| Research Article | ||
Open Vet J. 2024; 14(7): 1568-1576 Open Veterinary Journal, (2024), Vol. 14(7): 1568–1576 Research Article Morphological and molecular characterization of Dermanyssus gallinae (Acari: Gamasida: Dermanyssidae) isolates of Al- Baha provinceSamia Q. Alghamdi*Department of Biology, Faculty of Science, Al-Baha University, Al-Baha, Saudi Arabia *Corresponding Author: Samia Q. Alghamdi. Department of Biology, Faculty of Science, Al-Baha University, Al-Baha, Saudi Arabia. Email: sqassim [at] bu.edu.sa Submitted: 05/04/2024 Accepted: 19/06/2024 Published: 31/07/2024 © 2024 Open Veterinary Journal
AbstractBackground: An ectoparasite known as Dermanyssus gallinae feeds on infected blood with a high frequency in European chicken farms resulting in significant economic losses. Aim: The objective of the current work was to characterize D. gallinae, which infests laying hens on farms in Southern Al-Baha morphologically, and molecularly, and to determine the evolutionary relationship between the species. Methods: All mites that were morphologically recognized as D. gallinae were submitted to molecular analysis by PCR, which focused on the mitochondrial cytochrome oxidase subunit I (cox1) and internal transcribed spacers (ITS) of ribosomal DNA. Results: Morphological identification of the parasites uncovered three distinct features: a triangular anal shield, a broader than longer sternal shield, and a rounded posterior genitoventral shield. Each D. gallinae sample was amplified using a single band, measuring 550 bp for the cox1-targeting PCR, and 530 bp for the ITS-targeting PCR. The sequences of D. gallinae were added to the GenBank. Conclusion: At the molecular identification level, this research identifies D. gallinae in Al-Baha for the first time. The results collectively provide a foundation for further research to understand the epidemiology and the part of this superfamily in the epidemiology of certain zoonosis. Keywords: Demanyssus gallinae, Morphologically and molecularly identification, cox1 gene, ITS, Phylogenetic. IntroductionDermanyssus gallinae, De Geer, 1778 (Acari, Mesostigmata) (i.e., “poultry chicken mite” (PRM)) is a cosmopolitan nocturnal blood-sucking ectoparasite of primary poultry and other birds (Arends, 1991; Roy and Chauve, 2007). Dermanyssus gallinae has been involved as vectors of animal and human pathogens (Moro et al., 2005; Moro et al., 2007; Schiavone et al., 2022). Also, there have been several reports of additional poultry viruses, including those that cause chicken pox and avian spirochaetosis. Newcastle viruses, pullorum disease, fowl typhoid fever, and fowl cholera. In addition, this possible vectors red poultry mite D. gallinae is significant as a direct pest, as all developmental stages suck blood and reproduce (Abdel-Ghaffar et al., 2008; Valiente Moro et al., 2009). Human attacks have predominantly occurred during diurnal periods in professional environments or during nocturnal hours in residential dwellings. Due to the lack of an accurate diagnosis, symptoms can endure for an extended period of time (Barlaam et al., 2022). The frequency of infestations has been found to be higher in European cities where residents rarely interact with chickens (Cafiero et al., 2019). However, the infestation rate in Saudi, particularly in Al-Baha is approximately 11.76%. Therefore, one of the most harmful blood-feeding ectoparasites impelling chicken productivity is the poultry mite D. gallinae. This parasite also infects a wide variety of wild birds, producing stress, pain, and nervousness in the afflicted birds, finally become agitated and irritable. The most prevalent symptoms, which include cannibalism, feather pecking, sleep disturbances, and itching, might result in anaemia that can be fatal (Kilpinen et al., 2005). According to the reports, at least 28 different kinds of birds are parasitized by this mite (Øines and Brännström, 2011), and it is thought to be a big issue in poultry farms in the United States (Roy et al., 2010) Europe (Huong et al., 2014) and Japan (Murano, 2006). Theoretically, comparable to other species of mites that have been studied in (Qi et al., 2023; Van Leeuwen et al., 2010); the genetic composition of Dermanyssus populations may vary and this variation could be linked to the reduced susceptibility of D. gallinae to acaricides. Consequently, the dissemination of resistance could be facilitated by the movement of mites that are resistant to the different chicken farms. Previous study in Al-Baha has reported that infestation mostly caused by three ectoparasite species in chicken: lice, fleas, and mites; Four different species of chicken lice: Menacanthus sramineus, Menopon gallinae, Gonoides gigas, and Goniocotes gallinae were discovered. In contrast, one species of mite, Dermanyssus gallina, and one species of flea, Echidnophaga gallinacean, was also discovered (Gharsan and Elhassan, 2019). An examination of the genetic makeup of mite populations in areas where resistance to commonly used acaricides has been observed could provide valuable insights into the phenomenon of rising acaricide resistance. This, in turn, could lead to the development of novel ways to identify and manage populations that are resistant (Fujisawa et al., 2023). A global distribution of D. gallinae has been shown (Potenza et al., 2009), and studies examining its nucleotide sequence have been conducted in Europe, the US, Brazil, and Australia. To date, no molecular studies have assessed genetic variability patterns among worldwide populations of D. gallinae. Molecular data have long been recognized as an attractive marker for phylogenetic analysis (Marangi et al., 2009; Marangi et al., 2014). Nevertheless, data on the genetic identification of D. gallinae in Saudi Arabia is insufficient, including Al-Baha. This work involved the analysis of (cox1) gene and ITS sequences from mite samples taken in various locations in Al-Baha. These sequences were then compared with sequences obtained from other countries to determine the genetic diversity of D. gallinae. A lot of molecular markers have been used in several Acari over the past few years to investigate a range of topics, such as the definition of taxonomy, comprehension of population structure, identification of geographic origin, and even their ecological adaptation and food preferences (Yuan et al., 2024). Thus far, a number of genes with both nuclear and mitochondrial encoding have been preferentially amplified as molecular markers (Koç-İnak et al., 2024). Therefore, this work determines evolutionary relationship between species using mitochondrial cytochrome c oxidase subunit 1 (cox1) gene and internal transcribed space (ITS) as genetic markers. Materials and MethodsStudy area and red mite collectionIn August 2022, a total of 102 chickens (82 females and 20 male) were manually examined and selected from farm in Al-Baha city, South West Saudi Arabia. It is located at 19°51’43.1”N 41°34’18.6”E; (Fig. 1). The collected chickens were delivered to the Department of Biology, Faculty of Science, Al-Baha University. The poultry red mites were isolated from chickens using forceps. After that, the entire exterior of the body (the leg) was immersed in 70% ethyl alcohol and kept at −20°C. The concentrations of DNA were determined using a NanoDrop™ Lite Spectrophotometer at wavelength 260/280 nm (A260/A280) (Thermo Fisher Scientific, Wilmington, DE). Morphological identification of mitesThe chicken mite, also known as the red poultry mite (scientifically referred to as D. gallinae), was observed and analyzed using a stereomicroscope with a digital camera (Nikon SMZ18, Japan) at a magnification of 1×. Dermanyssus gallinae are characterized by Idiosoma broadly rounded posteriorly, wider body (2–3 mm), the genitoventral shield is equipped with one pair of setae and one pair of epigynal pores. The anal shield is equipped with three anal setae. The terms used are as follows: anal opening; anal shield; CxII, which refers to the second coxa; gs, which stands for genitoventral shield; LI–IV, which represents leg I–IV; and Pa, which denotes pedipalp. Its identification was based on its morphological characteristics, utilizing the morphological keys provided by Baker (1999). Furthermore, in total, 12 chicken was infested with mite collected from 102 chicken examined (an infestation rate of 11.76% of collected mite) and identified morphologically as D. gallinae.
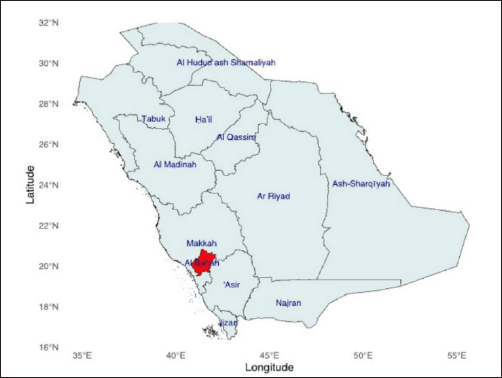
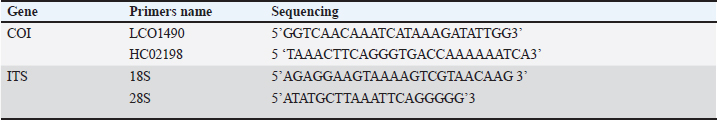
Fig. 1. Dermanyssus gallinae sampling site. Map of Al-Baha showing the location of the sampling sites that were used for PRM collection. Molecular identificationDNA extraction and amplification of (cox1) gene and ITS regions The genomic DNA (gDNA) of D. gallinae sample was extracted from indiviual D. gallinae sample. Dermanyssus gallinae was homogenized using a mini-prep DNA isolation kit (GeneAll® Exgene™ Clinic SV DNA Isolation Kit, Seoul, South Korea), that could use a few tweaks. Under particular conditions, D. gallinae samples were incubated for 16 hours on a heat-block using 200 µl of lysis buffer (CL) and 20 µl of proteinase K. Next, the steps that followed were carried out following the manufacturer’s directions. After being eluted into 30 µl of Tris buffer (pH=8.5, warmed to 70°C), the whole DNA was kept at −20°C until it was time for PCR amplification. Afterward, a fragment of mitochondrial gene cytochrome c oxidase subunit 1 (cox1) gene, and the ITS of the ribosomal DNA were amplified using the primers listed in Table 1. To determine the cox1 (cytochrome c oxidase subunit I) gene, the gDNA from each D. gallinae sample was utilized to amplify a specific region of the mitochondrial gene cox1, resulting in an amplicon size of 710 base pairs. The primers utilized in this investigation were LCOX1 GENECOX1 GENE490 and HC02198, as described by Folmer et al. in 1994. The PCR amplification was performed using a 50 µl reaction solution consisting of 2 µl of each primer (0.4 M final concentration), 20 µl of BioMix Red (2x concentration, Bioline, BIO-25006, Meridian Bioscience, Germany), and 5 µl of DNA template. The reaction was carried out in a thermal cycler (TECHNE TC-512, Chelmsford, Essex, CM1 3UP, England, UK). Water without any nucleases was used as a negative control. The PCR was conducted for a total of 40 cycles. Each cycle consisted of a denaturation phase at 94°C for 1 minute, followed by 5 cycles at 94°C for 1 minute, 45°C for 90 seconds, and 72°C for 90 seconds. The remaining 35 cycles were performed at 94°C for 1 minute, 50°C for 90 seconds. The final step was an extension at 72°C for 5 minutes (Folmer et al., 1994). The amplified segments of the target genes were subjected to bidirectional sequencing using the use of the primer sets shown in Table 1. The ITS of the ribosomal DNA (̴ 400 bp) was amplified from mite samples by PCRs primers of a fragment of 18S of the rDNA gene (Navajas et al., 1999). The polymerase chain reaction (PCR) was conducted using a 25-μl solution consisting of 10 μm of each oligonucleotide primer, 12.5 μl of BioMix Red, 2x (Bioline, BIO-25006) PCR Reaction Mix (Sigma, St. Louis, MO), and 5 μl of gDNA. The samples were subjected to denaturation at a temperature of 94°C for a duration of 4 minutes. Amplification was then performed for a total of 30 cycles, with each cycle consisting of 1 minute of denaturation at 93°C, 1 minute of annealing at 50°C, and 1 minute of extension at 72°C. The PCR result was observed using electrophoresis on a 1.5% agarose gel in 1x TBE buffer (SERVA Electrophoresis, Heidelberg, Germany). The gel was dyed with SYBR Safe DNA gel stain (Invitrogen, Life Technologies Corp, Carlsbad CA 92008, USA) and subjected to an electric field of 150 V for 30 minutes. The PCR products were purified using a PCR Purification Kit from QIA-quick, manufactured by QIAGEN Inc. in Valencia, CA. Every reaction included a positive control. The negative control was prepared using PCR-grade water. The PCR products underwent bi-directional sequencing at Macrogen Ltd. located in Seoul, South Korea. DNA sequencing and phylogenetic analysis The purified products of the cox1 and ITS regions were directly sequenced using the identical primers at the Macrogen sequencing facility (Macrogen Inc., Seoul, Republic of Korea). Sequences were obtained from mite samples of D. gallinae, representing the location farm in Al-Baha. The sequences were trimmed from each end of the sequences then create consensus sequence obtained using Bioedit software Hall (1999). A BLASTn search was conducted on the cleaned sequences against the NCBI GenBank database. The sequences used in this investigation were aligned with sequences from GenBank that were homologous and closely related based on the BLASTn results. The CLUSTALW multiple alignment tool in Bioedit was employed for this purpose (Thompson et al., 1997; Hall, 1999). Phylogenetic analyses were conducted using neighbor-joining approach in MEGA-X to compare the current sequences with from published sequences from gene bank (Kumar et al., 2018). By utilizing Kimura’s two-parameter model of substitution with 1000 replicates, we were able to estimate neighbor-joining bootstrap values, which allowed us to infer phylogenetic relationships in MEGA X. Bootstrap values of >70% and above were deemed to have strong support (Hillis and Bull, 1993). Table 1. Primer sets used to amplify the mitochondrial and nuclear genes.
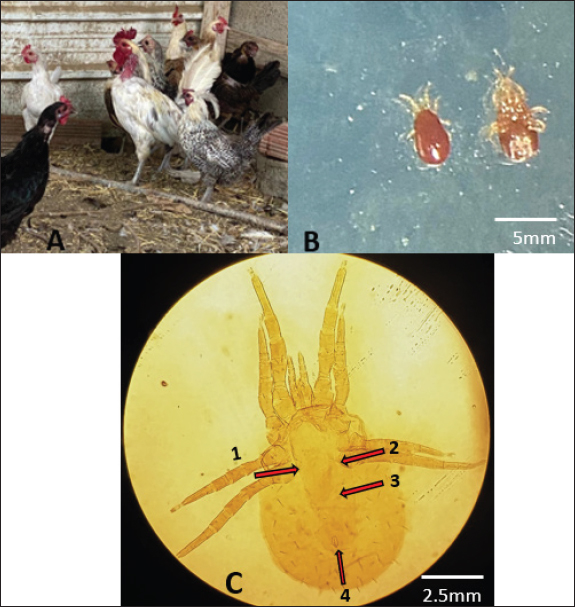
Ethical approvalThe specimens were obtained in accordance with ethical norms and a study protocol, with informed consent from Al-Baha University, in the Al-Baha province of Saudi Arabia. The infected animal was investigated after obtaining written approval from the owners of the private farms where they were located. ResultsInfestation rates of the collected D. gallinaeThe results of our study indicated that the infestation rate of the examined chickens with D. gallinae was 11.76% (the infested number/total number of examined chickens x100). Morphological identification of D. gallinaeFifty specimens of mites were first morphologically identified as D. gallinae using morphological keys (Baker, 1999). The morphological identification of the collected D. gallinae was carried out by meticulous examination under the stereomicroscope (Fig. 2). Generally, D. gallinae is characterized by Idiosoma broadly rounded posteriorly, wider body (2–3 mm), the genitoventral shield is equipped with one pair of setae and one pair of epigynal pores. The anal shield is equipped with three anal setae. The terms used are as follows: anal opening; anal shield; CxII, which refers to the second coxa; gs, which stands for genitoventral shield; LI-IV, which represents leg I-IV; and Pa, which denotes pedipalp. Eight samples of mites that were already morphologically confirmed as D. gallinae were chosen for molecular confirmation.
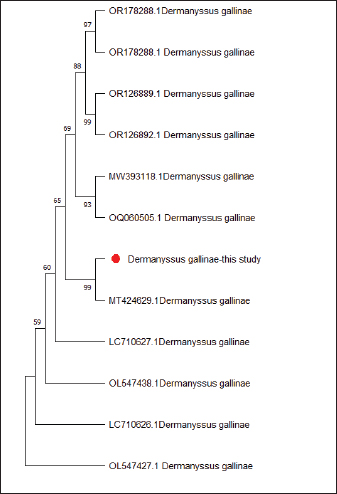
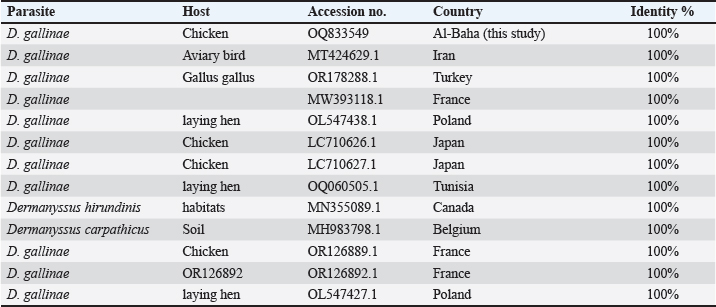
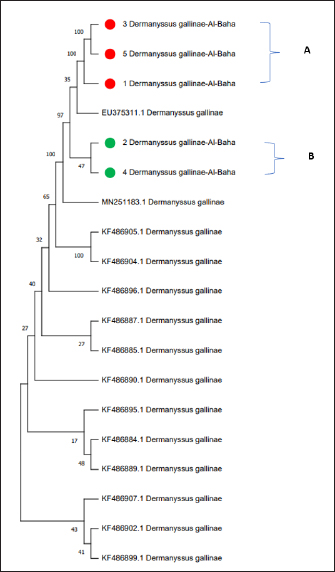
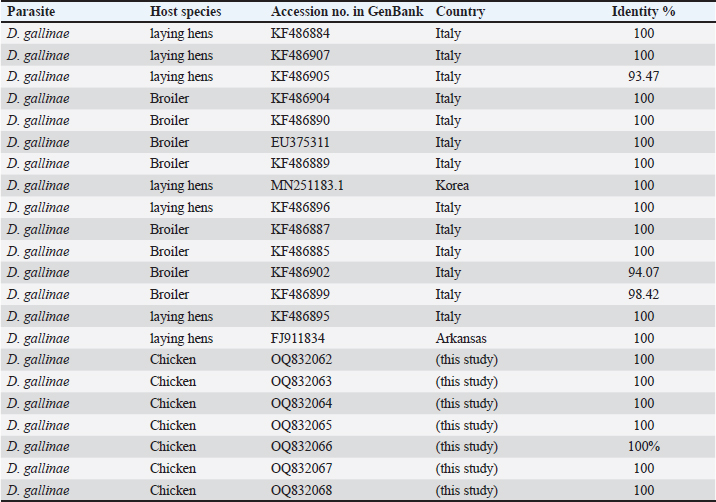
Fig. 2. (A) The poultry infested with D. gallinae. (B) Shows an adult D. gallinae observed under a stereomicroscope with a 40X magnification. (C) 1,2. The genitoventral shield of D. gallinae with one pair of setae; 3. Sternal shield (wider than longer) of D. gallinae; 4. Anal shield of D. gallinae with setae. Molecular identification, sequencing and phylogenetic analysisIn this study, D. gallinae was assembled from Al-Baha province. They were recognized based on (cox1) and (ITS), yielding a band of approximately 550 bp, and 530 bp in length, respectively (Fig. 3). The BLAST analysis results confirmed the morphological identification because the sequences that were extracted from the samples showed a high degree of similarity to those of D. gallinae, with an identity ranging from 99.57% to 100%. In the present study, we described the nucleotide sequences of the cox1 gene and ITS gene, which have been deposited in the GenBank database under the accession numbers (OQ833549) and (OQ832062- OQ832068), respectively. In this study, Figure 4 and Table 2 have shown the outcome obtained from BLAST analysis of cox1 gene revealed that D. gallinae sequence (GenBank accession number OQ833549) was similar to Iran (MT424629.1) with 99.57% similarity and 100%coverage. From ITS gene it was revealed that our sample are more closely related to the sequences from Italy (Accession number EU375311.1) (Fig. 5, Table 3). Dermanyssus gallinae is present in all 100 hits that the BLAST engine retrieved. Based on 530 bp, the phylogeny revealed that the Saudi Arabian D. gallinae sequences are more closely linked to Italy (EU375311.1) and Korea (MN251183.1). Fourteen ITS sequences from specimens collected in Al-Baha were used for phylogenetic analysis, and the results indicated that the sequences formed two distinct clades with bootstrap values of 100% and 97%.
Fig. 3. PCR amplification applying D. gallinae s samples using: ITS the internal transcribed spacers of the ribosomal; (M) a DNA ladder placed on the left sides of the gel; fragment sizes characterized in base pairs (530 bp). (Bands 1-4 D. gallinae samples); sample five negative control.
Fig. 4. Phylogenetic tree based on the analysis of the partial cytochrome c oxidase subunit I (cox1) gene of D. gallinae isolates. Only one sequence from the current investigation, indicated by a red circle, and 12 reference sequences representing D. gallinae were included in the analysis for comparative purposes. Table 2. Phylogenetic affiliation of partial coding sequences of cox 1 gene D. gallinae to sequences of closest D. gallinae relatives listed in the GenBank nucleotide sequence database.
DiscussionDermanyssus gallinae, poses a danger to the global poultry sector. PRM-infested chickens display a number of health issues, such as anemia, hypothenia, and decreased feed conversion, which lowers egg production and causes large financial losses (Fujisawa et al., 2023). Given that Orinthonyssus sylviarum, the northern fowl mite, and D. galline share the same host and habitat, so it is crucial to distinguish the two species based on their morphological characteristics (Di Palma et al., 2012). Consequently, molecular techniques are used for D. gallinae phylogenetic research (Salim et al., 2023). Some of the studies have shown that nucleotide sequences have been employed by numerous laboratories in the US, Europe, Brazil, Australia, and Japan to obtain molecular characteristics of D. gallinae. The mite migrates both within and between the nations, according to molecular epidemiological studies (Chu et al., 2015). The previous study in Al-Baha recorded the lowest infestation rate of 3.13% (Gharsan and Elhassan, 2019). Approximately D. gallinae with total infestation of 29.31% (17.24% in males and 12.07% in females) has been reported in the Hail region of Saudi Arabia (Abdelmageed et al., 2018). The differences in the prevalence may be due to the numerous factors, such as the season of inspection, population density, feeding fluctuations, personal care of birds, climate, and sanitation etc., In spite of the abundance of morphological investigations, genetic data on D. gallinae identification in Saudi Arabia is quite limited. However, our results of infestation rate disagree with the findings of Salim et al. (2023) carried out in Punjab region of Pakistan, where it was found that the infestation peak of D. gallinae was 44%. The rate of infestation of this species seems to differ between the countries. For instance, the infestation rates in Egypt, Algeria, and Tunisia were found to be 2.35% (Hassan et al., 2023), 1.18%, (Nahal et al., 2021), 24.14%, 74% (Gharbi et al., 2013) respectively.
Fig. 5. Phylogenetic tree based on the analysis of (ITS) gene of D. gallinae isolates. Sequences generated from the present study are shown in green and red circle, and 14 reference sequences representing D. gallinae were included in the analysis for comparative purposes. GenBank accession numbers and sample identification numbers (in red and green) are reported. ‘A’ and ‘B’: main clades. In the current investigation, the collected mites were identified using cox 1 region that is obtained from mitochondrial DNA fragments, and ITS region from nuclear. Based on morphology, the species being studied had been determined to be D. gallinae. The D. gallinae species has previously been registered in Genebank, as evidenced by the top hits returned by BLAST search, and the sequences from this work represent the first molecular data for this species that have been recorded. Molecular research, such as DNA amplification, sequencing, and phylogenetic analysis, is crucial in verifying the identification of D. gallinae. Katakam et al. (2010) carried out an identical type of investigation on D. gallinae, who extracted DNA from the adult D. gallinae of chicken and amplified it by (PCR-COX1 AND ITS) assay. Table 3. Phylogenetic affiliation of partial coding sequences of ITS gene D. gallinae to sequences of closest D. gallinae relatives listed in the GenBank nucleotide.
This is the first report that reveals the genetic identification of D. gallinae in Saudi Arabia. The evolutionary tree exhibits a strong species-level clustering trend (Figs. 4 and 5). The molecular characterization of D. gallinae (ITS) and, (coxI) was carried out on samples taken from chicken of various homes in Al-Baha. The sequence data that was acquired was uploaded to the NCBI repository. Both locally and worldwide, this research may have a major impact on the genetic information available on poultry red mites. ConclusionThis study is a significant milestone to characterize D. gallinae in Al-Baha genetically. A genetic study of the mitochondrial and ribosomal ITS regions classified all mites that were morphologically confirmed to be D. gallinae. Therefore, it is recommended that the molecular methodology may be used as the main methodological approach for accurately identifying D. gallinae. Overall, our findings provide a foundation for future research aimed at gaining a more comprehensive understanding of the epidemiology of this superfamily and its involvement in the epidemiology of certain zoonotic diseases. AcknowledgmentsThe author thanks the Deanship of Scientific Research at Al-Baha University for providing support in conducting this study (8/1442). FundingThe research work is supported by the fund from Deanship of Scientific Research at Al-Baha University (No. 8/1442). Data AvailabilityAll data are provided in the manuscript. Conflict of interestThe author declares that there is no conflict of interest. ReferencesAbdel-Ghaffar, F., Al-Quraishy, S., Sobhy, H. and Semmler, M. 2008. Neem seed extract shampoo, Wash Away Louse®, an effective plant agent against Sarcoptes scabiei mites infesting dogs in Egypt. Parasitol. Res. 104, 145–148. Abdelmageed, E., Abdelgadir, M., Babiker, M.Y. and AlRashidi, M. 2018. Survey of external parasites of house sparrows (passer domesticus) in Hail Region, Saudi Arabia. Adv. biores. 9, 1. Arends, J.J. 1991. External parasites and poultry pest. In: Disese of Poultry, pp: 718–720. Baker, A.S. 1999. Mites and ticks of domestic animals: an identification guide and information source. Stationery Office. Barlaam, A., Puccini, A., Caiaffa, M.F., Di Bona, D., Macchia, L. and Giangaspero, A. 2022. Dermanyssosis in the Urban context: when the one health paradigm is put into practice. Pathogens 11, 1396. Cafiero, M.A., Barlaam, A., Camarda, A., Radeski, M., Mul, M., Sparagano, O. and Giangaspero, A. 2019. Dermanysuss gallinae attacks humans. Avian Pathol. 48, S22–S34. Chu, T.T.H., Murano, T., Uno, Y., Usui, T. and Yamaguchi, T. 2015. Molecular epidemiological characterization of poultry red mite, D. gallinae, in Japan. J. Vet. Med. Sci. 77, 1397–1403. Di Palma, A., Giangaspero, A., Cafiero, M.A. and Germinara, G.S. 2012. A gallery of the key characters to ease identification of D. gallinae (Acari: Gamasida: Dermanyssidae) and allow differentiation from Ornithonyssus sylviarum (Acari: Gamasida: Macronyssidae). Parasites Vectors 5, 1–10. Folmer, O., Black, M., Hoeh, W., Lutz, R. and Vrijenhoek, R.C. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Marine Biol. Biotechnol. 3, 294–299. Fujisawa, S., Murata, S., Isezaki, M., Win, S.Y., Sato, T., Oishi, E., Taneno, A., Maekawa, N., Okagawa, T. and Konnai, S. 2023. Suppressive modulation of host immune responses by D. gallinae infestation. Poult. Sci. 102, 102532. Gharbi, M., Sakly, N. and Darghouth, M.A. 2013. Prevalence of D. gallinae (Mesostigmata: Dermanyssidae) in industrial poultry farms in North-East Tunisia. Parasite. 20, 3. Gharsan, F.N. and Elhassan, S. 2019. Prevalence of infection with ectoparasites among chickens of Albaha Region-Kingdom of Saudi Arabia. JAFT. 9, 1–6. Hall, T.A., 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In: Nucleic acids symposium series, pp: 95–98. Hassan, T.A., El-Gawady, H.M., El-Gayer, A.K., and Sallam, N.H. 2023. Morphological and molecular studies of ecto-and endoparasites infested chicken in Ismailia Province, Egypt. J. Adv. Vet. Res. 13, 352–359. Hillis, D.M. and Bull, J.J. 1993. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst. Biol. 42, 182–192. Huong, C.T.T., Murano, T., Uno, Y.,Usui, T. and Yamaguchi, T. 2014. Molecular detection of avian pathogens in poultry red mite (D. gallinae) collected in chicken farms. J. Vet. Med. Sci. 76, 1583–1587. Katakam, K.K., Nejsum, P., Kyvsgaard, N.C., Jørgensen, C.B. and Thamsborg, S.M. 2010. Molecular and parasitological tools for the study of Ascaridia galli population dynamics in chickens. Avian Pathol. 39, 81–85. Kilpinen, O., Roepstorff, A., Permin, A., Nørgaard-Nielsen, G., Lawson, L., and Simonsen, H. 2005. Influence of D. gallinae and Ascaridia galli infections on behaviour and health of laying hens (Gallus gallus domesticus). Br. Poult. Sci. 46, 26–34. Koç-İnak, N., Demırcı, B., Özbakiş-Becerıklısoy, G., Kandemır, B., Nalbantoğlu, S., and Akan, M. 2024. Screening of avian pathogens and genetic analysis of several mitochondrial genes in D. gallinae populations in Türkiye. Syst Appl Acarol. 29, 143–152. Kumar, S., Stecher, G., Li, M., Knyaz, C. and Tamura, K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547. Marangi, M., Cafiero, M.A., Capelli, G., Camarda, A., Sparagano, O. and Giangaspero, A. 2009. Evaluation of the poultry red mite, D. gallinae (Acari: Dermanyssidae) susceptibility to some acaricides in field populations from Italy. Exp. Appl. Acarol. 48, 11–18. Marangi, M., Cantacessi, C., Sparagano, O., Camarda, A. and Giangaspero, A. 2014. Molecular characterization and phylogenetic inferences of D. gallinae isolates in I taly within an European framework. Med. Vet. Entomol. 28, 447–452. Moro, C.V., Chauve, C. and Zenner, L. 2005. Vectorial role of some dermanyssoid mites (Acari, Mesostigmata, Dermanyssoidea). Parasite. 12, 99–109. Moro, C.V., Chauve, C. and Zenner, L. 2007. Experimental infection of Salmonella enteritidis by the poultry red mite, D. gallinae. Vet. Parasitol. 146, 329–336. Murano, T. 2006. Red mite, D. gallinae; ecology and latest problems in Japan. J Jpn Soc Poultry Dis (Japan). 42, 3. Nahal, A., Righi, S., Boucheikhchoukh, M. and Benakhla, A. 2021. Prevalence of ectoparasites in free-range backyard chicken flocks in northeast Algeria. Veterinarska Stanica. 52, 693–702. Navajas, M., Lagnel, J., Fauvel, G. and De Moraes, G. 1999. Sequence variation of ribosomal internal transcribed spacers (ITS) in commercially important Phytoseiidae mites. Exp. Appl. Acarol. 23, 851–859. Øines, Ø. and Brännström, S. 2011. Molecular investigations of cytochrome c oxidase subunit I (COI) and the internal transcribed spacer (ITS) in the poultry red mite, D. gallinae, in northern Europe and implications for its transmission between laying poultry farms. Med. Vet. Entomol. 25, 402–412. Potenza, L., Cafiero, M., Camarda, A., La Salandra, G., Cucchiarini, L., and Dachà, M. 2009. Characterization of D. gallinae (Acarina: Dermanissydae) by sequence analysis of the ribosomal internal transcribed spacer regions. Vet. Res. Commun. 33, 611–618. Qi, X., Li, H., Liu, X., Wang, B., Meng, J., Liu, Q., Sun, W. and Pan, B. 2023. Location of olfactory organs and architecture of gustatory organs in the poultry red mite, D. gallinae (Acari: Dermanyssidae). Zool. Anz. 303, 1–9. Roy, L. and Chauve, C. 2007. Historical review of the genus dermanyssus Dugès, 1834 (acari: mesostigmata: dermanyssidae). Parasite. 14, 87–100. Roy, L., Chauve, C.M. and Buronfosse, T. 2010. Contrasted ecological repartition of the northern fowl mite Ornithonyssus sylviarum (Mesostigmata: Macronyssidae) and the chicken red mite D. gallinae (Mesostigmata: Dermanyssidae). Acarologia. 50, 207–219. Salim, M.A., Lohrasb, S., Abrishami, S., Sahab, A., Babaei, Z., Heshmati, F. and Komeili, N. 2023. Red mite infestation in poultry: morphology, control and prevention. Worlds Poult. Sci. J. 2, 24–32. Schiavone, A., Pugliese, N., Otranto, D., Samarelli, R., Circella, E., De Virgilio, C. and Camarda, A. 2022. D. gallinae: the long journey of the poultry red mite to become a vector. Parasites Vectors. 15, 1–8. Thompson, J.D., Gibson, T.J., Plewniak, F., Jeanmougin, F. and Higgins, D.G. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25, 4876–4882. Van Leeuwen, T., Vontas, J., Tsagkarakou, A., Dermauw, W. and Tirry, L. 2010. Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: a review. Insect Biochem. Mol. Biol. 40, 563–572. Valiente Moro, C., De Luna, C.J., Tod, A., Guy, J.H., Sparagano, O.A. and Zenner, L. 2009. The poultry red mite (Dermanyssus gallinae): a potential vector of pathogenic agents. Control of poultry mites (Dermanyssus), pp: 93–104. Wang, Y., Liu, S.-S., Huang, P., Wang, Z.-J. and Xu, Y.-Q. 2021. Assessing the combined toxicity of carbamate mixtures as well as organophosphorus mixtures to Caenorhabditis elegans using the locomotion behaviors as endpoints. Sci. Total Environ. 760, 143378. Yuan, B., He, G. and Dong, W. 2024. The evolutionary characterization of Gamasida based on mitochondrial genes codon usage pattern. Parasitol. Res. 123, 30. | ||
| How to Cite this Article |
| Pubmed Style Samia Qasem Alghamdi. Morphological and molecular characterization of Dermanyssus gallinae (Acari: Gamasida: Dermanyssidae) isolates of Al- Baha province. Open Vet J. 2024; 14(7): 1568-1576. doi:10.5455/OVJ.2024.v14.i7.6 Web Style Samia Qasem Alghamdi. Morphological and molecular characterization of Dermanyssus gallinae (Acari: Gamasida: Dermanyssidae) isolates of Al- Baha province. https://www.openveterinaryjournal.com/?mno=195381 [Access: June 29, 2025]. doi:10.5455/OVJ.2024.v14.i7.6 AMA (American Medical Association) Style Samia Qasem Alghamdi. Morphological and molecular characterization of Dermanyssus gallinae (Acari: Gamasida: Dermanyssidae) isolates of Al- Baha province. Open Vet J. 2024; 14(7): 1568-1576. doi:10.5455/OVJ.2024.v14.i7.6 Vancouver/ICMJE Style Samia Qasem Alghamdi. Morphological and molecular characterization of Dermanyssus gallinae (Acari: Gamasida: Dermanyssidae) isolates of Al- Baha province. Open Vet J. (2024), [cited June 29, 2025]; 14(7): 1568-1576. doi:10.5455/OVJ.2024.v14.i7.6 Harvard Style Samia Qasem Alghamdi (2024) Morphological and molecular characterization of Dermanyssus gallinae (Acari: Gamasida: Dermanyssidae) isolates of Al- Baha province. Open Vet J, 14 (7), 1568-1576. doi:10.5455/OVJ.2024.v14.i7.6 Turabian Style Samia Qasem Alghamdi. 2024. Morphological and molecular characterization of Dermanyssus gallinae (Acari: Gamasida: Dermanyssidae) isolates of Al- Baha province. Open Veterinary Journal, 14 (7), 1568-1576. doi:10.5455/OVJ.2024.v14.i7.6 Chicago Style Samia Qasem Alghamdi. "Morphological and molecular characterization of Dermanyssus gallinae (Acari: Gamasida: Dermanyssidae) isolates of Al- Baha province." Open Veterinary Journal 14 (2024), 1568-1576. doi:10.5455/OVJ.2024.v14.i7.6 MLA (The Modern Language Association) Style Samia Qasem Alghamdi. "Morphological and molecular characterization of Dermanyssus gallinae (Acari: Gamasida: Dermanyssidae) isolates of Al- Baha province." Open Veterinary Journal 14.7 (2024), 1568-1576. Print. doi:10.5455/OVJ.2024.v14.i7.6 APA (American Psychological Association) Style Samia Qasem Alghamdi (2024) Morphological and molecular characterization of Dermanyssus gallinae (Acari: Gamasida: Dermanyssidae) isolates of Al- Baha province. Open Veterinary Journal, 14 (7), 1568-1576. doi:10.5455/OVJ.2024.v14.i7.6 |