| Research Article | ||
Open Vet. J.. 2024; 14(4): 1043-1050 Open Veterinary Journal, (2024), Vol. 14(4): 1043–1050 Original Research Anti-inflammatory effect of Faloak (Sterculia quadrifida R. Br) stem bark on TNF-α, IL-1β, and IL-6 in DENV-3-infected Wistar ratsAudrey Gracelia Riwu1, Jusak Nugraha2*, Erwin Astha Triyono3 and Djoko Agus Purwanto41Doctoral Program of Medical Science, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia 2Department of Clinical Pathology, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia 3Department of Internal Medicine, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia 4Department of Pharmaceutical Sciences, Faculty of Pharmacy, Universitas Airlangga, Surabaya, Indonesia *Corresponding Author: Jusak Nugraha. Department of Clinical Pathology, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: jusak-n [at] fk.unair.ac.id Submitted: 14/01/2024 Accepted: 25/03/2024 Published: 30/04/2024 © 2024 Open Veterinary Journal
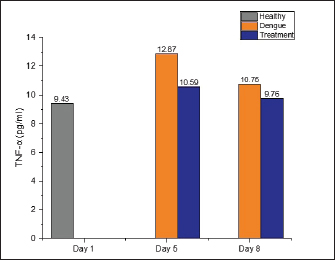
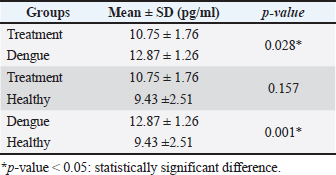
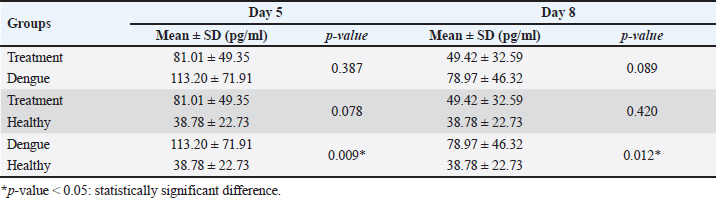
AbstractBackground: Dengue infection can trigger an immunological response that results in an inflammatory reaction, which acts as a defensive mechanism to protect the host. Dengue infection leads to an elevation in the release of pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6). These three cytokines have been shown to correlate with the development of thrombocytopenia and plasma leakage, which is related to the severity of the disease. Aim: This study aims to investigate the effect of faloak (Sterculia quadrifida R. Br) stem bark on TNF-α, IL-1β, and IL-6 levels in Wistar rats infected with dengue, specifically DENV-3. Methods: A group of 27 male Wistar rats (Rattus norvegicus) aged 2–3 months and weighting 200–300 g were divided into three distinct groups: healthy, dengue, and treatment (dengue infection and extract) groups. The rats in both the dengue and treatment groups were administered an injection of DENV-3 with a titer of 105 pfu at a dosage of 0.8 cc via the intraperitoneal route. The propagation of DENV-3 was initiated using C6/36 cells, and it underwent four passages. The extract was administered orally via a nasogastric tube at a dosage of 1,500 mg/kg body weight once daily for 7 days. The healthy group underwent blood sampling on the first day, whereas the dengue and therapy groups underwent blood sampling on the fifth and eighth, respectively. Results: Compared with the healthy group, TNF-α levels in the dengue and treatment groups showed significant differences on day 5 post-infection. The post hoc analysis revealed a statistically significant difference between the dengue-treatment and dengue-healthy groups. The IL-1β levels in the dengue and healthy groups significantly differed on days 5 and 8 post-infection compared to the healthy group. The treatment group had less of a decrease in IL-6 levels on days 5 and 8 than the dengue group. However, no statistically significant differences were observed. Conclusion: The stem bark of S. quadrifida shows potential as an anti-inflammatory agent in dengue infections, particularly in its ability to decrease levels of TNF-α and IL-1β. Keywords: Anti-inflammation, Dengue, Pro-inflammatory cytokine, Sterculia quadrifida R. Br, Wistar rats. IntroductionDengue is an arbovirus infection caused by one of four dengue virus serotypes (DENV1-DENV4), leading to a range of clinical symptoms varying from mild to severe. The worldwide incidence of this disease has seen a significant global escalation in recent decades. Most dengue infections are asymptomatic or exhibit minor symptoms that might resolve without medical intervention. In addition, many cases often go undiagnosed and are misclassified as other febrile illnesses, leading to an incomplete recording of the actual prevalence rate of severe dengue (Iyer and Thangam, 2022). The incidence rate, as reported to the World Health Organization (WHO), has exhibited a significant increase of up to eightfold during the past two decades. The number of instances observed in 2,000 amounted to 505,430, which then increased to 2.4 million cases in 2010 and escalated to 5.2 million cases in 2019. Based on prevalence surveys, the estimated number of individuals at risk of DENV infection is as high as 3.9 billion. Dengue has achieved endemic status in over 100 countries, with the Asian area contributing to over 70% of reported cases (WHO, 2023). DENV is transmitted to humans via the bite of the Aedes aegypti or Aedes albopictus mosquito. The virus undergoes replication within skin cells, specifically keratinocytes, and Langerhans cells, thus triggering the activation of the innate immune response to protect the host. DENV can stimulate the innate immune response, leading to the release of proinflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6). These cytokines are crucial in attracting and activating various immune cells involved in inflammatory reactions and initiating adaptive immune responses (Imad et al., 2020). The elevation of these three cytokines has been observed in cases of dengue infection and has been linked to the occurrence of plasma leakage, thrombocytopenia, and tissue damage in dengue infection (Butthep et al., 2012; Masood et al., 2018; Pan et al., 2019; Varghese et al., 2019;). Currently, targeted therapeutic interventions are absent for effectively managing dengue. The therapeutic approach employed for dengue fever patients is primarily supportive and symptomatic, aiming to alleviate the clinical manifestations of the disease (WHO, 2023). The utilization of herbal medicines as a therapeutic approach for various diseases is experiencing a significant increase among people living in tropical and subtropical areas. In addition, around 80% of the population in developing countries, including Asia and Africa, rely on herbal medicines as their primary source of medical therapy (Saleh and Kamisah, 2021). Hence, it is imperative to investigate the potential of utilizing traditional medicinal herbs as a viable resource for dengue therapy. Sterculia quadrifida R. Br is a traditional medicinal plant commonly used by Aboriginal People in North Queensland, Australia, and the people on Timor Island, Indonesia, for treating various health conditions (Saragih and Siswadi, 2019; Australia Tropical Rainforest Plants, 2020). The people of Timor Island commonly utilize the stem bark of S. quadrifida or Faloak (local name in Indonesia) to treat several medical medical conditions, such as hepatitis, kidney disease, rheumatism, lower back pain, and anemia (Saragih and Siswadi, 2019). The stem bark of S. quadrifida has been found to contain flavonoids, alkaloids, terpenoids, phenolic compounds, and saponins (Siswadi and Saragih, 2019). In addition, specific compounds such as (+)-catechin (Riwu et al., 2023), epicatechin (Dean et al., 2019), scopoletin (Munawaroh et al., 2020), and β-sitosterol (Lulan, 2020) have been identified. Prior research has established that the stem bark exhibits potent antioxidant activity (Amin et al., 2015; Dillak et al., 2019), and possesses immunomodulatory properties through the regulation of macrophage phagocytic activity, as well as the modulation of nuclear factor-kappaB and TNF-α (specifically in combination with Phyllanthus urinaria) (Winanta et al., 2019; Munawaroh et al., 2020; Rollando et al., 2020). No prior research investigations have been conducted on using S. quadrifida stem bark as an anti-inflammatory agent in dengue infections. Based on the background mentioned above, this study aims to investigate the effect of S. quadrifida stem bark extract on the levels of pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 in Wistar rats infected with DENV-3. Material and MethodsPlant materialThe stem bark of S. quadrifida was obtained from Kupang City, located in East Nusa Tenggara, Indonesia. The stem bark is extracted through the infusion method. The powdered simplicia is dissolved in distilled water and boiled to a temperature of 90°C for 15 minutes (Santosa et al., 2024). Afterward, the filtrate went through freeze-drying to get a crude extract. Virus propagationDENV-3 was propagated in C6/36 cells. C6/36 cells were cultured in a T-25 culture flask and incubated at 28°C. The cells were maintained in Minimum Essential Medium (MEM) (Sigma Aldrich) media supplemented with 10% fetal bovine serum (FBS) (Sigma Aldrich), 2% penicillin-streptomycin (Sigma Aldrich), and 0.5% amphotericin B (Gibco). Once confluent to 90%–95%, the cells were infected with 300 μl of the virus for 1 hour with agitation every 15 minutes. Following incubation, the inoculum was removed, and 5 ml of 2% MEM complete (2% FBS, 2% Penicillin-streptomycin, 0.5% Amphotericin B) was added and cultured for 5–7 days in a 28°C incubator while monitoring the Cytopathic Effect (Chen et al., 2012; Hitakarun et al., 2020). The virus was subjected to four rounds of passages on C6/36 cells, with each passage having an incubation period of 5–7 days. Plaque assay for virus titratingDENV titer was determined by plaque assay. C6/36 cells with a density of 1.5 × 105 were cultured on 24-well plates in 10% MEM (Sigma Aldrich) and incubated at 28°C for 1–2 days until confluent. Afterward, the medium was removed, and a six-fold dilution of the virus was added to the cells, with each well consisting of 200 μl. Then, the plates were incubated for 2 hours at 28°C and added with 1% CMC overlay media. Plates were incubated for 5–7 days, fixed in 3.7% formaldehyde, and stained with 1% crystal violet for 15–30 minutes. Plaques were counted manually, and titers were expressed as plaque form unit (pfu)/ml (Alkaff et al., 2019). Experimental unitThirty male Wistar rats (Rattus norvegicus) weighing between 200 and 300 g and aged 2–3 months were acquired from the Animal House, Faculty of Medicine-Public Health and Nursing, University of Gadjah Mada Yogyakarta. Three rats were utilized in the initial phase to confirm the infected model, while 27 were employed in the primary study. The 27 rats were divided into three groups: a healthy group (n=9), a dengue group (n=9), and a treatment group with dengue infection and administration of the extract (n=9). The extract was orally administered with a 1,500 mg/kg BW dose dissolved in 3 cc of distilled water. The rats were acclimated for a week in a controlled environment with temperatures ranging from 20°C–24°C and humidity levels ranging from 30%–70%. During the adaption phase, food and water were provided ad libitum. The lighting was controlled with a 12-hour cycle of light and darkness, starting from 06:00 to 18:00. Dengue infectionThe dengue infection model was initially established in three rats to improve the virus dose and injection method. The evaluation of infections includes assessing the NS-1 protein (Right Sign NS-1 Antigen Rapid Test). Rats were infected with DENV-3 by an intraperitoneal injection (Utama et al., 2023), with a viral titer of 1 × 105 pfu (Triyono, 2020) at a dosage of 0.8 cc. On days 1 and 3, blood samples were obtained via the retro-orbital vein. On the third day, NS-1 was confirmed to be positive. The viral dosage and injection technique employed in this stage was subsequently adopted as the protocol for the main study. Blood collectionBefore blood collection, all rats were administered an intramuscular injection of ketamine at a dose of 0.5 cc/kg to induce anesthesia. The healthy group was subjected to sampling on the initial day and subsequently euthanized. The dengue and treatment groups underwent blood sampling on the fifth and eighth days. Blood was obtained via the retro-orbital vein on the fifth day using a micro-capillary tube. Cardiac puncture was performed on the first and eighth days using a 23G needle. After blood collection on days 1 and 8, the sedated rats were euthanized by cutting the aorta. TNF-α, IL-1β, and IL-6 analysisSerum levels of TNF-α, IL-1β, and IL-6 were analyzed using Enzyme-Linked Immunosorbent Assay using kits from Abbkine EliKineTM (Abbkine Scientific Co., Georgia, USA) catalog numbers KTE9007, KTE9001, and KTE9004, respectively. The concentration of each cytokine is determined using a standard curve. Statistical analysisThe statistical analysis used one-way ANOVA (normal and homogenous data distribution) or Kruskal-Wallis (abnormal distribution) tests, followed by a post-hoc analysis using the least significant difference test or Pairwise Comparison (Kruskal-Wallis) with the SPSS 26 version. The results are presented as the mean value ± SD. A p-value less than 0.05 was determined statistically significant. Ethical approvalThe Ethics Committee of the Faculty of Medicine-Public Health and Nursing, Gadjah Mada University, has approved all treatments and experimental protocols. The approval number was KE/FK/1452/C/2022. ResultsEffect of S. quadrifida stem bark on TNF-αThe levels of TNF-α in the healthy, dengue, and treatment groups can be seen in Figure 1 with the healthy group being used as the reference value. The TNF-α in the dengue and treatment group were increased on day five compared to the healthy group and then decreased on day 8 after infection (Fig. 1). A statistically significant difference with a p-value <0.05 was only seen on day 5 after the infection. Table 1 displays the differences within the groups on the fifth day following infection. Significant differences were identified between the healthy-dengue group and the treatment-dengue group. Effect of S. quadrifida stem bark on IL-1βIL-1β levels in healthy, dengue, and treatment groups are shown in Figure 2. IL-1β levels in the healthy group were used as a reference value. Statistically significant differences were seen on days 5 and 8 after infection, with p-values of 0.028 and 0.038, respectively. Analysis of differences using post-hoc tests can be seen in Table 2. Statistically significant differences were seen solely between the healthy group and the dengue group, on both day 5 and day 8 following infection.
Fig. 1. The levels of TNF-α were measured in three groups: the healthy, dengue, and treatment group (dengue + S. quadrifida). TNF-α levels in the healthy group were used as reference values. On day 5 post-infection, there was a statistically significant difference with a p-value <0.05 (0.003). However, on day 8, no significant difference was seen [p-value > 0.05 (0.529)]. Table 1. Post-hoc analysis of TNF-α levels on day 5 post-infection
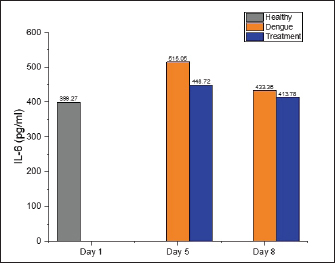
Effect of S. quadrifida stem bark on IL-6The levels of IL-6 in healthy, dengue, and treatment groups are displayed in Figure 3. On the fifth day after infection, the levels of IL-6 in the dengue group were observed to have increased to 515.05 pg/ml, whereas the healthy group and the treatment group had levels of 399.27 and 448.74 pg/ml, respectively. Nevertheless, no statistically significant difference was identified. On the eighth day post-infection, the levels of IL-6 in both the dengue and treatment groups were similar to the healthy group, indicating no significant. DiscussionThis current study demonstrated that TNF-α, IL-1β, and IL-6 levels were elevated in both the dengue and treatment groups compared to the healthy group on days 5 and 8 following infection. However, the rats in the treatment group administered S. quadrifida stem bark extract exhibited comparatively lower cytokine levels than those infected with dengue alone. Notably, significant reductions were observed in the levels of TNF-α on day five and IL-1β on days 5 and 8. TNF-α is a cytokine with pro-inflammatory properties that exhibits diverse actions and holds significance in the pathogenesis of several inflammatory disorders. This cytokine is primarily synthesized by monocytes/macrophages in significant amounts, as well as by other cell types, including mast cells, neutrophils, natural killer cells, fibroblasts, osteoclasts, T and B lymphocytes, but in smaller levels (Horiuchi, 2010). In dengue infection, TNF-α can stimulate the production of reactive oxygen intermediates and reactive nitrogen intermediates. In addition, it can trigger cell death through apoptosis, resulting in heightened vascular permeability and subsequent bleeding. This is supported by other research findings that indicate a positive correlation between elevated levels of TNF-α and enhanced vascular permeability in dengue (Masood et al., 2018).
Fig. 2. IL-1β levels in healthy, dengue, and treatment groups (dengue + S. quadrifida). IL-1β levels in the healthy group were used as a reference value. Statistically significant differences were seen on days 5 and 8 after infection, with p-values of 0.028 and 0.038, respectively.
Fig. 3. IL-6 levels on healthy, dengue, and treatment group. The healthy group’s IL-6 levels were employed as a standard to determine normal values. No significant differences were seen between the groups on days 5 and 8 following infection (day 5: p-value=0.227; day 8: p-value=0.528). Table 2. Post-hoc on IL-1β levels on days 5 and 8 post-infection.
The study observed an elevation in TNF-α levels in both the dengue and treatment groups compared to the healthy group on the fifth day after infection. This finding aligns with the research conducted by Costa et al. (2012), which demonstrated an elevation in serum TNF-α levels in C57BL/6 mice infected with DENV-3 on the fifth day. However, the treatment group exhibited slightly lower TNF-α levels than the dengue group on day 5 after infection, which was statistically significant (Table 1). These findings demonstrate that the stem bark extract of S. quadrifida can effectively decrease TNF-α levels in Wistar mice infected with DENV-3. IL-1β is a pro-inflammatory cytokine produced by macrophages using two mechanisms: activation of toll-like receptor, which results in the synthesis of pro-IL-1β, and activation of NLR family pyrin domain containing (NLRP3), which relies on caspase-1 and inflammasomes (Gabay et al., 2010). This cytokine can stimulate the activation of neutrophils and the production of cytokines. It also can activate T and B cells, which leads to the formation of antibodies. In addition, it has a role in angiogenesis by promoting the synthesis of vascular endothelial growth factor in conjunction with TNF-α and IL-6 (Maloney and Gao, 2015). The levels of IL-1β in patients with dengue infection showed a significant rise compared to healthy individuals, and this increase was linked to the severity of the disease. IL-1β has been demonstrated to enhance the permeability of blood vessels primarily through its interaction with TNF-α and interferon-γ, both of which are elevated in individuals with severe dengue (Tuyen et al., 2020). In the C57BL/6 mouse model infected with dengue, the levels of serum IL-1β were seen to increase on the fourth-day post-infection, along with IL-6 and TNF-α (Marques et al., 2018). Similar to this research, the present study observed a notable rise in IL-1β levels in the dengue and treatment groups on days 5 and 8 following infection compared to the healthy group. Furthermore, the mean IL-1β levels in the extract group were shown to be lower than those in the dengue group. Still, the difference was not statistically significant on either day 5 or day 8 following infection (Table 2). Nevertheless, the extract derived from the stem bark of S. quadrifida may possess the capability to reduce the levels of IL-1β. IL-6 is a pleiotropic cytokine that affects inflammation, immunological response, and hematopoiesis. IL-6 is crucial in immunological responses, as it promotes the generation of antibodies and effector T cells and the proliferation of both immune and non-immune cells (Tanaka et al., 2014). The IL-6 levels in dengue infection showed a comparable increase as TNF-α (Varghese et al., 2019). IL-6 has been identified for its role in regulating the elevation of C-reactive protein and secreted phospholipase A2 (Masood et al., 2018). In addition, IL-6 can produce prostaglandin E2, which leads to enhanced endothelial permeability. The levels of IL-6 exhibited an initial increase during the early phases of the disease and subsequently experienced significant elevation in conditions of shock (Butthep et al., 2012). The research study discovered that the levels of IL-6 in the treatment group were significantly lower than those in the dengue group on days 5 and 8 post-infection. However, the difference was not statistically significant. Nevertheless, there is a possibility that the extract obtained from the stem bark of S. quadrifida could decrease IL-6 levels. Previous studies have shown that the bark of S. quadrifida contains a variety of substances, including flavonoids (Saragih and Siswadi, 2019; Munawaroh et al., 2020). Flavonoids are compounds that show anti-inflammatory and antioxidant properties, thereby being important in preventing numerous chronic illnesses, including cancer and cardiovascular disease (Al-Khayri et al., 2022). Several Studies have indicated that flavonoids can inhibit PI3K, JNK, and NF-Kβ signaling pathways, leading to a reduction in the production of proinflammatory cytokines such as TNF-α, IL-1β, and IL-6 (Wright et al., 2015; Al-Khayri et al., 2022). Furthermore, several flavonoids have shown the ability to decrease the release of TNF-α in macrophages infected with DENV-2 and DENV-3, both in normal infection and antibody-dependent enhancement circumstances (Jasso-Miranda et al., 2019). A study conducted by Munawaroh et al. (2020) stated that the total flavonoid content of S. quadrifida stem bark extract was positively correlated with macrophage phagocytic activity, with a correlation coefficient (r2) of 0.61. These findings indicate that flavonoids account for 61% of macrophage phagocytic activity, with the remaining 39% attributed to other compounds, such as terpenoids. In addition, flavonoids exhibit antiviral properties by exerting an effect on the life cycles of various viruses, particularly those belonging to the Flaviviridae family, such as hepatitis C virus, ZIKV, and DENV (Badshah et al., 2021; Cateneo et al., 2021). The stem bark of S. quadrifida is also recognized for its presence of catechin derivative chemicals, including epicatechin (Dean et al., 2019) and (+)-catechin (Riwu et al., 2023). Catechin is a compound that can regulate NF-κB, a transcription factor that regulates the production of different inflammatory genes like pro-inflammatory cytokines (such as TNF-α and IL-6). In addition, it stimulates the release of the precursor IL-1β, which is necessary for activating inflammasomes and promoting the maturation of IL-1β (Liu et al., 2017). Meanwhile, catechin can inhibit NF-κB and FOXO3a by activating the AMPK/SIRT1 pathway, reducing the release of pro-inflammatory cytokines (Cheng et al., 2019). Furthermore, a study conducted by Yi et al. (2023) using in vitro approaches has demonstrated that catechin can inhibit the replication of DENV, particularly in the post-entry stages. In addition, it has been observed that catechin can reduce the titers of DENV-1, DENV-3, and DENV-4. The study conducted by Munawaroh et al. (2020) also mentioned the presence of scopoletin in S. quadrifida. Scopoletin is a derivative of coumarin, a class of compounds commonly found in medicinal plants, including Aster tataricus and Foeniculum vulgare. Scopoletin has been found to decrease proinflammatory cytokines and chemokines by modulating the signaling pathways of MAAPK, STAT-1, and NF-κB (Kim et al., 2004; Bak et al., 2022). According to the statement above, it may be concluded that S. quadrifide exhibits anti-inflammatory properties, probably due to flavonoids, catechin, and scopoletin. Meanwhile, the research findings indicated no significant differences, which could have been due to the method of extraction and solvent used in the current study. The utilization of water as a solvent and heating it to a high temperature of up to 90°C during the extraction process can lead to the degradation of molecules that impact the amounts of certain chemicals and antioxidant activity that may affect the anti-inflammatory effects produced. However, the extraction using the infusion process is considered to be safer, more convenient, and more applicable to the community (Hidalgo and Almajano, 2017; Réblová et al., 2017; Riwu et al., 2023; Xu et al., 2017). ConclusionThe findings of this study demonstrate that the stem bark of Sterculia quadrifida R. Br can exert an anti-inflammatory effect, particularly by decreasing the levels of TNF-α and IL-1β in cases of dengue infections. The presence of flavonoid compounds and secondary metabolite compounds, such as catechin and scopoletin, is thought to be the reason for this phenomenon. Hence, this extract has the potential to be a drug candidate for inhibiting exaggerated inflammatory responses in dengue infections and other infectious diseases. AcknowledgmentThe authors are grateful to the Laboratorium of Parasitology, Faculty of Medicine, Public Health-Nursing, Gadjah Mada University, and the Dengue Research Group of the Institute of Tropical Disease of Airlangga University for providing invaluable help in preparing the dengue virus. Conflict of interestThere is no conflict of interest in this study. FundingThis study was supported by the Directorate of Research, Technology, and Community Service, Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia for the Doctoral Dissertation Research Scheme (PPS-PDD) under contract 1177/UN3.LPPM/PT.01.03/2023. Author’s contributionAll authors conceptualized the idea and design of the study. AGR performed material preparation, data collection, and analysis. JN, EAT, and DAP supervised the entire research process. AGR drafted the manuscript under JN, EAT, and DAP supervision. All the authors read and approved the final manuscript. Data availabilityAll data are available in the manuscript. ReferencesAlkaff, A.H., Yohan, B., Tambunan, U.S.F. and Sasmono, R.T. 2019. Zika, chikungunya, and dengue viral infections in human peripheral blood mononuclear cells: cell susceptibility and gene expression. Med. J. Indones. 29, 129–135. Al-Khayri, J.M., Sahana, G.R., Nagella, P., Joseph, B.V., Alessa, F.M. and Al-Mssallem, M.Q. 2022. Flavonoids as potential anti-inflammatory molecules: a review. Molecules 27(9), 2901. Amin, A., Wunas, J. and Anin, Y.M. 2015. Uji Aktivitas Antioksidan Ekstrak Etanol Klika Faloak (Sterculia quadrifida R. Br) dengan Metode DPPH (2,2-diphenyl-1-picrylhydrazyl). Indonesian J. Phytopharm. 2(2), 111–114. Australian Tropical Rainforest Plants. 2020. Sterculia quadrifida R. Br. Available via https://apps.lucidcentral.org/rainforest/text/entities/sterculia_quadrifida.htm (Accessed 20 November 2023). Badshah, S.L., Faisal, S., Muhammad, A., Poulson, B.J., Emwas, A.H. and Jaremko, M. 2021. Antiviral activities of flavonoids. Biomed. Pharmacother. 140(11), 111596. Bak, S.G., Lim, H.J., Won, Y.S., Lee, S., Cheong, S.H., Lee, S.J., Bae, E.Y., Lee, S.W., Lee, S.J. and Rho, M.C. 2022. Regulatory effects of Lycium barbarum extract and isolated scopoletin on atopic dermatitis-like skin inflammation. Biomed. Res. Int. 2022, 2475699. Butthep, P., Chunhakan, S., Yoksan, S., Tangnararatchakit, K. and Chuansumrit, A. 2012. Alteration of cytokines and chemokines during febrile episodes associated with endothelial cell damage and plasma leakage in dengue hemorrhagic fever. Pediatr. Infect. Dis. J. 31(12), 232–238. Cateneo, A.H.D., Avila E.P., Mendes, L.A., de Oliveira, V.G., Ferraz, C.R., de Almeida, M.V., Frabasile, S., dos Santos, C.N.D., Verri, W.A., Bordignon, J. and Wowk, P.F. 2021. Flavonoids as molecules with Anti-Zika virus activity. Front Microbiol. 10, 710359. Chen, T.H., Lo, Y.P., Yang, C.F. and Chen, W.J. 2012. Additive protection by antioxidant and apoptosis-inhibiting effects on mosquito cells with dengue 2 birus infection. PLoS Negl. Trop. Dis. 6(4), e1613. Cheng, A.W., Tan, X., Sun, J.Y., Gu, C.M., Liu, C. and Guo, X. 2019. Catechin attenuates TNF-α induced inflammatory response via AMPK-SIRT1 pathway in 3T3-L1 adipocytes. PLoS One 14(5), e0217090. Costa, V.V., Fagundes, C.T., Valadão, D.F., Cisalpino, D., Dias, A.C., Silveira, K.D., Kangussu, L.M., Ávila, T.V., Bonfim, M.R., Bonaventura, D., Silva, T.A., Sousa, L.P., Rachid, M.A., Vieira, L.Q., Menezes, G.B., de Paula, A.M., Atrasheuskaya, A., Ignatyev, G., Teixeira, M.M. and Souza, D.G. 2012. A model of DENV-3 infection that recapitulates severe disease and highlights the importance of IFN-γ in host resistance to infection. PLoS Negl. Trop. Dis. 6, 1663. Dean, M., Handajani, R. and Khotib, J. 2019. Faloak (Sterculia quadrifida R.Br) stem bark extract inhibits hepatitis C virus JFH1. Orient. J. Chem. 35(1), 430–435. Dillak, H.I., Kristiani, E.B.E. and Kasmiyati, S. 2019. Secondary metabolites and antioxidant activity of ethanolic extract of faloak (Sterculia quadrifida). Biosaintifika 11(3), 296–303. Gabay, C., Lamacchia, C. and Palmer, G. 2010. IL-1 pathways in inflammation and human diseases. Nat. Rev. Rheumatol. 6(4), 232–241. Hidalgo, G.I. and Almajano, M.P. 2017. Red fruits: extraction of antioxidants, phenolic content, and radical scavenging determination: a review. Antioxidants 6(1), 7. Hitakarun, A., Ramphan, S., Wikan, N. and Smith, D. 2020. Analysis of the virus propagation profile of 14 dengue virus isolates in Aedes albopictus C6/36 cells. BMC Res. Notes. 13, 481. Horiuchi, T. 2010. Transmembrane TNF-α: structure, function, and interaction with anti-TNF agents. Rheumatology 249(7), 1215–1228. Imad, H.A., Phumratanaprapin, W., Phonrat, B., Chotivanich, K., Charunwatthana, P., Muangnoicharoen, S., Khusmith, S., Tantawichien, T., Phadungsombat, J., Nakayama, E., Konishi, E. and Shioda, T. 2020. Cytokine expression in dengue fever and dengue hemorrhagic fever patients with bleeding and severe hepatitis. Am. J. Trop. Med. Hyg. 102(5), 943–950. Iyer, S. and Thangam, SG. 2022. Pathophysiologic and prognostic role of proinflammatory and regulatory cytokines as a proinflammatory and regulatory cytokine in dengue fever. Indian J. Med. Microbiol. 40(2), 235–238. Jasso-Miranda, C., Herrera-Camacho, I., Flores-Mendoza, L.K., Dominguez, F., Vallejo-Ruiz, V., Sanchez-Burgos, G.G., Pando-Robles, V., Santos-Lopez, G. and Reyes-Leyva, J. 2019. Antiviral and immunomodulatory effects of polyphenols on macrophages infected with dengue virus serotypes 2 and 3 enhanced or not with antibodies. Infect. Drug Resist. 12, 1833–1852. Kim, H.J., Jang, S.I., Kim, Y.J., Chung, H.T., Yun, Y.G., Kang, T.H., Jeong, O.S. and Kim, Y.C. 2004. Scopoletin suppresses pro-inflammatory cytokines and PGE2 from LPS-stimulated cell line, RAW 264.7 cells. Fitoterapia 75(3-4), 261–266. Liu, T., Zhang, L., Joo, D. and Sun, A.C. 2017. NF-κB signaling in inflammation. Sig. Transduct. Target. Ther. 2, 17023. Lulan, T.Y.K. 2020. Exploration of the chemical constituents and bioactivity of Sterculia quadrifida R. Br and Dipterocarpus littoralis Blume. Two plant species endemic to Indonesia. Ph.D Thesis, Sepuluh November Institute of Technology, Surabaya, Indonesia. Maloney, J.P. and Gao, L. 2015. Proinflammatory cytokines increase vascular endothelial growth factor expression in alveolar epithelial cells. Mediators Inflamm. 2015, 387842. Marques, R.E., Besnard, A.G., Maillet, I., Fagundes, C.T., Souza, D.G., Ryffel, B., Teixeira, M.M., Liew, F.Y. and Guabiraba, R. 2018. Interleukin-33 contributes to disease severity in Dengue virus infection in mice. Immunology 55(4), 477–490. Masood, K.I., Jamil, B., Rahim, M., Islam, M., Farhan, M. and Zahra, H. 2018. Role of TNF-α, IL-6, and CXCL10 in dengue disease severity. Iran J. Microbiol. 10(3), 202–207. Munawaroh, R, Setyowati, E.P, Murwanti, R. and Hertiani, T. 2020. Investigation of immunomodulatory active compounds from faloak (Sterculia quadrifida R.Br.) bark fractions. Int. J. Pharm. Res. 12(1), 497–502. Pan, P., Zhang, Q., Liu, W., Wang, W., Yu, Z., Lao, Z., Zhang, W., Shen, M., Wan, P., Xiao, F., Shereen, M.A., Zhang, W., Tan, Q., Liu, Y., Liu, X., Wu, K., Liu, Y., Li, G. and Wu, J. 2019. Dengue virus infection activates interleukin-1β to induce tissue injury and vascular leakage. Front Microbiol. 10, 2637. Réblová, Z. 2012. Effect of temperature on the antioxidant activity of phenolic acids. Czech J. Food Sci. 30(2), 171–175. Riwu, A.G., Nugraha, J., Purwanto, D.A. and Triyono, E.A. 2023. Determination of (+)-catechin and antioxidant activity in faloak (Sterculia quadrifida R. Br) stem bark infusion. Sci. Technol. Indones. 8(1), 59–65. Rollando, R., Warsito, W., Masruri, M. and Widodo, W. 2020. Potential therapeutic use of Sterculia quadrifida R.Br and Sterculia foetida Linn.: review. Asian J. Plant Sci. 19(4), 325–334. Saleh, M.S.M. and Kamisah, Y. 2021. Potential medicinal plants for the treatment of dengue fever and severe acute respiratory syndrome-coronavirus. Biomolecules 11(42), 1–25. Santosa, A., Purnawarman, T., Mustika, A.A., Rahma, A. and Sutardi, L.N. 2024. Effectiveness of Malay rose apple (Syzygium malaccense) infusion as antidiarrheal in mice (Mus musculus). Curr. Biomed. 2(1), 21–28. Saragih, G. and Siswadi. 2019. Antioxidant activity of plant parts extracts from Sterculia quadrifida R. Br. Asian J. Pharm. Clin. Res. 12(7), 143–148. Tanaka, T., Narazaki, M. and Kishimoto, T. 2014. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 6(10), a016295. Triyono, E.A. 2020. The mechanism of the effect of Monascus jmbA rice on increased platelet count in Wistar rats infected with dengue virus serotype 3. Infect. Dis. Rep. 12(1), 8720. Tuyen, T.T., Viet, N.T., Hang, N.T., Giang, N.T., Anh, D.D., Anh, D.T., Hung, H.V., Quyet, D., Toan, N.L., Cam, T.D. and Van Tong, H. 2020. Proinflammatory cytokines are modulated in Vietnamese patients with dengue fever. Viral. Immunol. 33(7), 514–520. Utama, I., Merati, T., Bakta, M. and Jawi, M. 2023. BALB/c Mice as animal model in dengue infection research: role of endothelial activation. J. Biomed. Transl. Res. 9(2), 72–76. Varghese, A., Balu, P., Saravanakumar, R., Muthu, J. and Vineela, K. 2019. Salivary interleukin-6 levels among polycystic ovary syndrome patients with and without chronic periodontitis—a comparative study. Contemp. Clin. Dent. 10(3), 498–501. WHO. 2023. Dengue and severe dengue fact sheet 2023. Geneva, Switzerland: WHO. Available via https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (Accessed 20 November 2023). Winanta, A., Hertiani, T., Purwantiningsih. and Siswadi. 2019. In vivo immunomodulatory activity of faloak bark extract (Sterculia quadrifida R.Br). Pak. J. Biol. Sci. 22(12), 590–596. Wright, B., Watson, K.A., McGuffin, L.J., Lovegrove, J.A., and Gibbins, J.M. 2015. GRID and docking analyses reveal a molecular basis for flavonoid inhibition of Src family kinase activity. J. Nutr. Biochem. 26(11), 1156–1165. Xu, D.P., Li, Y., Meng, X., Zhou, T., Zhou, Y., Zheng, J., Zhang, J.J. and Li, H.B. 2017. Natural antioxidants in foods and medicinal plants: extraction, assessment and resources. Int. J. Mol. Sci. 18(1), 96. Yi, B., Chew, B.X.Z., Chen, H., Lee, R.C.H., Fong, Y.D., Chin, W.X., Mok, C.K. and Chu, J.J.H. 2023. Antiviral activity of catechin against dengue virus infection. Viruses 15(6), 1377. | ||
| How to Cite this Article |
| Pubmed Style Riwu AG, Nugraha J, Triyono EA, Purwanto DA. Anti-inflammatory effect of Faloak (Sterculia quadrifida R. Br) stem bark on TNF-α, IL-1β, and IL-6 in DENV-3-infected Wistar rats. Open Vet. J.. 2024; 14(4): 1043-1050. doi:10.5455/OVJ.2024.v14.i4.11 Web Style Riwu AG, Nugraha J, Triyono EA, Purwanto DA. Anti-inflammatory effect of Faloak (Sterculia quadrifida R. Br) stem bark on TNF-α, IL-1β, and IL-6 in DENV-3-infected Wistar rats. https://www.openveterinaryjournal.com/?mno=185265 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i4.11 AMA (American Medical Association) Style Riwu AG, Nugraha J, Triyono EA, Purwanto DA. Anti-inflammatory effect of Faloak (Sterculia quadrifida R. Br) stem bark on TNF-α, IL-1β, and IL-6 in DENV-3-infected Wistar rats. Open Vet. J.. 2024; 14(4): 1043-1050. doi:10.5455/OVJ.2024.v14.i4.11 Vancouver/ICMJE Style Riwu AG, Nugraha J, Triyono EA, Purwanto DA. Anti-inflammatory effect of Faloak (Sterculia quadrifida R. Br) stem bark on TNF-α, IL-1β, and IL-6 in DENV-3-infected Wistar rats. Open Vet. J.. (2024), [cited January 25, 2026]; 14(4): 1043-1050. doi:10.5455/OVJ.2024.v14.i4.11 Harvard Style Riwu, A. G., Nugraha, . J., Triyono, . E. A. & Purwanto, . D. A. (2024) Anti-inflammatory effect of Faloak (Sterculia quadrifida R. Br) stem bark on TNF-α, IL-1β, and IL-6 in DENV-3-infected Wistar rats. Open Vet. J., 14 (4), 1043-1050. doi:10.5455/OVJ.2024.v14.i4.11 Turabian Style Riwu, Audrey Gracelia, Jusak Nugraha, Erwin Astha Triyono, and Djoko Agus Purwanto. 2024. Anti-inflammatory effect of Faloak (Sterculia quadrifida R. Br) stem bark on TNF-α, IL-1β, and IL-6 in DENV-3-infected Wistar rats. Open Veterinary Journal, 14 (4), 1043-1050. doi:10.5455/OVJ.2024.v14.i4.11 Chicago Style Riwu, Audrey Gracelia, Jusak Nugraha, Erwin Astha Triyono, and Djoko Agus Purwanto. "Anti-inflammatory effect of Faloak (Sterculia quadrifida R. Br) stem bark on TNF-α, IL-1β, and IL-6 in DENV-3-infected Wistar rats." Open Veterinary Journal 14 (2024), 1043-1050. doi:10.5455/OVJ.2024.v14.i4.11 MLA (The Modern Language Association) Style Riwu, Audrey Gracelia, Jusak Nugraha, Erwin Astha Triyono, and Djoko Agus Purwanto. "Anti-inflammatory effect of Faloak (Sterculia quadrifida R. Br) stem bark on TNF-α, IL-1β, and IL-6 in DENV-3-infected Wistar rats." Open Veterinary Journal 14.4 (2024), 1043-1050. Print. doi:10.5455/OVJ.2024.v14.i4.11 APA (American Psychological Association) Style Riwu, A. G., Nugraha, . J., Triyono, . E. A. & Purwanto, . D. A. (2024) Anti-inflammatory effect of Faloak (Sterculia quadrifida R. Br) stem bark on TNF-α, IL-1β, and IL-6 in DENV-3-infected Wistar rats. Open Veterinary Journal, 14 (4), 1043-1050. doi:10.5455/OVJ.2024.v14.i4.11 |