| Research Article | ||
Open Vet. J.. 2024; 14(7): 1553-1560 Open Veterinary Journal, (2024), Vol. 14(7): 1553–1560 Research Article Arterial and venous supply of the Harderian gland in hensPamela Bejdić1*, Liljana Amidžić2 and Benjamin Čengić31Department of Basic Science of Veterinary Medicine, University of Sarajevo-Veterinary Faculty, Sarajevo, Bosnia and Herzegovina 2Department of Human Genetics, Faculty of Medicine, University of Banja Luka, Banja Luka, Bosnia and Herzegovina 3Department for Clinical Sciences of Veterinary Medicine, University of Sarajevo-Veterinary Faculty, Sarajevo, Bosnia and Herzegovina *Corresponding Author: Pamela Bejdić. Department of Basic Science of Veterinary Medicine, University of Sarajevo-Veterinary Faculty, Sarajevo, Bosnia and Herzegovina. Email: pamela.bejdic [at] vfs.unsa.ba Submitted: 04/03/2024 Accepted: 19/06/2024 Published: 31/07/2024 © 2024 Open Veterinary Journal
AbstractBackground: The Harderian gland in domestic birds is a major paraocular excretory gland that has an important role in tear production as well as in the immune protection of the conjunctiva surface. Aim: The aim of this research was to investigate the arterial and venous supply of the gland in hens and provide valuable and useful information for future research. Methods: The research was conducted on 26 adult hens, provenience of Lohmann Brown. For the identification and determination of blood vessels, we used the vascular corrosion cast technique in conjunction with the transmission electron microscope (TEM). Results: The casts showed that the gland receives the arterial supply via branches of a. ophthalmotemporalis and a. nasalis communis and these arteries are accompanied by the corresponding veins. Ultrastructural analyses showed the presence of fenestrated capillaries, which indicates the possibility for permeability of larger molecules. Conclusion: The present research gives important and detailed information about the arterial and venous supply of the Harderian gland in hens that may serve as guidelines for future vascular and morphological investigations. Keywords: Birds, Harderian gland, Vascularization, Arteries, Vein. IntroductionThe Harderian gland was first described by Johann Jacob Harder in 1694 who identified it in deer and described it as a large intraorbital structure that probably moistens the surface of the eye (Payne, 1994; Olcese and Wesche, 1989). Since then, numerous studies have been conducted with the aim of describing the morphology and function of this gland in various animal species (Burns and Maxwell, 1979; Buzzell, 1996; Mobini, 2012; Dubey et al., 2014; Domínguez-Pérez, 2019; Beheiry, 2020; Mahmoud et al., 2022; Oliveira et al., 2023). The morphology of this gland has been described in amphibians, reptiles, birds, and mammals (Payne, 1994; Olcese and Wesche, 1989). The gland has not been found in fish, aquatic urodele amphibia (except crocodiles and cetaceans), and in some mammals such as bats, cows and horses, terrestrial carnivores, and higher primates (Payne, 1994; Mahmoud et al., 2022). The function of the gland is quite variable among the animal species. At first, it is considered that its main function is to lubricate the surface of the conjunctiva, but besides this simple role, recent research shows that depending on the animal species, the gland also acts as an organ of immune responses, a source of thermoregulatory lipids, a source of pheromones, as a photoprotective organ, accessory salivary gland and as a part of olfactory (Vomeronasal) and chemosensory organ (Payne, 1994; Domínguez-Pérez, 2019; Mahmoud et al., 2022; Oliveira et al., 2023). In domestic birds, the Harderian gland has been described as a major exocrine paraocular gland, situated behind the eyeball, in the ventral and posterior-medial part of the orbit (Wight et al., 1971, Burns and Maxwell, 1979; Mobini, 2012; Bejdić et al., 2014). Its function is that together with the lacrimal gland lubricates, moistens, and cleans the surface of the conjunctiva. In addition to this exocrine role, in domestic birds, the Harderian gland is also an important part of the immune barrier CALT-Conjunctiva-associated lymphoid tissue (Shirama et al., 1996; Ohshima and Hiramatsu, 2002, Khan, 2007; Davison et al., 2008; Bejdić et al., 2018). Although numerous anatomical, histological, histochemical, and ultrastructural studies have been conducted the data about vascularization of the gland are still very scarce. In one of the first reports, it is mentioned the gland in fowls is supplied by a. ophthalmotemporalis, which is the branch of the external ophthalmic artery, while venous drainage goes via the ophthalmic vein (Wight et al., 1971). However, the arterial and venous supply of the gland was not presented in detail in this as well as in recently conducted research on cephalic and intraorbital vascularization in chickens (Sedlmayr, 2002; Porter and Witmer, 2016). Bearing in mind that the Harderian gland plays an important role in tear production as well as in the immune system of birds, the main aim of this study was to investigate its arterial and venous supply and provide valuable and useful information for future research. Materials and MethodsAnimalsThe research was conducted on adult laying hens, provenience of Lohmann Brown. The hens were hatched on a local poultry farm in standard laying cages. At the end of production cycles, when the animals reached 18 months of age, the flock was excluded from production and sent to the slaughterhouse. Here, we randomly selected the 26 dead birds and transported them to the Department of Basic Veterinary Science, University of Sarajevo-Veterinary Faculty where the research was conducted. Corrosive cast techniqueFor identification and determination of arterial and venous supply of the Harderian gland, we applied the vascular corrosive cast technique according to Tompsett (1956). Ten birds were used for the investigation of arterial blood supply, ten for venous supply, and six for ultrastructural characteristics of blood vessels. To obtain the cast of the blood vessels, we use an 18% solution of Vinylite plastic mass, dissolved in absolute acetone (p.a). Half of this mass was colored red (Biodur Paste Rot, AC50), and the other half in blue color (Biodur Farbpaste Blau, AC52). The red-colored Vinylite was used for the injection of a. carrotis communis, while the blue was used for the injection of v. jugularis. The Vinylite mass was injected into the blood vessels with a plastic syringe until the maximum resistance was reached. All samples were then immersed in cold water for 24 hours, to complete the curing process of the Vinylite. Later, five bird heads injected with red and five injected with blue-colored Vinylete were dissection in situ, and the other half of the birds from the selected group were transferred into the macerator (Schaerer, Weber-Bern), where the water temperature was maintained at a constant level of 37°C. After two weeks macerated samples were washed under a gentle stream of tap water and immersed in 6% hydrogen peroxide solution for 48 hours. In this way, we obtained a corroded cast of arterial and venous blood vessels with preserved bones of the cranium. Macerated, corroded, and dissected in situ samples were observed and photographed under a magnifying glass. For better visualization of arterial and venous blood vessels that supply the Harderian gland, on macerated samples, we removed with scissors the vessels that supply the eye bulb. Transmission electron microscopy (TEM)For the ultrastructural part of the research, the complete gland from six birds was dissected and placed in the 2.5% glutaraldehyde (Serva, Heidelberg, Germany) dissolved in 0.085 M sodium cacodylate buffer (pH 7.4). After 24 hours the samples were washed in phosphate-buffered saline (PBS) (Karnovsky, 1965) and transported to the Laboratory of Electron Microscopy, Department of Pathology, Clinical Center of the University of Banja Luka for further ultrastructural preparation and examination. The glandular tissue was prepared for semi-thin (0.6–0.9 µm) and ultra-thin (0.06–0.09 µm) sections by standard procedure for ultrastructural microscopy based on the sample <1 mm3. From each animal, three ultrathin sections were made. The post-fixation was performed in 1% osmium tetroxide (Serva, Heidelberg, Germany) for one hour. Semi-thin sections were stained with 1% toluidine blue (Merck, Darmstadt, Germany) to identify desirable areas for further analysis. The ultra-thin section was mounted on copper grids (Agar Scientific Ltd., Cambridge, UK) and stained with a 2% water solution of uranyl acetate (Merck, Darmstadt, Germany) and lead citrate prepared by Reynold's procedure All samples were analyzed under the TEM - Option 9 S2 (Germany). Ethical approvalThe research is following the legislative, Official Journal B&H No. 34/02. of November 22. The research material is obtained from the slaughterhouse so additional ethical approvement is not needed for this experiment. ResultsThe Harderian gland is situated behind the eyeball in the rostroventral part of the orbit. Its anterior part lies near the medial margin of the orbit, while the posterior part reaches the point where n. opticus penetrates the eyeball. The casts show that the gland receives the arterial supply via two arterial branches. One of them emerges from a. ophthalmotemporalis, and the second from a. nasalis communis (Figs. 1 and 2). The branch that originates from the a. ophthalmotemporalis, emerges from the anastomose connection of this artery with the a. ethmoidea. After separation, this arterial branch goes in the naso-dorsal direction and near the central narrowing of the gland gives two smaller branches. One of them goes subcapsularly towards the upper margin of the gland, while the second branch goes towards its lower margin. These arteries give many smaller branches that follow the capsular septa and by them pass into the deeper part of the glandular tissue. The artery that separates from a. nasalis communis supplies the anterior part of the gland and its main duct. Above the anterior part of the gland, this artery gives two branches. The one, shorter pierces the connective tissue capsule of the gland and goes in the posterior direction, following the upper margin of the gland, while the second, longer branch goes along the primary excretory duct and follows it until it opens at the base of the third eyelid. Above the gland a. nasalis communis anastomose with a. ethmoidalis.
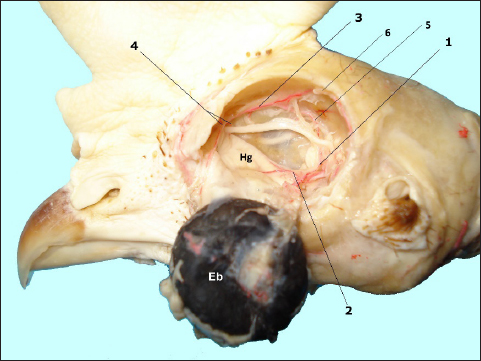
Fig. 1. In situ dissected the Harderian gland (Hg) with corroded arterial blood vessels showing: enucleated eye bulb (Eb), a. ophtalmotemporalis (1) and its branch that supply the gland (2), a. nasalis communis (3) and its two branches (4), a. ethmoidalis (5) and a. supraorbitalis (6).
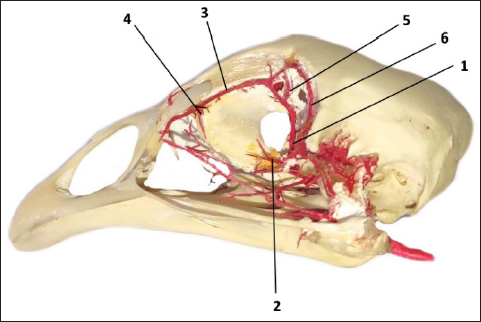
Fig. 2. The arterial vascular cast sowing: a. ophtalmotemporalis (1) and its branch that supply the Harderian gland (2), a. nasalis communis (3) and its two branches (4) for the gland and its primary duct, a. ethmoidalis (5) and a. supraorbitalis (6).
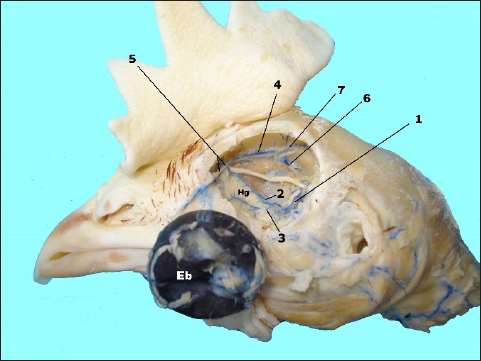
Fig. 3. In situ dissected the Harderian gland (Hg) with corroded venous blood vessels showing: enucleated eye bulb (Eb), v. ophtalmotemporalis (1) and its two branches that supply the gland (2 and 3), v. nasalis communis (4) and its two branches (5), v. ethmoidalis (6) and v. supraorbitalis (7).
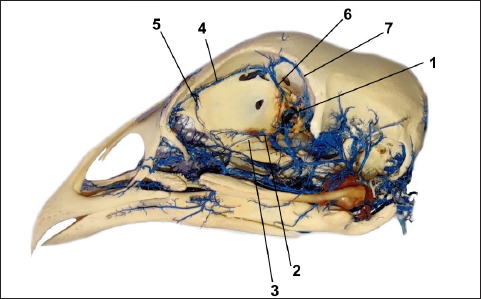
Fig. 4. The vein vascular cast sowing: v. ophtalmotemporalis (1) and its two branches that supply the Harderian gland (2), v. nasalis communis (3) and its two branches (4) for the gland and its primary duct, v. ethmoidalis (5) and v. supraorbitalis (6). The arteries are accompanied by the corresponding veins, with the difference that the veins are numerous (Figs. 3 and 4). Two of them drain the main part of the gland and these veins are visible as they pass the subcapsular, one along the upper and the second along the lower margin of the gland. Both veins emerge from the glandular tissue at the level of its central narrowing and enter separately into the v. ophthalmotemporalis. The anterior part of the gland and its main duct is drained by one vein, which gives two branches. One of the branches drains the primary excretory duct following its course, while the second much shorter branch drains the anterior part of the gland. Before entering into the common nasal vein, both branches join.
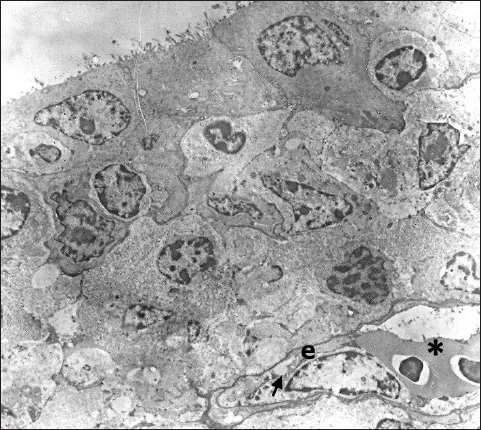
Fig. 5. In the subepithelial region of the primary duct of the Harderian gland is visible one capillary containing two erythrocytes (*). The capillary endothelium (e) is thin and poses fenestra (arrow) TEM; 2880X. Ultrastructural analysis shows that the tissue of the Harderian gland has capillaries with a thin endothelium and occasionally visible fenestrae (Fig. 5). The fenestrated capillaries are identified in the subepithelial region, as well as in the deeper part of the glandular tissue, where they come into close contact with lymphocytes and plasma cells (Figs. 6 and 7). DiscussionDifferent research shows that the structure of the Harderian gland and its function is quite variable among animal species (Olcese and Wesche, 1989; Payne, 1994; Domínguez-Pérez, 2019; Mahmoud et al., 2022; Oliveira et al., 2023). However, the studies regarding the blood supply of this gland still are very limited in all species. In fowls and sparrows (Passer domesticus), it is reported that the Harderian gland receives the arterial supply via the ophthalmotemporal branch of the external ophthalmic artery, while venous blood is collected and drained by the ophthalmic vain (Wight et al., 1971; Payne, 1994). In Nomina Anatomica Avium, these blood vessels are named as aa. and vv. glandulae membranae nictitantis (Baumel et al., 1993). The results of our research partially coincide with these findings. By careful analysis of arterial vascular casts, we identified the one branch that separates from the a. ophthalmotemporalis, but besides this, we also found the second artery that comes from the a. nasalis communis. The branch that separates from the common nasal artery supplies the anterior part of the gland and its primary duct and it is not mentioned in Nomina Anatomica Avium or any previous research (Richards, 1967, 1968; Wight et al., 1971; Baumel et al., 1993; Payne, 1994). Following the course of venous blood vessels, unlike Wight et al. (1971), we identified three veins that drain the gland. Two of them drain the main part of the gland and both inflows separately into the v. ophthalmoteporalis, while the third, enters into the v. nasalis communis. As for arteries, we did not find any data in the present literature regarding the drainage of the gland by the branches of the common nasal vein. In a recently conducted study of cephalic vascularization in turkey and turkey vulture birds, the main intraorbital blood vessels are shown in figures, but the arteries and veins that supply the Harderian gland are not labeled or described in the manuscript (Arad et al., 1989; Porter and Witmer, 2016). Comparing the vascular pattern of this research with our findings we can say that the vascularisation of the gland is very similar between these bird species. Also, comparing the methodology of the research, we can say that both, the corrosive cast technique and CT scanning with the use of contrast media can be equally useful for the display and general study of the distribution of blood vessels, with the fact that CT scanning is practically more convenient and easier to perform. On the other side for fine, ultrastructural research of blood vessels, the use of TEM is still irreplaceable. Ultrastructural research of the Harderian gland conducted on small mammals (common tree shrew and hamster) shows that the gland possesses two capillary types, small fenestrated and irregular sinusoidal (Pradidarcheep et al., 2003; Menendez-Pelaez et al., 2012). Our research by TEM shows that the gland in hens possesses only fenestrated capillaries and this confirms the previous findings of Wight et al. (1971).
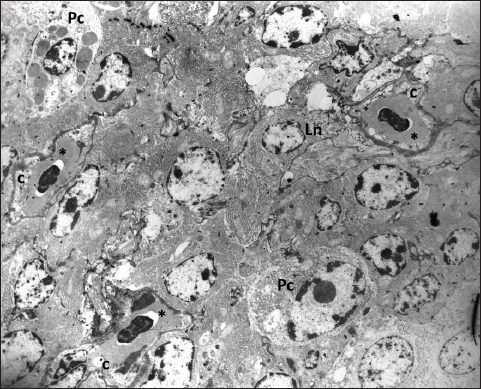
Fig. 6. The fenestrated capillaries (c) containing erythrocytes are present in the stroma of the gland where they are surrounded by lymphocytes (Ln) and mature plasma cells (Pc); TEM, 3000X.
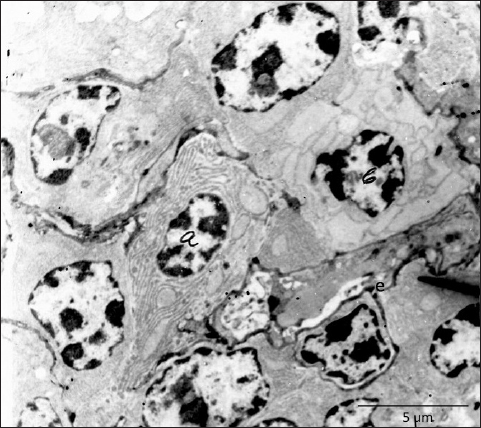
Fig. 7. Near the endothelium of fenestrated capillary (e) are visible two plasma cells, one with stacked laminae of rough endoplasmic reticulum (a) and one with highly dilated cisternae (b), TEM, 3660X. In conclusion, we can say that the present research demonstrates a detailed arterial and venous supply of the Harderian gland in hens and provides useful information that may serve as guidelines for future vascular and morphological investigations. The ultrastructural findings of fenestrated capillaries indicate that this gland has the possibility for permeability of larger molecules, and this remains a challenge for further research. AcknowledgmentsThe research was supported by Lush Prize 2022 and the authors appreciate their help and support. Conflict of interestThe authors certify that there are no financial and personal relationships or conflicts of interest with other people or organizations with the content of the present manuscript. FundingThe research was funded by the Lush Prize 2022. Authors' contributionsPamela Bejdić created and performed the main part of the experimental research. Ljiljana Amidžić prepared and worked on the interpretation of the results of the material for TEM. Benjamin Čengić prepared the photographs of the corrosive cast as well as the final preparation of the manuscript. Data availabilityAll data are provided in the manuscript. ReferencesArad, Z., Midtgard, U. and Bernstein M.H. 1989. Thermoregulation in turkey vultures vascular anatomy, arteriovenous heat exchange, and behavior. Condor. 91, 505–514. Baumel, J., King, A.S., Breazile, J.E., Evans, H.E. and Vanden Berge, J.C. 1993. Handbook of avian anatomy: nomina anatomica avium, 2nd ed. Cambridge, MA: Nuttall Ornithological Club. Systema Cardiovasculare, pp: 407–476. Beheiry, R.R., Ali, S.A., Aref, M. and Emam, H. 2020. Harderian gland of flying and non-flying birds: morphological, histological, and histochemical studies. J. Basic Appl. Zool. 81, 35. Bejdić, P., Avdić, R., Amidzić, L., Ćutahija, V., Tandir, F. and Hadžiomerović N. 2014. Developmental changes of lymphoid tissue in the Harderian gland of laying hens. Macedonian Vet. Rev. 37, 83–88. Bejdić, P., Avdić, R., Amidžić, Lj., Ćutahija, V., Tandir, F, Hadžiomerović, N., Katica A. and Mlaćo, N. 2018. Ultrastructure of plasma cells in Harderian gland of laying hens. Anat. Histol. Embryol. 47(1), 46–50. Burns, R.B. and Maxwell, M.H. 1979. The structure of the Harderian and lacrimal gland ducts of the turkey, fowl and duck. A light microscope study. J. Anatom. 128(2), 285–292. Buzzell, G.R. 1996. The Harderian gland: perspectives. Microscopy Res. Tech. 34(1), 2–5. Davison, F., Kaspers, B. and Schat, K.A. 2008. Avian immunology. Elsevier Press Ltd.: Amsterdam, Boston, Heidelberg, London, New York, Oxford, Paris, San Diego, San Francisco, Syngapore, Sydeney, Tokyo, pp: 38–39. Domínguez-Pérez, D., Durban, J., Agüero-Chapin, G., López, J.T., Molina-Ruiz, R., Almeida, D., Calvete, J.J., Vasconcelos, V. and Antunes, A. 2019. The Harderian gland transcriptomes of Caraiba andreae, Cubophis cantherigerus and Tretanorhinus variabilis, three colubroid snakes from Cuba. Genomics. 111(6), 1720–1727. Dubey, S., Verma, R. and Haldar, C. 2014. Electron microscopic study of Harderian gland of tropical bird Indian jungle bush quail perdicula asiatica. J. Endocrinol. Reprod. 18, 41–46. Karnovsky, M.J. 1965. A Formaldehyde-Glutaraldehyde Fixative of High Osmolality for Use in Electron Microscopy. J. Cell Biol. 27, 137–138A. Khan, M.Z.I., Jahan, M.R., Islam, M.N., Haque, Z., Islam, M.R. and Kon, Y. 2007. Immunoglobulin (Ig)-containing plasma cells in Harderian gland in broiler and native chickens of Bangladesh. Tissue Cell. 39(3), 141–149. Mahmoud, F.A., Gaber, S.A., Mahmoud, A.S. and Gamal, A. 2022. Anatomical, scanning electron microscopy, histological and histochemical studies of the orbital glands of the Egyptian agama Trapelus mutabilis. J. Basic Appl. Zool. 83(1), 1–9. Menendez-Pelaez, A., Tolivia, D., Rodriguez-Colunga, M.J. and Reiter, R.J. 1990. Ultrastructure of the blood vessels in the Harderian gland of the hamster (Mesocricetus auratus): existence of sinusoids. J. Morphol. 204(3), 257–263. Mobini, B. 2012. Histological and histochemical studies on the Harderian gland in native chickens. Vet. Med. Czech. 57(8), 404–409. Ohshima, K. and Hiramatsu, K. 2002. Immunohistochemical localization of three different immunoglobulin classes in the Harderian gland of young chickens. Tissue Cell. 34, 129–133. Olcese, J. and Wesche, A. 1989. The Harderian gland. Mini review. Contp. Biochem. Physiol. 93(4), 655–665. Oliveira, L., Nachtigall, P.G., Vialla, V.L., Campos, P.F., Costa-Neves, A.D., Zaher, H., Silva, N.J.D. Jr, Grazziotin, F.G., Wilkinson, M. and Junqueira-de-Azevedo, I.L.M. 2023. Comparing morphological and secretory aspects of cephalic glands among the New World coral snakes brings novel insights on their biological roles. Toxicon. 234, 1–20. Payne, A.P. 1994. The Harderian gland: a tercentennial review. J. Anatom. 185, 1–49. Porter, W.R. and Witmer, L.M. 2016. Avian cephalic vascular anatomy, sites of thermal exchange, and the rete ophthalmicum. Anatom. Rec. 299(11), 1461–1486. Pradidarcheep, W., Asavapongpatana, S., Mingsakul, T., Poonkhum, R., Nilbunga, S. and Somana, R. 2003. Microscopic anatomy of the orbital Harderian gland in the common tree shrew (Tupaia glis). J. Morphol. 255(3), 328–336. Richards, S.A. 1967. Anatomy of the arteries of the head in the domestic fowl. J. Zool. 152, 221–234. Richards, S.A. 1968. Anatomy of the veins of the head in the domestic fowl. J. Zool. 154, 223–234. Sedlmayr, J.C. 2002. Anatomy, evolution, and functional significance of cephalic vasculature in Archosauria. Ph.D. dissertation. Athens, OH: Ohio University. Shirama, K., Satoh, T., Kitamura, T. and Yamada, J. 1996. The avian Harderian gland: Morphology and immunology. Microscopy Res. Tech. 34, 16–27. Tompsett, D.H. 1956. Anatomical techniques. E&S Livingstone: Edinburgh, London. Wight, P.A., Burns, R.B., Rothwell, B. and Mackenzie, G.M. 1971. The Harderian gland of the domestic fowl. I. Histology, with reference to the genesis of plasma cells and Russell bodies. J. Anatom. 110(2), 307–315. | ||
| How to Cite this Article |
| Pubmed Style Bejdić P, Amidžić L, Čengić B. Arterial and venous supply of the harderian gland in hens. Open Vet. J.. 2024; 14(7): 1553-1560. doi:10.5455/OVJ.2024.v14.i7.4 Web Style Bejdić P, Amidžić L, Čengić B. Arterial and venous supply of the harderian gland in hens. https://www.openveterinaryjournal.com/?mno=192992 [Access: January 24, 2026]. doi:10.5455/OVJ.2024.v14.i7.4 AMA (American Medical Association) Style Bejdić P, Amidžić L, Čengić B. Arterial and venous supply of the harderian gland in hens. Open Vet. J.. 2024; 14(7): 1553-1560. doi:10.5455/OVJ.2024.v14.i7.4 Vancouver/ICMJE Style Bejdić P, Amidžić L, Čengić B. Arterial and venous supply of the harderian gland in hens. Open Vet. J.. (2024), [cited January 24, 2026]; 14(7): 1553-1560. doi:10.5455/OVJ.2024.v14.i7.4 Harvard Style Bejdić, P., Amidžić, . L. & Čengić, . B. (2024) Arterial and venous supply of the harderian gland in hens. Open Vet. J., 14 (7), 1553-1560. doi:10.5455/OVJ.2024.v14.i7.4 Turabian Style Bejdić, Pamela, Liljana Amidžić, and Benjamin Čengić. 2024. Arterial and venous supply of the harderian gland in hens. Open Veterinary Journal, 14 (7), 1553-1560. doi:10.5455/OVJ.2024.v14.i7.4 Chicago Style Bejdić, Pamela, Liljana Amidžić, and Benjamin Čengić. "Arterial and venous supply of the harderian gland in hens." Open Veterinary Journal 14 (2024), 1553-1560. doi:10.5455/OVJ.2024.v14.i7.4 MLA (The Modern Language Association) Style Bejdić, Pamela, Liljana Amidžić, and Benjamin Čengić. "Arterial and venous supply of the harderian gland in hens." Open Veterinary Journal 14.7 (2024), 1553-1560. Print. doi:10.5455/OVJ.2024.v14.i7.4 APA (American Psychological Association) Style Bejdić, P., Amidžić, . L. & Čengić, . B. (2024) Arterial and venous supply of the harderian gland in hens. Open Veterinary Journal, 14 (7), 1553-1560. doi:10.5455/OVJ.2024.v14.i7.4 |