| Research Article | ||
Open Vet. J.. 2024; 14(8): 1866-1876 Open Veterinary Journal, (2024), Vol. 14(8): 1866–1876 Research Article Clinical and diagnostic evaluation of a wild ocelot (Leopardus pardalis) specimenMaría Flórez Solarte1, Juliana Loaiza1, Marcela Eraso1, Cristina Úsuga-Monroy1, Horwald A.B. Llano1, Andrea Pizarro2, Diana Stasiukynas2 and Juan Felipe Zapata1*1Grupo de Investigación en Medicina Veterinaria (GINVER), Corporación Universitaria Remington, Medellín, Colombia 2Panthera Colombia, Bogota, Colombia *Corresponding Author: Juan Felipe Zapata. Grupo de Investigación en Medicina Veterinaria (GINVER), Corporación Universitaria Remington, Medellín, Colombia. Email: juan.zapata [at] uniremington.edu.co Submitted: 22/04/2024 Accepted: 25/07/2024 Published: 31/08/2024 © 2024 Open Veterinary Journal
ABSTRACTBackground: Monitoring the health of wild animals under the principles of one health contributes to the prevention of diseases and the preservation of human and animal health, thus contributing to the conservation of species. Aim: The current study describes the clinical and paraclinical status of an ocelot (Leopardus pardalis) captured in Buriticá, Antioquia, Colombia, for research purposes with the aim of contributing to the construction of animal health reference values in the wild, considering the scarcity of published data for the country on capture, management, and paraclinical parameters related to this species. Methods: For this, hematological parameters, blood chemistry, urine cytochemical analyses, and coprological examinations were carried out. Results: The hematological values of the captured individual do not show relevant differences concerning those reported in the literature in both captive and free specimens. However, differences were identified between the reference blood chemistry and urine cytochemical values between reports of animals in captivity and the wild, revealing the need to develop reference standards for animals in the wild that guarantee adequate management of these species and favor their conservation. A possible picture of renal failure and multiple parasitic infections of epidemiological importance was found. Conclusion: This study reports for the first time a urine infection by Capillaria sp. and an infection by Dicrocoelium spp. in fecal matter for the species. Keywords: Wild feline, Capillaria spp., Dicrocoelium spp., South America, Leopardus pardalis. IntroductionUnder the One Health principles, monitoring the health of wild populations is crucial to understanding and managing threats to the health of domesticated fauna, wildlife, and humans (Zinsstag et al., 2018). Hence, the conservation science approaches are expanding beyond understanding the behavior, abundance, and habitat use of wild animals to approaches extended from one-health and one-wellness approaches with the goal of understanding and minimizing impacts related to disease transmission, hybridization phenomena, public health problems, environmental pollution, animal welfare, and conservation (Deem et al., 2001). Therefore, it is pertinent to understand and standardize clinical and paraclinical parameters, whether physiological or genetic, that allow establishing reference values to define the health status of wild animals in the wild (Barnes et al., 2008; Widmer et al., 2016). In Colombia, few studies document the health status of various species, particularly, carnivores in their natural habitats. Due to their extensive habitat requirements and low population densities, among carnivores, felines are particularly susceptible to habitat loss and fragmentation processes that expose them to different risks associated with diverse anthropic processes (Anile et al., 2019). One of these is the transmission of pathogens from domesticated animals to wild felines and the effects that these pathogens can have on individuals and populations, potentially contributing to the decline and extinction of species (Aguirre et al., 2002; Carver et al., 2016). Among the main limitations associated with the study of felids in the wild are their reserved habits, low population densities, and difficulties associated with capture processes, such as high implementation costs (Barea-Azcón et al., 2007; Mccarthy et al., 2013; Widmer et al., 2017). In the case of ocelots in the wild, there is little published data on the capture and handling processes. On the other hand, the identification of species through morphological strategies has been one of the most used techniques because the physical descriptors used are easily observable and are expressed in the same way regardless of the environment. However, the use of morphological characters is of little use for the differentiation of cryptic species (Volobouev et al., 2002; Jörger et al., 2014) and in the case of species conservation, morphological identification is difficult to apply due to the scarcity of intact specimens. In this sense, the application of molecular methods such as “DNA barcoding” is more precise and efficient for identification (Li et al., 2017), and also allows us to understand the evolution, ecology, and biodiversity of multiple species (Hebert et al., 2003). In Colombia, no reports of paraclinical parameters and molecular identification related to this species have been made because research approaches to date have been focused on understanding behavior, abundance, patterns, and habitat use (Diaz-Pulido and Payán Garrido, 2010, García et al 2019). Accordingly, this study reports the clinical, paraclinical, and molecular identification analysis of a female ocelot captured in Buriticá, Antioquia, Colombia. We collected data related to hematological parameters, blood chemistry, urine cytochemical analysis, and coprological examinations to identify possible factors that could be affecting the population health of these felines in highly fragmented anthropic landscapes. The findings obtained will significantly contribute to data collection, which will be essential in the future for the development of reference intervals specific to these species. Through reviews and statistical analyses, these data will aid statisticians and scientists in establishing normal ranges of health parameters, thereby enabling a better understanding and conservation of wildlife. This information will be essential to detect possible trends or emerging problems in their well-being and will allow comparisons to be established based on the recorded data. Furthermore, it will facilitate the identification of factors that may influence the health of wild animals and guide strategies for the conservation and management of wildlife. Materials and MethodsStudy areaThis study was carried out in the western region of the Department of Antioquia, Colombia, near Serranía de Buriticá, which is located in the north of Cordillera Occidental (western mountain range) and is of ecosystem importance as it corresponds to one of the transit routes for the biological diversity that disperse between Nudo de Paramillo and the Darién region in the north and the Andean forests and the biogeographic region of Chocó towards the south and west, characterized by a predominance of Andean Humid Forest ecosystems (Corantioquia, 2022). The study area is represented by a heterogeneous landscape that includes riparian forests, high and low secondary vegetation, crop mosaics, monoculture pastures, bare and degraded lands, road networks, infrastructure and urban fabric, and a mining extraction zone that has biotic influence in an area of approximately 2,948 hectares ranging from the Cauca River at 600 m a.s.l., up to 2,200 m a.s.l. in the municipal borders of Buriticá. Capture methodologyTo capture ocelot individuals, 19 Havahart® traps (model 1079) designed for medium and large animals were used, equipped with very high frequency (VHF) radio signal transmitters that alert the presence of the animal in the trap. The installation and baiting of all traps took approximately one week. Each trap was checked periodically every 24 hours to maintain the bait, accumulating a total of 380 trap nights before the capture. Various types of baits were used: olfactory, visual, and live animals in adjacent cages to the capture trap, specifically production chickens. These chickens, which were not consumed, were monitored by the team’s veterinarians, and their use was carried out according to the protocol approved by the relevant bioethics committees. Visual baits consisted of objects that reflected light, such as compact discs (CDs) or Christmas ornaments, which were hung from a branch or the adjacent vegetation at a height of at least 40 cm above the trap. These objects, when reflecting light, created flashes near the trap and attracted the attention of the felines, who approached the trap drawn by the flashes. Olfactory baits consisted of gauzes soaked with sardine and tuna oil placed inside perforated Falcon tubes (with at least three perforations on each side). These tubes were placed inside the trap compartments, so the feline was attracted by the smell but could not access the contents of the tube. The gauzes were placed inside these tubes to prevent ants and other insects from easily accessing and carrying away the gauze from the trap. For trap placement, aspects related to identifying feline pathways in the study area were considered. To this end, camera traps were installed three months before the capture sessions began in areas where the species of interest had been recorded the previous year through a systematic survey covering the entire study area. Using the obtained records, behavior patterns and routes of the individuals were evaluated, and a pre-selection of the most suitable areas was made. Once these locations were defined, the field team’s response capacity was also assessed, considering criteria such as the time needed to move with the equipment. This was done to select areas with better access, allowing for quicker response times, minimizing the time the animal spent in the trap, and preserving the health of the live baits. Manipulation and anesthesiaAn anesthetic protocol was carried out according to the weight of the individual, applying an intramuscular injection using a combination of dissociative medications (Ketamine), alpha 2 agonists (Metomidine), and Propofol. Clinical-physical evaluationThis assessment followed the occupational health protocol for sampling and handling animals and the basic guide for best practices during the capture and handling of wildlife of the science-based NGO Panthera Colombia. In this procedure, the information collected was recorded in the data collection instrument called “clinical history wild animals” prepared by project researchers, including information on the date, place of capture, species, age, body condition, state of hydration/dehydration, as well as information related to the physical examination per system and biometrics measurement, photographic records and notes on the samples taken from the individual. SamplingOnce an adequate anesthetic plane was achieved (Fig. 1), samples, biological data, and vital signs were recorded and monitored every 5–10 minutes throughout the procedure. The blood sample was obtained by venipuncture of the right femoral vein for hematological and molecular analysis in tubes with EDTA and blood chemistry in a yellow cap tube. The urine sample was taken using a sterile probe placed in a gray-capped tube with boric acid additive. The feces sample was collected directly from the rectum and the traps and were stored at 2°C–4°C until analysis for a maximum of 5 days. A portion of these samples was placed in a Ziploc® plastic bag and subsequently frozen at −20°C, and another part was placed in plastic bottles with 70% alcohol in a 1:1 ratio and refrigerated at 4°C until analysis. At the end of the procedure, an anesthetic reverser (Atipamizol-Revertidine®) was used to accelerate recovery. Subsequently, the individual was released. Paraclinical analysisA hemoleukogram was carried out using 10 µl of blood with EDTA that was passed through an auto hematology analyzer BC-2800 Vet (Shenzhen Mindray Animal Medical Technology Company Ltd., Shenzhen, China), and a peripheral blood smear was made, which was colored with Wright’s stain and read in an optical microscope under the 100X objective with immersion oil. The blood chemistry analysis used serum separated and frozen at −20°C until analysis in a chemical analyzer Mindray BS-120 (Shenzhen Mindray Animal Medical Technology Company Ltd., Shenzhen, China). A urine sample was taken, and parameters such as leukocytes, nitrites, urobilinogen, proteins, pH, blood, ketone bodies, bilirubin, and glucose were analyzed using a Mission® brand urine reagent strip. Density was measured by refractometry with a portable CHILABX® veterinary refractometer. Subsequently, the sample was centrifuged at 1,500 rpm for 5 minutes, and the sediment was analyzed in an optical microscope with 10X and 40X objectives. Fecal samples were examined using two methods: centrifugal-flotation in Sheather’s solution (d=1.203 g/cm3) as described by Ogassawara et al. (1986), and centrifugal-sedimentation with diethyl ether (Ferreira et al., 1962). Both methods are qualitative, and the results are expressed as the presence or absence of parasites. The quantitative analysis was interpreted according to the number of crosses, established as follows: one cross (+) for a mild infection with 1–5 parasitic structures per field; two crosses (++) for a moderate infection with 6–10 parasitic structures per field, and three crosses (+++), for a high infection with 11 to 20 parasitic structures per field (Estrada et al., 2014).
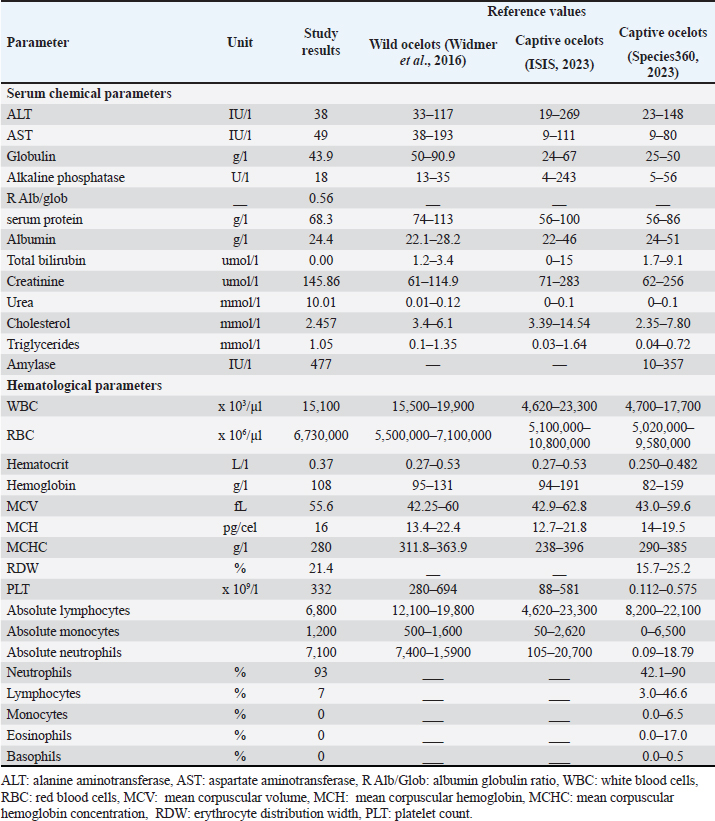
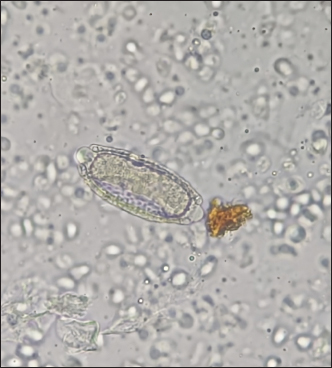
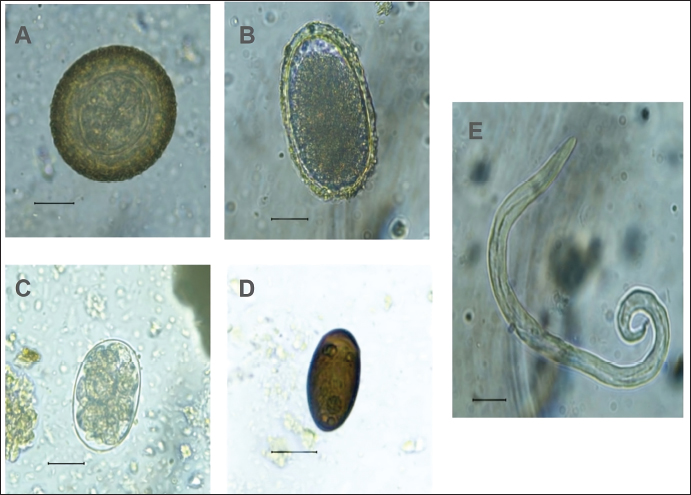
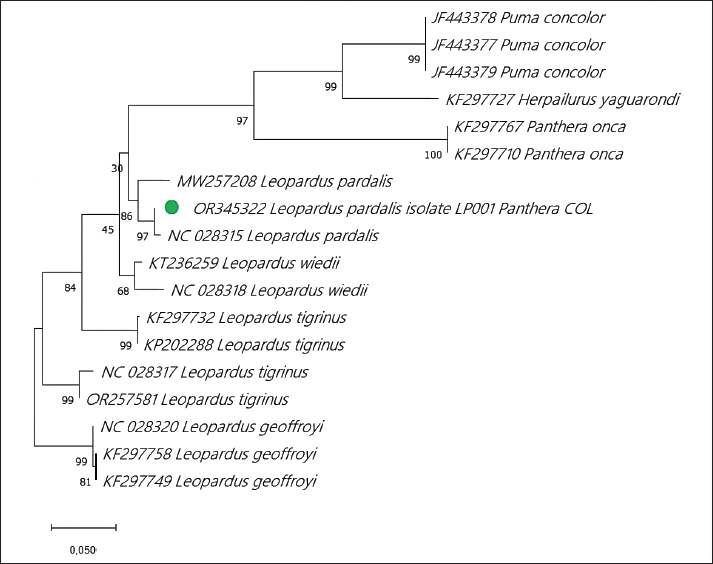
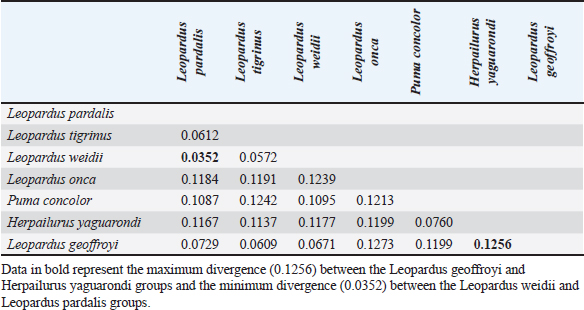
Fig. 1. Anesthetized ocelot during clinical evaluation, sample collection, and anesthetic monitoring. DNA extractionDNA was extracted from the blood sample. The entire volume contained in the EDTA microtainer was transferred to a 1.5 ml tube and incubated at 55°C for 20 min with 25 μl of proteinase K and 250 μl of BLU buffer. The extraction was continued according to the manufacturer’s instructions (HigherPurity™ Blood DNA Extraction Kit - Canvax Biotech). The sample was preserved in 30 μl of elution buffer. The sample was quantified in a NanoDrop™ 2000/2000c spectrophotometer (Thermo Fisher Scientific), where the quality and quantity of the sample were verified. Molecular identificationThe PCR for the molecular identification of the Leopardus pardalis specimen was performed by amplifying the cytochrome oxidase I (COI) gene fragment. Endpoint PCR was carried out in a final volume of 50 μl with 150 ng of DNA, 0.25 μl of Taq DNA polymerase, 0.5 μl of 10X buffer, 0.5 μl of dNTPs, and 0.5 μl 10 mM of each Chmf4 (TYT CWA CWA AYC AYA AAG AYA TCG G) and Chmr4 (ACY TCR GGR TGR CCR AAR AAT CA) oligonucleotide (Che et al., 2012). The reaction conditions were: 2 minutes at 94°C, 35 cycles of 30 seconds at 94°C, 30 seconds at 53°C, 48 seconds at 72°C, and a final extension for 1 minutes at 72°C. At the end of the reaction, a 959 bp COI gene fragment was obtained, verified in a 1.5% agarose gel, and visualized under a UV lamp (Labnet International, Inc.). Sequencing and phylogeny analysisThe PCR product was sent to a commercial company (Macrogen Inc., Korea) for sequencing using the Sanger method. The same company purified the product. For the phylogenetic analysis of the partial sequence of the COI gene, 17 sequences of felines representative of Colombia and South America were obtained. The sequences were aligned using the CLUSTAL W. Phylogenetic analysis was performed using the General Time Reversible substitution model with Gamma distribution (GTR+G), selected as the best substitution model based on the Akaike Information Criterion (AIC), and bootstrap values set to 1,000 replicates. Pairwise distance analyses were conducted using the Maximum Composite Likelihood model. All analysis were performed in MEGA v.11 software. Ethical approvalThis project was endorsed by the Bioethics Committee for Animal Research (CIBA) of Corporación Universitaria Remington (CUR) in its session on December 7, 2022, based on Minute 11 of the same year. In addition, the capture and collection of mammals in the selected localities is covered by the Framework permit for the Collection of Specimens of Wild Species of Biological Diversity for Non-Commercial Scientific Research Purposes granted by the National Environmental License Authority (ANLA, for its acronym in Spanish) with Resolution 00652 of April 13, 2020. ResultsIn the municipality of Buriticá, near Quebrada Chachafrutal (6°41’06.4”N 75°53’18.2”W), an adult female ocelot was captured (Leopardus pardalis). Its total measurement from head to the end of the tail was 108 cm; its height was 41 cm, with a body condition of 1, based on a scale from 1 to 5 (being 1: excessively thin, 2: underweight, 3: ideal weight, 4: overweight, and 5. Obese), a weight of 10.73 kg, with dry and opaque mucous membranes, and an estimated dehydration percentage of 4%–6%. The physical examination per system showed normality except for: i) the genitourinary system, where vaginal discharge was evident, and ii) in the integumentary system and annexes, a lesion was observed in nail no. 3 of the left anterior limb (LAL), and diffuse hypertrichosis was evidenced in the first two-thirds of the tail and at the level of the left ileum due to friction with the Havahart® trap. The peripheral blood smear analysis showed structures compatible with apicomplex protozoan hemoparasites and normal-shaped red blood cells and platelets in clusters. The hematology and blood chemistry examinations were performed in the Veterinary Diagnostic Unit of the CUR in Medellín. The values recorded were compared with reference values obtained from the only report of paraclinical parameters reported in the literature on ocelots in the wild (Widmer et al., 2016) and contrasted with the reference ranges established by the International Species Information System (ISIS, 2023) and Species 360 Global information serving conservation (Species360, 2023), which are elaborated from samples of captive ocelots (Table 1). The macroscopic appearance of the urine was turbid, yellow in color, pH 6.5, and with a density of 1020; the chemical examination determined a high presence of proteins (++++), blood in a small amount (+), ketone bodies in low amount 15 (+), positive for leukocytes (+++), and nitrites (++), negative for urobilinogen, glucose, and bilirubin. In the sediment, the following was observed: bacteria in moderate quantity, epithelial cells 4–6 per high power fields (HPF), tall cells 2–4 per HPF, mucus in scarce quantity (+), erythrocytes 1–3 per HPF, leukocytes countless. Additionally, eggs compatible with Capillaria spp. are observed; 54 eggs were counted in the entire plate (Fig. 2). In the coproparasitological analysis, four parasitic genera of the Nematoda class were found: Ancylostoma sp. (59.93 x 41.35 μm) (+++), Ascaridea (90.78 x 52.78 μm) (+), Toxocara cati (68.96 x 63.20 μm) (++), eggs of Dicrocoelium sp. (45.1 x 24.1 μm) (+++), and nematode larvae (290 μm) (+) (Fig. 3) were registered. The COI gene analysis as a method to identify animal species delivered a sequence of 663 base pairs registered in GenBank with access code OR345322 (Marin-Villa et al., 2023). The sequence of the present study OR345322 was aligned in a clade with two sequences from Leopardus pardalis, this clade presented good branch support greater than 86%. Furthermore, the pairwise distance between our sequence (OR345322) with respect to the sequences of Leopardus pardalis NC028315 and MW257208 was 0.0034 and 0.022, respectively. As seen in Table 2, the lowest genetic distance (0.0352) occurred between the Leopardus pardalis and Leopardus weidii groups; while the greatest genetic distance occurred between the Leopardus geoffroyi and Herpailurus yaguarondi groups (0.1256). These data are consistent with those presented in the phylogenetic tree (Fig. 4). All of the above allowed us to confirm that the captured individual corresponds to a specimen of Leopardus pardalis (Fig. 4). Table 1. Hematological and serum chemical parameters measured in an adult wild ocelot (Leopardus pardalis) captured in a rural area of the municipality of Buriticá, Antioquia Department, Colombia, in 2023. Reference intervals established in free-ranging/wild (Widmer et al., 2016) and captive ocelots (ISIS, 2023; Species360, 2023) are included for comparison. Dashes indicate unavailable data.
Fig. 2. Egg compatible with Capillaria spp. in a urine sample of Leopardus pardalis. DiscussionThe Leopardus lineage comprises seven species of small spotted felines distributed throughout South and Central America. These species are closely related, and although some literature reports indicate that mitochondrial cytochrome B sequencing is not sufficient to differentiate between sister species since interspecific distances are lower than intraspecific diversity (García-Alaníz et al., 2010); however, the use of genetic markers (COI, COII, COIII, Cytb, ND1-6, 4L, ATP6, ATP8, lrRNA, srRNA) depends on the species to be identified, for which an analysis of previous genetic reports must be carried out (Janczewski et al., 1995; Masuda et al., 1996; Adrados et al., 2019). In the present study, the genetic identity of the individual (Leopardus pardalis) was established and validated based on the use of the COI marker. This mitochondrial and universal marker has been used more frequently due to its advantages such as: high variability, durability, and small size. (Linacre et al., 2011). According to the branch supports of the phylogenetic tree (Fig. 4) and the pairwise distance analysis (Table 2), it was possible to establish that the sample OR345322 corresponds to the Leopardus pardalis clade.
Fig. 3. Coproparasitological findings. Parasites found in Leopardus pardalis. (A) Toxocara cati, (B) Ascaridea, (C) Ancylostoma, D) Dicrocoelium spp., and E) a larva in feces sample. The hematological profile showed that no altered values were identified in relation to the morphology of the red blood cells or their hemoglobin concentration. Regarding white blood cells, a slight leukopenia was identified at the expense of incipient lymphopenia and neutropenia. However, the study did not consider that these differences are biologically relevant. These findings contribute to the development of reference patterns of wild animals, contributing to the findings previously described in Brazil that resemble the reference values established for animals in captivity (Widmer et al., 2016; ISIS, 2023; Species 360, 2023). Blood chemistry analyses in the literature report differences between the reference values of animals in captivity compared to animals in the wild (Widmer et al., 2016; ISIS, 2023; Species 360, 2023). In the case of the ocelot in this report, most results are within the normal ranges established for animals in captivity. However, when comparing the results of the analytes of the feline in this work with the reference values established for ocelots in the wild, a hypoglobulinemia value of 43.9 g/l was found (Ref. Values 50–90.9 g/l in Widmer et al. (2016)); creatinine with a value of 145.86 µmol/l (61–114.9 µmol/l in Widmer et al. (2016)) and urea with a value of 10.01 µmol/l (0.01–0.12 µmol/l in Widmer et al. (2016)) were elevated. Moreover, cholesterol levels were also elevated (24.57 mmol/dl) when compared to the reference values in the wild (Ref. Values 3.4–6.1 mmol/L in Widmer et al. (2016)) and in captivity (Ref. Values 3.39–14.54 mmol/L in ISIS (2023), and 2.35–7.80 mmol/L in Species360 (2023)). Furthermore, an elevated amylase value was registered compared to the reference values in captivity. With these results, kidney failure is suspected since elevated urea and creatinine values associated with a decreased urinary density with moderate dehydration are clinical indicators of a kidney function disorder. However, the current study did not carry out follow-up, monitoring, or treatment of the ocelot. Therefore, a diagnosis of the health status of the animal could not be made (Sparkes et al., 2016).
Fig. 4. Phylogenetic analysis of the COI gene using maximum likelihood. The evolutionary history was inferred using the substitution model (GTR+G) to model evolutionary rate differences between sites. Support values are derived from 1,000 bootstrap replicates. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The bar indicates 0.020 substitutions per site. The green circle corresponds to the LP001 sequence. Evolutionary analyses were performed using the MEGA v11 software. Table 2. Estimates of evolutionary divergence over sequence pairs between groups. The number of base substitutions per site from averaging over all sequence pairs between groups are shown. Analyses were conducted using the maximum composite likelihood model. This analysis involved 18 nucleotide sequences. Codon positions included were 1st+2nd+3rd+Noncoding. All ambiguous positions were removed for each sequence pair (pairwise deletion option). There were a total of 663 positions in the final dataset. Evolutionary analyses were conducted in the MEGA v11 software.
In the evaluation of the peripheral blood smear tinted with Wright’s stain, structures compatible with apicomplex protozoan hemoparasites were observed in the form of a ring inside the erythrocytes, which possibly correspond to Cytauxzoon spp., reported in wild felines in some studies (Davis, 1929; Ketz-Riley et al., 2003; Luaces et al., 2005; André et al., 2011; Alvarado-Rybak et al., 2016; Wang et al., 2017). In Brazil, there are reports of infection by Cytauxzoon spp. in ocelots (André et al., 2009, 2011). In the case of ocelots in Colombia, Cytauxzoon spp. has been identified in reports in the literature but without molecular confirmation (Ayala et al., 1973). Since smear tests are the only evidence, it is important to clarify that direct observations of blood smears do not allow species identification and molecular techniques are needed. Likewise, Wright’s stain is neither diagnostic nor indicated for the morphological identification of hemoparasites. Nematodes such as Ancylostoma, Ascaridea, and Toxocara cati are common in mammals that have been extensively studied. However, the report of Dicrocoelium spp. in humans and domesticated and wild carnivores is until now considered an atypical finding, making this report the first documented case of Dicrocoeliosis spp. in ocelots. These infections can occur due to the consumption of infected livers or coprophagy of ruminant feces, as reported in the literature (Burger et al., 2006; Mitchell et al., 2017; Moure et al., 2017). Capillaria spp. are widely distributed parasites and can be found in numerous domestic and wild carnivores (Bédard et al., 2002; Fiorello et al., 2006; Whitehead, 2009; Guimarães et al., 2020; Alam, 2023). However, this constitutes the first report of this parasite in the bladder of a wild ocelot, and this infection may contribute significantly to the kidney failure that is evident in the blood chemistry analysis carried out since, in other studies, this type of infection in other mammals has been reported related to mild renal failure (Bédard et al., 2002; Rossi et al., 2011). It is also important to mention that the habitat of the ocelot in this study has strong anthropogenic pressures, such as livestock and mining, which can also affect the health status of the animal. Although no association between the environment and the health of ocelots has been reported, the manifestation of chronic kidney failure has been documented in humans living in mining environments (Afrifa et al., 2017; Coulibaly et al., 2020). ConclusionLike other small feline species, the ocelot (Leopardus pardalis) has received little attention in health research, which is why these findings have important implications for the conservation of this species. The information obtained about its health expands knowledge about the clinical parameters of this species in wild conditions, information that enriches the literature and that, through future reviews, will contribute to the elaboration of guides for the clinical management of this species and the formulation of more effective conservation strategies. In addition to the findings and contributions of this study, which enrich the information on the parameters and analytes of blood chemistry and hematology, this study reports, for the first time, infections by Capillaria sp. and Dicrocoelium sp. for the species in the wild. This study highlights the need to continually monitor the health and genetics of ocelot populations to guarantee their long-term survival and thus favor protection strategies for this species and its habitat. Likewise, this report contributes to monitoring infectious diseases and their circulation among different species, including humans, as an expanded public health strategy under a One Health vision to prevent and manage emerging infectious diseases that invites reflecting on considering health states and the ecology of diseases. Interactions between species are a variable of interest and a determining factor in public health and conservation approaches. AcknowledgmentThe researchers express their gratitude to the Research Vice-Rectory of Corporación Universitaria Remington, the Deanship of the Veterinary Medicine Faculty, Panthera’s Small Cat Program, and the environmental team of Zijin-Continental Gold for their support during the project. Similarly, we acknowledge and appreciate the assistance of Maricruz Jaramillo, Victor Quiroz, Jacobo Chamorro, Valentina Martínez, María Clara Vasquez, and Juliana Jaramillo in the field during capture and monitoring. Many thanks to Laurel Seyries for sharing her knowledge and training the local team. We also extend our appreciation to Professor Clara Susana Arias for her initial efforts within the project from the Faculty of Veterinary Medicine of CUR. Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionsM.F.S.: Research, processing of chemical and hematological samples, information analysis, writing, review; J.L.: Conceptualization, processing of chemical and hematological samples, data analysis, review, and editing; M.E.: Conceptualization, processing of chemical, hematological samples, and diagnosis of hemoparasites, data analysis, review, and editing; C.U.M.: Conceptualization, molecular studies, data analysis, review, and editing; HA.B.Ll.: Conceptualization, parasitological studies, data analysis, review, and editing; A.P.: Monitoring, capture, field studies, clinical analysis; D.S.: Conceptualization, review, editing, project administration; and J.F.Z.: Information analysis, writing, review, editing, project administration. FundingThis study was conducted within a solidarity project framework of Corporación Universitaria Remington [code no. 4000000368]. Data availabilityAll information and data related to this study is explicitly detailed in the manuscript. ReferencesAdrados, B., Zanin, M., Silveira, L., Villalva, P., Chávez, C., Keller, C., González-Borrajo, N., Harmsen, B J., Rubio, Y. and Palomares, F. 2019. Non-invasive genetic identification of two sympatric sister-species: Ocelot (Leopardus pardalis) and margay (L. wiedii) in different biomes. Conserv. Genet. Resour. 11(2), 203–217. Afrifa, J., Essien-Baidoo, S., Ephraim, R.K.D., Nkrumah, D. and Dankyira, D. O. 2017. Reduced egfr, elevated urine protein and low level of personal protective equipment compliance among artisanal small scale gold miners at Bibiani-Ghana: a cross-sectional study. BMC Public Health 17(1), 601. Aguirre, E. By A.A., Ostfeld, R.S., Tabor, G.M., House, C. and Pearl, M.C. (Eds.). 2002. Conservation Medicine: Ecological Health in Practice. Oxford, UK: Oxford University Press. Alam, H.M. 2023. Chapter 2-Parasites in the urogenital tract of dogs and cats. En T. Rana (Ed.), Organ-Specific Parasitic Diseases of Dogs and Cats. Cambridge, MA: Academic Press, pp: 33–51 Alvarado-Rybak, M., Solano-Gallego, L. and Millán, J. 2016. A review of piroplasmid infections in wild carnivores worldwide: Importance for domestic animal health and wildlife conservation. Parasit. Vectors 9(1), 538. André, M.R., Adania, C.H., Machado, R.Z., Allegretti, S.M., Felippe, P.A.N., Silva, K.F., Nakaghi, A.C.H. and Dagnone, A.S. 2009. Molecular detection of Cytauxzoon spp. In asymptomatic Brazilian wild captive felids. J. Wildl. Dis. 45(1), 234–237. André, M.R., Adania, C.H., Teixeira, R.H.F., Allegretti, S.M. and Machado, R.Z. 2011. Molecular and serological detection of babesia spp. In neotropical and exotic carnivores in Brazilian Zoos. J. Zoo. Wildl. Med. 42(1), 139–143. Anile, S., Devillard, S., Ragni, B., Rovero, F., Mattucci, F., and Valvo, M.L. 2019. Habitat fragmentation and anthropogenic factors affect wildcat Felis silvestris silvestris occupancy and detectability on Mt Etna. Wildlife Biol. 2019(1), 1–13. Ayala, S.C., D’Alessandro, A., Mackenzie, R. and Angel, D. 1973. Hemoparasite infections in 830 wild animals from the Eastern Llanos of Colombia. J. Parasitol. 59(1), 52–59. Barea-Azcón, J.M., Virgós, E., Ballesteros-Duperón, E., Moleón, M. and Chirosa, M. 2007. Surveying carnivores at large spatial scales: a comparison of four broad-applied methods. Biodiver. Conserv. 16(4), 1213–1230. Barnes, T.S., Goldizen, A.W. and Coleman, G.T. 2008. Hematology and serum biochemistry of the brush-tailed rock-wallaby (Petrogale penicillata). J. Wildl. Dis. 44(2), 295–303. Bédard, C., Desnoyers, M., Lavallée, M.-C. and Poirier, D. 2002. Capillaria in the bladder of an adult cat. Can. Vet. J. 43(12), 973–974. Burger, N.C., Nesvadba, J., Nesvadba, Z., Busato, A. and Gottstein, B. 2006. The incidence of dicrocoelium dendriticum in Emmental. Berl. tierarztl. Wschr. 119(7–8), 324–329. Carver, S., Bevins, S.N., Lappin, M.R., Boydston, E.E., Lyren, L.M., Alldredge, M. and VandeWoude, S. 2016. Pathogen exposure varies widely among sympatric populations of wild and domestic felids across the United States. Ecol. Appl. 26(2), 367–381. Che, J., Chen, H.M., Yang, J.X., Jin, J.Q., Jiang, K., Yuan, Z.Y., Murphy, R.W. and Zhang, Y.P. 2012. Universal COI primers for DNA barcoding amphibians. Mol. Ecol. Resour. 12(2), 247–258. Corantioquia. 2022. Ruta declaratoria de área protegida regional Serranía de Buriticá. Available via https://www.corantioquia.gov.co/wp-content/uploads/2023/06/Cartilla-digital-DRMI-Serrania-de-Buritica_VF.pdf Coulibaly, G., Sanou, G., Sanon, M., Lengani, A.H.Y., Bonzi, J.Y. and Semde, A. 2020. Clinical, paraclinical, and evolutionary profiles of kidney failure in gold miners hospitalized in a nephrological service in a Sub-Saharan African Country. Int. J. Nephrol. 2020, 4282969. Davis, L.J. 1929. On a piroplasm of the Sudanese wild cat (Felis ocreata). Trans. R. Soc. Trop. Med. Hyg. 22(6), 535–537. Deem, S.L., Karesh, W.B. and Weisman, W. 2001. Putting theory into practice: wildlife health in conservation. Conserv. Biol. 15(5), 1224–1233. Diaz-Pulido, A. and Payán Garrido, E. 2010. Densidad de ocelotes (Leopardus pardalis) en los llanos colombianos. Mastozool. Neotrop. 17(1), 63–72. Fabián Estrada, M.B., Otárola Mayhua, J. and Tarqui Terrones, K. 2014. Manual de procedimientos de laboratorio para el diagnóstico de los parásitos intestinales del hombre. Ferreira, L., Morteo, R. and Silva, J. 1962. Padronização de técnicas para o exame parasitológico das fezes. J. Bras. Med. 6(2), 241–257. Fiorello, C.V., Robbins, R.G., Maffei, L. and Wade, S.E. 2006. Parasites of free-ranging small canids and felids in the Bolivian Chaco. J. Zoo. Wildl. Med. 37(2), 130–134. García-R, S., Botero-Cañola, S., Sánchez-Giraldo, C. and Solari, S. 2019. Habitat use and activity patterns of Leopardus pardalis (Felidae) in the Northern Andes, Antioquia, Colombia. Biodiver. 20(1), 5–19. García-Alaníz, N., Naranjo, E.J. and Mallory, F.F. 2010. Hair-snares: a non-invasive method for monitoring felid populations in the Selva Lacandona, Mexico. Trop. Conserv. Sci. 3(4), 403–411. Guimarães, A., Aguilera, V.C.O., Gomes, D.P.P., Zanesco, E.V., Oliveira, Á.F.X., Stocco, N.V., Andrade, G.F.P., Souza, N.C., Souza, H.J.M. and Baldani, C.D. 2020. Urinary capillariosis in a cat from Rio de Janeiro, Brazil-Clinical, morphological and phylogenetic characterization. Vet. Parasitol. 20, 100409. Hebert, P.D., Cywinska, A., Ball, S.L., and DeWaard, J.R. 2003. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. B. Biol. Sci. 270(1512), 313–321. ISIS. 2023. Ocelot (Leopardus pardalis) physiological reference ranges (both sexes, all ages). Animal Diversity Web. Available via https://animaldiversity.org/accounts/Leopardus_pardalis/ Janczewski, D.N., Modi, W.S., Stephens, J.C. and O’Brien, S.J. 1995. Molecular evolution of mitochondrial 12S RNA and cytochrome b sequences in the pantherine lineage of Felidae. Mol. Biol. Evol. 12(4), 690–707. Jörger, K.M. and Schrödl, M. 2013. How to describe a cryptic species? Practical challenges of molecular taxonomy. Front. Zool. 10, 1–27. Ketz-Riley, C.J., Reichard, M.V., Van Den Bussche, R.A., Hoover, J.P., Meinkoth, J. and Kocan, A.A. 2003. An intraerythrocytic small piroplasm in wild-caught Pallas’s Cats (Otocolobus manul) from Mongolia. J. Wildl. Dis. 39(2), 424–430. Li, J., Cui, Y., Jiang, J., Yu, J., Niu, L., Deng, J. and Yue, B. 2017. Applying DNA barcoding to conservation practice: a case study of endangered birds and large mammals in China. Biodiversity. Conser. 26(3), 653–668. Linacre, A., Gusmão, L., Hecht, W., Hellmann, A.P., Mayr, W.R., Parson, W., Prinz, M., Schneider, P.M., y Morling, N. 2011. ISFG: recommendations regarding the use of non-human (animal) DNA in forensic genetic investigations. Forensic Sci. Int. Genet. 5(5), 501–505. Luaces, I., Aguirre, E., García-Montijano, M., Velarde, J., Tesouro, M.A., Sánchez, C., Galka, M., Fernández, P. and Sainz, Á. 2005. First report of an intraerythrocytic small piroplasm in Wild Iberian Lynx (Lynx pardinus). J. Wildl. Dis. 41(4), 810–815. Marin-Villa, J., Úsuga-Monroy, C., Bedoya-LLano, H., Flores-Solarte, M J., Loaiza-Escobar, M.J., Erazo-Cadena, M. and Pizarro-Correal, A. 2023. Leopardus pardalis isolate LP001_Panthera_COL cytochrome c oxidase subunit I (COX1) gene, partial cds; mitochondrial (2551780950; OR345322) [dataset]. GenBank; NCBI Nucleotide Database. Available via http://www.ncbi.nlm.nih.gov/nuccore/OR345322.1 Masuda, R., Lopez, J.V., Slattery, J.P., Yuhki, N. and O’Brien, S.J. 1996. Molecular phylogeny of mitochondrial cytochrome B and 12S rRNA sequences in the Felidae: Ocelot and domestic cat lineages. Mol. Phylogenet. Evol. 6(3), 351–365. Mccarthy, J., Belant, J., Breitenmoser, C., Hearn, A. and Ross, J. 2013. Live trapping carnivores in tropical forests: Tools and techniques to maximise efficacy. Raffles Bull. Zool. 28, 55–66. Mitchell, G., Cuthill, G., Haine, A., Zadoks, R., Chaudhry, U., Skuce, P. and Sargison, N. 2017. Evaluation of molecular methods for the field study of the natural history of Dicrocoelium dendriticum. Vet. Parasitol. 235, 100–105. Moure, Z., Zarzuela, F., Espasa, M., Pou, D., Serre-Delcor, N., Treviño, B., Bocanegra, C., Molina, I., Pumarola, T. and Sulleiro, E. 2017. Dicrocoelium dendriticum: an unusual parasitological diagnosis in a reference international health unit. Am. J. Trop. Med. Hyg. 96(2), 355–357. Ogassawara, S., Benassi, S., Larsson, C.E. and Hagiwara, M.K. 1986. Prevalência de endoparasitas em gatos na cidade de São Paulo. Revista da Faculdade de Medicina Veterinária e Zootecnia da Universidade de São Paulo, 23(1):39–46. Rossi, M., Messina, N., Ariti, G., Riggio, F. and Perrucci, S. 2011. Symptomatic capillaria plica infection in a young European cat. J. Feline. Med. Surg. 13(10), 793–795. Sparkes, A.H., Caney, S., Chalhoub, S., Elliott, J., Finch, N., Gajanayake, I., Langston, C., Lefebvre, H.P., White, J. and Quimby, J. 2016. ISFM consensus guidelines on the diagnosis and management of feline chronic kidney disease. J. Feline. Med. Surg. 18(3), 219–239. Species360. 2023. Conservation case studies and collaboration. Species360. Available via https://species360.org/serving-conservation/conservation-case-studies/ Terborgh, J., Lopez, L., Nuñez, P., Rao, M., Shahabuddin, G., Orihuela, G., Riveros, M., Ascanio, R., Adler, G.H., Lambert, T.D. and Balbas, L. 2001. Ecological meltdown predator-free forest fragments. Science 294(5548), 1923–1926. Volobouev, V.T., Aniskin, V.M., Lecompte, E. and Ducroz, J.F. 2002. Patterns of karyotype evolution in complexes of sibling species within three genera of African murid rodents inferred from the comparison of cytogenetic and molecular data. Cytogenet. Genome Res. 96(1–4), 261–75. Wang, J.-L., Li, T.-T., Liu, G.-H., Zhu, X.-Q. and Yao, C. 2017. Two tales of cytauxzoon felis infections in domestic Cats. Clin. Microbiol. Rev. 30(4), 861. Whitehead, M. 2009. Urinary capillariosis in a cat in the UK. Vet. Rec. 165(25), 757. Widmer, C.E., Matushima, E.R. and de Azevedo, F.C.C. 2016. Clinical evaluation, hematology, and serum chemistry of ocelots (Leopardus pardalis) in the atlantic forest of Brazil. J. Wildl. Dis. 52(4), 916–921. Widmer, C.E., Perilli, M.L.L., Matushima, E.R. and Azevedo, F.C.C. 2017. Live-trapping Ocelots (Leopardus pardalis): traps, baits, injuries, immobilization and costs. Biota Neotrop. 17, e20150125. Zinsstag, J., Crump, L., Schelling, E., Hattendorf, J., Maidane, Y.O., Ali, K.O., Muhummed, A., Umer, A.A., Aliyi, F., Nooh, F., Abdikadir, M.I., Ali, S. M., Hartinger, S., Mäusezahl, D., de White, M.B.G., Cordon-Rosales, C., Castillo, D.A., McCracken, J., Abakar, F. and Cissé, G. 2018. Climate change and one health. FEMS Microbiol. Lett. 365(11), fny085. | ||
| How to Cite this Article |
| Pubmed Style Solarte MF, Loaiza J, Eraso M, Úsuga-monroy C, Llano HA, Pizarro A, Stasiukynas D, Zapata JF. Clinical and diagnostic evaluation of a wild ocelot (Leopardus pardalis) specimen. Open Vet. J.. 2024; 14(8): 1866-1876. doi:10.5455/OVJ.2024.v14.i8.15 Web Style Solarte MF, Loaiza J, Eraso M, Úsuga-monroy C, Llano HA, Pizarro A, Stasiukynas D, Zapata JF. Clinical and diagnostic evaluation of a wild ocelot (Leopardus pardalis) specimen. https://www.openveterinaryjournal.com/?mno=194929 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i8.15 AMA (American Medical Association) Style Solarte MF, Loaiza J, Eraso M, Úsuga-monroy C, Llano HA, Pizarro A, Stasiukynas D, Zapata JF. Clinical and diagnostic evaluation of a wild ocelot (Leopardus pardalis) specimen. Open Vet. J.. 2024; 14(8): 1866-1876. doi:10.5455/OVJ.2024.v14.i8.15 Vancouver/ICMJE Style Solarte MF, Loaiza J, Eraso M, Úsuga-monroy C, Llano HA, Pizarro A, Stasiukynas D, Zapata JF. Clinical and diagnostic evaluation of a wild ocelot (Leopardus pardalis) specimen. Open Vet. J.. (2024), [cited January 25, 2026]; 14(8): 1866-1876. doi:10.5455/OVJ.2024.v14.i8.15 Harvard Style Solarte, M. F., Loaiza, . J., Eraso, . M., Úsuga-monroy, . C., Llano, . H. A., Pizarro, . A., Stasiukynas, . D. & Zapata, . J. F. (2024) Clinical and diagnostic evaluation of a wild ocelot (Leopardus pardalis) specimen. Open Vet. J., 14 (8), 1866-1876. doi:10.5455/OVJ.2024.v14.i8.15 Turabian Style Solarte, María Flórez, Juliana Loaiza, Marcela Eraso, Cristina Úsuga-monroy, Horwald A.b. Llano, Andrea Pizarro, Diana Stasiukynas, and Juan Felipe Zapata. 2024. Clinical and diagnostic evaluation of a wild ocelot (Leopardus pardalis) specimen. Open Veterinary Journal, 14 (8), 1866-1876. doi:10.5455/OVJ.2024.v14.i8.15 Chicago Style Solarte, María Flórez, Juliana Loaiza, Marcela Eraso, Cristina Úsuga-monroy, Horwald A.b. Llano, Andrea Pizarro, Diana Stasiukynas, and Juan Felipe Zapata. "Clinical and diagnostic evaluation of a wild ocelot (Leopardus pardalis) specimen." Open Veterinary Journal 14 (2024), 1866-1876. doi:10.5455/OVJ.2024.v14.i8.15 MLA (The Modern Language Association) Style Solarte, María Flórez, Juliana Loaiza, Marcela Eraso, Cristina Úsuga-monroy, Horwald A.b. Llano, Andrea Pizarro, Diana Stasiukynas, and Juan Felipe Zapata. "Clinical and diagnostic evaluation of a wild ocelot (Leopardus pardalis) specimen." Open Veterinary Journal 14.8 (2024), 1866-1876. Print. doi:10.5455/OVJ.2024.v14.i8.15 APA (American Psychological Association) Style Solarte, M. F., Loaiza, . J., Eraso, . M., Úsuga-monroy, . C., Llano, . H. A., Pizarro, . A., Stasiukynas, . D. & Zapata, . J. F. (2024) Clinical and diagnostic evaluation of a wild ocelot (Leopardus pardalis) specimen. Open Veterinary Journal, 14 (8), 1866-1876. doi:10.5455/OVJ.2024.v14.i8.15 |