| Short Communication | ||
Open Vet. J.. 2024; 14(8): 2079-2084 Open Veterinary Journal, (2024), Vol. 14(8): 2079–2084 Short Communication Effect of GnRH treatment as a potential solution for ovarian disorders in dairy cows infected with foot and mouth disease in Indonesian smallholder farmsJoko Susilo1,2, Erif Maha Nugraha Setyawan1, Slamet Hartanto3, Michael Haryadi Wibowo4 and Agung Budiyanto1*1Department of Reproduction, Obstetrics, and Gynecology, Faculty of Veterinary Medicine, Gadjah Mada University, Yogyakarta, Indonesia 2Lampung Disease Investigation Center, Lampung, Indonesia 3Research Center for Sustainable Production System and Life Cycle Assessment, National Research and Innovation Agency (BRIN), Banten, Indonesia 4Department of Microbiology, Faculty of Veterinary Medicine, Gadjah Mada University, Yogyakarta *Corresponding Author: Agung Budiyanto. Department of Reproduction, Obstetrics, and Gynecology, Faculty of Veterinary Medicine, Gadjah Mada University, Yogyakarta, Indonesia. Email: budiyanto [at] ugm.ac.id Submitted: 22/02/2024 Accepted: 12/07/2024 Published: 31/08/2024 © 2024 Open Veterinary Journal
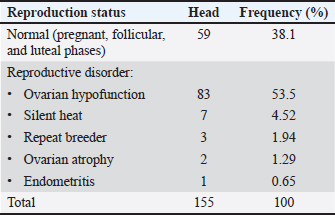
ABSTRACTBackground: The outbreak of foot and mouth disease (FMD) in Indonesia induces reproductive disorders in dairy cows that lead to economic losses to smallholder dairy farms. Aim: The study was to assess the influence of FMD on reproductive traits and evaluate the effect of gonadotropin hormone-releasing hormone (GnRH) administrations on the reproductive performance in FMD-infected dairy cows. Methods: The study was conducted in Jemowo village, Taman Sari sub-district, Boyolali district, Central Java, Indonesia. A total of 155 cows were used to identify the reproductive disorders on FMD-infected dairy cows aged 2–10 years old. Cows were raised in similar conditions and fed diets. A single dose of 2 ml GnRH was injected intramuscularly into 96 ovarian disorder cows. Reproductive performance was measured by service per conception (S/C), conception rate (CR), and pregnancy rate (PR). A descriptive study was conducted to demonstrate the results. Results: The study showed that 61.9% of FMD-infected cows had reproductive disorders, whereby 53.5% ovarian hypofunction, 4.52% silent heat, 1.94% repeat breeder, 1.29% ovarian atrophy, and 0.65% endometritis. FMD-infected cows injected with GnRH had a 98% reproductive recovery rate. Moreover, the S/C, CR, and PR of cows injected with GnRH were 2.02%, 51%, and 85%. Conclusion: GnRH administrations enhanced the reproductive traits of FMD-infected dairy cows indicated by the improvement of CR and PR. Keywords: Dairy cow, Foot and mouth disease, Gonadotropin-releasing hormone, Indonesia, Reproductive disorder. IntroductionFoot and mouth disease (FMD) is an acute and rapidly spreading disease that leads to significant financial losses (Grubman and Baxt, 2004). Attribute to the Apthovirus genus in the Picornaviridae family (MacLachlan and Dubovi, 2011), it presents an incubation period of 2–14 days, with mortality rates varying from 1% to 5% in adult cattle to surpassing 20% in calves (WOAH, 2022). Clinical symptoms of FMD encompass anorexia, fever, and lesions on the mouth, hooves, and udder (Knipe and Howely, 2001), along with diminished milk production (Adjid, 2020; Sudarsono, 2022). Moreover, FMD inhibits growth, reduces milk yield, impairs fertility, and elevates mortality in dairy cattle (Paton, 2018). FMD is classified as a strategic infectious animal disease in Indonesia (MARI, 2013). Although Indonesia attained FMD-free status in 1986, the disease re-emerged in 2022, swiftly spreading to 22 provinces and resulting in significant economic loss. The outbreak affected 570,137 cattle, with a morbidity rate of 1.04% and a mortality rate of 0.02%. Additionally, it necessitated the forced slaughter of 12,650 cattle by November 2022 (MARI, 2022). However, there is no data on the impact of FMD on reproductive performance, and no strategy for the recovery of reproductive performance in smallholder dairy cattle has been implemented in Indonesia to date. Moreover, gonadotropin-releasing hormone (GnRH) is a neuropeptide that plays a pivotal role in regulating vertebrate reproduction (Mohammadzadeh, 2019). Administration of GnRH induces the activation of GnRH receptors in the pituitary, leading to the release of LH and the initiation of luteinization (Schneider et al., 2006). Also, GnRH administration facilitates the luteinization process of the corpus luteum (Besbaci et al., 2020; López-Gatius and Garcia-Ispierto, 2020; García-Guerra et al., 2020) and enhances progesterone levels post-ovulation (Roth et al., 2021). These result in normal reproductive function in cows indicated by the estrus cycle within 18–21 days (Crowe et al., 2018). Based on the aforementioned, we hypothesized that GnRH administrations have potential effects to enhance the reproductive performance of FMD-infected dairy cows. Thus, the study was to assess the influence of FMD on the reproductive traits in dairy cows and to examine the impact of GnRH injections on the ovarian disorder of FMD-infected dairy cows. Materials and MethodsAnimals and locationAll subjects in the study were dairy cows that had been infected with foot-and-mouth disease (FMD) 1 month prior, aged from 2 to 10 years old, and owned by smallholder farmers in Jemowo village, Tamansari, Boyolali district, Central Java. All cows were reared under identical conditions. The research was performed from October 2022 to March 2023. Identification of reproductive disordersA total of 155 head dairy cows were used for the identification of ovarian disorders during and one month after FMD infection. The assessments were conducted through farmer interviews and rectal palpation. No signs of estrus behaviour as reported by farmers were used as indicators of potential reproductive disorders. Furthermore, no follicular activity in the ovaries detected by rectal palpation confirmed the irregular reproductive cycle. Experimental treatmentGnRH treatments were administered to 96 ovarian disorders FMD-infected dairy cows at 1 month after FMD infection with some cows showed recovery signs and others still displaying mild clinical symptoms. The GnRH was Gonasyl, a commercial product from Agroveta, Kalbe Co., Indonesia, containing gonadorelin (acetate) at a concentration of 50 mg/ml. All cows received a single intramuscular injection of 2 ml GnRH in accordance with the manufacturer’s instructions. Estrus detection and artificial inseminationEstrus signs were observed after the treatment (Fig. 1). Visual assessments were conducted to identify estrus signs, including the presence of a reddish and enlarged vulva, warm palpability, restlessness, engagement in mounting behavior with other cows, and remaining silent when mounted by others. Cows displaying signs of estrus were subjected to artificial insemination at the optimal time. Pregnancy evaluationThe assessment of pregnancy was performed at 60 days using ultrasound. Service per conception (S/C), conception rate (CR), and pregnancy rate (PR) were indicators to measure artificial insemination (AI) success. S/C was derived from the ratio of artificial inseminations to successfully pregnant cows. CR represented the proportion of cows achieving pregnancy with a single artificial insemination. PR was determined by dividing the number of pregnant cows by the total number of inseminated cows. Statistical analysisDescriptive analysis was used in this study. Data were presented in numbers and percentages. Ethical approvalAll protocols followed the ethical procedure approved by the Ethics Committee of the Faculty of Veterinary Medicine, Gadjah Mada University, Indonesia (71/EC-FKH/Int./2023). Results and DiscussionReproductive disorders of FMD-infected cowsThe present study showed that clinical symptoms of foot-and-mouth disease (FMD) in dairy cattle included fever, low appetite, lameness, hypersalivation, and the presence of lesions in the mouth, tongue, and hooves. Reproductive disorders were identified in 96 out of 155 (61.9 %) FMD-infected cows including ovarian hypofunction (53.5%), silent heat (4.52%), repeat breeder (1.94%), ovarian atrophy (1.29%), and endometritis (0.65%) (Table 1). Based on farmer interviews, all of the sampled cows had no these reproductive disorders before FMD infection. Thus, we suggest that FMD infection was the primary factor of reproductive disorders in cows in the present study. In this investigation, the prevalence of reproductive disorders was notably high. It is higher than previous studies, whereby the studies of Khan et al. (2016) and Hadush et al. (2016) found that FMD induces reproductive abnormalities in cattle with incident rates at 33.85% and 37.85%. Moreover, Tesfaye and Shamble (2013) discovered that 40.25% of cows have reproductive disorders attributed to the effects of FMD. Anoestrus emerged as the primary reproductive abnormality observed in FMD-infected cows in the present study. The incidence of anoestrus was 57.4% in FMD-infected cows with conditions such as ovarian hypofunction, silent heat, ovarian atrophy, and endometritis. Our result was higher than the finding of Sarder et al. (2010), which revealed that the prevalence of anoestrus crossbred dairy cows infected by FMD in Bangladesh is 20.4%. Similarly, Khan et al. (2016) reported the incidence of anoestrus dairy cows infected by FMD in India is 31.79%. Table 1. Reproductive status of FMD-infected cows.
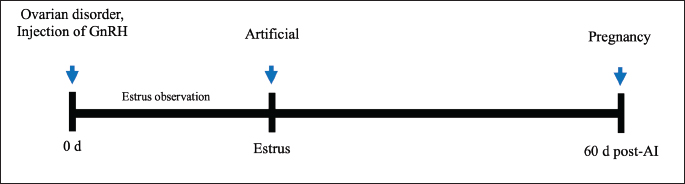
Fig. 1. Illustration of the study of GnRH injection on reproductive performance in FMD-infected cows.
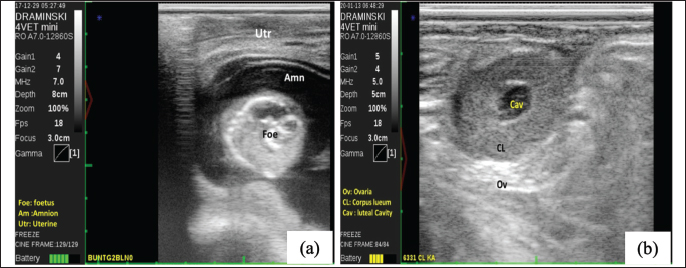
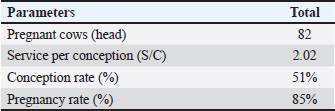
Fig. 2. Displaying pregnancy test using ultrasound (USG) at 60 days post-AI in FMD-infected cows (Foe: Foetus, Amn: amnion, Utr: Uterine) (a); depicting corpus luteum (CL) at normal oestrus cycle (Cav: luteal cavity, Ov: ovarium) (b). The prevalence of inactive ovaries (ovarian hypofunction and ovarian atrophy) was recorded at 54.84% in the present study. This incidence was higher than the research of Zaher and Ahmed (2008) conducted on FMD-affected buffaloes in Egypt, whereby the incidence of inactive ovaries was 31.89%. The inactive ovarian incidence in FMD-infected dairy cows was attributed to low nutritional intake during the infection. Low body condition scores and inadequate nutritional intake cause hypofunction and ovarian atrophy. Hermadi et al. (2017) found that ovarian atrophy was prolonged ovarian hypofunction. FMD reduces the appetite of cows because of the lesions in the mouth and tongue resulting in a negative energy balance (NEB). Furthermore, NEB leads to a decrease in the reproductive performance and the fertility of cows (Roche et al., 2017; Bisinotto et al., 2018; Đuričić et al., 2019). Likewise, Ahmed et al. (2006) found that NEB affected by inadequate nutritional intake caused inactive ovaries in FMD-affected Egyptian buffaloes. Effect of treatment on reproductive performance of FMD-infected cowsAdministrations of gonadotropin hormone-releasing hormone (GnRH) effectively induced estrus in all cows with ovarian hypofunction and silent heat in this study. A total of 94 heads of cows (97.9%) showed normal estrus after the treatment. However, estrus signs were not present in cows with ovarian atrophy. No prior research has documented the optimal timing for GnRH injection post-FMD infection. Therefore, our findings suggest that administering GnRH one month after FMD infection is an effective strategy for restoring reproductive function in cows. The pregnancy test was performed at 60 days post artificial insemination with ultrasound to show the fetus and amnion (Fig. 2). Our study revealed that the S/C in FMD-infected cows post-treatment was 2.02 (Table 2). Moreover, CR and PR were 51 % and 85 % in FMD-infected cows post-treatment. Table 2. Effect of GnRH administrations on reproductive performance of FMD-infected cows.
The present investigation showed that GnRH administration successfully triggers estrus activity, resulting in an 85% PR. Our study is in line with the finding of Burnett et al. (2019), where GnRH administration in low-estrus cows results in a 40.0% PR, compared to 30.8% in untreated cows. Likewise, Roth et al. (2021) reported that GnRH treatment during the dry season, especially in the fall, increases the PRs of cows. Moreover, the administration of 200-μg GnRH increases the ovulatory response, leading to a 54.6% PR in cows (Ariciniega et al., 2020). López-Gatius and Garcia-Ispierto (2020) demonstrated that GnRH treatment effectively maintains pregnancy in sub-fertile cows. Furthermore, Besbaci et al. (2020) documented GnRH-treated cows group has higher PRs than the untreated group. In addition, GnRH injection in Ovsynch protocol, combined with split-time artificial insemination, increases total estrus response and PRs in cows (Bishop et al., 2017). Thus, our study confirms the finding of Long et al. (2022) study, whereby GnRH administration is an effective method for managing cows with ovarian disorders. Furthermore, it contributes to the normalization of the ovarian cycle (Jeengar et al., 2014). GnRH treatment proves very effective for treating ovarian disorders in cows (Ari et al., 2017; Naglis, 2019). GnRH, a neuropeptide produced by the hypothalamus, is a preeminent regulator for physiological processes and reproductive functions in mammals (Mohammadzadeh, 2019). it stimulates the synthesis and release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary (Kadokawa et al., 2014). Moreover, GnRH also acts as a regulator of gonadotropin secretion and LH/FSH-releasing hormone (Hafez et al., 2000). It is well-documented that GnRH effectively induces estrus in cows, whereby its actions are facilitated by GnRH receptors in follicular and corpus luteum tissues (Ramakrishnappa et al., 2005). Exogenous GnRH injections activate pituitary GnRH receptors, triggering the release of LH (Schneider et al., 2006). Thus, we suggest that GnRH administration effectively induced normal estrus in FMD-infected cows by modulating LH secretion. The S/C of FMD-infected cows post-treatment in this study was 2.02. our finding was similar to the study of Pramono et al. (2010) found that the S/C of dairy cows in Yogyakarta is 1.98 ± 0.91. Similarly, Kusmayadi and Hadist (2023) discovered that S/C was 1.8 ± 0.85 for dairy cows in Pengalengan and Kertasari, West Java. Wirando et al. (2023) also recorded that the S/C of dairy cows in Banyuwangi, East Java is 2.15 ± 0.28. In contrast, Setyorini et al. (2022) reported that S/C is 1.4–1.7 for dairy cows in the Baturraden and Sleman regencies, Indonesia. Moreover, the present found that the CR of FMD-infected cows post-treatment was 51 %. It is better than the report of Yekti et al. (2019), whereby the CR of crossbred Ongole cows is 43.75%. ConclusionThe study revealed that 98% of FMD-infected cows had normal estrus after the injection of GnRH. The treatments resulted in a CR (51 %) and PR (85 %) in FMD-infected cows. Thus, the administration of GnRH effectively improves the reproductive performance of FMD-infected cows. However, a larger sample is necessary in further investigation to validate our results. AcknowledgmentsThe authors are thankful to the Ministry of Agriculture Republic Indonesia for funding the research and publication support. The authors thank drh. Hasan Abdullah Sanyata Head of Lampung Investigation Centre, drh. Enny Saswiyanti, M.Sc. for contribution to qRT-PCR assay and ELISA. In particular, the authors are grateful to the Head of the Microbiology Laboratory, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta along with the laboratory technical assistant team, Disease Investigation Center Lampung, for providing the necessary support during the study. FundingThis study was supported and funded by the Ministry of Agriculture, Indonesia. Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionsJS designed and conducted the research, analyzed the data, and wrote the manuscript. EMNS, MHW, and AB supervised the research. SH and AB reviewed and revised the manuscript. Data availabilityAll data are provided in the manuscript. ReferencesAdjid, R.M.A. 2020. Foot and mouth disease: an exotic animal disease that must be alert of entry into Indonesia. Wartazoa 30(2), 61–70. Ahmed, W.M., El-Khadrawy, H.H. and Abd El-Hamed, A.R. 2006. Applied investigations on ovarian inactivity in buffaloes. In the Proceeding of Third International Conference of Veterinary Research Division, pp:1–15. Ari, U.C., Pancarci, S.M., Kaçar, C., Güngör, O., Lehimcioğlu, N.C., Öztürkler, Y. and Yildiz, S. 2017. Effect of progestagen application during ovsynch protocol on pregnancy rates of lactating-grazing cows. Kafkas Univ. Vet. Fak. 23, 319–32. Ariciniega, T.V., Leao, I.M.R., Galvan, E.A., Cunha, T.O., El Azzi, M.S., Cook, N.B. and Martins, J.P.N. 2020. Effect of a high dose of gonadorelin hydrochloride at the first gonadotropin-releasing hormone of the breeding-Ovsynch of a fertility program on ovulation rate and pregnancies per AI in first-service lactating Holstein cows. J. Dairy Sci. 106(12), 9718–9732. Besbaci, M., Abdelli, A., Minviel, J.J., Belabdi, I., Kaidi, R. and Raboisson, D. 2020. Association of pregnancy per artificial insemination with gonadotropin-releasing hormone and human chorionic gonadotropin administered during the luteal phase after artificial insemination in dairy cows: a meta-analysis. J. Dairy Sci. 103, 2006–2018. Bishop, B.E., Thomas, J.M., Abel, J.M., Poock, S.E., Ellersieck, M.R., Smith, M.F and Patterson, D.J. 2017. Split-time artificial insemination in beef cattle: III. Comparing fixed-time artificial insemination to split-time artificial insemination with delayedadministration of GnRH in postpartum cows. Theriogenology 99, 48–52. Bisinotto, R.S., Greco, L.F., Ribeiro, E.S., Martinez, N., Lima, F.S., Staples, C.R., Thatcher, W.W. and Santos, J.E.P. 2018. Influences of nutrition and metabolism on fertility of dairy cows. Anim. Reprod. 9(3), 260–272. Burnett, T.A., Madureira, A.M., Bauer, J.W., Gomes, W.A. and Cerri, R.L. 2019. Interaction of estrus expression and progesterone on the impact of GnRH administration at the time of AI on pregnancy and ovulation rates. J. Dairy Sci. 102(1), 275. Crowe, M.A., Hostens, M. and Opsomer, G. 2018. Reproductive management in dairy cows—the future. Ir. Vet. J. 71(1), 1–13. Đuričić, D., Vince, S., Lojkić, M., Jelušić, S., Turk, R., Valpotić, H., Gračner, D., Maćešić, N., Folnožić, I., Šostar, Z. and Samardžija, M. 2019. Effects of dietary clinoptilolite on reproductive performance, serum progesterone and insulin-like growth factor-1 concentrations in dairy cows during pregnancy and lactation. Pol. J. Vet. Sci. 23(1), 69–75. García-Guerra, A., Sala, RV., Carrenho-Sala, L., Baez, G.M., Motta, J.C.L., Fosado, M., Moreno, J.F. and Wiltbank, M.C. 2020. Postovulatory treatment with GnRH on day 5 reduces pregnancy loss in recipients receiving an in vitro produced expanded blastocyst. Theriogenology 141, 202–210. Grubman, M.J. and Baxt, B. 2004. Foot-and-mouth disease. Clin. Microbiol. Rev. 17(2), 465–93. Hadush, A., Abdella, A. and Regassa, F. 2016. Major prepartum and postpartum reproductive problems of dairy cattle in Central Ethiopia. J. Vet. Med. Anim. Health. 5(4), 118–123. Hafez, E.S.E., Jainudeen, M.R. and Rosnina, Y. 2000. Hormones, Growth Factors, and Reproduction. In Reproduction in Farm Animals. Eds., Hafez, B. and Hafez, E.S.E. Philadelphia, MD: Lippincott Williams & Wilkins, pp: 31–54. Hermadi, H.A., Mas’ud, H. and Suherni, S. 2017. The ovarian hypofunction: a case in cow management therapy. In the Proceeding of the 1st International Conference in One Health (ICOH 2017), pp: 311–316. Jeengar, K., Chaudhary, V., Kumar, A.V., Raiya, S.A., Gaur, M.S. and Purohit, G.N. 2014. Ovarian cysts in dairy cows: old and new concepts for definition, diagnosis and therapy. Anim. Reprod. 11, 63–73. Kadokawa, H., Pandey, K., Nahar, A., Nakamura, U. and Rudolf, F.O. 2014. Gonadotropin-releasing hormone (GnRH) receptors of cattle aggregate on the surface of gonadotrophs and are increased by elevated GnRH concentrations. Anim. Reprod. Sci. 150(3-4), 84–95. Khan, M.H., Manoj, K. and Pramod, S. 2016. Reproductive disorders in dairy cattle under semi-intensive system of rearing in North-Eastern India. Vet. World 9(5), 512–8. Knipe, D.A. and Howely, D.M. 2001. Fields Virology. Philadelphia, PA: Welter Kluwer Health. Kusmayadi, T. and Hadist,I. 2023. Milk production and reproductive performance of Holstein Friesian dairy cattle in the working area of South Bandung Pangalengan animal husbandry cooperative (KPBS) Bandung Regency. Bull. Anim. Sci. 47(1), 1–6. Long, S.T., Toan, N.C., Gioi, P.V. and Hang, P.T. 2022. Factors associated with the odds of pregnancy for dairy cattle after treatment of ovarian disorders in Northern Vietnam. Trop. Anim. Sci. J. 45(3), 277–283. López-Gatius, F. and Garcia-Ispierto, I. 2020. Treatment with an elevated dose of the GnRH analogue dephereline in the early luteal phase improves pregnancy rates in repeat-breeder dairy cows. Theriogenology 155, 12–6. MacLachlan, J. and Dubovi, E.J. 2011. Epidemiology and control of viral diseases. In: Fenner’s Veterinary Virology. Eds., MacLachlan, J. and Dubovi, E.J. London, UK: Elsevier Inc, pp: 125–148. Ministry of Agriculture of the Republic of Indonesia (MARI). 2013. Peraturan Nomor 51/Permentan/OT.140/5/2013 tentang Pelarangan Pemasukan Media Pembawa Penyakit Mulut dan Kuku dari Negara Republik Rakyat China ke Dalam Wilayah Negara Republik Indonesia. Jakarta, Indonesia: Ministry of Agriculture of the Republic of Indonesia. Ministry of Agriculture of the Republic of Indonesia (MARI). 2022. FMD Outbreak Management and Prevention Information. Available via https://siagapmk.crisis-center.id/index.php (Accessed 26 March 2023). Mohammadzadeh, M. 2019. The impacts of eCG administration, 3 days before Ovsynch on the treatment of inactive ovary of dairy cows. Rev. Med. Vet. 170(4-6), 110–116. Naglis, G. 2019. Prevalence, diagnostics and treatment of ovarian follicular cysts in dairy cows. Trakia J. Sci. 4, 353–357. Paton, D.J., Gubbins, S. and King, D.P. 2018. Understanding the transmission of foot-and-mouth disease virus at different scales. Curr. Opin. Virol. 28, 85–91. Pramono, A., Kustono and Hartadi, H. 2010. Reproductive performance of dairy cows in Yogyakarta province based on balanced ration given. In the Proceeding of the 5th International Seminar on Tropical Animal Production Community Empowerment and Tropical Animal Industry, pp: 535–540. Ramakrishnappa, N., Rajamahendran, R., Lin, Y.M. and Leung, P.C. 2005. GnRH in non-hypothalamic reproductive tissues. Anim. Reprod. Sci. 88(1-2), 95–113. Roche, J.R., Burke, C.R., Crookenden, M.A., Heiser, A., Loor, J.L., Meier, S., Mitchell, M.D., Phyn, C.V.C. and Turner, S.A. 2017. Fertility and the transition dairy cow. Reprod. Fertil. Dev. 30(1), 85–100. Roth, Z., Kressel, Y.Z., Lavon, Y., Kalo, D. and Wolfenson, D. 2021. Administration of GnRH at onset of estrus, determined by automatic activity monitoring, to improve dairy cow fertility during the summer and autumn. Animals 11(8), 2194. Sarder, M.J.U., Moni, M.I.Z. and Aktar, S. 2010. Prevalence of reproductive disorder of crossbreed cows in the Rajshahi District of Bangladesh. SAARC J. Agric. 8(2), 65–75. Schneider, F., Tomek, W. and Gründker, C. 2006. Gonadotropin-releasing hormone (GnRH) and its natural analogues: a review. Theriogenology 66(4), 691–709. Setyorini, Y.W., Kurnianto, E. and Sutopo, S. 2022. Estimation of genetic superiority and reproductive performance of dairy cows at different rearing locations. J. Sain. Peternakan. Indonesia. 17(3), 134–140. Sudarsono, R.P.E. 2022. Epidemiological study of suspected occurrence of foot and mouth disease in Lamongan Regency. J. Basic Med. Vet. 11(1), 56–63. Tesfaye, D. and Shamble, A. 2013. Reproductive health problems of cows under different management systems in Kombolcha, Northeast Ethiopia. Adv. Biomed. Res. 7, 104–108. Wirando, Doloksaribu, L., Dewantari, M., Kayana, I.G.N. and Mahardika, I.G. 2023. Perfomance of Frisian Holstein cows at Sumberbulu Dairy Farm in Banyuwangi East Java. Majalah Imiah Peternakan 26(1), 37–48. World Organisation of Animal Health (WOAH). 2022. Foot and mouth disease. Available via https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.01.08_FMD.pdf (Accessed 12 November 2023). Yekti, A.P.N., Octaviani, E.A., Kuswati and Susilawati, T. 2019. Increasing of conception rate with artificial insemination using double dose sexing semen on Ongole crossbred cow. JTAPRO 20(2), 135–140. Zaher, K.S. and Ahmed, W.M. 2008. Impact of foot and mouth disease on oxidative status and ovarian activity in Egyptian buffaloes. World J. Zool. 3(1), 01–07. | ||
| How to Cite this Article |
| Pubmed Style Susilo J, Setyawan EMN, Hartanto S, Wibowo MH, Budiyanto A. Effect of GnRH treatment as a potential solution for ovarian disorders in dairy cows infected with foot and mouth disease in Indonesian smallholder farms. Open Vet. J.. 2024; 14(8): 2079-2084. doi:10.5455/OVJ.2024.v14.i8.37 Web Style Susilo J, Setyawan EMN, Hartanto S, Wibowo MH, Budiyanto A. Effect of GnRH treatment as a potential solution for ovarian disorders in dairy cows infected with foot and mouth disease in Indonesian smallholder farms. https://www.openveterinaryjournal.com/?mno=191871 [Access: October 20, 2025]. doi:10.5455/OVJ.2024.v14.i8.37 AMA (American Medical Association) Style Susilo J, Setyawan EMN, Hartanto S, Wibowo MH, Budiyanto A. Effect of GnRH treatment as a potential solution for ovarian disorders in dairy cows infected with foot and mouth disease in Indonesian smallholder farms. Open Vet. J.. 2024; 14(8): 2079-2084. doi:10.5455/OVJ.2024.v14.i8.37 Vancouver/ICMJE Style Susilo J, Setyawan EMN, Hartanto S, Wibowo MH, Budiyanto A. Effect of GnRH treatment as a potential solution for ovarian disorders in dairy cows infected with foot and mouth disease in Indonesian smallholder farms. Open Vet. J.. (2024), [cited October 20, 2025]; 14(8): 2079-2084. doi:10.5455/OVJ.2024.v14.i8.37 Harvard Style Susilo, J., Setyawan, . E. M. N., Hartanto, . S., Wibowo, . M. H. & Budiyanto, . A. (2024) Effect of GnRH treatment as a potential solution for ovarian disorders in dairy cows infected with foot and mouth disease in Indonesian smallholder farms. Open Vet. J., 14 (8), 2079-2084. doi:10.5455/OVJ.2024.v14.i8.37 Turabian Style Susilo, Joko, Erif Maha Nugraha Setyawan, Slamet Hartanto, Michael Haryadi Wibowo, and Agung Budiyanto. 2024. Effect of GnRH treatment as a potential solution for ovarian disorders in dairy cows infected with foot and mouth disease in Indonesian smallholder farms. Open Veterinary Journal, 14 (8), 2079-2084. doi:10.5455/OVJ.2024.v14.i8.37 Chicago Style Susilo, Joko, Erif Maha Nugraha Setyawan, Slamet Hartanto, Michael Haryadi Wibowo, and Agung Budiyanto. "Effect of GnRH treatment as a potential solution for ovarian disorders in dairy cows infected with foot and mouth disease in Indonesian smallholder farms." Open Veterinary Journal 14 (2024), 2079-2084. doi:10.5455/OVJ.2024.v14.i8.37 MLA (The Modern Language Association) Style Susilo, Joko, Erif Maha Nugraha Setyawan, Slamet Hartanto, Michael Haryadi Wibowo, and Agung Budiyanto. "Effect of GnRH treatment as a potential solution for ovarian disorders in dairy cows infected with foot and mouth disease in Indonesian smallholder farms." Open Veterinary Journal 14.8 (2024), 2079-2084. Print. doi:10.5455/OVJ.2024.v14.i8.37 APA (American Psychological Association) Style Susilo, J., Setyawan, . E. M. N., Hartanto, . S., Wibowo, . M. H. & Budiyanto, . A. (2024) Effect of GnRH treatment as a potential solution for ovarian disorders in dairy cows infected with foot and mouth disease in Indonesian smallholder farms. Open Veterinary Journal, 14 (8), 2079-2084. doi:10.5455/OVJ.2024.v14.i8.37 |