| Research Article | ||
Open Vet. J.. 2024; 14(4): 1029-1042 Open Veterinary Journal, (2024), Vol. 14(4): 1029–1042 Original Research Customized modified therapies for ovarian cyst and persistent follicle followed with TAI in early postpartum dairy cowsNajmi M. Mariol1*, Atef B. Mahdy2, Hussein A. Amer2, Ali A. Aghwider3 and Abubakr M. Hazzaa21Department of Surgery and Theriogenology, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya 2Department of Theriogenology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 3Department of Physiology, Faculty of Veterinary Medicine, Azzaytuna University, Tarhuna, Libya *Corresponding Author: Najmi M. Mariol. Department of Surgery and Theriogenology, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya. Email: drnajmi2000 [at] gmail.com Submitted: 12/01/2024 Accepted: 18/03/2024 Published: 30/04/2024 © 2024 Open Veterinary Journal
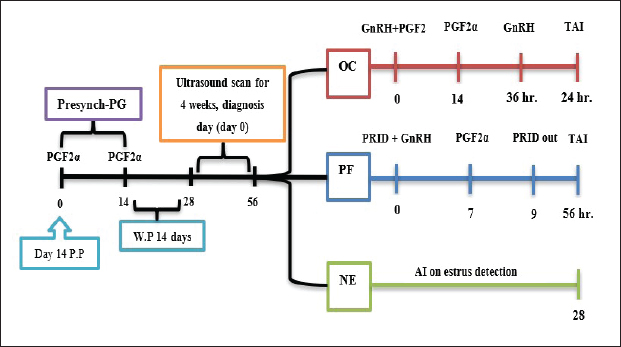
AbstractBackground: Postpartum ovarian dysfunction [ovarian cyst (OC) and persistent follicle (PF)] has been an important issue. Finding effective hormonal treatments to improve reproductive performance in dairy cows has become a necessity. Aim: Improve reproductive performance and ovarian activity in postpartum cows with specific customized treatment for OC and PFs Methods: The study included 48 cows at 14 days P.P, which received two dosages of 500 μg IM cloprostenol, 14 days apart as presynchronization protocol. Ultrasound ovarian scans 14 days after the last injection for 4 weeks. The cows were divided into three groups according to ovarian status: OC (n=14), PF (n=12), and NE (n =22). In the OC group, received 500 μg IM cloprostenol and 100 μg IM cystoriline, a second dose of cloprostenol 14 days later and a second dose of cystoriline 36 hours later, and AI after 24 hours (GnRH+ PG/PG/GnRH). In the PF group, was fitted with progesterone-releasing intravaginal device (PRID) for 9 days; the same day, they received 100 μg cystoreline then 500 μg cloprostenol 7 days later, after PRID removal AI 56 hours later (PRID + GnRH/PG). In the NE group, artificial insemination was implemented until 28 days depending on estrus detection. Results: The ovarian activity was greatly affected by the customized treatments, leading to enhanced follicular and luteal activity, particularly after the PGF2α injection. The OC and PF groups showed substantial estrus responses of 71.43% and 75.02%, respectively, during AI time. While the NE group had an ovulation rate of 54.5% and a pregnancy rate of 31.8%, the treatment groups showed marked improvements in reproductive performance. The ovulation rates in the OC and PF groups were 71.43% and 75% and the pregnancy rates at the 1st artificial insemination were 64.28% and 66.7%. Conclusion: Improving reproductive performance and minimizing the time to first service are possible advantages of early case-specific treatment for postpartum cows with OC and PFs. Keywords: Postpartum cow, Ovarian cyst, Persistent follicle, TAI. IntroductionRecent studies reveal common pre-service/post-partum ovarian issues, including energy intake and postpartum diseases, with only 51% of high-producing dairy cows showing normal cyclicity 50 days postpartum (Opsomer et al., 2000; Abraham, 2017). Hormonal synchronization and reproductive management have been associated with differences in reproductive performance in previous studies (Negrón-Pérez et al., 2019; Civiero et al., 2021; Pfeifer et al., 2021; Fricke and Wiltbank, 2022; Fury and Trevor, 2022; Mounir and Aspinas, 2022; Haile et al., 2023). The duration of follicular dominance, diestrus progesterone, proestrus length, and estradiol exposure have been found to impact ovarian dynamics, fertilization, embryo quality, and uterine environment (Crowe, 2008; Chauhan et al., 2021; Sayid, 2021). Functional infertility, influenced by management, sickness, and genetics, affects cow performance and breeding, affecting ovaries, oestrous behavior, and uterine function, with sterility rare and subfertility widespread (Parkinson et al., 2009). When they affect a significant percentage, become a major issue, especially as luteal deficiencies, cystic ovary, persistent corpus luteum (CL), anestrus, non-detected estrus, and repeat breeding (Vanholder et al., 2006; Schlafer and Miller, 2007; Kumar et al., 2020; Pérez-Marín and Quintela, 2023). Ultrasonography and hormonal measurements have identified anovulatory conditions, characterized by deviation but not ovulatory size, are a significant issue in cows, affecting GnRH/LH pulses. These conditions, primarily due to changes in hypothalamus responsiveness to estradiol, can be treated by increasing progesterone concentrations (Wiltbank et al., 2002; BorŞ and BorŞ, 2020; Kumar et al., 2020). Low progesterone concentration in plasma may contribute to follicular pathologies persistence (Roth et al., 2012). It is still not entirely clear what process gives rise to ovarian dysfunction in postpartum dairy cattle, but many authors started to figure out and find different ways to treat and prevent such issues. One of the most popular therapies are used the GnRH and PGF2α combination or synergistically with progesterone in the form of progesterone-releasing intravaginal device (PRID) or CIDR, different protocols planned to get different results, and try to increase postpartum dairy cattle fertility and decrease the hazer of anestrus problems. Many therapeutic programs have been developed to improve fertility (López-Gatius, 2022; Tschopp et al., 2022; Amin et al., 2023; Martins et al., 2023; Rojas Canadas et al., 2023; Uddin et al., 2023). The study objective was to identify specific therapies for anestrus issues in early-postpartum dairy cows that were caused by ovarian cysts (OC) and persistent follicles (PFs). Customized modified therapies are intended for each case to investigate the effect of the treatment on ovarian activity and reproductive performance after timed artificial insemination (TAI). Materials and MethodsExperimental animalsFour commercial dairy farms in Alzawia (60 km west of Tripoli, 30.54 N0 latitude) from August 2022 to March 2023. Only 48 out of 83 cows participated in this experiment. All dairy cows were fed a complete milking cow feed, water, and minerals in partially shaded open stalls. The animals were ranged from 3 to 6 years old. The average cow milked 30 l three times a day. Experimental designThe cows were presynchronized by the second week after birth, depending on the birth order. Two doses of cloprostenol 500 μg IM. (Synchromate, Bremer Pharma, GMBH, Cloprostenol 250 μg/ml IM), 14 days apart. Transrectal ultrasonography (a portable machine B-mode scanner. RKU10 Handheld, equipped with a 5.0–7.5 MHz transducer Vet. Palm. Ultrasound KEEBOMEM, CHINE), examination 14 days following the second presynchronization dosage of cloprostenol for 4 weeks or until AI or starting treatment, both ovaries were scanned. The cows were then divided into three groups according to their ovarian status (Fig. 1): (i) OC group, (ii) PF group, and (iii) Natural estrus group (NE). OC groupThere were 14 cows in this group that were diagnosed with OC after two successive ultrasounds and revealed follicular structures larger than 25 mm, without CL, and without any estrus symptoms. In this group, the cows received 500 μg IM., cloprostenol and 100 μg IM., cystoriline (Gonadoreline, GnRH diacetate tetrahydrate 50 μg/ml for parenteral injection, Ceva Sante animale, 10, va. De La Ballastiere, 33500 libourne, France) at cyst diagnosis, a second dose of cloprostenol 14 days later and second dose of cystorelin 36 hours., insemination was undertaken 24 hours. after the second cystorelin dose (GnRH + PG/PG/GnRH protocol) (Fig. 1).
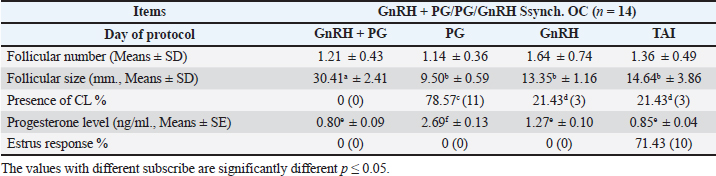
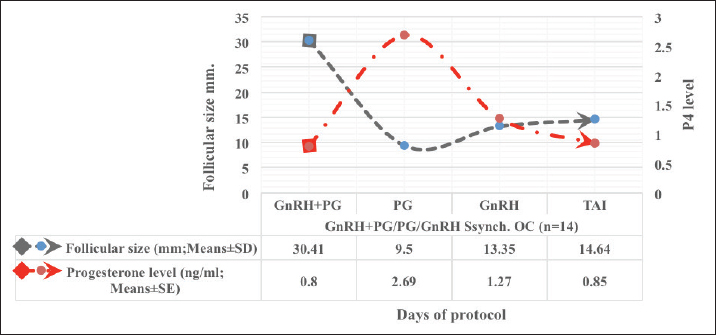
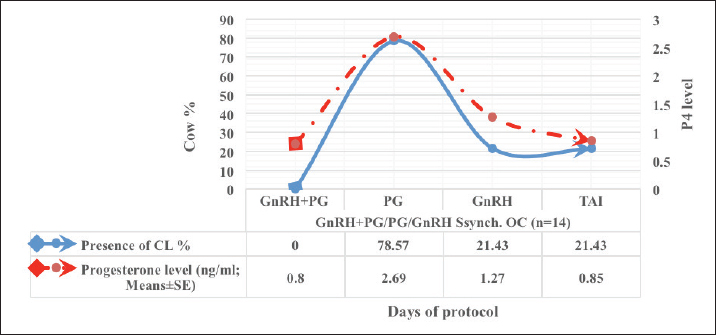
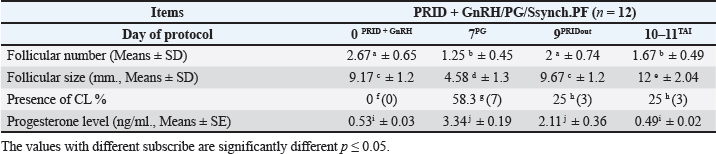
Fig. 1. Experimental design. PF groupThe cows in this group (n=12) were considered to have a PF when a follicular structure ≤15 mm, was detected in two consecutive ultrasonography examinations 7 days apart, in the absence of a CL or cyst, without any signs of estrus noted during the time of observation. These cows were fitted with a (PRID containing 1.55 g progesterone, PRID, DELTA; Ceva Sante animale, Z.I. Tres-22600 Loudeac, France). PRID was maintained in the vagina for 9 days, at the same day of PRID insertion, the cows received 100 μg cystoreline and 500 μg cloprostenol IM., 7 days later. These cows were inseminated 56 hours., after PRID removal (PRID + GnRH/PG protocol) (Fig. 1). NE groupIncluding (n=22), cows showing NE between days 0 and 28. Estrus was confirmed by palpation per rectum and the cows were inseminated at this time (Fig. 1). Ovulation rate was evaluated by ultrasound on days 11–14 following the first AI, by detecting a CL in the ovaries. If a cow returned to estrus during 30 days, re-insemination was done without medication. Pregnancy diagnoses were conducted on day 45 following insemination by ultrasound and confirmed on day 70. Progesterone was determined using The Eagle Biosciences Progesterone ELISA Assay Kit (enzyme-linked immunoassay kit) is intended for the direct quantitative determination of progesterone to investigate ovarian activity during postpartum. The sensitivity of the test is 0.1 ng/ml, dynamic range of 0.3–60 ng/ml. Statistical analysisThe data were analyzed using descriptive statistics for estrus response, ovulation rate, return rate, pregnancy rate (1st and 2nd AI), and ovarian activity. were analyzed by t-test and chi-squared test. The level of significance was set p ≤ 0.05. The statistical analysis was carried out by using SPSS.2007, version 16. Ethical approvalIn accordance with the National Institutes of Health for the Care and Use of Animals’ guidelines, the Zagazig University ethical committee approved the study. ResultsAccording to ovarian findings in Table 1, the cows had OC, after enrolled in the GnRH + PG/PG/GnRH protocol. The follicular size significantly changed from 30.41 ± 2.41 mm at the start of the treatment to 9.59 ± 0.59 mm at PGF2α injection, to start increasing toward the time of GnRH injection and TAI to reach 13.35 ± 1.16 and 14.64 ± 3.86 mm (Fig. 2). The presence of CL was high at the time of PGF2α injection (78.57%) compared with the absence of CL at the beginning of the protocol. Also, the progesterone level was low at starting the treatment (0.80 ± 0.09 ng/ml) and increased at the time of PGF2α injection to reach 2.69 ± 0.13 ng/ml, then returned to decreasing toward the TAI to become 0.85 ± 0.04 ng/ml with a significant difference in between (p < 0.05) (Fig. 3). There was no difference in the follicular number through the treatment. The estrus response was 71.43% at the time of TAI, cows did not show estrus signs during the other days of protocol steps. The presence of CL at the time of PGF2α injection and the size of follicles at the time of TAI during the GnRH + PG/PG/GnRH protocol in Table 2 were found to have a significant effect (p ≤ 0.05) on ovulation and pregnancy rates. It was found the cows had CL at the time of PGF2α injection and the follicle greater than 10 mm at the time of TAI had ovulation and pregnancy rates (90.91% and 81.82%), with an overall 78.57% ovulation rate and 64.28% pregnancy rate. The return rate was low, with an overall 14.28% (2/14). The overall pregnancy rate per second round of AI was 14.28% (Fig. 4). The effect of the PRID + GnRH/PG protocol on the follicular dynamics of the cows having PFs was obviously noticeable, as indicated by the significant difference (p ≤ 0.05) in Table 3. It was found the follicular number decreased at the time of PGF2α injection (day 7 of protocol; 1.25 ± 0.45) and the time of TAI (days 10 and 11 of protocol; 1.67 ± 0.49) compared with the day 0 of starting treatment (PRID + GnRH Day; 2.67 ± 0.65) and day of PRID out (day 9 of protocol; 2 ± 0.74). Also, the follicular size decreased at the time of PGF2α to 4.58 ± 1.3 mm on day 7 in return to day 0 of PRID insertion and GnRH injection was 9.17 ± 1.2 mm, and then the size of follicles became 9.67 ± 1.2 mm at PRID removal to become 12 ± 2.04 mm at the time of TAI (Fig. 5). Table 1. Effect of GnRH + PG/PG/GnRH protocol on follicular activity and luteal activity during the days of protocol in OC group.
Fig. 2. Effect of GnRH + PG/PG/GnRH protocol on follicular size and progesterone level in OC group.
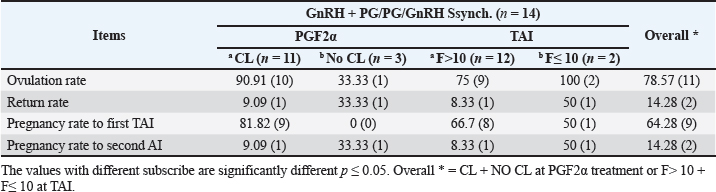
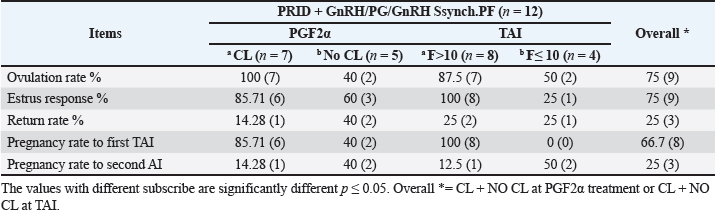
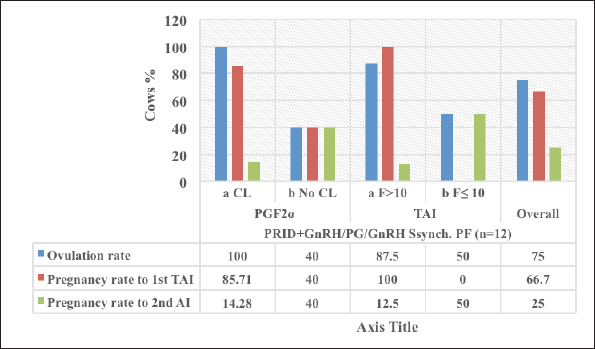
Fig. 3. The luteal activity of the GnRH + PG/PG/GnRH protocol in OC group. The luteal activity was found also significantly (p ≤ 0.05) affected by this protocol, which found the presence of CL was high on the day of PGF2α injection (day 7: 58.3%) compared with the day of stating the protocol, day PRID out, and the day of TAI (0%, 25%, and 25%, respectively). The level of progesterone was found to significantly differ (p ≤ 0.05) among the different days of the protocol. It was found the P4 level was low at the beginning of the treatment (0.53 ± 0.03 ng/ml.) and then started to increase coincidence with PRID insertion until the day of PGF2α injection and day of PRID removal (3.34 ± 0.19 and 2.11 ± 0.36 ng/ml) and the level at the TAI was at 0.49 ± 0.02 ng/ml (Table 3 and Fig. 6). The results in Table 4 show the effect of the presence or absence of CL after PGF2α injection and the size of the follicles (F> 10, F< 10) at the time of TAI on ovulation rate, estrus response, return rate, and pregnancy rate using the PRID + GnRH/PG protocol. The table reveals a significant interaction (p ≤ 0.05) in the two variances in the parameters. It was found that the ovulation rate was high in cows with CL at PGF2α injection (100%), and at the size of the follicle more than 10 mm at TAI (87.5%), with an overall 75%. The estrus response was also found in 100% of cows with follicles larger than 10 mm and in 85.72% of cows that had CL at the time of PGF2α injection. The pregnancy rate was high in both groups (CL/PGF2α and F> 10/TAI); it was 85.71% and 100%, respectively, compared with the other two groups, with an overall 66.7% (Fig. 7). The return rate and pregnancy rate after the second round of AI did not differ in both groups (p > 0.05). Table 2. Effect of GnRH + PG/PG/GnRH protocol on ovulation, return, and pregnancy rate according to presence or absent of CL and preovulatory follicle in OC group.
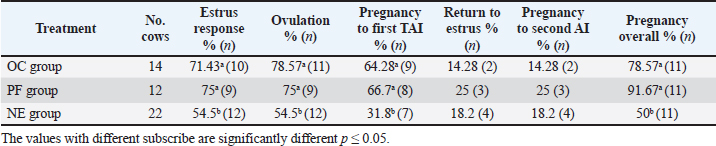
Fig. 4. Effect of GnRH + PG/PG/GnRH protocol on ovulation and pregnancy rate according to presence or absent of CL and preovulatory follicle size in OC group. Table 5 shows the reproductive performance following different treatments of the specific synchronization protocols. The table reveals that the estrus response (71.43% and 75%) and ovulation rate (78.57% and 75%) in OC and PF groups, respectively, were found significantly higher (p ≤ 0.05) versus the NE group (54.5% and 54.5%, respectively). The pregnancy rate at the first round (1st TAI) was significantly higher in the two groups of treatment (OC: 64.28% and PF: 66.7%), compared with the NE group (31.8%). The return rate and pregnancy rate at the second round of AI did not differ among the groups. The overall pregnancy rate was significantly higher in the OC and PF groups (78.57% and 91.67%) compared with the NE group (50%). Table 3. Effect of PRID + GnRH/PG protocol on follicular dynamic and luteal activity during the days of protocol in PFs group.
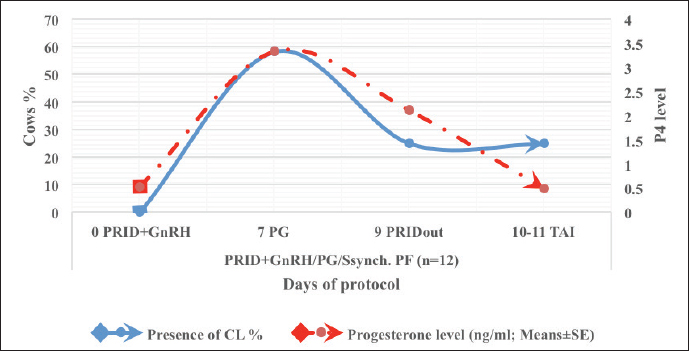
Fig. 5. The effect of PRID + GnRH/PG protocol on the progesterone level and follicular size in PFs group.
Fig. 6. The luteal activity of the PRID + GnRH/PG protocol in PFs group. Table 4. Effect of PRID + GnRH/PG/GnRH protocol on ovulation, return, and pregnancy rate according to presence or absent of CL and preovulatory follicle size in PFs group.
Fig. 7. Effect of PRID + GnRH/PG/GnRH protocol on ovulation and pregnancy rate according to presence or absent of CL and preovulatory follicle size in the PFs group. DiscussionThe functional causes of infertility typically affect single animals within a herd, but when considered together, they represent an important contributor to infertility. In addition, when they affect a significant percentage of a particular subgroup within a herd, they frequently indicate the presence of other issues. According to extensive studies, dairy cattle have 2.7%–15.1% OC, peaking 14–40 days postpartum. The cause of OC in dairy cows is unclear, most researchers attribute OC to hypothalamic-pituitary-gonadal axis LH release changes and an altered hypothalamic-pituitary oestrogen feedback mechanism may produce abnormal LH secretion. OC symptoms vary by luteinization, in 62%–85% of cases, anestrus is most frequent, especially postpartum, intermittent estrus, nymphomania, wide pelvic ligament relaxation, and masculine characteristics are symptoms (Vanholder et al., 2006; Mimoune et al., 2021; Rodríguez et al., 2022). The risk of spontaneous recovery from early postpartum cysts is higher in cows with lesser productivity, 80% of cows with OC in their first lactation spontaneously healed, compared to 30% in older cows (López-Gatius et al., 2002). Researchers recommend commencing therapy quickly after diagnosis for cost. Treating multiparous cows with OC early postpartum is advantageous, but primiparous cows should wait till the end of pre-service to allow spontaneous healing (López-Gatius et al., 2002; Brito and Palmer, 2004). Table 5. Comparative of reproductive performance following the different treatment of the Specific synchronization.
Transrectal ultrasound was performed 14 days post-presynchronization (44–62 P.P.). Cows were ultrasounds weekly for 4 weeks or until AI or treatment began. Two sequential ultrasounds found an OC in cows with a follicular structure over 25 mm, no CL, and no estrus, 14 of 48 cows were registered. Following the diagnosis of the cyst, the cows received GnRH + PG/PG/GnRH therapy. The follicular size decreased from 30.41 ± 2.41 to 9.59 ± 0.59 mm at PGF2α injection, gradually rising to 13.35 ± 1.16 mm at GnRH injection and 14.64 ± 3.86 mm at TAI. Comparing the absence of CL at the commencement of the treatment to its significantly high presence at PGF2α injection (78.57%). At first, progesterone levels were low (0.80 ± 0.09 ng/ml), increased after PGF2α injection (2.69 ± 0.13 ng/ml), and subsequently decreased throughout TAI (0.85 ± 0.04 ng/ml). Therapy did not affect the follicle count. At TAI, 71.43% of cows showed estrus, on other protocol days, none did. It was an ultrasonographic and progesterone analysis that was effective in providing a description of the follicles’ growth, regression, and luteal activity during the period of recovery until TAI (Amer and Badr, 2008; Ahmed et al., 2019; Ali et al., 2023). The ovulation and pregnancy rate during the GnRH + PG/PG/GnRH protocol were shown to be significantly affected by the existence of CL at the time of PGF2α injection and the size of follicles at the time of TAI. The total ovulation rate was 78.57%, and the pregnancy rate was 64.28% among the cows that had CL at the time of PGF2α injection and among those with follicles larger than 10 mm at the time of TAI. Overall, the rate of return was low, just 14.28% (2/14). The overall pregnancy rate per second round of AI was 14.28%. It was interesting to note the correlation between the presence of CL, level of progesterone and follicular growth, the P4 level <1 ng/ml during the follicular growth phase or absence of CL at PGF2α injection result in low pregnancy rate per AI (Carvalho et al., 2018; Hölper et al., 2023b). Our study comes in agreement with Gundling et al. (2015), investigation of dairy cow fertility after two OC treatments. The study variables were treatment success, pregnancy outcome, and cost. The study comprised 130 German Holsteins with OC on days 55–60 P.P. Group 1 cows received cloprostenol and buserelin acetate (GnRH) on day 0, PGF2α on day 14, and GnRH on day 16. Group 2 received standard ovsynch. TAI occurred 20–24 hours later. OC were absent in control cows. The recovery rate was more successful in group 1 (66.2%) than group 2 (23.1%). The results reveal better OC cured, more pregnancy rate, and cheaper. Overconditioned and bigger OC cows had poor treatment and pregnancy results. The study comes in according to results recorded by Lopez-Gatius and Lopez-Bejar (2002), who employed ovsynch protocols comparable to those used in this investigation and likewise obtained a higher cure rate with the modified ovsynch than the standard procedure (89% vs. 53%, p=0.001). Synergistic effects of GnRH and PGF2α on follicular cyst luteinization were postulated by the authors as a potential reason. This may help endogenous and exogenous GnRH luteinize another follicular cyst and/or ovulate. Although one could have anticipated greater outcomes for luteal cysts due to the luteolytic impact of the first PGF2α application, the favorable treatment outcome of the modified ovsynch protocol was seen regardless of the type of cyst. The luteolytic action of PGF2α in luteal cysts was shown to be unaffected by the co-administration of GnRH and PGF2α, found by Dinsmore et al. (1990); however, as compared to cows that only got GnRH, the reproductive performance of those received both GnRH and PGF2α was the same, so treating all cows with GnRH and PGF2α simultaneously was not recommended. In contrast, Naor et al. (2007) recommended the use of GnRH in conjunction with PGF2α to treat infertility and disorders characterized by imbalanced LH and FSH output, such as polycystic ovary syndrome or hormone-dependent diseases. This supports our work in this study by using GnRH and PGF2α synergically. In comparable research by Amer and Badr (2008), GnRH and PGF2α delivered to cows with cystic ovaries 7 days apart resulted in the recruitment of new healthy follicles, synchronization of ovulation, and a considerably greater conception rate (with than without CIDR). This fact must be kept in mind before choosing the hormonal protocol, which supports the action designed in this research, which recommended the use of GnRH and PGF2α as therapeutic choices. The recent study comes in line with what was reviewed by Dhara and Sharma (2019), who mentioned that OC, on the other hand, seem to respond less well to therapy with GnRH alone. Cysts are more likely to resolve when GnRH is combined with cloprostenol, and ovulation, estrus, and pregnancy rates are all much greater. However, GnRH is presently frequently used as a treatment for OC, followed by PGF2α 7–10 days later. Moreover, Prostaglandin F2α is utilized to treat luteinized cysts due to its luteolytic action, resulting in estrous signs within 2–3 days. This is the best-luteinized cyst therapy, within 7 days of therapy, 75% of cows were in estrus, and 66% were pregnant at first estrus. PGF2α luteolytic dosages are recommended for luteal cyst therapy, resulting in estrus within 3–5 days (BorŞ and BorŞ, 2020). In support of our study, Yimer et al. (2018) discussed that follicular cysts, in contrast to luteal cysts, are characterized by low plasma progesterone values (<1 ng/ml). As a result, the progesterone profile is useful for distinguishing follicular OC from luteal ones. In addition, transrectal ultrasonography facilitates distinguishing between the two cysts with pinpoint accuracy. Since follicular cysts and ovarian inactivity (the presence of little or no follicular development) share a low progesterone level, distinguishing between the two would be difficult without the use of ultrasound. Therefore, follicular cysts, luteal cysts, and ovarian inactivity may be accurately distinguished by a combination of progesterone analysis and ultrasound. From our perspective, the treatment should be based on an accurate diagnosis, or it is better to use a treatment that includes both conditions together without specifying. In another way, Mimoune et al. (2021) suggested that treatment with GnRH is widespread. PGF2 effectively treats luteal cysts. However, when first-line hormone treatment fails, alternatives must be considered. Thus, the Ovsynch regimen with progestogen may cure OC. In the dry-off and postpartum stages, reducing sickness and stress and improving dietary quality may decrease OC development and occurrence. Medical prevention may utilize the same drugs as treatment (GnRH, PGF2α). Gad et al. (2022) evaluated the effectiveness of alternative treatments on Holstein dairy cows. Single GnRH injection and PRID resulted in the greatest pregnancy rate (66.67%) in cows with cystic ovarian, whereas GnRH and hCG resulted in the lowest pregnancy rate (30.0%). Another study discussed by De Rensis et al. (2010) pointed out that, when it comes to cystic cows, the therapeutic impact of administering either hCG or GnRH is comparable; however, recovery occurs more rapidly in response to hCG therapy compared to GnRH treatment during the warm season. However, before using hCG, one must keep in mind that hCG has the potential to provoke an immunological response after repeated administration. However, the pregnancy rates with scheduled inseminations after therapy with the Ovsynch treatment are low, despite the fact that the Ovsynch treatment seems to be the most utilized among the several treatments that have been indicated for the treatment of OC (Fricke and Wiltbank, 1999; Bartolome et al., 2000; Meyer et al., 2007; BorŞ and BorŞ, 2020). Moreover, Meena et al. (2022), identified the most OC (87.5%) in Holstein Friesian crossbred cows, 37.5% aged 5%–6% and 40% in their third parity. Ovsynch, progesterone injection, and potassium iodide for 7 days had the greatest effect on third-lactation cows. The medication caused 47.5% of cow pregnancies, 35% in the first insemination and 12.5% in the second. A study by Kawate et al. (2011), indicates that adding a CIDR to the Ovsynch protocol did not significantly impact plasma estradiol-17β and progesterone levels during and after treatment, or on conception following timed AI in dairy cows with cystic ovarian disorders. The study suggests that decreased plasma estradiol-17β levels during the second GnRH injection in dairy cows with cystic ovarian disorders may imply impaired luteal development and reduced conception rates. Disruption of the 17-estradiol-LH positive feedback loop promotes persistent and post-calving follicular cysts. Low peripheral blood progesterone is the main cause of prolonged follicles and cysts. This ovarian disfunction can affect cows of any age, although it is most frequent in the second through fifth lactation, between 1 and 4 months post-calving (Pankratova et al., 2019). Acute progesterone therapy regresses produced PFs, enhancing future conception rates, while the dominant follicles that remain decrease fertility. The phrase “PFs” is often used in the beef cattle industry to describe dominant follicles that remain dominant for an exceptionally long time. Smaller than average OC, and induced PFs may be a contributing factor to anestrus (López-Gatius et al., 2001). Several scientists think that functional (but not morphological) atresia is caused by PFs, which are dominating follicles in the extended resting period (Pankratova et al., 2019). The outcomes of ultrasonography are shown by this statement. In recent study, cows were regarded as having a PF, if two separate ultrasonography tests 7 days apart revealed a follicular structure ≤15 mm in size, without a CL or cyst, and no estrus. The PRID + GnRH/PG treatment significantly improved follicular dynamics in cows with PFs. The follicular number decreased during PGF2α injection (day 7; 1.25 ± 0.45) and TAI (days 10 and 11; 1.67 ± 0.49) compared to PRID + GnRH Day (day 0; 2.67 ± 0.65) and PRID out (day 9; 2 ± 074). The follicular size fell from 9.17 ± 1.2 mm on day 0 of PRID insertion and GnRH injection to 4.58 ± 1.3 mm on day 7 of PGF2α, then increased to 9.67 ± 1.2 mm at PRID removal and 12 ± 2.04 mm at TAI. All of the persisting follicles in the cows in this investigation regressed after progesterone treatment. The PF shrank, and then a new one grew and ovulated in its place. Treatment with progesterone likely decreased the frequency of LH pulses, enabling the dominant follicle to atresia. It seems that the PF simply requires a brief decrease in LH production to go through atresia. The temporal decrease in follicular dimensions and the presence of follicular regression markers suggested that the dominant follicle had regressed. This result was in agreement with Anderson and Day (1994), who found that PFs in cows are more likely to disappear after receiving an acute dose of progesterone. Progesterone treatment boosted fertility in heifers and postpartum cows when employed in a 14-day Melengesterol acetate in estrus synchronization program. When given to animals with a high propensity for developing persistent dominant follicles, progesterone had the greatest effect on fertility. The protocol in this study affected luteal activity, with a high percentage of CL presence on day 7 of PGF2α injection (58.3%) compared to days of stating the protocol, PRID out, and TAI (0, 25, and 25%). The levels of progesterone varied considerably (p ≤ 0.05) over the regimen days. P4 levels were low at the onset of treatment (0.53 ± 0.03 ng/ml) and increased with PRID insertion and at PGF2α injection (3.34 ± 0.19), and PRID removal 2.11 ± 0.36 ng/ml, to reach 0.49 ± 0.02 ng/ml level at TAI. This study agreed with Stock and Fortune (1993), who pointed out in their study that minor changes in the endocrine milieu are crucial for achieving, maintaining, and losing follicular dominance. Peripheral progesterone levels seem to alter the hormonal milieu that determines the dominant follicle destiny. More importantly, our results strongly suggest that plasma progesterone affects follicular growth and demise through negative feedback on LH pulse frequency and that the dominant follicle controls estradiol production by increasing LH pulse frequency. The sudden change in the progesterone level from a low level at the start of the protocol to a high level at the PRID insertion, followed by a sudden change to a low level of P4 at PRID out. The importance of inducing atresia in persistent ovarian follicles was identified. The release of LH was seen in reaction to abrupt changes in progesterone levels. Rest assured, there is further evidence supporting the positive relationship between LH pulse frequency and estradiol. These results were in line with those achieved by McDowell et al. (1998) and Pereira et al. (2020). A recent study by Hölper et al. (2023a) confirmed that postpartum synchronization cows need progesterone, indicating Low P4 levels during follicular maturation in TAI-treated lactating dairy cows are concerning. An increase in LH pulse frequency and early initiation of meiosis due to low P4 levels damage oocyte quality. In addition, low blood P4 levels before ovulation may impact endometrial morphology and secretory processes, resulting in increased PGF2α release after TAI. Another study by McDougall (2010) shows that TAI can speed up anestrous cow pregnancy. After adding P4 to Ovsynch, first-service conception increased by 12%. The study examined the impact of PGF2α injection in the presence or absence of CL and follicle size (F> 10, F< 10) on ovulation, estrus response, return rate, and pregnancy rate utilizing the PRID + GnRH/PG regimen. The study found a significant interaction between the two variances in the parameters. Results indicate high ovulation rates in cows with CL at PGF2α injection (100%), 87.5% at follicle size exceeding 10 mm, with an overall 75%. Estrus response was seen in all cows with follicles above 10 mm and in 85.72% of cows with CL at the time of PGF2α injection. Both CL/PGF2α and F> 10/TAI groups had high pregnancy rates (85.71% and 100%, respectively) compared to the other two groups, with an overall 66.7%. Both groups had similar return and pregnancy rates following the second AI round. The pregnancy rate in this study was found to be lower than what Anderson and Day (1994) documented (92%–93%), progesterone treatment given to animals with persistent dominant follicles in a 14-day Melengesterol acetate estrus synchronization program increases postpartum cow fertility. Moreover, Helguera et al. (2018), examined how initial GnRH levels and AI time in a 5-day Co-synch plus CIDR operation affected P/AI and pregnancy loss. The outcome was 55%–56%, which appears lower than this study. However, 75%–91% of ovulation was within the range. After CIDR removal, TAI lasted 66–72 hours, compared to 56 hours in this study. At the same time López-Gatius et al. (2001), found Progesterone, GnRH, and PGF2α can be used to synchronize and timing inseminate cows with PFs. Treatment with GnRH and PGF2α yields modest results. PRID therapy boosted ovulation (85%), pregnancy (34%), and lower follicular persistence (22% 40 days after treatment) provided the best results. Pregnancy rates were comparable to lactating dairy cows in spontaneous estrus. These findings were in agreement with our results. By adding progesterone to the synchronization procedure, animals without estrous cycles can become cyclic. If given before the first postpartum ovulation, some progestins can block or lengthen the luteal phase. Many investigations have shown that progestins may reduce estrus return variance after estrous synchronization. Although synchronizing estrus with a PRID improves LH pulse frequency and follicular growth rate, it is frequently the most expensive element of the process (Yoshimura et al., 2022). PRID successfully treats inactive ovaries in dairy cattle without affecting their metabolic or physiological state (Zulu et al., 2000). In addition, after CL regression, progestin therapy of heifers or cows produces prolonged follicles, which contribute to reduced fertility (Epperson et al., 2020). This study overcomes the issue by injecting corporate GnRH during PRID insertion and PGF2α 7 days later for improved responsiveness and fertility. By recruiting a new dominant follicle, the GnRH injection corrected fertility loss caused by a long-lasting follicle. This strategy of synchronizing follicle growth with CL regression could boost fertility in postpartum dairy cows, which have larger anovulatory and pre-ovulatory follicles during the oestrous cycle. These concepts were in agreement with Thatcher et al. (1996). Also, with Tschopp et al. (2022), when lactating dairy cows that have been synchronized with estradiol/P4, adding GnRH on Day 0 and a second dose of PGF2 the day before P4 device removal increases P/AI. In another study using different strategies by Oosthuizen et al. (2020), Presynchronization and TAI were compared for pregnancy rates. In the CIDR removal regimen, prostaglandin F2α was administered 7 days before removal, followed by a second GnRH injection during TAI. Presynchronization and prolonged exogenous progesterone exposure altered estrus expression and increased fertility. Also, Tiwari et al. (2019), observed that the progesterone-based Ovsynch regimen and GnRH after AI therapy increased breeder cow conception. Progesterone levels were lower and slower in these animals. Lactating dairy cows benefit from exogenous progesterone. As an extension of what has been listed in the discussion above, the reproductive performance of specific synchronization therapy procedures. According to the data, the treatment groups had a far larger estrus response than the NE group. The ovulation rates in the customized therapy groups were significantly higher than those in the NE group and significantly more cows in these groups got pregnant after the first timed TAI. Our data clearly demonstrate that postpartum presynchronized cows respond better to a specific oestrous synchronization technique based on ovarian status. These findings suggest that it would be profitable to identify specific therapies for each anestrus problem case in early postpartum dairy cows. Based on the current findings, it is recommended to manage postpartum cows with ovarian dysfunction, such as OC and PFs, earlier in the postpartum period. A customized therapy protocol can be developed for each case after diagnosis. This will minimize the time to first service and improve reproductive performance. AcknowledgmentsNone. Conflict of interestThe authors declare that there is no conflict of interest. FundingThis study was funded by the Libyan government. Authors’ contributionAB and AH designed the experiments, NM performed the experiments and wrote the first draft of the manuscript, and HAS revised and edited the manuscript. AA analyzed the data. Data availabilityAll data are provided in the manuscript. ReferencesAbraham, F. 2017. An overview on functional causes of infertility in cows. JFIV Reprod. Med. Genet. 5(2), 203. Ahmed, M.U., Rahman, M.M., Bhattacharjee, J. and Bhuiyan, M.M.U. 2019. Ultrasound guided diagnosis of anoestrus and its treatment in postpartum crossbred Holstein-Friesian cows. Bangladesh Vet. 36(1-2), 33–41. Ali, Z., Sohail, M., Ameen, Y., Hamidullah, I.A. and Malik, M. 2023. Ultrsonography: a tool for management of reproductive disorders in dairy cows. Vet. Sci. Res. Rev. 9(1), 18–24. Amer, H. and Badr, A. 2008. Hormonal profiles associated with treatment of cystic ovaries with GnRH and PGF2αwith and without CIDR in dairy cows. Appl. Biol. Sci. 2(1), 51–56. Amin, Y.A., Mahmoud, A.E.Z., Ali, R.A., Fouad, S.S., Shanab, O., Ibrahim, R.M., Farrag, F., Shukry, M., Ibrahim, S.F., Fericean, L. and Mohamed, R.H. 2023. Treatment of inactive ovaries of holstein dairy cows by epidural injection of GnRH analogue (Receptal) and its impact on the reproductive hormones, oxidant/antioxidant profile and micro and macro-elements profile. J. Anim. 13(4), 653. Anderson, L.H. and Day, M.L. 1994. Acute progesterone administration regresses persistent dominant follicles and improves fertility of cattle in which estrus was synchronized with melengestrol acetate. J. Anim. Sci. 72(11), 2955–2961. Bartolome, J.A., Archbald, L., Morresey, P., Hernandez, J., Tran, T., Kelbert, D., Long, K., Risco, C. and Thatcher, W. 2000. Comparison of synchronization of ovulation and induction of estrus as therapeutic strategies for bovine ovarian cysts in the dairy cow. Theriogenology 53(3), 815–825. BorŞ, S.-I. and BorŞ, A. (2020). Ovarian cysts, an anovulatory condition in dairy cattle. J. Vet. Med. Sci. 82(10), 1515–1522. Brito, L. and Palmer, C. (2004). Cystic ovarian disease in cattle. Large Anim. Rev. 4(10), 1–4. Carvalho, P.D., Santos, V.G., Giordano, J.O., Wiltbank, M.C. and Fricke, P.M. 2018. Development of fertility programs to achieve high 21-day pregnancy rates in high-producing dairy cows. Theriogenology 114, 165–172. Chauhan, J., Hadiya, K., Dhami, A. and Sarvaiya, N. 2021. Ovarian dynamics, plasma endocrine profile and fertility response following synchronization protocols in crossbred cows with cystic ovaries. Indian J. Anim. Res. 55(2), 127–133. Civiero, M., Cabezas-Garcia, E.H., Ribeiro-Filho, H.M.N., Gordon, A.W. and Ferris, C.P. 2021. Relationships between energy balance during early lactation and cow performance, blood metabolites, and fertility: a meta-analysis of individual cow data. J. Dairy Sci. 104(6), 7233–7251. Crowe, M. 2008. Resumption of ovarian cyclicity in post-partum beef and dairy cows. Reprod. Domest. Anim. 43, 20–28. De Rensis, F., López-Gatius, F., García-Ispierto, I. and Techakumpu, M. 2010. Clinical use of human chorionic gonadotropin in dairy cows: an update. Theriogenology 73(8), 1001–1008. Dhara, S. and Sharma, M. 2019. Cystic ovarian disease in dairy cow. Theriogenology 9(1), 27–34. Dinsmore, R.P., White, M.E. and English, P.B. 1990. An evaluation of simultaneous GnRH and cloprostenol treatment of dairy cattle with cystic ovaries. Can. Vet. J. 31(4), 280. Epperson, K.M., Rich, J.J.J., Zoca, S.M., Northrop, E.J., Perkins, S.D., Walker, J.A., Rhoades, J.R. and Perry, G.A. 2020. Effect of progesterone supplementation in a resynchronization protocol on follicular dynamics and pregnancy success. Theriogenology 157, 121–129. Fricke, P. and Wiltbank, M. 1999. Effect of milk production on the incidence of double ovulation in dairy cows. Theriogenology 52(7), 1133–1143. Fricke, P. and Wiltbank, M. 2022. Symposium review: the implications of spontaneous versus synchronized ovulations on the reproductive performance of lactating dairy cows. J. Dairy Sci. 105(5), 4679–4689. Fury, M. and Trevor, T. 2022. Chapter 7 - Fertility monitoring of cattle. In Digital agritechnology. Ed., Mottram, T. Cambridge, MA: Academic Press, pp: 143–173. Gad, M., Elbaz, H.T., El-Razek, A., Zaghloul, A. and Genedy, T. 2022. Different therapeutic approaches for treatment of cystic ovarian disease (COD) and its effect on conception rate in Holstein dairy cows. J.C.V.R. 4(1), 175–181. Gundling, N., Drews, S. and Hoedemaker, M. 2015. Comparison of two different programmes of ovulation synchronization in the treatment of ovarian cysts in dairy cows. Reprod. Domest. Anim. 50(6), 893–900. Haile, S.M., Abebe, B.K. and Tesfa, T.W. 2023. Efficiency evaluation of two estrus synchronization protocols in estrus response and conception rate of dairy cows in the Dalocha district, Ethiopia. Heliyon 9(1), e12781. Helguera, I.L., Whittaker, P., Behrouzi, A., Mapletoft, R.J. and Colazo, M.G. 2018. Effect of initial GnRH and time of insemination on reproductive performance in cyclic and acyclic beef heifers subjected to a 5-d Co-synch plus progesterone protocol. Theriogenology 106, 39–45. Hölper, M., Bretzinger, L., Randi, F., Heuwieser, W. and Borchardt, S. 2023a. Effect of a progesterone-releasing intravaginal device (PRID) for 8 days during a modified Ovsynch protocol on pregnancy outcomes in lactating Holstein cows. JDS Communications. 4(4), 303–307. Hölper, M., Bretzinger, L., Randi, F., Heuwieser, W. and Borchardt, S. 2023b. Effect of dose and frequency of prostaglandin F2α treatments during a 7-day Ovsynch protocol with an intravaginal progesterone releasing device on luteal regression and pregnancy outcomes in lactating Holstein cows. J. Dairy Sci. 106(1), 755–768. Kawate, N., Watanabe, K., Uenaka, K., Takahashi, M., Inaba, T. and Tamada, H. 2011. Comparison of plasma concentrations of estradiol-17β and progesterone, and conception in dairy cows with cystic ovarian diseases between Ovsynch and Ovsynch plus CIDR timed AI protocols. J. Rep. Develop. 57(2), 267–272. Kumar, P., Rajanna, R. and Sunitha, R. (2020). Anoestrus in bovines: a review article. J. Pharm. Innov. 9(9), 458–460. López-Gatius, F. 2022. Ovarian response to prostaglandin F2α in lactating dairy cows: a clinical update. J. Rep. Develop. 68(2), 104–109. Lopez-Gatius, F. and Lopez-Bejar, M. 2002. Reproductive performance of dairy cows with ovarian cysts after different GnRH and cloprostenol treatments. Theriogenology 58(7), 1337–1348. López-Gatius, F., Santolaria, P., Yániz, J., Fenech, M. and López-Béjar, M. 2002. Risk factors for postpartum ovarian cysts and their spontaneous recovery or persistence in lactating dairy cows. Theriogenology 58(8), 1623–1632. López-Gatius, F., Santolaria, P., Yániz, J., Rutlant, J. and López-Béjar, M. 2001. Persistent ovarian follicles in dairy cows: a therapeutic approach. Theriogenology 56(4), 649–659. Martins, J.P.N., Cunha, T.O., Martinez, W. and Schmitt, J.S. 2023. Presynchronization with prostaglandin F2α and gonadotropin-releasing hormone simultaneously improved first-service pregnancy per artificial insemination in lactating Holstein cows compared with Presynch-14 when combined with detection of estrus. J. Dairy Sci. 106(7), 5115–5126. McDougall, S. 2010. Effects of treatment of anestrous dairy cows with gonadotropin-releasing hormone, prostaglandin, and progesterone. J. Dairy Sci. 93(5), 1944–1959. McDowell, C.M., Anderson, L.H., Kinder, J.E. and Day, M.L. 1998. Duration of treatment with progesterone and regression of persistent ovarian follicles in cattle2. J. Anim. Sci. 76(3), 850–855. Meena, M., Meena, S., Meena, M. and Purohit, G. 2022. Field approach to treat ovarian cysts in dairy cows. Pharma Innov. J. 7(SP-11), 2250–2253. Meyer, J., Radcliff, R., Rhoads, M., Bader, J., Murphy, C. and Lucy, M. 2007. Timed artificial insemination of two consecutive services in dairy cows using prostaglandin F2α and gonadotropin-releasing hormone. J. Dairy Sci. 90(2), 691–698. Mimoune, N., Azzouz, M.Y., Khelef, D. and Kaidi, R. 2021. Ovarian cysts in cattle: a review. Vet. Stanica. 52(5), 587–603. Mounir, A. and Aspinas, C. 2022. A review of the diversity of the genital tract microbiome and implications for fertility of cattle. J. Anim. 12(4), 460. Naor, Z., Jabbour, H.N., Naidich, M., Pawson, A.J., Morgan, K., Battersby, S., Millar, M.R., Brown, P. and Millar, R.P. 2007. Reciprocal cross talk between gonadotropin-releasing hormone (GnRH) and prostaglandin receptors regulates GnRH receptor expression and differential gonadotropin secretion. Mol. Endocrinol. 21(2), 524–537. Negrón-Pérez, V.M., Fausnacht, D.W. and Rhoads, M.L. 2019. Invited review: management strategies capable of improving the reproductive performance of heat-stressed dairy cattle. J. Dairy Sci. 102(12), 10695–10710. Oosthuizen, N., Fontes, P.L.P., Porter, K. and Lamb, G.C. 2020. Presynchronization with prostaglandin F2α and prolonged exposure to exogenous progesterone impacts estrus expression and fertility in beef heifers. Theriogenology 146, 88–93. Opsomer, G., Gröhn, Y.T., Hertl, J., Coryn, M., Deluyker, H. and de Kruif, A. 2000. Risk factors for post partum ovarian dysfunction in high producing dairy cows in Belgium: a field study. Theriogenology 53(4), 841–857. Pankratova, A., Aminova, A., Kozyrev, S. and Al-Azawi Nagham, M. 2019. Role of reproductive hormones in ovarian pathology in cows. Plant Arch. 19(suppl 1), 24–30. Parkinson, T., Smith, K., Paccamonti, D., Pycock, J., Peltoniemi, O., Kemp, B. and England, G. 2009. Subfertility and infertility. In Arthur’s veterinary reproduction and obstetrics E-book. Eds., Arthur, G.H., Noakes, D.E., Parkinson, T.J. and England, G.C.W. pp: 391. Pereira, M.H.C., Wiltbank, M.C., Guida, T.G., Lopes, F.R., Cappellozza, B.I. and Vasconcelos, J.L.M. 2020. Evaluation of presynchronization and addition of GnRH at the beginning of an estradiol/progesterone protocol on circulating progesterone and fertility of lactating dairy cows. Theriogenology 147, 124–134. Pérez-Marín, C.C. and Quintela, L.A. 2023. Current insights in the repeat breeder cow syndrome. J. Anim. 13(13), 2187. Pfeifer, L.F.M., Rodrigues, W.B. and Nogueira, E. 2021. Relationship between body condition score index and fertility in beef cows subjected to timed artificial insemination. Livest. Sci. 248, 104482. Rodríguez, F.M., Cattaneo Moreyra, M.L., Huber, E., Gareis, N.C., Etchevers, L., Ortega, H.H., Salvetti, N.R. and Rey, F. 2022. An altered expression of components of the IGF system could contribute to follicular persistence in Holstein cows. Res. Vet. Sci. 143, 99–106. Rojas Canadas, E., Battista, S.E., Kieffer, J.D., Wellert, S.R., Mussard, M.L. and Garcia-Guerra, A. 2023. GnRH dose at initiation of a 5-day CO-Synch + P4 for fixed time artificial insemination in suckled beef cows. Anim. Reprod. Sci. 250, 107210. Roth, Z., Biran, D., Lavon, Y., Dafni, I., Yakobi, S. and Braw-Tal, R. 2012. Endocrine milieu and developmental dynamics of ovarian cysts and persistent follicles in postpartum dairy cows. J. Dairy Sci. 95(4), 1729–1737. Sayid, A. 2021. Fertilization failure and early embryonic mortality as a major cause of reproductive failure in cattle: a review. W.S.N. 158, 59–71. Schlafer, D. and Miller, R. 2007. Pathology of the ovary (nondevelopmental lesions). In Jubb, Kennedy, and Palmer’s pathology of domestic animals. Ed., Maxie, M.G., vol. 3, pp: 444–450. Stock, A. and Fortune, J. 1993. Ovarian follicular dominance in cattle: relationship between prolonged growth of the ovulatory follicle and endocrine parameters. Endocrinology 132(3), 1108–1114. Thatcher, W., de la Sota, R.L., Schmitt, E., Diaz, T., Badinga, L., Simmen, F., Staples, C. and Drost, M. 1996. Control and management of ovarian follicles in cattle to optimize fertility. Reprod. Fertil. Develop. 8(2), 203–217. Tiwari, I., Shah, R., Kaphle, K. and Gautam, M. 2019. Treatment approach of different hormonal therapy for repeat breeding dairy animals in Nepal. Arch. Vet. Sci. 2(3), 28–40. Tschopp, J.C., Macagno, A.J., Mapletoft, R.J., Menchaca, A. and Bó, G.A. 2022. Effect of the addition of GnRH and a second prostaglandin F2α treatment on pregnancy per artificial insemination in lactating dairy cows submitted to an estradiol/progesterone-based timed-AI protocol. Theriogenology 188, 63–70. Uddin, A.H.M.M., Petrovski, K.R., Song, Y., Garg, S. and Kirkwood, R.N. 2023. Application of exogenous GnRH in food animal production. J. Anim. 13(12), 1891. Vanholder, T., Opsomer, G. and de Kruif, A. 2006. Aetiology and pathogenesis of cystic ovarian follicles in dairy cattle: a review. Reprod. Nutr. Dev. 46(2), 105–119. Wiltbank, M.C., Gümen, A. and Sartori, R. 2002. Physiological classification of anovulatory conditions in cattle. Theriogenology 57(1), 21–52. Yimer, N., Haron, A.W. and Yusoff, R. 2018. Determination of ovarian cysts in cattle with poor reproductive performance using ultrasound and plasma progesterone profile. Vet. Med. Open J. 3, 1–9. Yoshimura, N., Morita, Y., Yamamoto, M., Higashine, C., Takebayashi, K., Kumegawa, T., Higashiyama, Y., Niimi, M., Tanihara, F. and Otoi, T. 2022. Relationship between GnRH-induced LH increase profiles in the serum and vaginal mucus of Japanese Black beef cows. Arch. Anim. Breed. 65(3), 353–356. Zulu, V.C., Nakao, T., Yamada, K., Moriyoshi, M., Nakada, K. and Sawamukai, Y. 2000. Clinical response of inactive ovaries in dairy cattle after PRID treatment. J. Rep. Develop. 46(6), 415–422. | ||
| How to Cite this Article |
| Pubmed Style Mariol NM, Mahdy AB, Amer HA, Aghwider AA, Hazzaa AM. Customized modified therapies for ovarian cyst and persistent follicle followed with TAI in early postpartum dairy cows. Open Vet. J.. 2024; 14(4): 1029-1042. doi:10.5455/OVJ.2024.v14.i4.10 Web Style Mariol NM, Mahdy AB, Amer HA, Aghwider AA, Hazzaa AM. Customized modified therapies for ovarian cyst and persistent follicle followed with TAI in early postpartum dairy cows. https://www.openveterinaryjournal.com/?mno=185110 [Access: January 09, 2026]. doi:10.5455/OVJ.2024.v14.i4.10 AMA (American Medical Association) Style Mariol NM, Mahdy AB, Amer HA, Aghwider AA, Hazzaa AM. Customized modified therapies for ovarian cyst and persistent follicle followed with TAI in early postpartum dairy cows. Open Vet. J.. 2024; 14(4): 1029-1042. doi:10.5455/OVJ.2024.v14.i4.10 Vancouver/ICMJE Style Mariol NM, Mahdy AB, Amer HA, Aghwider AA, Hazzaa AM. Customized modified therapies for ovarian cyst and persistent follicle followed with TAI in early postpartum dairy cows. Open Vet. J.. (2024), [cited January 09, 2026]; 14(4): 1029-1042. doi:10.5455/OVJ.2024.v14.i4.10 Harvard Style Mariol, N. M., Mahdy, . A. B., Amer, . H. A., Aghwider, . A. A. & Hazzaa, . A. M. (2024) Customized modified therapies for ovarian cyst and persistent follicle followed with TAI in early postpartum dairy cows. Open Vet. J., 14 (4), 1029-1042. doi:10.5455/OVJ.2024.v14.i4.10 Turabian Style Mariol, Najmi M., Atef B. Mahdy, Hussein A. Amer, Ali A. Aghwider, and Abubakr M. Hazzaa. 2024. Customized modified therapies for ovarian cyst and persistent follicle followed with TAI in early postpartum dairy cows. Open Veterinary Journal, 14 (4), 1029-1042. doi:10.5455/OVJ.2024.v14.i4.10 Chicago Style Mariol, Najmi M., Atef B. Mahdy, Hussein A. Amer, Ali A. Aghwider, and Abubakr M. Hazzaa. "Customized modified therapies for ovarian cyst and persistent follicle followed with TAI in early postpartum dairy cows." Open Veterinary Journal 14 (2024), 1029-1042. doi:10.5455/OVJ.2024.v14.i4.10 MLA (The Modern Language Association) Style Mariol, Najmi M., Atef B. Mahdy, Hussein A. Amer, Ali A. Aghwider, and Abubakr M. Hazzaa. "Customized modified therapies for ovarian cyst and persistent follicle followed with TAI in early postpartum dairy cows." Open Veterinary Journal 14.4 (2024), 1029-1042. Print. doi:10.5455/OVJ.2024.v14.i4.10 APA (American Psychological Association) Style Mariol, N. M., Mahdy, . A. B., Amer, . H. A., Aghwider, . A. A. & Hazzaa, . A. M. (2024) Customized modified therapies for ovarian cyst and persistent follicle followed with TAI in early postpartum dairy cows. Open Veterinary Journal, 14 (4), 1029-1042. doi:10.5455/OVJ.2024.v14.i4.10 |