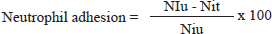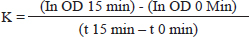| Research Article | ||
Open Vet. J.. 2024; 14(8): 1794-1800 Open Veterinary Journal, (2024), Vol. 14(8): 1794–1800 Research Article The immunostimulatory effects of fucoidan on the cellular and humoral immune response in Wistar ratsRochmah Kurnijasanti1, Giftania Wardani2, Muhammad Rais Mustafa3 and Sri Agus Sudjarwo1*1Department of Basic Veterinary Medicine, Faculty of Veterinary Medicine, Airlangga University, Surabaya, Indonesia 2Program Study of Pharmacy, Faculty of Medicine, Hang Tuah University, Surabaya, Indonesia. 3Department of Pharmacology, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia *Corresponding Author: Sri Agus Sudjarwo. Department of Basic Veterinary Medicine, Faculty of Veterinary Medicine, Airlangga University, Surabaya, Indonesia. Email: ags158 [at] yahoo.com Submitted: 17/03/2024 Accepted: 06/07/2024 Published: 31/08/2024 © 2024 Open Veterinary Journal
ABSTRACTBackground: Natural product active ingredients are currently being studied rigorously worldwide and offer a viable substitute for traditional immunotherapy for various medical disorders. Aim: The objective of the study was to investigate the immunostimulatory properties of fucoidan in albino Wistar rats. Methods: For the current study, forty rats were divided into five groups of rats that were used in good condition. In-vivo experiments of fucoidan were carried out in Wistar albino rats, such as the cyclophosphamide-caused myelosuppression, the delayed-type hypersensitivity (DTH) response, the phagocytic activity, the haemagglutinating antibody (HA) titer, and the neutrophil adhesion test. Results: The phagocytic index increased significantly in response to Fucoidan in a dose-dependent manner, as well as enhanced DTH reaction, and HA titer caused by sheep red blood cells sheep red blood cells. Additionally, fucoidan decreased myelosuppression in rats after cyclophosphamide treatment and enhanced neutrophil adhesion with nylon fiber. Conclusion: These findings imply that fucoidan has immunostimulant properties and could potentially utilised to treat immune-depression diseases Keywords: Fucoidan, Immunostimulant, Humoral immune response, Cellular immune response. IntroductionImmunomodulatory drugs have the potential to modify several aspects of the immune system, including humoral and cellular immune responses. They may either activate or inhibit these components. A complicated mechanism regulates the pathophysiology and pathogenesis of several immune system-related disorders. Immunostimulation is the term for the set of processes that the body uses to fight against infections (Wardani et al., 2018; Strzelec et al., 2023). The thymus and spleen are two organs where immunological response-related cells originate. The body is protected from bacteria, parasites, viruses infections, and toxins by the immune system, which is a complex network of chemicals, cells, tissues, and organs. This immune system is essential because they are the first protection of the body against antigens such as microorganisms, tenacious particles, damaged cells, and cellular debris (Casas-Rodriguez et al., 2022; Yuandani et al., 2022; Ogbue et al., 2023). In the innate arm of the immune system, neutrophils are necessary for effector cells. These cells are in the continuous process of searching the organism for signs of microbial disease. Cellular immunity was assessed using the neutrophil adhesion test methodology (Sudjarwo et al., 2018; Aljutaily, 2022). The titer of hemagglutination antibodies in the humoral immune response to the sheep red blood cells (SRBCs) antigen significantly increased, indicating that the formulation may be able to activate lymphocytes, especially B lymphocytes and other cells linked to the humoral immune response. Following an interaction between B lymphocytes and antigens, plasma cells produce antibodies, which stimulate B lymphocyte proliferation and differentiation into cells that secrete antibodies. The produced antibodies were designed to bind to the antigen, neutralize it, or facilitate phagocyte recognition, ultimately leading to the antigen’s elimination (Hamid et al., 2020; Hamid et al., 2021; Elhusseiny et al., 2022). The carbon clearance assay was used to measure macrophage activity concurrently (Sudjarwo et al., 2018). Pathologies affecting humans may result from phagocytic process abnormalities. More immune cells become activated as a result of the production of several cytokines, which are reliant on macrophages. The carbon clearance test evaluates how quickly carbon particles are eliminated from blood after being injected systemically as ink. A preset formula was used to calculate the clearance rate. Like macrophages, phagocytic cells get rid of carbon particles (Diez-Quijada et al., 2022; Hattori et al., 2023). T cells production of lymphokines activates immune cells, which in turn triggers the delayed-type hypersensitivity (DTH) response and attracts more macrophages. Against pathogenic agents, foreign grafts, cancerous tissues, and DTH reactions, cell-mediated immune response plays a crucial role in defense (Jantan et al., 2019; Thirumalaikumaran et al., 2024). Nowadays, cyclophosphamide, a commonly used chemotherapeutic medication, has been employed in conjunction with cancer immunotherapies as an immunostimulatory or antiangiogenic/antivasculogenic agent. The main adverse effects of cyclophosphamide therapy are immunosuppression and myelosuppression, which happen as a result of the drug’s suppression of the growth of healthy immune cells (Noh et al., 2019; Park et al., 2020; Van Pham et al., 2021). Many artificial and natural immunomodulatory drugs have recently been available to control specific and non-specific immune responses. Natural product-based immunomodulators can be used as an alternative to conventional immunotherapy for the prevention and treatment of various diseases. Used as an immunostimulant to increase the body’s immune response in microbial diseases, and as an immunosuppressant in autoimmune diseases and organ transplants to suppress the immune system (Shukla et al., 2022; Gond et al., 2022). Worldwide, scientists are devoting their attention to investigating therapeutic natural products and plant-derived compounds that have the potential to modify certain immune responses. In this context, research focusing on the discovery of new drugs as candidates for natural product-based immunostimulatory agents has developed rapidly. Natural products have been utilized widely to strengthen immunity, restore homeostasis, and condition body tissues to boost body resistance against infection. It has been found that several natural products used in traditional medicine contain immunomodulatory qualities. Certain natural products can boost humoral as well as cellular immunity (Porwal et al., 2021; Zebeaman et al., 2023; Kadiyska et al., 2023). Fucoidan is one natural product with immunostimulant properties. Sulfated polysaccharide fucoidan is found in marine invertebrates and brown algae. It is a heteropolysaccharide mostly made up of sulfate esters and fucose. Bioactive macromolecules known as polysaccharides possess advantageous pharmacological properties such as anti-tumor, antioxidant, anti-diabetic, and immunomodulatory actions. Fucoidan can stimulate the immune system due to its polysaccharide structure. L-fucose is the primary fucoidan sugar component with immunomodulatory properties. As a result, it has been discovered to possess a wide range of bioactivities, such as immune-regulatory, antioxidant, anti-inflammatory, and antiviral as well as anticancer, antidiabetic, and anti-hepatic damage properties (Wang et al., 2019; Jayawardena et al., 2022; Du et al., 2022). This investigation aimed to demonstrate the immune-stimulating properties of fucoidan on specific humoral and cellular immunity markers in rats. Materials and MethodsExperimental animalA male Wistar albino rat, weighing between 225 and −250 g, was obtained for experimental purposes from Gadjah Mada University, Yogyakarta, Indonesia. They were kept in a plastic cage with an air conditioning room, 12-hour light and dark cycles, and a temperature control of 26oC ± 2oC. The rat was fed commercial rat chow and had unrestricted access to tap water. Fucoidan was obtained from Sigma Chemical, Company Ltd. (St. Louis, MO). In vivo experiments were performed on Wistar albino rats utilizing the neutrophil adhesion test (Wardani et al., 2018), haemagglutinating antibody (HA) tester (Sudjarwo et al., 2018), DTH reaction (Hamid et al., 2020), phagocytic activity (Casas-Rodriguez et al., 2022), and cyclophosphamide-induced myelosuppression (Aljutaily, 2022). The humoral response was measured in rats sensitized to SRBCs using primary and secondary antibody titers, and the cell-mediated immune response was evaluated in the same rats using a DTH reaction. The rate of carbon clearance was used to gauge the reticuloendothelial system’s (RESs) neutrophil recruitment and phagocytic activity. Analysis was also carried out to determine how fucoidan affects hematological indicators due to administration of cyclophosphamide. Preparing SRBCsSheep offered for sacrifice in the neighborhood slaughterhouse provided fresh blood. SRBCs were adjusted to a concentration of 0.5×109 cells/ml for immunization and challenge after being cleansed three times with 0.9% normal saline to remove pyrogens. Neutrophil adhesion testCellular immunity was assessed using the neutrophil adhesion method (Wardani et al., 2018). The neutrophil count decreases when blood is incubated with nylon fibers (NF) because the neutrophils adhere to the fibers. In the experiment, there were four groups consisting of six rats each. For 14 days, the rats in the fucoidan group received dosages of 75, 150, and 300 mg/kg BW, whereas the rats in the control group received 10 ml/kg BW. Blood samples were taken on the fourteenth day of the fucoidan treatment by puncturing the retro-orbital plexus into heparinized vials. Subsequently, the total leukocyte cell (TLC) and differential leukocyte cell (DCL) counts were determined. Following the preliminary count, the incubated blood samples were analyzed again using TLC and DLC, respectively, to ascertain the neutrophil index. Using the following formula, the % neutrophil adhesion was determined:
The neutrophil index of untreated blood samples is denoted by NIu, while the neutrophil index of treated blood samples is represented by NIt. Hemagglutinating antibody (HA) titerThe HA titer method was described by Sudjarwo et al (2018). There were four groups in the experimental models, each with six rats. On day 0, the rats in every group received an intraperitoneal injection of 0.1 ml of a suspension of 0.5×109 cells of SRBCs. In the treatment groups, fucoidan at varied doses (75, 150, and 300 mg/kg BW) was administered orally for seven days, whereas the control group of rats received 10 ml/kg BW of normal saline. On the seventh day, blood samples were taken from each rat’s heart to make the serum. Blood samples were centrifuged, and serum was extracted. The hemagglutination method was used to measure the antibody levels in 86 wells (12×8 flat-bottomed titer plates). A mixture of 25 µl serum and 25 µl normal saline was initially added to each well microtiter plate. The 25 µl diluted serum was then taken out of the first well and put into the second well to dilute the antibodies. Following the addition of 25 µl of 1×108 SRBC to each well, the microtiter plates were incubated for an hour at 37oC and then were checked for agglutination. DTH responseHamid et al (2020) method was used to calculate the DTH response. On day 0 of the immunization process involved injecting intraperitoneally 0.1 ml of a suspension of 0.5×109 cells of SRBC into the rats in each group. Rats were split into treatment and control groups. The control groups received 10 ml/kg BW of normal saline, while the treatment group received 75, 150, and 300 mg/kg BW of fucoidan orally for eight days. All of the rats received an SRBC challenge (0.5 x 109 cells) in the left hind foot pad on the seventh day of immunization. To control for nonspecific edema, the same volume of normal saline was injected into the right foot pad. The increase in footpad thickness was observed 24 hours following the challenge.
Phagocytic response (carbon clearance methods)The effect of fucoidan treatment on the macrophage was evaluated using a carbon clearance assay (Casas-Rodriguez et al., 2022). Rat is divided into control and treatment groups. Oral fucoidan at varying dosages (75, 150, and 300 mg/kg BW) was administered to treatment groups, while the control groups received 10 ml/kg BW of normal saline for 7 days. On the seventh day, an intravenous injection of 5 ml/kg BW of carbon suspension (1:50 dilution of Indian ink, Hi-Media Laboratories Pvt, Ltd., Mumbai, India) was administered. At 0 and 15 minutes after injecting colloidal carbon ink, blood samples were taken from the retro-orbital plexus, and lysed in 2 ml of 0.1% w/v sodium carbonate solution. Using spectrophotometry, the optical density was determined at 650 nm in wavelength. The phagocytic index was used to express the results:
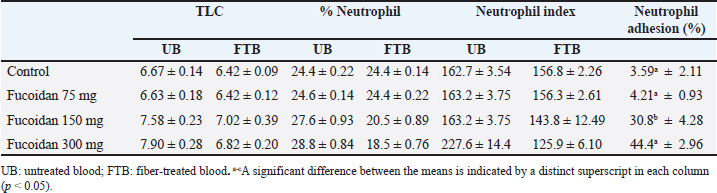
where the optical densities at time t 15 and t 0, respectively, are represented by OD 15 min and OD 0 min. Cyclophosphamide-induced myelosuppressionThis study aimed to investigate how fucoidan might inhibit myelosuppression induced by cyclophosphamide (Aljutaily, 2022). Five groups of Wistar rats, each with six rats made up the control groups, the cyclophosphamide groups, and the fucoidan groups. The rats in the control groups only received a saline solution, the rats in the cyclophosphamide group only received intraperitoneally cyclophosphamide at doses of 30 mg/kg BW, and the fucoidan group received orally fucoidan at doses of 75, 150, and 300 mg/kg BW for eleven days, and also received intraperitoneally cyclophosphamide at doses of 30 mg/kg BW on the eighth, ninth and tenth days, one hour after received fucoidan. On day eleven of the experiment, blood samples were taken and their hematological characteristics were examined. Analytical statisticsOne-way analysis of variance (ANOVA) and post hoc Tukey test was used to statistically assess the data, which were presented as mean and standard error mean (SEM), p < 0.05 indicated statistical significance in the difference. Ethical approvalAll trials were examined and approved by the Faculty of Veterinary Medicine at Airlangga University’s Ethical Approval Committee assessed this work, and it was given ethical approval under No. 1.KEH.081.06.2023. ResultsEffect of fucoidan on neutrophil adhesionThe number of neutrophils decreased when incubated with NFs fibers because the neutrophils adhered to the fibers. Table 1 shows how fucoidan affects neutrophil activation in the neutrophil adhesion assay. Administration of fucoidan only at doses of 300 mg/kg BW significantly elevated neutrophil adhesion when the data were compared to rats in the control group; however, this effect was not observed at dosages of 75 or 150 mg/kg. This data suggests that fucoidan has immunostimulant effects. Table 1. Effect of fucoidan on neutrophil adhesion in rats
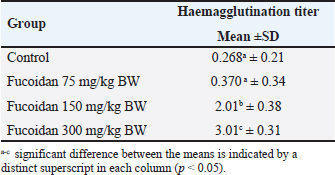
Table 2. Effect of fucoidan on haemagglutination titer
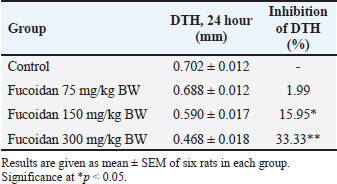
Table 3. Effect of fucoidan on DTH.
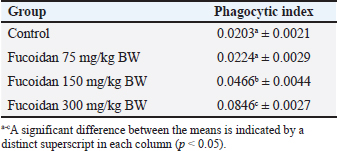
Table 4. Effect of fucoidan on carbon clearance test in rats.
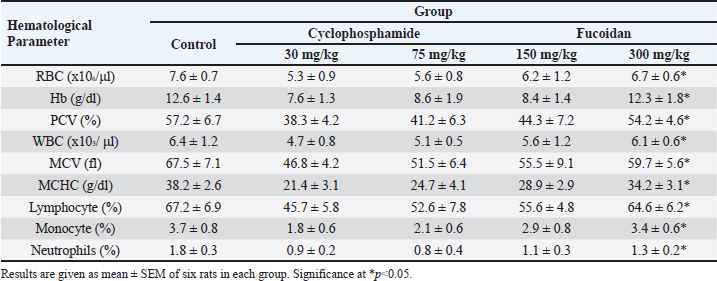
Effect of fucoidan on humoral immunityRat antibody production was assessed in response to oral fucoidan therapy using the SRBC haemagglutination titer. The humoral antibody titer value is shown in Table 2. Administration of fucoidan only at doses of 300 mg/kg BW resulted in a significant increase in haemagglutination antibody titer when compared with rats in the control groups. Effect of fucoidan on cell-mediated immunity (CMI)The DTH reaction, or foot pad reaction, was utilized to assess the cell-mediated immune response of the fucoidan. Table 3 shows that fucoidan significantly and dose-dependently boosted the DTH reactivity of rats. Administration of fucoidan only at doses of 300 mg/kg BW significantly elevated DTH reactivity when compared with control groups. The rat-increased DTH response to a cell-dependent antigen showed that fucoidan had activated T cells. Effect of fucoidan on phagocytic activityThere is a correlation between increased phagocytic activity and the speed at which carbon particles are removed. RES phagocytic activity was assessed by removing carbon particles from the bloodstream. The phagocytic index is shown in Table 4. When fucoidan is administered orally, the RES’s cells eliminate more carbon in a dose-dependent manner. Fucoidan demonstrated a significant phagocytic index when it was administered at doses of 300 mg/kg BW, but not at 75 and 150 mg/kg BW when compared with rats in the control groups. These results suggest the phagocytic activity of fucoidan. Effect of fucoidan on myelosuppression caused by cyclophosphamideCyclophosphamide, administered intraperitoneally at a dose of 30 mg/kg BW, significantly decreases hemoglobin, white blood cells, red blood cells, and platelets. When cyclophosphamide and fucoidan are administered together in a dose-dependent manner, bone marrow activity is restored more than when cyclophosphamide is administered alone. Hemoglobin, red blood cells (RBCs), WBCs, and platelet count were all markedly elevated by fucoidan at doses of 300 mg/kg BB in comparison to the group treated with cyclophosphamide (Table 5). Table 5. Effect of fucoidan on cyclophosphamide-induced myelosuppression.
DiscussionNumerous natural compounds have been shown to have immunomodulatory qualities recently, and they often work by promoting both specific and nonspecific immunity (Aipire et al., 2020; Cholaraj and Venkatachalam, 2023). Many natural products are used in conventional medicine to stimulate the immune system. Although some of them can also suppress the immune system, through humoral and cellular immunity (Wardani et al., 2018; Yuandani et al., 2022; Aljutaily, 2022). The primary line of defense against infectious and non-infectious diseases is the immune system. Strong immune systems are made up of components that work in harmony with one another; if this harmony is upset, the immune system cannot defend the body from dangerous substances (Shukla et al., 2022; Gond et al., 2022). For a variety of disorders, immunomodulation with natural products can be used in place of traditional immunotherapy, particularly when the immunological response is compromised, and the host defense system must be activated. Natural immunostimulants can be used to overcome low immunity due to drugs or disease. Immunological system-boosting medications are desperately needed to counteract the immunosuppressive effects of stress, long-term illnesses, and ailments involving compromised immunological responses (Gond et al., 2022; Zebeaman et al., 2023; Kadiyska et al., 2023). The natural substance has become widely employed as an immunostimulatory in recent years. Even though natural products have been studied for a variety of pharmacological uses; however, it is still uncertain whether fucoidan can stimulate the immune system (Porwal et al., 2021; Shukla et al., 2022). Through the experiment, we were able to show how fucoidan modulates immunity in the cellular and humoral immunity model. According to the current study’s findings, fucoidan is a powerful immunostimulant that boosts both the specific and nonspecific immune systems. The neutrophil’s ability to adhere to other cells is one of their initial responses to both physical and immunological injury. Neutrophils in blood samples from different populations were tested for their ability to stick to NFs, a feature known as cell adherence. Pathogen removal is the principal duty of neutrophils, a vital component of the innate immune system. Certain inflammatory mediators produced by the infection site regulate the ability of neutrophils to locate and destroy microorganisms. The neutrophil is an end cell that cannot divide but is capable of several different reactions, including chemotaxis, exocytosis, phagocytosis, and both extracellular and intracellular death. The neutrophil counts decreased when neutrophils were incubated with NFs, and this was correlated with the neutrophils’ “adhesion” to the fibers (Sudjarwo et al., 2018; Van Pham et al., 2021). In the current investigation, a notable rise in the percentage of neutrophils was elicited by fucoidan. This shows that fucoidan has the potential to strengthen the body’s defenses against microorganism infections. The purpose of the indirect haemagglutination test was to verify how fucoidan affected the humoral immune system. It comprises B cells that interact with antigens, multiply, and differentiate into cells that produce antibodies (Jantan et al., 2019; Thirumalaikumaran et al., 2024). The rise in antibody titer in rats is proof positive that fucoidan can enhance the response of humoral immune to SRBC. This suggests that certain subsets of B and T cells, which are involved in the production of antibodies, have heightened sensitivity. An immunostimulatory response was attained by humoral immunity, as demonstrated by elevated levels of fucoidan HA titers. The humoral immunity component of the adaptive immune system is mediated by B cells and plasma cells, which produce antibodies such as IgM and IgG. These immunoglobulins play a crucial function in complement activation, opsonization, and neutralization of foreign substances. A component of CMI is effector function, such as T cells and the lymphokines they generate. CMI responses are necessary for DTH reactions, foreign graft infection, immunity, and defense against harmful microorganisms (Hamid et al., 2021; Elhusseiny et al., 2022). The increased DTH reaction in rats in response to T cell-dependent antigen thus revealed the stimulatory effect of fucoidan on T cells. In this investigation, fucoidan strengthened the rat’s immune system. There were stimulatory effects on cellular and humoral immunity. The fucoidan responded to the DHT test at all doses, but only at 300 mg/kg did this reaction become statistically significant. This phenomenon may arise from the fact that fucoidan stimulates the B lymphocyte and macrophage subsets involved in the manufacture of antibodies, hence enhancing the humoral response. Sensitized T-lymphocytes may be the cause of the increased DTH during the CMI reactions. They become lymphocytes in response to the antigen, secreting a range of chemicals, including proinflammatory lymphokines, which attract additional scavenger cells to the reaction site. An increase in response to DTH suggests that fucoxanthin has stimulated the lymphocytes and accessory cell types needed for this reaction to be expressed. Using the carbon clearance assay, drug activity in the RES was investigated. Phagocytic cells comprise the diffuse RES. RES cells play a major role in the elimination of particulates from the circulation. When intravenous injection into blood vessels, the ink contains colloidal carbon particles, an exponential equation that controls how quickly macrophages remove carbon from the blood. The process of phagocytosis is how some bodily cells, referred to as phagocytes, take up and eliminate microorganisms, cancerous cells, inorganic particles, and tissue fragments (Wardani et al., 2018; Yuandani et al., 2022). The phagocytic index significantly increased upon fucoidan administration and the rate at which the RES cells eliminated carbon increased in a dose-dependent manner. This suggests that the RES was more active in rats given fucoidan treatment. The myelosuppressive effect of cyclophosphamide can be inhibited by administering fucoidan, which is indicated by a rise in the overall quantity of WBC, hemoglobin, red blood cells, and platelets (Park et al., 2020; Van Pham et al., 2021). The current study’s findings suggest that fucoidan can increase bone marrow activity. The organ most harmed by immunosuppressive therapy is the bone marrow, which is also susceptible to cytotoxic drugs like cyclophosphamide. Thrombocytopenia and leucopenia are caused by the reduction of stem cells and the bone marrow’s incapacity to produce new blood cells. The overall WBC count increased when the fucoidan was administered, while the cytotoxic medication cyclophosphamide decreased it. This suggests that the fucoidan can promote bone marrow activity. ConclusionBased on our findings, we may conclude that fucoidan has the capacity to trigger both cellular and humoral immune responses, and that the impact is dose-dependent. Fucoidan efficiently enhances cellular and humoral immunity, as well as nonspecific immune responses. AcknowledgmentThe authors are grateful to the Faculty of Veterinary Medicine and Airlangga University, Surabaya, Indonesia. FundingThis research was supported by the Faculty of Veterinary Medicine and funded by Airlangga University, Surabaya, Indonesia, through the International Research Collaboration Grant number No 1531/UN3.15/PT/2021. Author contributions W.G., S.A.S., and M.R.M conceptualization. W.G and R.K contributed to the animal study. W.G analyzed data and draft preparation. S.A.S and M.R.M writing review and editing. All authors have read and agreed to the published version of the manuscript. Conflict of interestAll authors declare no conflict of interest. Data availabilityThe datasets used and analyzed during this study are included in the article. ReferencesAipire, A., Mahabati, M., Cai, S., Wei, X., Yuan, P., Aimaier, A., Wang, X. and Li, J. 2020. The immunostimulatory activity of polysaccharides from Glycyrrhiza uralensis. Peer J. 29(8), e8294. Aljutaily, T. 2022 Evaluating the nutritional and immune potentiating characteristics of unfermented and fermented turmeric camel milk in cyclophosphamide-induced immunosuppression in rats. Antioxidants 11, 792–812. Casas-Rodriguez, A., Guzmán-Guillén, R., Molina-Hernández, V., Albaladejo, R.G., Cameán, A.M and Jos, A. 2022. Immunomodulatory effects of pure cylindrospermopsinin rats orally exposed for 28 days.Toxins 14, 144. Cholaraj, R. and Venkatachalam, R. 2023. The effect of polysaccharide from Padina boergesenii on Aeromonas hydrophila resistance and growth, biochemical, digestive enzymes, non-specific immune response in Oreochromis niloticus, Bioact. Carbohydr. Diet. Fibre. 30, 100357. Du, B., Zhao, Q., Cheng, C., Wang, H., Liu, Y., Zhu, F. and Yang, Y. 2022. A critical review on extraction, characteristics, physicochemical activities, potential health benefits, and industrial applications of fucoidan. eFood 3, e19–36. Elhusseiny, S.M., El-Mahdy, T.S., Elleboudy, N.S., Farag, M.M.S. and Yassien, M.A. 2022. Immunomodulatory activity of extract from five edible basidiomycetes mushrooms in Wistar albino rats. Sci. Rep. 12, 12423. Diez-Quijada, L., Casas-Rodriguez, A., Guzmán-Guillén, R., Molina-Hernández, V., Albaladejo, R.G.; Cameán, A.M. and Jos, A. 2022. Immunomodulatory effects of pure cylindrospermopsin in rats orally exposed for 28 days. Toxins 14, 144–152. Gond, S.P., Sahu, S., Rawat,s., Rajendiran, A. and Singh, A. 2022. Immunomodulatory natural product: review. Asian J. Pharm. Clin. Res. 5, 5–9. Hamid, K.M., Kalgo, M.U., Isyaku, A., Tijjani, M., Shehu, A.A. and Umahi, N.P.F. 2020. Immunostimulatory activity of aqueous leaves extract of Cassia occidentalis on innate and adaptive immune response in mice. Fudma. J. Sci. 4(4), 108–115. Hamid, K.M., Isah, S.Y., Kalgo, M.U. and Yahaya, I.S. 2021. Immunostimulatory activity of aqueous extract of Polyherbal formulation on Th1/Th2 cytokine secretion and cell-mediated immune response in rats. Saudi J. Med. Pharm. Sci. 7(1), 64–70. Hattori, K., Takagi, H., Ogata, Y., Yamada, Y., Horiba, H. and Tanaka, H. 2023. Immunomodulatory effect of a subcritical water extract of Ganoderma. Biomed. Rep. 18(1), 1–10. Jantan, I., Ilangkovan, M., Haque, M.A. and Arshad, L. 2019. An insight into the modulatory effects and mechanisms of action of Phyllanthus species and their bioactive metabolites on the immune system. Front. Pharmacol. 10, 1–19. Jayawardena, T.U., Nagahawatta, D.P., Fernando, I.P.S., Kim, Y.T., Kim, J.S., Kim, W.S., Lee, J.S. and Jeon, Y.J. 2022. A review on fucoidan structure, extraction techniques, and its role as an immunomodulatory agent. Mar. Drugs 20, 755–774. Kadiyska, T., Tourtourikov, I., Dabchev, K. and Zoumpourlis, V. 2023. Herbs and plants in immunomodulation (review). Inter. J. Funct. Nut. 4, 1–16. Noh, E., Kim, J., Lee, H.Y., Song, H., Joung, S.O. and Lee, Y. 2019. Immuno-enhancement effects of Platycodon grandiflorum extracts in splenocytes and a cyclophosphamide-induced immunosuppressed rat model. BMC Complement Alternat. Med. 19, 322–334. Ogbue, C.O., Onyegbule, F.A., Ezugwu, C.O., Nchekwube., I.H.M. and Ajaghaku, A.A. 2023. Immunostimulatory and immunorestorative effect of leaf extract and fractions of Musanga cecropioides on immunocompetent and experimentally immunocompromised mice. Clin. Complement Med. Pharmacol. 3(1), 100075–87. Park, Y.M., Lee, H.Y., Shin, D.Y., Lee, Y.H., Yang, Y.J., Lee, H.S., Kim, M.G. and Le, Y. 2020. Immunostimulatory activity of black rice bran in cyclophosphamide-induced immunosuppressed rats. Nat. Prod. Commun. 15(7), 1–12. Porwal, O., Ozdemir, M., Kala, D. and Anwer, E.T. 2021. A review on medicinal plants as potential sources of natural immunomodulatory action. J. Drug Deliv. Ther. 11(6), 324–331. Shukla, K., Singh, S.K., Pandey, S., Gupta, P.K. and Kumar, D. 2022. Potential immunomodulatory activities of plant product. South Afria. J. Bot. 149, 937–943. Strzelec, M., Detka, J., Mieszczak, P., Sobocin´ ska, M.K. and Majka, M. 2023. Immunomodulation-a general review of the current state-of-the-art and new therapeutic strategies for targeting the immune system. Front. Immunol. 14, 1127704. Sudjarwo, S.A., Wardani, G., Eraiko, K and Koerniasari. 2018. The potency of nanoparticle of pinus merkusii as immunostimulatory on male wistar albino rat. Int. J. Nutr. Pharmacol. Neurol. Dis. 8, 10–15. Thirumalaikumaran, R., Prema, S. and Vijayaraghavalu, S. 2024. Influence of Stachydrine on immunomodulation in mice. Int. J. Pharma. Sci. Res. 14(8), 3957–3964. Van Pham, A.T., Luong, M.H., Dhin, H.T.T, Mai, T.P and Luong, L.H. 2021. Immunostimulatory effect of Moringa oleifera extract on cyclophosphamide-induced immunosuppressed mice. J. Herbs. Spices. Med. Plants. 27(4), 1–12. Wang, Y., Xing, M. and Cao, C. 2019. Biological activities of fucoidan and the factors mediating its therapeutic effects: a review of recent studies. Mar. Drugs 17(3), 183–201. Wardani, G., Mahmiah, A. and Sudjarwo, S.A. 2018. Immunostimulatory activity of chitosan nanoparticles on wistar albino rats. Pharmacog. J. 10(5), 892–898. Yuandani, Y., Jantan, I., Laila, L., Marianne, M., Septama, A.W., Lintang, N., Almadani, P., Friti, A. and Aini, S. 2022. Immunomodulatory effect of combined ethanol extract of Curcuma mangga and Picria felterrae on cellular and humoral-mediated immunity in Wistar rats and mice. Evid. Based Complement Alternat. Med. 2022, 1791165. Zebeaman, M., Tadesse, M.G., Bachheti, R.K., Gabeyhu, R. and Chaubey, K.K. 2023. Plants and plant-derived molecules as natural immunomodulators. Biomed. Res. Int. 2023, 771129. | ||
| How to Cite this Article |
| Pubmed Style Kurnijasanti R, Wardani G, Mustafa MR, Sudjarwo SA. The immunostimulatory effects of fucoidan on the cellular and humoral immune response in Wistar rats. Open Vet. J.. 2024; 14(8): 1794-1800. doi:10.5455/OVJ.2024.v14.i8.7 Web Style Kurnijasanti R, Wardani G, Mustafa MR, Sudjarwo SA. The immunostimulatory effects of fucoidan on the cellular and humoral immune response in Wistar rats. https://www.openveterinaryjournal.com/?mno=194485 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i8.7 AMA (American Medical Association) Style Kurnijasanti R, Wardani G, Mustafa MR, Sudjarwo SA. The immunostimulatory effects of fucoidan on the cellular and humoral immune response in Wistar rats. Open Vet. J.. 2024; 14(8): 1794-1800. doi:10.5455/OVJ.2024.v14.i8.7 Vancouver/ICMJE Style Kurnijasanti R, Wardani G, Mustafa MR, Sudjarwo SA. The immunostimulatory effects of fucoidan on the cellular and humoral immune response in Wistar rats. Open Vet. J.. (2024), [cited January 25, 2026]; 14(8): 1794-1800. doi:10.5455/OVJ.2024.v14.i8.7 Harvard Style Kurnijasanti, R., Wardani, . G., Mustafa, . M. R. & Sudjarwo, . S. A. (2024) The immunostimulatory effects of fucoidan on the cellular and humoral immune response in Wistar rats. Open Vet. J., 14 (8), 1794-1800. doi:10.5455/OVJ.2024.v14.i8.7 Turabian Style Kurnijasanti, Rochmah, Giftania Wardani, Muhammad Rais Mustafa, and Sri Agus Sudjarwo. 2024. The immunostimulatory effects of fucoidan on the cellular and humoral immune response in Wistar rats. Open Veterinary Journal, 14 (8), 1794-1800. doi:10.5455/OVJ.2024.v14.i8.7 Chicago Style Kurnijasanti, Rochmah, Giftania Wardani, Muhammad Rais Mustafa, and Sri Agus Sudjarwo. "The immunostimulatory effects of fucoidan on the cellular and humoral immune response in Wistar rats." Open Veterinary Journal 14 (2024), 1794-1800. doi:10.5455/OVJ.2024.v14.i8.7 MLA (The Modern Language Association) Style Kurnijasanti, Rochmah, Giftania Wardani, Muhammad Rais Mustafa, and Sri Agus Sudjarwo. "The immunostimulatory effects of fucoidan on the cellular and humoral immune response in Wistar rats." Open Veterinary Journal 14.8 (2024), 1794-1800. Print. doi:10.5455/OVJ.2024.v14.i8.7 APA (American Psychological Association) Style Kurnijasanti, R., Wardani, . G., Mustafa, . M. R. & Sudjarwo, . S. A. (2024) The immunostimulatory effects of fucoidan on the cellular and humoral immune response in Wistar rats. Open Veterinary Journal, 14 (8), 1794-1800. doi:10.5455/OVJ.2024.v14.i8.7 |