 0.14, 1.69 ± 0.16, 2.05 ± 0.17, and 2.71 ± 0.22, respectively (Table 7). A maximum of 15.0 µg/g for meat and edible offal is the maximum permissible limit of Cu in meat and offal as established by EOS (EOS, 2010). No sample exceeded that limit. Table 6. Fitness of the examined samples of chicken meat according to their Cd residues.
Table 7. Concentrations of Cu (mg/kg) in the examined samples.
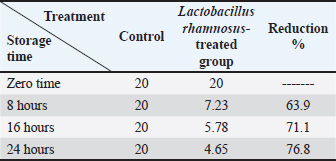
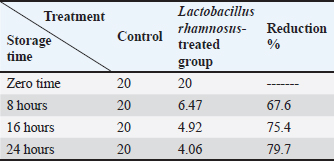
Effects of L. rhamnosus culture on experimentally contaminated chicken fillet with Pb and CdThe findings shown in Table 8 showed that the L. rhamnosus strain culture (1 × 107) effectively reduced Pb. After 8, 16, and 24 hours of incubation, Pb content showed a decline; the reduction rates were 63.9%, 71.1%, and 76.8%, respectively. Likely, Cd residues were significantly reduced by 67.6%, 75.4%, and 79.7%, respectively (Tables 8 and 9). Table 8. Influence of L. rhamnosus on the levels of Pb experimentally inoculated to chicken fillets.
Table 9. Influence of L. rhamnosus on the levels of Cdexperimentally inoculated to chicken fillets.
DiscussionLeadAccording to Garcia-Leston et al. (2010), lead is a non-essential element that has a well-known history of having detrimental health effects, such as neurotoxicity and nephrotoxicity. Concerning the Pb concentrations that were found in the tested samples of chicken giblets and flesh. According to earlier findings from Egypt and around the world, these values were comparable to previous reports (ElBayomi et al., 2018, El-Wehedy et al., 2018, Elsharawy, 2019). A number of other researchers (Iwegbue et al., 2008, Sadeghi et al., 2015, Chowdhury et al., 2022) reported higher Pb levels. Other reports showed that meat samples yielded lower values (Mohammed et al., 2013, Onyeka and David, 2015). Lead toxicity results in neuropathy, and lead encephalopathy, which manifests as agitation, ataxia, seizures, and altered states of consciousness in children. Other conditions that could arise include ovarian atrophy, renal toxicity, liver apoptosis, hemolytic anemia, and atherosclerosis (Khalafalla et al., 2011). CadmiumAccording to Khalafalla et al. (2011), cadmium is an ostensibly non-essential metal that is missing at birth but accumulates in the body as people age. This process is known as tissue-specific bioaccumulation. The levels of Cd residues found in the gizzards, liver, breast, and thigh meat samples that were tested were greater than those found in previous publications (Ghimpeteanu et al., 2012; Hamasalim and Mohammed, 2013). Prior studies found almost identical amounts (Onyeka and David, 2015; Shaltout et al., 2018). Although the results of the current study were not as high as those reported in a number of papers (Badis et al., 2014; Mottalib et al., 2018). Liver samples had the highest Cd concentrations found in the tested samples. Muscle samples had the lowest amounts of Cd concentrations found in them. The meats with the highest concentrations of cadmium were the liver, gizzard, thigh, and breast. Cadmium lowers the glomerular filtration rate and produces nephrotoxicity, glucosuria, aminoacid uria, and severe respiratory symptoms. It can also induce lung damage, liver injury, and hypertension. Significant changes were observed in the liver and gizzard detoxifying enzymes when exposed to a teratogenic dosage of cadmium chloride. It was stated that Cd is the cause of the Itai-Itai sickness, osteoporosis, and osteomalacia (Faten et al., 2014). CopperHuman metabolism also requires Cu, but excessive Cu consumption can result in convulsions, vomiting, cramping in the muscles, and even death (Fu and Wang, 2011). Copper is a crucial component of many different enzymes in both people and animals. It primarily builds up in the muscle and liver where it serves as a vital element, but when concentrations of Cu exceed safe bounds, it can be hazardous to both humans and animals over the long term. Cu residues found in the samples under examination matched those previously documented in chicken giblets and flesh (Darwish et al., 2018). Conversely, a few publications (Canli and Atli, 2003; Iwegbue et al., 2008; Chowdhury et al., 2011; Imran et al., 2015; Jayanthi et al., 2019) reported greater quantities of copper. Meat and edible offal poultry have been found to have lower copper concentrations (Akan et al., 2010). Effects of L. rhamnosus culture on experimentally contaminated chicken fillet with Pb and CdHussien and Nosir (2017) asserted that there was a direct connection between public health and the harmful impacts of heavy metals in the food chain and the environment's pollution with these harmful pollutants. Probiotics are one of the many trials that have been done in an effort to regulate these elements and their main issue. Some microbes, like lactic acid bacteria (LAB), have the ability to move and hold metal ions within their cells. As a result, a lot of research has been done lately on the interactions between metal ions and LAB in an effort to develop the usage of these bacteria for novel biotechnology processes as well as for health purposes and as probiotics. Food safety and quality can be enhanced by using LAB that has a high absorption potential for necessary and hazardous metal ions, according to a preliminary study conducted in model aqueous solutions (Mrvčić et al., 2012). The information gathered showed that the L. rhamnosus strain successfully decreased the levels of Pb and Cd in the chicken samples that were experimentally infected. The outcomes surpassed the findings of Halttunen et al. (2007), who reported that Pb and Cd removal ranged from 39.7% to 69.6% and 22.1% to 49.1%, respectively, by four different bacterial strains (L. rhamnosus LC705, L. rhamnosus GG, Propionibacterium freudenreichii shermanii JS, and Bifidobacterium-breve Bbi99/E8). In contrast to Enterococcus faecium E and EF031, which bound 77.3% to 98.1% of Cd and 66.9% to 98.9% of Pb, M74 bound 53.5% to 91% of Cd and 42.9% to 93.1% of Pb. Topcu and Bulat (2010) reported a lower decrease rate. Furthermore, the ability of two strains of Saccharomy cerevisiae (ATCC 24858, ATCC 834) to bind metals, specifically Cd, was examined by Park et al. (2003). They claimed that S. cerevisiae ATCC 834 could bind more Cd because it had a larger specific surface area. Hussien and Nosir (2017) also used a probiotic strain (Enterococcus facium E980) to reduce Cd and Pb to 79.4% and 75.0%, respectively. ConclusionThe human body needs both trace elements for essential processes. The goal of this study was to clarify the residual Pb, Cd, and Cu concentrations detected in chicken meat and edible offal. All heavy metal concentrations were found to be high in the muscle, gizzard, and liver, respectively, and the majority were within allowable levels. The use of the probiotic strain L. rhamnosus effectively reduced Pb and Cd from the experimentally contaminated chicken samples. AcknowledgmentNone. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis research received no specific grant. Author contributionH.M.K. performed the experiments, analyzed the data, and wrote the manuscript; G.A.K. worked on measurement and statistical analysis and edited the manuscript; S.S. collected the samples. G.A.K., S.S., and N.Y.M. supervised the work. All authors reviewed and approved the manuscript. Data availabilityAll data will be available upon a reasonable request. ReferencesAdejumo, O.E., Pius, S.F., Odion, J.E., Silva, B.O. and Fajemirokun, T.O. 2016. High cadmium levels in cured meat products marketed in Nigeria-implications for public health. Asian. Pac. J. Cancer. Prev. 17, 4. Adewuyi, G.O. and Opasina, M.A. 2010. Physicochemical and heavy metal assessment of leachates from Aperin Abardoned Dumpsite in Ibadan city, Nigeria. Environ. J. Chem. 7, 1278–1283. Ahmed, M.K., Shaheen, N., Islam, M.S., Al-Mamun, M.H., Islam, S., Mohiduzzaman, M. and Bhattacharjee, L. 2015. Dietary intake of trace elements from highly consumed cultured fish (Labeo rohita, Pangasius pangasius and Oreochromis mossambicus) and human health risk implications in Bangladesh. Chemosphere 128, 284–292. Akan, J.C., Abdulrahman, F.I., Sodipo, O.A. and Chiroma, Y.A. 2010. Distribution of heavy metals in the liver, kidney and meat of beef, mutton, caprine and chicken from Kasuwan Shanu Market in Maiduguri Metropolis, Borno State, Nigeria. Res. J. Appl. Sci. Eng. Technol. 2, 743–748. Al-Hwaiti, M. and Al-Khashman, O. 2015. Health risk assessment of heavy metals contamination in tomato and green pepper plants grown in soils amended with phosphogypsum waste materials. Environ. Geochem. Health. 37, 287–304. Alturiqi, A.S. and Albedair, L.A. 2012. Evaluation of some heavy metals in certain fish, meat and meat products in Saudi Arabian markets. Egypt. J. Aquatic. Res. 38, 45–49. Arslan, B., Djamgoz, M.B. and Akün, E. 2016. A Review on exposure pathways, accumulation, mobility and transmission into the human food chain. Rev. Environ. Contam. Toxicol. 243, 27–51. Association of Official Analytical Chemists “AOAC” 2006. Official methods of analysis, 31th ed. Washington, DC: Academic Press, Vol. 114, pp: 30–35. Badis, B., Rachid, Z. and Esma, B. 2014. Levels of selected heavy metals in fresh meat from cattle, sheep, chicken and camel produced in Algeria. Ann. Res. Rev. Biol. 4, 1260–1267. Canli, M. and Atli, G. 2003. The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, and Zn) levels and the size of six Mediterranean fish species. Environ. Pollut. 121, 129–136. Chowdhury, M., Abir, M., Nesha, M., Fardous, Z., Rahman, H. and Bari, L. 2022. Assessment of toxic heavy metals and trace elements in poultry feeds, consumer chickens and eggs in Bangladesh. Asian-Austral. J. Biosci. Biotechnol. 6, 128–141. Chowdhury, M.Z.A., Siddique, Z.A., Hossain, S.M.A., Kazi, A.I., Ahsan, A.A., Ahmed, S. and Zaman, M.M. 2011. Determination of essential and toxic metals in meats, meat products and eggs by spectrophotometric method. J. Bangl. Chem. Soc. 24, 165–172. Darwish, W.S., Atia, A.S., Khedr, M.H. and Eldin, W.F.S. 2018. Metal contamination in Quail meat: residues, sources, molecular biomarkers, and human health risk assessment. Environ. Sci. Pollut. Res. 25, 20106–20115. ElBayomi, R., Darwish, W., Elshahat, S. and Hafez, A. 2018. Human health risk assessment of heavy metals and trace elements residues in poultry meat retailed in Sharkia Governorate, Egypt. Slov. Vet. Res. 55, 211–230. Elsharawy, N. 2019. Control of cadmium, copper, iron and lead residues in chicken meat and their offal. J. Food. Nutr. Sci. 6, 62–67. El-Wehedy, S.E., Darwish, W.S., Tharwat, A.E. and Hafez, A.E. 2018. Estimation and health risk assessment of toxic metals and antibiotic residues in meats served at hospitals in Egypt. J. Vet. Sci. Technol. 9, 1–5. EOS. 2010. Egyptian Organization for Standardization and Quality Control. Maximum level for certain contaminants in food stuffs. ES No. 7136/2010. Faten, S., Hassan, M., Amira, M. and Enas, A. 2014. Heavy metals residues in some chicken meat products. Benha. Vet. Med. J. 27, 256–263. Feldman, D., Ganon, J., Haffman, R. and Simpson, J. 2003. The solution for data analysis and presentation graphics, 2nd ed. Berkeley, CA: Abacus Lancripts, Inc. Forti, E., Salovaara, S., Cetin, Y., Bulgheroni, A., Pfaller, R.W. and Prieto, P. 2011. In vitro evaluation of the toxicity induced by nickel soluble and particulate forms in human airway epithelial cells. Toxicol. In Vitro. 25, 454–461. Fu, F. and Wang, Q. 2011. Removal of heavy metal ions from wastewaters: a review. J. Environ. Manag. 92, 407–418. Garcia-Leston, J., Mendez, J., Pasaro, E. and Laffon, B. 2010. Genotoxic effects of lead: an updated review. Environ. Intern. 36, 623–636. Ghimpeteanu, O.M., Das, K., Militaru, M. and Scippo, M.L. 2012. Assessment of heavy metals and mineral nutrients in poultry liver using inductively coupled plasma- mass spectrometer (ICP-MS) and direct mercury analyzer (DMA). Bull. UASMV. Vet. Med. 69, 285–266. Halttunen, T., Salminen, S. and Tahvonen, R. 2007. Rapid removal of lead and cadmium from water by specific lactic acid bacteria. Int. J. Food. Microbiol. 114, 30–35. Halttunen, T., Salminen, S., Jussi, M., Raija, T. and Kalle, L. 2008. Reversible surface binding of cadmium and lead by lactic acid and bifidobacteria. Int. J. Food Microbiol. 125(2), 170–175. Hamasalim, H.J. and Mohammed, H.N. 2013. Determination of heavy metals in exposed corned beef and chicken luncheon that sold in Sulaymaniah markets. Afr. J. Food. Sci. 7, 178–182. Hariri, E., Abboud, M., Demirdjian, S., Korfali, S., Mroueh, M. and Taleb, R. 2015. Carcinogenic and neurotoxic risks of acrylamide and heavy metals from potato and corn chips consumed by the Lebanese population. J. Food. Compos. Anal. 42, 91–97. Hussien, H. and Nosir, S. 2017. Assessment of heavy metals residues in some food stuffs and its biocontrol by probiotic strain Enterococcus facium (E980) in an experimental model. Alex. J. Vet. Sci. 52, 54. Imran, R., Hamid, A. and Amjad, R. 2015. Estimation of the heavy metal concentration in the poultry meat being produced in Kasur. J. Biodivers. Environ. Sci. 7, 62–75. Iwegbue, C.M.A., Nwajei, G.E. and Iyoha, E.H. 2008. Heavy metal residues of chicken meat and gizzard and turkey meat consumed in southern Nigeria. Bulg. J. Vet. Med. 11, 275–280. Jayanthi, R., Appa, R.V., Narendra, R., Sivakumar, P., Sriram, T. and Robinson, J.J. 2019. Analysis of essential heavy metals in ready-to-eat chicken meat products of Chennai city. J. Entomol. Zool. Stud. 7, 427–432. Khalafalla, A., Fatma, H., Schwagele, F. and Mariam, A. 2011. Heavy metal residues in beef carcasses in Beni-Suef abattoir, Egypt. Vet. Ital. 47, 351–361. Lei, M., Zhang, Y., Khan, S., Qin, PF. and Liao, B. 2010. Pollution, fractionation and mobility of Pb, Cd, Cu, and Zn in garden and paddy soils from a Pb/Zn mining area. Environ. Monitor. Assess. 168, 215–222. Mohammed, A.I., Kolo, B. and Geidam, Y.A. 2013. Heavy metals in selected tissues of adult chicken layers (Gallus spp). ARPN. J. Sci. Technol. 3, 518–522. Mottalib, M.A., Zilani, G., Suman, T.I., Ahmed, T. and Islam, S., 2018. Assessment of trace metals in consumer chickens in Bangladesh. J. Health. Pollut. 8, 181208. Mrvčić, J., Stanzer, D., Šolić, E. and StehlikTomas, V. 2012. Interaction of lactic acid bacteria with metal ions: opportunities for improving food safety and quality. World. J. Microbiol. Biotechnol. 28, 2771–2782. Onyeka, O. and David, O.D. 2015. Assessment of selected heavy metal residues in the kidney, liver, muscle and gizzard of chickens raised within Enugu metropolis. Int. J. Environ. Pollut. Res. 3, 62–66. Park, J.K., Lee, J.W. and Jung, J.Y. 2003. Cadmium uptake capacity of two strains of Saccharomy cescerevisiae cells. J. Enzyme. Microb. Technol. 33, 371–378. Rahman, M.A., Rahman, M.M., Reichman, S.M., Lim, R.P. and Naidu, R. 2014. Heavy metals in Australian grown and imported rice and vegetables on sale in Australia: health hazard. Ecotoxicol. Environ. Saf. 100, 53–60. Sadeghi, A., Hashemi, M., Jamali-Behnam, F., Zohani, A., Esmaily, H. and Dehghan, A.A. 2015. Determination of chromium, lead and cadmium levels in edible organs of marketed chickens in Mashhad, Iran. J. Food. Qual. Hazards. Control. 2, 134–138. Shaltout, F., Ahmed, A.M. and Ahmed, M. 2018. Heavy metal residues in chicken cuts up and processed chicken meat. Benha. Vet. Med. J. 34, 473–483. Solomon, I.P., Oyebadejo, S.A. and Uyanga, V.A. 2014. Effect of heavy metals in feeding dumpsite forage (Calapo—Calopogonium mucunoides) on heamatological profile of rabbits (Oryctolagus cuniculus). Asian. J. Biomed. Pharm. Sci. 4, 35–43. Tao, Y., Yuan, Z., Xiaona, H. and Wei, M. 2012. Distribution and bioaccumulation of heavy metals in aquatic organisms of different trophic levels and potential health risk assessment from Taihu Lake, China. Ecotoxicol. Environ. Saf. 81, 55–64. Topcu, A. and Bulat, T. 2010. Removal of cadmium and lead from aqueous solution by Enterococcus faecium strains. J. Food. Sci. 75(1), T13–T17. Tsoumbaris, P. and Papadopoulou, T.H. 1994. Heavy metals in common foodstuff: quantitative analysis. Bull. Eniron. Cntam. Toxicol. 53, 61–66. Veglio, F., Beolchini, F. and Gasbarro, A., 1997. Biosorption of toxic metals: an equilibrium study using free cells of Arthrobacter sp. Process. Biochem. 32, 99–105. | ||
| How to Cite this Article |
| Pubmed Style Kamouh HM, Kirrella GA, Shafik S, Mostafa NY. Heavy metals load in chicken meat and its reduction by probiotic strains. Open Vet. J.. 2024; 14((1) (Zagazig Veterinary Conference)): 564-570. doi:10.5455/OVJ.2024.v14.i1.51 Web Style Kamouh HM, Kirrella GA, Shafik S, Mostafa NY. Heavy metals load in chicken meat and its reduction by probiotic strains. https://www.openveterinaryjournal.com/?mno=182432 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i1.51 AMA (American Medical Association) Style Kamouh HM, Kirrella GA, Shafik S, Mostafa NY. Heavy metals load in chicken meat and its reduction by probiotic strains. Open Vet. J.. 2024; 14((1) (Zagazig Veterinary Conference)): 564-570. doi:10.5455/OVJ.2024.v14.i1.51 Vancouver/ICMJE Style Kamouh HM, Kirrella GA, Shafik S, Mostafa NY. Heavy metals load in chicken meat and its reduction by probiotic strains. Open Vet. J.. (2024), [cited January 25, 2026]; 14((1) (Zagazig Veterinary Conference)): 564-570. doi:10.5455/OVJ.2024.v14.i1.51 Harvard Style Kamouh, H. M., Kirrella, . G. A., Shafik, . S. & Mostafa, . N. Y. (2024) Heavy metals load in chicken meat and its reduction by probiotic strains. Open Vet. J., 14 ((1) (Zagazig Veterinary Conference)), 564-570. doi:10.5455/OVJ.2024.v14.i1.51 Turabian Style Kamouh, Heba M., Ghada A. Kirrella, Saleh Shafik, and Nader Y. Mostafa. 2024. Heavy metals load in chicken meat and its reduction by probiotic strains. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 564-570. doi:10.5455/OVJ.2024.v14.i1.51 Chicago Style Kamouh, Heba M., Ghada A. Kirrella, Saleh Shafik, and Nader Y. Mostafa. "Heavy metals load in chicken meat and its reduction by probiotic strains." Open Veterinary Journal 14 (2024), 564-570. doi:10.5455/OVJ.2024.v14.i1.51 MLA (The Modern Language Association) Style Kamouh, Heba M., Ghada A. Kirrella, Saleh Shafik, and Nader Y. Mostafa. "Heavy metals load in chicken meat and its reduction by probiotic strains." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 564-570. Print. doi:10.5455/OVJ.2024.v14.i1.51 APA (American Psychological Association) Style Kamouh, H. M., Kirrella, . G. A., Shafik, . S. & Mostafa, . N. Y. (2024) Heavy metals load in chicken meat and its reduction by probiotic strains. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 564-570. doi:10.5455/OVJ.2024.v14.i1.51 |