| Research Article | ||
Open Vet. J.. 2024; 14(4): 1059-1071 Open Veterinary Journal, (2024), Vol. 14(4): 1059–1071 Original Research Discolored urine in sheep and goats: Clinical, etiological, hematobiochemical, sonographic and postmortem findingsMohamed Tharwat1,2, Yamen Hegazy3,4 and Abdulrahman A. Alkheraif5*1Department of Clinical Sciences, College of Veterinary Medicine, Qassim University, Buraidah, Saudi Arabia 2Department of Animal Medicine, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 3Department of Clinical Sciences, College of Veterinary Medicine, King Faisal University, Al-Ahsa, Saudi Arabia 4Department of Animal Medicine, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafrelsheikh, Egypt 5Department of Pathology and Laboratory Diagnosis, College of Veterinary Medicine, Qassim University, Buraidah, Saudi Arabia *Corresponding Author: Abdulrahman A. Alkheraif. Department of Pathology and Laboratory Diagnosis, College of Veterinary Medicine, Qassim University, Buraidah, Saudi Arabia. Email: alkheraif [at] qu.edu.sa Submitted: 20/01/2024 Accepted: 09/03/2024 Published: 30/04/2024 © 2024 Open Veterinary Journal
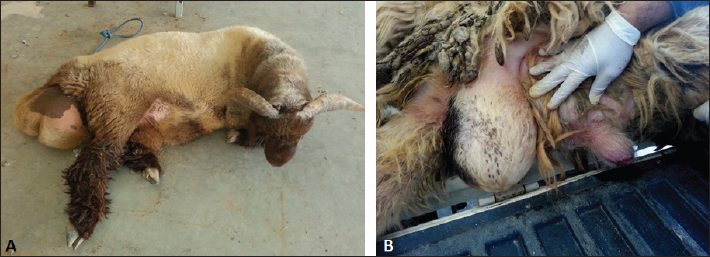
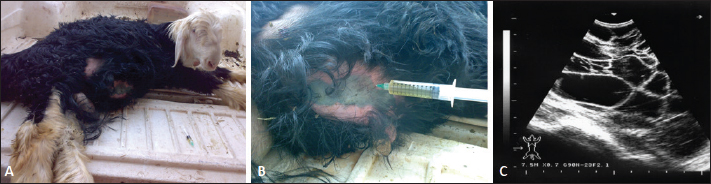
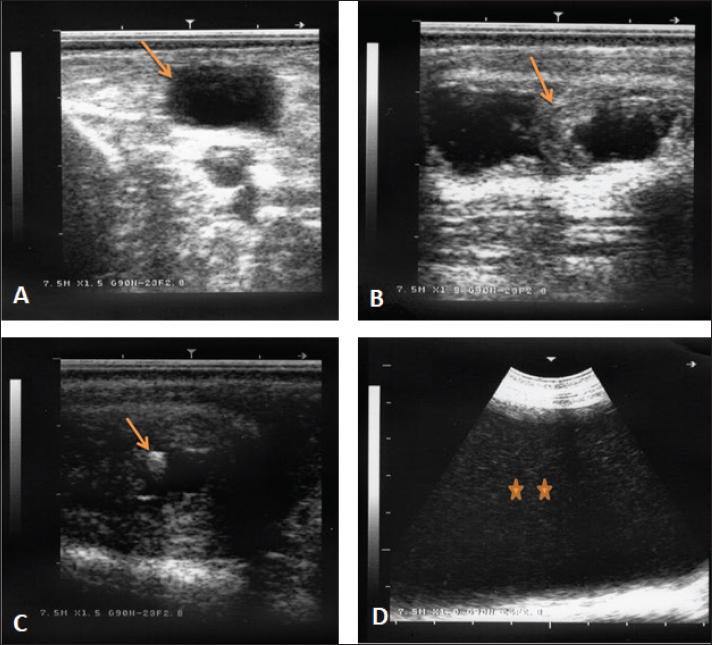
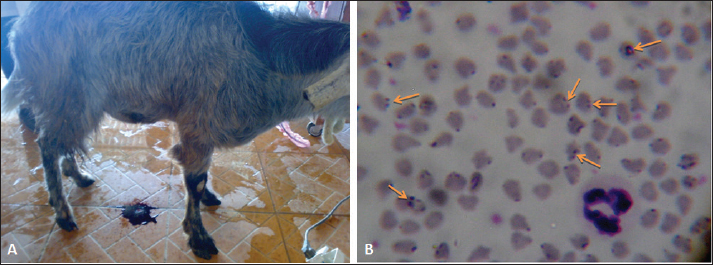
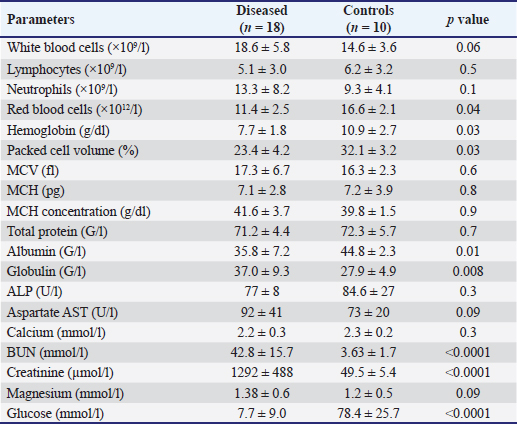
AbstractBackground: Bloody urine is classified in farm animals as hematuria, hemoglobinuria, and myoglobinuria. In small ruminants, discolored urine is reported due to several etiologies which is sometimes fatal. Of these causes are babesiosis, bacillary hemoglobinuria, copper toxicity, and hypophosphatemia. Aim: This study was designed to investigate the clinical, etiological, hematobiochemical, ultrasonographic, and pathological findings in rams and bucks with red urine syndrome. Methods: Eighteen male animals (nine rams and nine bucks) of 6 months to 3 years were examined. Parallel, 10 healthy controls were used. They were admitted due to red urine, voiding of only urine drops, straining during the act of urination, grunting during urination, ventral abdominal edema, and abdominal distension. The duration of the disease ranged from 2 to 30 days. A history of chronic copper toxicosis was informed in two bucks and a ram. Two blood samples were collected from diseased as well as from controls in EDTA tubes (for complete blood count testing) and in plain tubes (for serum collection). Results: Hematuria was found in 11 animals (seven bucks and four rams) while hemoglobinuria was detected in seven animals (five bucks and two rams). Sonographic findings in diseased animals included ruptured urinary bladder in 3, ruptured urethra in 5, penile calculi, uroperitoneum in 6, distended urinary bladder in 7, hydronephrosis in 5, echogenic deposits in the bladder in 3, and ventral urine accumulation in four animals. Laboratory evaluation of a Geimsa-stained blood smear confirmed the infection with Babesia in three bucks and a ram. Hemolytic anemia was marked in two bucks and a ram due to chronic copper toxicity. Biochemical abnormalities included hypoalbuminemia, hyperglobulinemia, increased blood urea nitrogen and creatinine concentration, and hyperglycemia. Postmortem examination was carried out on six animals (four rams and two bucks). Conclusion: Discolored urine in rams and bucks in this study resulted from hematuria due to urinary calculi and pelvic abscessation or from hemoglobinuria due to Babesia infection or due to copper toxicity. Hemolytic anemia was the outstanding hematological finding and hypoalbuminemia, hyperglobulinemia, increased blood urea nitrogen (BUN) and creatinine, and hyperglycemia were the characteristic biochemical findings. Sonography of the urinary tract was very helpful in assessing the renal parenchyma, urinary bladder, and abdominal cavity for the verification of urolithiasis, hydronephrosis, intact or ruptured urinary bladder, uroperitoneum, and perforated urethra. Keywords: Goat, Pathology, Ruminant, Sheep, Ultrasound. IntroductionThe red water syndrome is categorized in farm animals as hematuria, hemoglobinuria, and myoglobinuria. Hematuria is usually seen as a result of urinary bladder tumors (Di Loria et al., 2012; Ali et al., 2018; Al-Sobayil et al., 2018; Tharwat et al., 2018a,b). The syndrome of hemoglobinuria is usually detected due to babesiosis (Mtshali and Mtshali, 2013; Mahmmod, 2014; Bal et al., 2016; He et al., 2021), infection by Clostridium hemolyticum (Takagi et al., 2009; Shinozuka et al., 2011; Navarro et al., 2017), decreased blood phosphorus level (Grünberg, 2014; Abramowicz et al., 2022) and intoxication by water (Kawahara et al., 2016). In equines, discolored urine is also found in animals with hematuria, hemoglobinuria, or myoglobinuria (Schumacher, 2007; Delvescovo et al., 2022). Urinary calculi are also a cause of hematuria in the equines (Duesterdieck-Zellmer, 2007). In addition, hemoglobinuria is reported in horses with copper toxicity (Belli et al., 2021). In ruminants, urinary calculi are one of the most important urinary tract disorders, and one of the most emergencies (Tharwat and Al-Sobayil, 2016; Tharwat et al., 2017; Tharwat, 2021a, b, 2023; Almundarij and Tharwat, 2023). In rams and bucks, the process of urolithiasis is one of the highly fatal conditions (Nwaokorie et al., 2015; Videla and van Amstel, 2016; Jones et al., 2018; Oman et al., 2019). In small ruminants, babesiosis, theileriosis, and anaplasmosis are caused by tick-borne blood hemoparasites Babesia, Theileria, and Anaplasma (Galon et al., 2022a). Vector-borne apicomplexan protozoa, Babesia and Theileria are the main causative agents for piroplasmoses (Esmaeilnejad et al., 2020; Villanueva-Saz et al., 2022). Babesiosis is considered in tropical and subtropical regions as an important economic disease (Kage et al., 2019). Several species of Babesia can infect sheep and goats. However, Babesia ovis and Theileria ovis are the most common causative pathogens (Esmaeilnejad et al., 2020; Aydın et al., 2022; Galon et al., 2022b). Of the blood parasites, babesiosis is usually accompanied by anorexia, fever, polypnea, icterus, hemolytic anemia, hemoglobinuria, anemia, diarrhea, and in acute clinical cases, could be terminated by death (Galon et al., 2022b; Villanueva-Saz et al., 2022; Ulucesme et al., 2023). Other causes of discolored urine in sheep include bacillary hemoglobinuria caused by C. hemolyticum infection (Randhawa et al., 1995). The disease in the later report was manifested clinically by elevated rectal temperature, constipation, hemoglobinuria, ataxia, and finally recumbency. Copper toxicity is also considered a cause of hemoglobinuria in sheep and goats (Bozynski et al., 2009; Borobia et al., 2022). Hemoglobinuria was also reported in lambs due to nutritional hypophosphatemia (Alidadi et al., 2005). This study was designed to investigate the clinical, etiological, hematobiochemical, ultrasonographic, and pathological findings in rams and bucks with red urine syndrome. Materials and MethodsAnimals, history, clinical examination, and blood samplingAnimal Care and Welfare Committee of the Deanship of Scientific Research at Qassim University, Kingdom of Saudi Arabia approved the study design. Eighteen male animals (nine rams and nine bucks) of 6 months to 3 years were examined at the Veterinary Hospital of the University of Qassim, Saudi Arabia between 2019 and 2023. They were admitted due to red urine, voiding of only urine drops, straining during the act of urination, grunting during urination, ventral abdominal edema, and abdominal distension. The duration of the disease ranged from 2 to 30 days. A history of chronic copper toxicosis was informed in two bucks and a ram. Just after entrance, all animals underwent a full examination including rectal temperature, pulse and respiratory rates, mucus membranes, and cardiopulmonary and digestive systems. Special attention was made for the examination of the kidneys, urinary bladder, urethra, and penile body. Ten clinically healthy animals (five rams and five bucks) were assigned as a control group. Samples of blood were collected from diseased as well as from healthy controls in EDTA tubes (4 ml for complete blood count testing) and in plain tubes (6 ml for serum collection). Evaluation of hematobiochemical measurementsThe EDTA sample was subjected to a complete blood count [total and differential leukocytic count, erythrocyte count, hematocrit (HCT), hemoglobin (Hg), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and MCH concentration (MCHC)] using the VetScan HM5, Abaxis, California, USA. The serum concentrations of total protein, albumin, globulin, BUN, creatinine, calcium, magnesium, and glucose were measured using an automated biochemical analyzer (VetScan VS2, Abaxis, USA). The VetScan VS2 analyzer also measured the serum activity of aspartate aminotransferase (AST) and alkaline phosphatase (ALP). Ultrasonographic and postmortem examinationsSonographic imaging of the urinary system was carried out using 3.5, 5.0, and 7.5 MHz transducers (SonoScape, Sonoscape Medical Corporation, China). The right kidney was scanned either in the upper part of the right flank or in the 1th and 12th intercostal spaces high on the right side. The left kidney was imaged both from the caudal region of the left flank or transrectal. The urinary bladder was imaged transrectally and the penile body was scanned transcutaneously. Sonographic examination of the urinary tract and the abdomen was carried out as reported (Tharwat et al., 2012; Tharwat and Al-Sobayil, 2017; Ali et al., 2019; Tharwat, 2021a, b; Sadan et al., 2023; Tharwat and Al-Hawas, 2024a,b). Postmortem examination was carried out on six animals (four rams and two bucks). The thoracic and abdominal cavities were examined in detail. Special attention was given to the kidneys, ureters, urinary bladder, and urethra. Statistical analysisData are presented as means ± SD and were analyzed statistically using the SPSS statistical package, version 25, 2017. Student’s t test was used for comparisons, and the significance was set at p ≤ 0.05. Ethical approvalAnimals were maintained and treated according to the Laboratory Animal Control Guidelines of Qassim University, which basically conforms to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health in the USA (NIH publications No. 86 to 23, revised 1996). ResultsThe clinical presentation of the eighteen rams and bucks with red urine is displayed in Table 1. With a duration ranging from two to thirty days, clinical findings included discolored red urine, urine dribbling, straining and grunting during urination, abdominal distension, and ventral abdominal edema. Hematuria was found in 11 (61.1%) animals (seven bucks and four rams) (Fig. 1) while hemoglobinuria was detected in 7 (38.9%) animals (five bucks and two rams) (Figs. 2 and 3). Other clinical findings included dribbling of urine in 10 (55.6%) and abdominal distension in 4 (22.2%) animals. Penile and perpetual edema and scrotal enlargement were found in 4 (22.2%) animals (Fig. 4) and ventral abdominal edema in 5 (27.8%) (Fig. 5). Table 1 shows also the sonographic results of the rams and bucks with red urine. These findings included ruptured urinary bladder in 3 (16.7%) (Fig. 6), ruptured urethra in 5 (27.8%) (Figs. 4 and 5), penile calculi in 4 (22.2%) (Fig. 6), uroperitoneum in 6 (33.3%) (Figs. 1 and 6), distended urinary bladder in 7 (38.9%) (Fig. 1), hydronephrosis in 5 (27.8%) (Fig. 2), echogenic deposits in the bladder in 3 (16.7%) (Fig. 3), and ventral urine accumulation in 4 (22.2%) animals (Fig. 5). Laboratory evaluation of a Geimsa-stained blood smear confirmed the infection with B. ovis in three bucks and a ram (Fig. 7). Hemolytic anemia was marked in two bucks and a ram due to chronic copper toxicity (Fig. 8). The means ± SD of the hematobiochemical parameters in rams and bucks with red urine are shown in Table 2. Significant hematological alterations included decreased erythrocytes, Hg, and HCT (p < 0.05). Other parameters that included total leukocytic count, lymphocytes, neutrophils, MCV, MCH, and MCHC did not differ significantly between diseased and control groups (p > 0.05). In regard to the biochemical variables, hypoalbuminemia (p=0.01), hyperglobulinemia (p=0.008), increased BUN and creatinine concentration ( p < 0.0001), and hyperglycemia ( p < 0.0001) were detected. Other parameters including total proteins, AST, ALP, calcium, and magnesium did not show significant levels between diseased and healthy animals (p > 0.05). Table 1. Clinical presentation and ultrasonographic findings in 18 male sheep and goats with discolored urine.
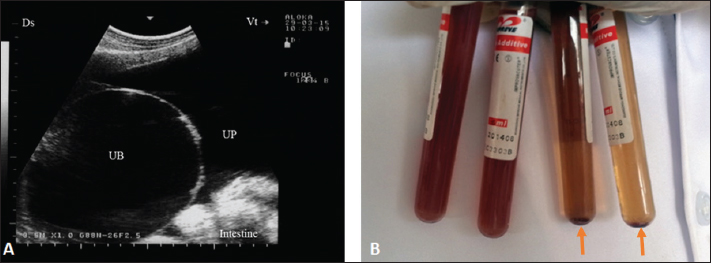
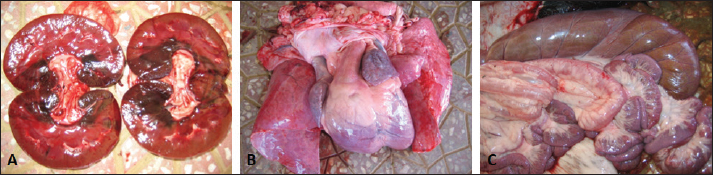
Fig. 1. Ultrasonographic finding in a buck with uroperitoneum due to urethral obstruction. Image A shows distended urinary bladder (UB) with uroperitoneum (UP). Image B shows abdominal fluid; centrifugation of the 2 tubes on the right led to precipitation (arrows). The postmortem findings in six animals (four rams and two bucks) are also recorded in Table 1. A retroperitoneal abscess adhered to the bladder, hydropericardium, bilateral hydronephrosis, and ulcerated bladder mucosa in a ram (Fig. 2). In a buck, hemorrhagic renal parenchyma, cardiac hypertrophy, hepatomegaly, and congested viscera were found (Fig. 9). In another ram, massive uroperitoneum, collapsed bladder, and bilateral hydronephrosis were recorded (Fig. 10).
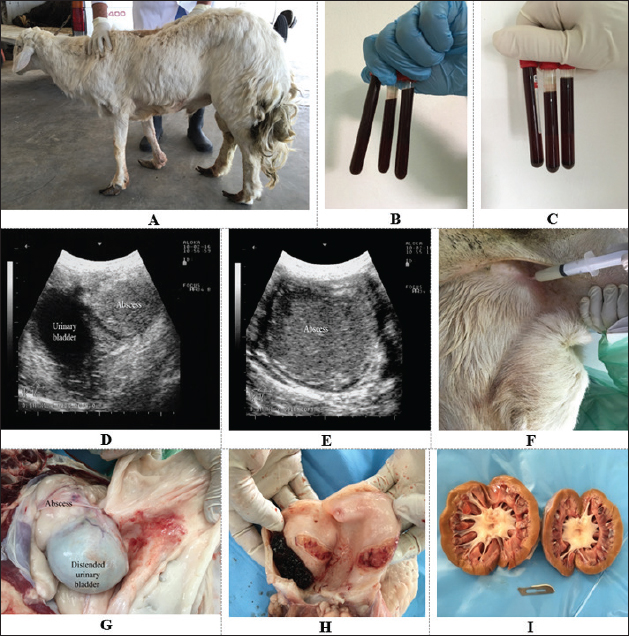
Fig. 2. A ram with hemoglobinuria for 10 days (A). The urine sample was dark red (B) and had no sediment after centrifugation (C). Ultrasonographic findings included a retroperitoneal abscess close to the urinary bladder (D) that had hyperechoic contents (E). Image (F) shows the retroperitoneal abscessation that was confirmed by ultrasound-guided aspiration. Image (G) shows where a distended urinary bladder with a retroperitoneal abscess adheres to it. Image (H) shows a blood clot within the bladder with ulceration of the bladder wall. Image (I) shows bilateral hydronephrosis. DiscussionThe majority of diseased cases in this study were admitted with a history of disclosed red urine that was proved to be hematuria. The etiologies in such cases were attributed in most cases to urolithiasis that led to either rupture of the urinary bladder or perforation of the urethra. One ram with a retroperitoneal abscess and bladder mucosal ulceration was the only one who was referred for hematuria without urolithiasis. The condition of obstructive urolithiasis is common in male sheep and male goats and its consequences are completely undesirable (Nwaokorie et al., 2015; Videla and van Amstel, 2016; Jones et al., 2018; Oman et al., 2019). The uroliths of calcium phosphate and amorphous magnesium calcium phosphate are considered the common calculi types in small ruminants (Jones et al., 2018).
Fig. 3. A buck with hemoglobinuria. The urinary bladder was scanned transrectally and no evidence of inflammation and only a little urine sediment was found (A). Image (B) shows a urine sample before centrifugation and image (C) shows a urine sample after centrifugation where no sediment was formed.
Fig. 4. Ultrasonographic finding in a ram with ruptured urethra due to urethral obstruction by a calculus. The animal was admitted depressed with a history of dribbling of red urine (A). Image (B) shows ventral edema and scrotal and perpetual enlargement.
Fig. 5. Ruptured urethra in a ram. The urine accumulated subcutaneously in the ventral abdomen and within muscles leading to tissue gangrene (A). Image (B) shows urine aspiration from the subcutaneous and muscular tissues which was detected clearly by ultrasound (C).
Fig. 6. Ultrasonographic finding in a buck with ruptured bladder due to urethral obstruction by a calculus. Image (A) shows a ruptured and collapsed urinary bladder within the pelvic cavity (arrow). Image (B) shows a dilated urethra with increased wall thickness (arrow). Image (C) shows a calculus within the urethra characterized by acoustic enhancement with distal acoustic shadowing (arrow). Image (D) shows massive abdominal effusions (stars). Different predisposing factors share a role in the formation of urinary calculi. Of these factors are the tortuous and narrow urethra, the sigmoid flexure formation, and the urethral process. Therefore, females are rarely affected. This is the reason why all affected animals in this study were males, most likely because females have a short and wider urethra that permits the passage of calculi. Breed, sex, age, restriction of water, season, and geographical position are also contributing factors (Sickinger, 2019). Hypovitaminosis A resulted in desquamation of the urinary tract epithelial tissue and early castration resulting in more narrowing and underdevelopment of the urethra are also very important predisposing agents. A calculus is primarily developed when a nidus is formed by urinary tract sediment, mucoproteins, cells, casts, or bacteria followed by minerals precipitation especially when urine is concentrated (Videla and van Amstel, 2016).
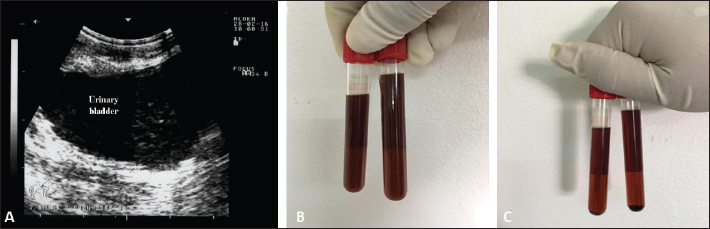
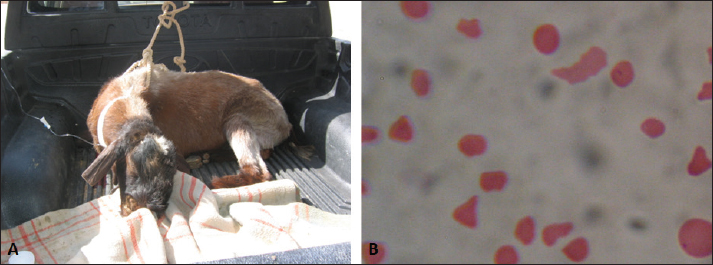
Fig. 7. Babesia infection in a buck with fever and hemoglobinuria (A). Image (B) shows multiple infections with the intracellular parasite (arrows) identified in buck erythrocytes.
Fig. 8. Hemolytic anemia in a buck due to injection with copper over dosage. The buck was admitted depressed and in a recumbent position (A). Image (B) shows ruptured erythrocytes indicating severe hemolytic anemia. Seven of the diseased animals in this study had pigmenturia or more accurately hemoglobinuria. Of these seven, three bucks and a ram were confirmed to be Babesia-infected. It was reported that babesiosis is manifested clinically in sheep and goats by inappetence, harried reparation, jaundice, anemia, diarrhea, and hemoglobinuria (Galon et al., 2022b; Villanueva-Saz et al., 2022; Ulucesme et al., 2023). From the case history, the remaining three animals (two bucks and a ram) had chronic toxicity with copper. It was reported that copper toxicosis results in hemolysis and hemoglobinuric nephrosis in goats (Bozynski et al., 2009). In sheep, chronic toxicity by copper results also in severe hemolysis, jaundice, hemoglobinuria, anemia, and collapse within 24–48 hours (Borobia et al., 2022). In small ruminants, ultrasonography provides the field veterinarian with immediate, non-invasive, and inexpensive results about the general condition of the animals. Through repeat scanning, the clinician can also visualize the progression of the disease and can ass the treatment effectiveness (Scott, 2016). In addition, ultrasonographic evaluation of the urinary system in sheep and goats was documented to be a highly valuable technique. It is especially important for the early detection of renal disorders and their progression, expecting the prognosis and treatment follow-up in different urinary disorders (Tharwat, 2021a, b). During the current investigation, ultrasonography was very helpful in rams and bucks affected with red urine syndrome due to urolithiasis, babesiosis, and copper toxicosis. The technique was especially helpful in verification cases with ruptured urinary bladder, ruptured urethra, urethral calculi, uroperitoneum, distended urinary bladder, hydronephrosis, detection of echogenic deposits in the bladder, and evaluation of the extension of urine in the ventral abdominal region. Unfortunately, only six of the eighteen diseased rams and bucks with poor prognosis were examined postmortem. At necropsy, different consequences for the red urine syndrome that were detected by ultrasonography antemortem were confirmed postmortem. These findings included retroperitoneal abscessation, hydropericardium, hydronephrosis, uroperitoneum, and ruptured bladder. Lesions that were not detected by sonography as ulceration of the urinary bladder mucosa, hemorrhagic renal parenchyma, cardiac hypertrophy, hepatomegaly, and congested viscera were also found. Table 2. Hematobiochemical parameters in 18 rams and bucks with red urine versus controls.
Fig. 9. Postmortem findings in a buck with hemoglobinuria and dark red urine. Necropsy findings revealed hemorrhagic kidneys (A), cardiac hypertrophy (B), and congested intestines.
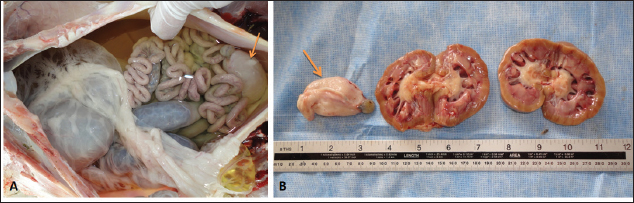
Fig. 10. Postmortem findings of a ram with ruptured bladder. Massive amounts of free urine were found in the abdominal cavity where viscera are floating (A). The urinary bladder was shrieked and collapsed (arrow). Image (B) shows bilateral hydronephrosis and the ruptured bladder arrow). ConclusionDiscolored urine in rams and bucks in this study resulted from hematuria due to urinary calculi and pelvic abscessation or from hemoglobinuria due to Babesia infection or due to copper toxicity. Hemolytic anemia was the outstanding hematological finding and hypoalbuminemia, hyperglobulinemia, increased BUN and creatinine, and hyperglycemia were the characteristic biochemical findings. Sonography of the urinary tract was very helpful in assessing the renal parenchyma, urinary bladder, and abdominal cavity for the verification of urolithiasis, hydronephrosis, intact or ruptured urinary bladder, uroperitoneum, and perforated urethra. Unfortunately, this study had a small number of cases, and therefore, it may be supported by another study with large numbers of rams and bucks with red urine syndrome. AcknowledgmentsThe authors would like to thank the Deanship of Scientific Research, Qassim University, for funding the publication of this project. Conflict of interestThe authors declare that there is no conflict of interest. Author ContributionsConceptualization, design, and practical work: MT, MT, and AAA; formal analysis and interpretation of data: MT and YH; writing-original draft preparation: MT; review and editing: YH and AAA. All authors revised and approved the final manuscript for publication. FundingPublication of this research was funded by the Deanship of Scientific Research, Qassim University, Saudi Arabia. Data availabilityAll data supporting the findings of this study are available within the manuscript and no additional data sources are required. ReferencesAbramowicz, B., Kurek, Ł., Chałabis-Mazurek, A. and Lutnicki, K. 2022. Changes to blood parameters after postparturient hemoglobinuria in 11 Holstein-Friesian cows. Vet. Clin. Pathol. 51, 101–106. Ali, A., Dear, R., Osman, S., Tharwat, M., Al-Sobayil, F. and Elshahed, M. 2019. Scrotal enlargement in rams and bucks in Qassim region, central of Saudi Arabia: clinical and ultrasonographic findings and seroprevalence of brucellosis. Trop. Anim. Health Prod. 51, 2109–2114. Ali, A., Derar, D., Al-Sobayil, F., Tharwat, M., Ahmed, F. and Khodeir, M. 2018. Adenocarcinoma in the genital tract of female dromedary camels. J. Camel Pract. Res. 25, 181–187. Alidadi, N., Bazargani, T.T. and Mashhadi, A. G. 2005. Lamb hemoglobinuria due to nutritional hypophosphatemia. Can. Vet. J. 46, 947–948. Almundarij, T. and Tharwat M. 2023. Impact of intestinal and urinary tracts obstruction on oxidative stress biomarkers in dromedary camels. Int. J. Vet. Sci. 12, 422–427. Al-Sobayil, F., Ali, A., Derar, D., Tharwat, M., Ahmed, F. and Khodeir, M. 2018. Tumors in dromedary camels: prevalence, types and locations. J. Camel Pract. Res. 25, 189–197. Aydın, N., Vatansever, Z. and Arslan, M.Ö. 2022. Molecular epidemiology of babesia and Theileria species in sheep in Kars Region of Turkey. Turkiye Parazitol. Derg. 46, 20–27. Bal, M.S., Mahajan, V., Filia, G., Kaur, P. and Singh, A. 2016. Diagnosis and management of bovine babesiosis outbreaks in cattle in Punjab state. Vet. World. 9, 1370–1374. Belli, C.B., Fernandes, W.R., Torres, L.N., Sucupira, M.C.A., de Sá, L.R.M, Maiorka, P.C., Neuenschwander, H.M., de Barros, A.M.C. and Baccarin, R.Y.A. 2021. Copper toxicity in horses: does it exist? J. Equine Vet. Sci. 106, 103752. Borobia, M., Villanueva-Saz, S., Ruiz de Arcaute, M., Fernández, A., Verde, M.T., González, J.M., Navarro, T., Benito, A.A., Arnal, J.L., De Las Heras, M. and Ortín, A. 2022. Copper poisoning, a deadly hazard for sheep. Animals 12, 2388. Bozynski, C.C., Evans, T.J., Kim, D.Y., Johnson, G.C., Hughes-Hanks, J.M., Mitchell, W.J., Rottinghaus, G.E., Perry, J. and Middleton, J.R. 2009. Copper toxicosis with hemolysis and hemoglobinuric nephrosis in three adult Boer goats. J. Vet. Diagn. Invest. 21, 395–400. Delvescovo, B. 2022. Discolored urine in horses and foals. Vet. Clin. North. Am. Equine Pract. 38, 57–71. Di Loria, A., Piantedosi, D., Cortese, L., Roperto, S., Urraro, C., Paciello, O., Guccione, J., Britti, D. and Ciaramella, P. 2012. Clotting profile in cattle showing chronic enzootic haematuria (CEH) and bladder neoplasms. Res. Vet. Sci. 93, 331–335. Duesterdieck-Zellmer, K.F. 2007. Equine urolithiasis. Vet. Clin. North Am. Equine Pract. 23, 613–629. Esmaeilnejad, B., Rajabi, S., Tavassoli, M., Rashnavadi, M., Seif, F., Aligolzadeh, A. and Khoshnejad, A. 2020. Evaluation of inflammatory biomarkers in goats naturally infected with Babesia ovis. Parasitol. Res. 119, 4151–4158. Galon, E.M., Ybañez, R.H., Macalanda, A.M., Estabillo, G.R., Montano, M.T.R., Veedor, M.D., Garvida, A., Fabon, R.J., Callanta, M.R., Labutong, K.J., Tumwebaze, M.A., Byamukama, B., Ji, S., Zafar, I., Ybañez, A. and Xuan, X. 2022a. First Molecular Identification of Babesia, Theileria, and Anaplasma in Goats from the Philippines. Pathogens 11, 1109. Galon, E.M., Zafar, I., Ji, S., Li, H., Ma, Z. and Xuan, X. 2022b. Molecular reports of ruminant Babesia in southeast Asia. Pathogens 11, 915. doi:10.3390/pathogens11080915. Grünberg, W. 2014. Treatment of phosphorus balance disorders. Vet. Clin. North Am. Food Anim. Pract. 30, 383–408. He, L., Bastos, R.G., Sun, Y., Hua, G., Guan, G., Zhao, J. and Suarez, C. E. 2021. Babesiosis as a potential threat for bovine production in China. Parasit. Vect. 14, 460. Jones, M.L., Dominguez, B.J. and Deveau, M.A. 2018. An experimental model for calcium carbonate urolithiasis in goats. J. Vet. Intern. Med. 32, 1268–1273. Kage, S., Mamatha, G.S., Lakkundi, J.N., Shivashankar, B.P. and D'Souza, P.E. 2019. Detection of incidence of Babesia spp. in sheep and goats by parasitological diagnostic techniques. J. Parasit. Dis. 43, 452-457. Kawahara, N., Ofuji, S., Abe, S., Tanaka, A., Uematsu, M. and Ogata, Y. 2016. Water intoxication in adult cattle. Jpn. J. Vet. Res. 64, 159–164. Mahmmod, Y. 2013. Natural Babesia bovis infection in water buffaloes (Bubalus bubalis) and crossbred cattle under field conditions in Egypt: a preliminary study. J. Arthropod Borne Dis. 8, 1–9. Mtshali, M.S. and Mtshali, P.S. 2013. Molecular diagnosis and phylogenetic analysis of Babesia bigemina and Babesia bovis hemoparasites from cattle in South Africa. BMC Vet. Res. 9, 154. Navarro, M.A., Dutra, F., Briano, C., Romero, A., Persiani, M., Freedman, J.C., Morrell, E., Beingesser, J. and Uzal, F.A. 2017. Pathology of naturally occurring bacillary hemoglobinuria in cattle. Vet. Pathol. 54, 457–466. Nwaokorie, E.E., Osborne, C.A., Lulich, J.P., Fletcher, T.F., Ulrich, L.K., Koehler, L.A. and Buettner, M.T. 2015. Risk factors for calcium carbonate urolithiasis in goats. J. Am. Vet. Med. Assoc. 247, 293–299. Oman, R.E., Reppert, E.J., Streeter, R.N. and Jones, M. 2019. Outcome and complications in goats treated by perineal urethrostomy for obstructive urolithiasis: 25 cases (2010–2017). J. Vet. Intern. Med. 33, 292–296. Randhawa, S.S., Sharma, D.K., Randhawa, C.S., Gill, B.S., Brar, R.S. and Singh, J. 1995. An outbreak of bacillary haemoglobinuria in sheep in India. Trop. Anim. Health Prod. 27, 31–36. Sadan, M., Tharwat, M. and El-Deeb W. 2023. Deep Swellings in sheep and goats: clinical, ultrasonographic and post-mortem findings. Int. J. Vet. Sci. 12, 793–801. Schumacher, J. 2007. Hematuria and pigmenturia of horses. Vet. Clin. North Am. Equine Pract. 23, 655–675. Scott, P. 2016. Practical use of ultrasound scan in small ruminant medicine and surgery. Vet. Clin. North Am. Food Anim. Pract. 32, 181–205. Shinozuka, Y., Yamato, O., Hossain, M.A., Higaki, T., Ishikawa, I., Ichiba, S. and Takagi, M. 2011. Bacillary hemoglobinuria in Japanese black cattle in Hiroshima, Japan: a case study. J. Vet. Med. Sci. 73, 255–258. Sickinger, M. 2019. Therapeutische Möglichkeiten bei obstruktiver Urolithiasis des kleinen Wiederkäuers [therapeutic options of obstructive urolithiasis in small ruminants]. Tierarztl Prax Ausg G Grosstiere Nutztiere. 47, 111–115. Takagi, M., Yamato, O., Sasaki, Y., Mukai, S., Fushimi, Y., Yoshida, T., Mizukami, K., Shoubudani, T., Amimoto, K., Chuma, T., Shahada, F., Endo, Y. and Deguchi, E. 2009. Successful treatment of bacillary hemoglobinuria in Japanese Black cows. J. Vet. Med. Sci. 71, 1105–1108. Tharwat M. 2021a. Obstructive urolithiasis in dromedary camels: clinical, ultrasonographic and postmortem findings. J. Camel Pract. Res. 28, 85–93. Tharwat, M. 2021b. Clinical, ultrasonographic and postmortem findings in sheep and goats with different urinary tract disorders. Vet. World 14, 1879–1887. Tharwat M. 2023. Changes in acid-base balance, blood gases and hemato-biochemical parameters in Arabian camels with different urinary tract disorders. Int. J. Vet. Sci. 12, 724–729. Tharwat, M. and Al-Hawa A. 2024a. Liver diseases in sheep and goats: parallel sonographic and pathologic findings. Int. J. Vet. Sci. 13, 284–290. Tharwat, M. and Al-Hawas, A. 2024b. Suppurative pyelonephritis in a caprine buck: clinical, laboratory, ultrasonographic and pathologic findings. Int. J. Vet. Sci. 13, 479–483. doi:10.47278/journal.ijvs/2023.097. Tharwat, M. and Al-Sobayil F. 2016. Ultrasonographic findings in camels (camelus dromedarius) with different urinary affections. J. Camel Pract. Res. 23, 301–308. Tharwat, M. and Al-Sobayil, F. 2017. Diagnostic ultrasonography in goats with contagious caprine pleuropneumonia caused by Mycoplasma capricolum subsp. Capripneumoniae. BMC Vet. Res. 13, 263. Tharwat, M., Al-Sobayil, F., Ali, A., Derar, D. and Khodeir M. 2017. Renal cell carcinoma in a female aranian camel: clinical, hematobiochemical, ultrasonographic and pathologic findings. J. Camel Pract. Res. 24, 61–66. Tharwat, M., Al-Sobayil, F., Hashad, M. and Buczinski, S. 2012. Transabdominal ultrasonographic findings in goats with paratuberculosis. Can. Vet. J. 53, 1063–1070. Tharwat, M., Sadan, M., El-Shafaey, E., Al-Hawas, A. and Saeed, EMA. 2018a. Unilateral nephrectomy in a female dromedary camel with pyelonephritis caused by Staphylococcus lugdunensis. Pak. Vet. J. 38, 116–118. Tharwat, M., Sadan, M., El-Shafaey, E.S., Saeed, E.H. and Al-Hawas, A. 2018b. Bilateral renal abscessation and chronic active pyelonephritis in a male camel (Camelus dromedarius) caused by Escherichia coli. J. Vet. Med. Sci. 80, 778–783. Ulucesme, M.C., Ozubek, S., Karoglu, A., Turk, Z.I., Olmus, I., Irehan, B. and Aktas, M. 2023. Small ruminant piroplasmosis: high prevalence of Babesia aktasi n. sp. in goats in Türkiye. Pathogens 12, 514. Videla, R. and van Amstel, S. 2016. Urolithiasis. Vet. Clin. North Am. Food Anim. Pract. 32, 687–700. Villanueva-Saz, S., Borobia, M., Fernández, A., Jiménez, C., Yzuel, A., Verde, M.T., Ramo, M.Á., Figueras, L. and Ruíz, H. 2022. Anaemia in sheep caused by Babesia and Theileria haemoparasites. Animals 12, 3341. | ||
| How to Cite this Article |
| Pubmed Style Tharwat M, Hegazy Y, Alkheraif AA. Discolored urine in sheep and goats: Clinical, etiological, hematobiochemical, sonographic and postmortem findings. Open Vet. J.. 2024; 14(4): 1059-1071. doi:10.5455/OVJ.2024.v14.i4.13 Web Style Tharwat M, Hegazy Y, Alkheraif AA. Discolored urine in sheep and goats: Clinical, etiological, hematobiochemical, sonographic and postmortem findings. https://www.openveterinaryjournal.com/?mno=186560 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i4.13 AMA (American Medical Association) Style Tharwat M, Hegazy Y, Alkheraif AA. Discolored urine in sheep and goats: Clinical, etiological, hematobiochemical, sonographic and postmortem findings. Open Vet. J.. 2024; 14(4): 1059-1071. doi:10.5455/OVJ.2024.v14.i4.13 Vancouver/ICMJE Style Tharwat M, Hegazy Y, Alkheraif AA. Discolored urine in sheep and goats: Clinical, etiological, hematobiochemical, sonographic and postmortem findings. Open Vet. J.. (2024), [cited January 25, 2026]; 14(4): 1059-1071. doi:10.5455/OVJ.2024.v14.i4.13 Harvard Style Tharwat, M., Hegazy, . Y. & Alkheraif, . A. A. (2024) Discolored urine in sheep and goats: Clinical, etiological, hematobiochemical, sonographic and postmortem findings. Open Vet. J., 14 (4), 1059-1071. doi:10.5455/OVJ.2024.v14.i4.13 Turabian Style Tharwat, Mohamed, Yamen Hegazy, and Abdulrahman A. Alkheraif. 2024. Discolored urine in sheep and goats: Clinical, etiological, hematobiochemical, sonographic and postmortem findings. Open Veterinary Journal, 14 (4), 1059-1071. doi:10.5455/OVJ.2024.v14.i4.13 Chicago Style Tharwat, Mohamed, Yamen Hegazy, and Abdulrahman A. Alkheraif. "Discolored urine in sheep and goats: Clinical, etiological, hematobiochemical, sonographic and postmortem findings." Open Veterinary Journal 14 (2024), 1059-1071. doi:10.5455/OVJ.2024.v14.i4.13 MLA (The Modern Language Association) Style Tharwat, Mohamed, Yamen Hegazy, and Abdulrahman A. Alkheraif. "Discolored urine in sheep and goats: Clinical, etiological, hematobiochemical, sonographic and postmortem findings." Open Veterinary Journal 14.4 (2024), 1059-1071. Print. doi:10.5455/OVJ.2024.v14.i4.13 APA (American Psychological Association) Style Tharwat, M., Hegazy, . Y. & Alkheraif, . A. A. (2024) Discolored urine in sheep and goats: Clinical, etiological, hematobiochemical, sonographic and postmortem findings. Open Veterinary Journal, 14 (4), 1059-1071. doi:10.5455/OVJ.2024.v14.i4.13 |