| Research Article | ||
Open Vet. J.. 2024; 14(8): 1808-1818 Open Veterinary Journal, (2024), Vol. 14(8): 1808–1818 Research Article Detection of Trypanosoma lewisi from rodents residing in the densely populated residential regions along the coastal areas of Banyuwangi Sub District, IndonesiaApril Hari Wardhana1,2*, Frenky Laksana Putra3, Aditya Yudhana1,4, Dyah Haryuningtyas Sawitri1, Ening Wiedosari1, Mujiyanto Mujiyanto5, Swastiko Priyambodo6, Mufasirin Mufasirin2,3, Penny Humaidah Hamid7, Yudhi Ratna Nugraheni8, Aan Awaludin9, Priyono Priyono10, Alan Payot Dargantes11 and Makoto Matsubayashi121Veterinary Medicine Study Program, Department of Health and Life Sciences, Faculty of Health, Medicine, and Life Sciences, Universitas Airlangga, Surabaya, Indonesia 2Department of Parasitology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 3Department of Health and Life Sciences, Faculty of Health Science, Medicine and Life Sciences, Universitas Airlangga, Banyuwangi, Indonesia 4Research Group for Animal Biomedical and Conservation, Universitas Airlangga, Surabaya, Indonesia 5Research Center for Public Health and Nutrition, Organization for Health, National Research and Innovation Agency, Cibinong, Indonesia 6Faculty of Agriculture, IPB University, Bogor, Indonesia 7Faculty of Agriculture, Universitas Sebelas Maret, Surakarta, Indonesia 8Department of Parasitology, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia 9Department of Animal Science, Politeknik Negeri Jember, Jember Regency, Indonesia 10Research Center for Behavioral and Circular Economics, Research Organization of Governance, Economy, Community Welfare, National Research and Innovation Agency, Jakarta, Indonesia 11Department of Veterinary Immunology, Osaka Metropolitan University, Osaka, Japan 12Department of Immunology and Epidemiology, Osaka Metropolitan University, Osaka, Japan *Corresponding Author: April Hari Wardhana. Research Center for Veterinary Science, Organization for Health, National Research and Innovation Agency, Cibinong, Indonesia. Email: Wardhana24id [at] yahoo.com Submitted: 02/04/2024 Accepted: 06/07/2024 Published: 31/08/2024 © 2024 Open Veterinary Journal
ABSTRACTBackground: Extensive attention has been devoted to studies of Trypanosoma lewisi in rodents ever since it became recognised as a zoonotic pathogen known as atypical human trypanosomiasis. Regrettably, although T. lewisi infections of small mammals remain significant public health concerns for humans, there is a lack of comprehensive study in Indonesia. Aim: The aim of the study was to detect T. lewisi from rodents residing in the densely populated residential regions along the coastal areas of Banyuwangi Sub District. Methods: A total of 169 rodents were captured across three villages of Kampung Mandar, Lateng and Kepatihan, using rat single live traps. After being euthanized and identified, the blood samples were collected from each rodent via cardiac puncture. Subsequently, the samples were subjected to native (direct blood microscopic examination), microscopic blood smear examination, and molecular analyses utilizing TRYP1S-TRYP1R (623 bp) and LEW1S-LEW1R (220 bp). Results: The results demonstrated that two species of rodents were successfully captured: Rattus norvegicus (65.68%) and Rattus tanezumi (34.32%). Based on the native and microscopic blood smear examinations, the prevalence of T. lewisi across three villages was 23.08% and 24.26% for molecular analysis employing both primers, respectively. The highest prevalence was found in Kampung Mandar Village (31.18%), followed by Kepatihan (16.67%) and Lateng Villages (15.71%). Conclusion: Statistical analysis revealed that T. lewisi was more prevalent in R. tanezumi compared to R. norvegicus. In terms of sex, no statistically significant distinction was observed between female and male infected rodents of either species (p > 0.05), indicating both species can serve as a source of T. lewisi for humans in the surveyed villages. Keywords: Banyuwangi, Public health, Tropical disease, Trypanosoma lewisi, Zoonosis. IntroductionRodents are small cosmopolitan and synanthropic mammals harbouring more than 143 genera of infectious agents, including 14 viruses, 31 bacteria, 83 parasites, and 15 fungi. They are found worldwide and there are 2,277 species identified globally (Issae et al., 2023). In addition to serving as definitive and intermediate hosts for ectoparasites (vector-borne diseases), these animals can transmit a wide range of microbial pathogens both directly and indirectly, including at least 85 zoonotic pathogens (Hardgrove et al., 2021). Ecologically speaking, rodents are most likely to harbour pathogens (Zhang et al., 2022). In terms of one health concept involving the interconnectedness of humans, animals and the environment, it is critical to mitigate the risk associated with rodent reservoirs, especially the escalating risk of spillover at the expanding human-animal interface and the potential expansion of host ranges due to climate change (Kelly et al. 2020). The presence of rodents in urban environments substantially influences the potential for zoonotic pathogens to be transmitted to humans (Azimi et al., 2021; Babyesiza et al., 2024). Due to their function as carriers and reservoirs of pathogenic agents in residential areas, rodents are a significant source of concern for human health (Griffiths et al., 2022). Trypanosoma lewisi is a flagellated blood protozoan parasite in rodents and grouped as obligatory rodent parasites distributed worldwide (Hong et al., 2017). Even though this species is considered to be non-pathogenic to most rodents, it is recognized as a zoonotic pathogen and human infections, including fatal cases as reported in Asia and Africa, known as atypical human trypanosomiasis (Truc et al., 2013; Kumar et al., 2022; Jain et al., 2023). The vector of transmission for this parasite is a flea, Xenopsylla cheopis (X. cheopis), that coexists with rodents. Because fleas can harbor and develop parasites, they have the potential to disseminate rapidly throughout an area. Silva et al. (2010) stated that T. lewisi can cause sporadic and opportunistic flea-borne infection in primates, including humans. Several studies involving T. lewisi in rodents have been documented. Rodríguez et al. (2010) reported that the prevalence of T. lewisi in Rattus rattus spread in Italy was greater than Rattus norvegicus by 54% and 4%, respectively. In Malaysia, Shafiyyah et al. (2012) presented data that T. lewisi infected 1.5% of rats distributed in the traditional market. In addition, Pumhom et al. (2014) studied the prevalence of T. lewisi in three countries and found various infection rates i.e. 16.7% in Thailand, 9.5% in Camboja and 12.4% in Myanmar. Likewise, Nguyen et al. (2022) demonstrated a significant abundance of T. lewisi in rodents (62.5%) captured in hospitals, markets, and train stations. All of the aforementioned investigations demonstrated that T. lewisi can spread in any environment containing rodents. Nevertheless, the investigation of T. lewisi infections in Indonesia is limited, particularly molecular analysis. The studies were restricted to some cities only, such as Malang, South Sulawesi, Banjarnegara, and Surabaya. In a recent study, Wardhana et al. (2024) investigated T. lewisi in rodents captured inside the house, outside the house and in cattle pens located in Aceh and Jakarta employing molecular analysis. The finding revealed that rodents captured inside the house typically had a higher prevalence compared to those outside the house. In addition, rodents trapped in the cattle pens also showed positive T. lewisi. Those studies indicated that investigation of the prevalence T. lewisi is fundamentally needed to provide a comprehensive description of rodents that freely carry the pathogen zoonotic agents and coexist with humans. Banyuwangi is among the sites of interest to investigate T. lewisi in Indonesia. Due to its proximity to the shore, this region possesses an extensive coastline. The region exhibits significant economic potential due to its substantial influx of tourists. The coastal regions in Banyuwangi are predominantly inhabited by individuals who rely on fishing for their livelihood. Generally, they have a relatively dense population, but their income and awareness of healthy living are comparatively low. Consequently, littoral regions are perceived as densely populated, typically slum-like communities (Kharisma, 2020; Monica et al., 2023). The aim of this study was to investigate the prevalence of T. lewisi in Banyuwangi Sub District based on three methods, namely direct blood smear, stained thin blood smear microscopic examination and molecular analyses with polymerase chain reaction (PCR). The study employed rodent traps situated in close proximity to densely populated housing complexes in the coastal regions of Kampung Mandar, Lateng, and Kepatihan villages of Banyuwangi Sub District. Materials and MethodsSites of trapping and sample sizeA total of 169 rodents were captured in Kampung Mandar, Lateng, and Kepatihan Villages in the Banyuwangi Sub-District coastal area, consisting of 93, 70, and 6 samples, respectively. In terms of a geographical standpoint, Kampung Mandar Village shares a southern border with Kepatihan Village and a northern border with Lateng Village (Fig. 1). The single rat live traps constructed of wire, which had the following dimensions: length (34 cm), width (20 cm), and height (15 cm), were used to trap rodents. Rats were captured in May – June 2023. The traps were installed daily in multiple dwellings situated in densely populated coastal regions for a duration of two weeks. The traps are deposited in the afternoon at approximately 5 p.m. and collected at 7 a.m. Salted fish or leftover processed foods (e.g., chicken meat) were utilised as bait in the traps.
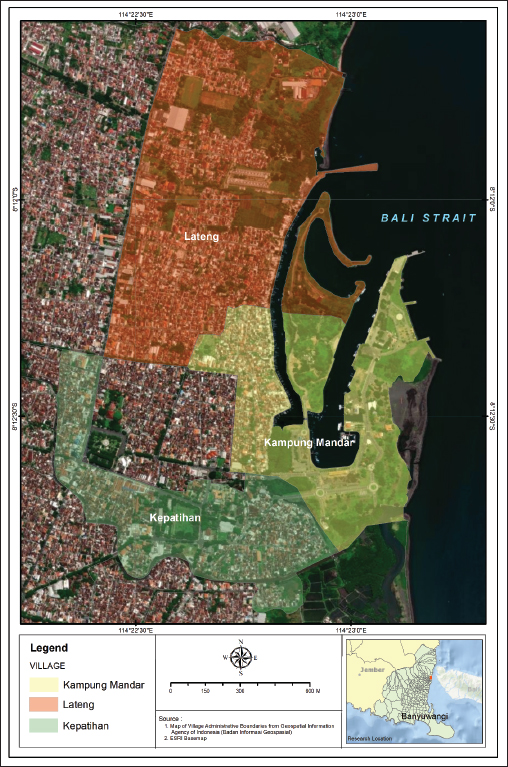
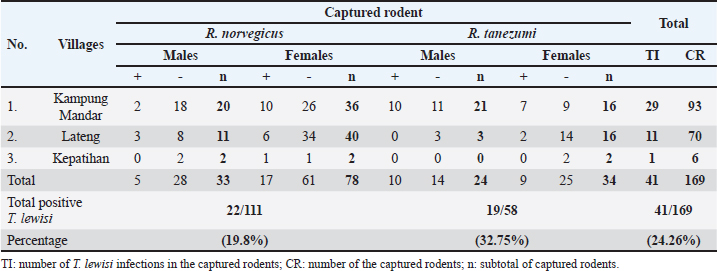
Fig. 1. Locations of sampling: Kampung Mandar, Lateng and Kepatihan Villages located in the coastal region of Banyuwangi Sub District. Rodent identificationRodents were restrained manually by gently grabbing their neck scruff before euthanasia to ensure safe and effective administration of intramuscular injection. The rat is put into an anesthesia tube. Rodents were euthanized intramuscularly using ketamine and xylazine. The identification of captured rodents was based on external morphological attributes, sex and various anatomical components were measured. The body weight (BW) of euthanized rodents was determined using a digital precision weight balance. Additionally, external morphometric measurements were taken using a vernier calliper. Five specific external morphometric parameters were measured, which included head and body length (HB), tail length (L), hind-foot length (HF), ear length (E), and skull length (S). A standard reference was utilised to compare the measurement results (Herbreteau et al., 2011; Ramdani and Prasetyo, 2011; Yuliadi et al., 2016). Blood collection, direct and stained thin blood smear microscopic examinationsAfter being euthanized and identified, blood samples were collected from each rodent with the cardiac puncture. Blood samples collected varied based on rodent size, with small rodents giving 1–2 ml and larger rodents giving 2–3 ml. The volume was adjusted to minimalize stress during collection. The blood samples obtained were poured into an Ethylenediaminetetraacetic acid (EDTA) tube, swiftly shaken, and stored at −20oC for further analysis (Herbreteau et al., 2011). The direct microscopic examination was carried out by taking 3 ml of the fresh blood sample from an EDTA tube and dropping it on the object glass, subsequently covering it with a cover glass. The presence of Trypanosoma spp. was screened in a direct magnification of 400 times under a microscope. The presence of the T. lewisi protozoa with the trypomastigote shape indicated a positive sample. This transparent protozoon causes erythrocytes to move erratically and rapidly through the blood. In addition, a thin blood smear was prepared by dropping 5 mL of the fresh blood samples onto a glass slide, followed by making a blood smear using a cover glass and stained using Dip Quick (eosin and methylene blue) staining (MDT IR®, Indonesia). Briefly, before being fixed in methanol for 3 minutes, the blood smear was dried at room temperature. Then, the fixed smear was put in eosin for 3 minutes and in methylene blue for 3 minutes. After that, the stained blood smear was rinsed using running water and dried at room temperature. The presence of T. lewisi was observed under a microscope at 400-time magnification (Tanthanathipchai et al., 2023). Identification of the morphological characteristics of the parasites that were compatible with T. lewisi was based on the description of Hoare (1972). DNA extractionDNA extraction was obtained from whole blood samples (approximately 300 ml) utilising the Genomic DNA Mini Kit (Geneaid, Taiwan) following the guidelines provided by the manufacturer. Each DNA extraction sample was put in a 1.5 ml Eppendorf tube and labelled with the sample identification number. All samples were kept at −20oC until further use for PCR analysis (Wardhana et al., 2024). PCR analysisTwo primers of DNA were employed to identify T. lewisi using a conventional PCR, namely TRYP1S-TRYP1R and LEW1S-LEW1R, with 623 and 220 bp products, respectively. Both primers were to amplify the DNA fragment of the internal transcribed spacer 1 region (ITS1)/ribosomal DNA (Desquesnes et al., 2002; Desquesnes et al., 2011). Thermal Cycler Biometra Tone PCR brand equipment was utilised for targeted DNA amplification. The following sequences of the primers are TRYP1R (5’-GGA AGC CAA GTC ATC CAT CG-3’), TRYP1S (5’-CGT CCC TGC CAT TTG TACA CA-3’), LEW1S (5’-ACC ACC ACA CGC TCT CTT CT-3’) and LEW1R (5’-TGT ATG TGC GTG CTT GTT CA-3’). For both primers, each reaction consisted of 25 ml of PCR mixture containing My Taq TM HS Mix Bioline (Meridian Bioscience, UK), primers, DNA template, and DNA water. The PCR cycling of both primers was: pre-denaturation of the sample (95°C, 1 minute, 1 cycle); denaturation (95°C, 15 seconds, 35 cycles); annealing (58°C, 15 seconds, 35 cycles); extension (72°C, 15 seconds, 35 cycles); and final extension (72°C, 10 minutes, 1 cycle). The PCR products were observed on a 1.5% TAE (Tris-acetate-EDTA) agarose gel alongside a 1,000 bp DNA ladder. The products were stained using Fluoro® Safe gel staining (1stBase) and the gels were electrophorized using 100 V for 30 minutes and then visualised using the GelDoc Transluminator (Cleaver) machine (Wardhana et al., 2024). Data analysisAll data obtained from the observation were tabulated in Microsoft Excel and analysed statistically with the chi-square test using SPPS Program version 23. We conducted data analysis with a 95% confidence interval and a significance level of p < 0.05 to identify any significant associations or differences between variables. An association between variables was considered significant if the p-value was less than 0.05. Ethical approvalThe Ethical Clearance Committee of the Faculty of Veterinary Medicine, University of Gadjah Mada, Yogyakarta, Indonesia, reviewed and approved this study with certificate No. 055/EC-FKH/Eks/2023. ResultsA total of 169 rodents were captured across three villages situated along the coast of the Banyuwangi Sub District. Based on external morphological observations, two species of rodents, R. norvegicus and Rattus tanezumi, were present in this study. In this study, the comparison of discovered rodent species was conducted descriptively, presenting the percentage of each species found in the studied areas. Kampung Mandar Village had a greater capture rate of rodents (55.03%) than Lateng (41.42%) and Kepatihan (3.55%). The percentages of discovered rodent species in the study sites are presented in Table 1. The quantity of R. norvegicus captured in all three subdistricts was greater (65.68%) than in R. tanezumi (34.32%). The distribution of these numbers was as follows: in Kampung Mandar Village, R. norvegicus comprised 56 individuals, while R. tanezumi comprised 37 individuals; in Lateng Village, R. norvegicus comprised 51 individuals and 19 R. tanezumi; and in Kepatihan Village, 4 R. norvegicus and 2 R. tanezumi (Table 1). Table 1. Prevalences of T. lewisi infection based on species and sex of rodents captured in the three villages along the coast in Banyuwangi Sub District.
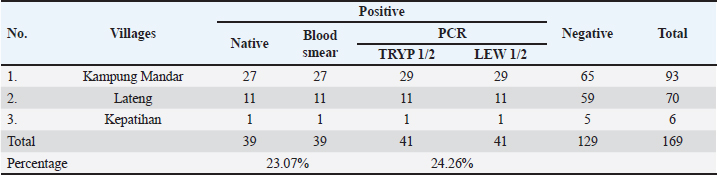
Table 2. Prevalences of T. lewisi infection in captured rodents analyzed using native and PCR methods.
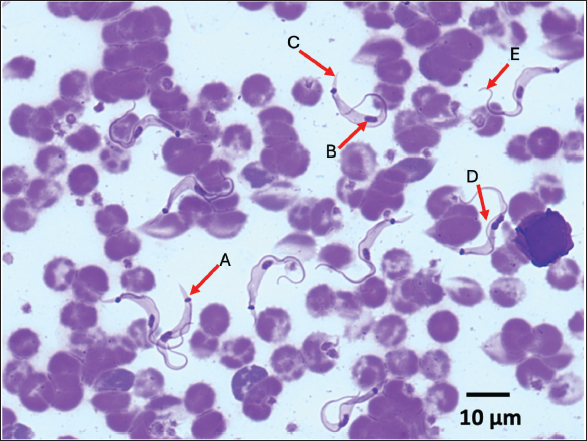
The prevalence of T. lewisi infection in rodents as determined by PCR assay, was found to be 24.46% (41/169) across three sites. The study employed various diagnostic methodologies, each of which yielded slightly different results (Table 2). Figure 2 illustrates the morphological characteristics of T. lewisi. The parasite possesses a long, thin posterior end with a sub-terminal oval kinetoplast, the nucleus is in the anterior part of the body, the shape of the posterior part is pointed, and part of the flagellum is free. Across all villages surveyed, the incidence proportion of T. lewisi based on PCR assay using primer LEW1R-LEW1S was greater on R. tanezumi (32.76%) than on R. norvegicus (19.82%) (Table 1). As compared to TRYP1R-TRYP1S, the primer LEW1R-LEW1S (220 bp) exhibited greater sensitivity. The direct blood and stained thin blood smear microscopic examinations yielded identical results (23.08%; 39/169) marked by trypomastigote shape with flagella, an undulating membrane, kinetoplast, a nucleus, and a pointed posterior end. The molecular analysis produced more precise results. The identical outcomes produced by the two DNA primers indicated that both primers exhibited a comparable degree of sensitivity in detecting T. lewisi in rodents. DiscussionLocation of trappingIn terms of a geographical standpoint, Kampung Mandar Village shares a southern border with Kepatihan Village and a northern border with Lateng Village. The selection of three villages as sites for wild rodent capture was influenced by several factors, including the proximity of the settlements to urban areas and dense population (Dewi et al., 2020), the geography characterised by river crossings, and the historical status of the area as a slum residential zone (Kharisma, 2020). Rodents may potentially establish new habitats in urban residential areas and slum regions (Garcia et al., 2019). Slum settlements are typically characterised by inadequate sanitation, dense populations, and unclean drainage systems. Furthermore, an overabundance of waste that causes environmental pollution potentially establishes an optimal environment conducive to the proliferation and survival of rodents (Dewi et al., 2020; Setiati et al., 2021). The conditions described above were observed in the three subdistricts where rodents were captured for this research.
Fig. 2. The morphological features of T. lewisi are with long thin posterior end, a sub terminal oval kinetoplast (A), an anterior portion of the body housing the nucleus (B), a pointed posterior portion (C), undulating membrane (D) and a free flagellum (E). According to the Banyuwangi District Central Statistics Agency (2023), Kampung Mandar Village has the highest population density in the district at 34,783 people/Km2. Following closely behind are Lateng and Kepatihan, with population densities of 9,365 and 13,668 people/km2, respectively. The villages are traversed by rivers that ultimately empty into the sea. In general, some residents continue to throw trash into the river, resulting in a relatively filthy appearance of the river. As a result of this condition, the rodent population in the region has increased. Setiati and Fatmawati (2023) documented a significant high rodent population in riverside areas. Field observations in the present study revealed the presence of numerous rodents in ditches and river areas, suggesting a comparatively sizable rodent population, particularly in Kampung Mandar and Lateng Villages. Species of rodentsThe capture of these rodents served as an indicator of the potential correlation between human activities and the presence of rodents in these areas (Tanthanathipchai et al., 2023). Garcia et al. (2019) reported that in impoverished areas surrounding the Venezuelan city of Maracay, R. norvegicus predominated (90.53%; 86/95) than R. tanezumi (9.47%; 9/95). Feng and Himsworth (2014) state that R. norvegicus prefers urban environments and regions traversed by rivers. In addition, Setiati et al. (2021) provided identical data that the abundance of R. norvegicus was greater than that of R. tanezumi in river areas. According to a separate report, R. tanezumi thrives in densely populated, close-knit residential areas because the environment is conducive to developing into a commensal rodent. Infection of T. lewisiThe prevalence of T. lewisi infection in Banyuwangi is comparatively lower than the findings reported by Garcia et al. (2019), who performed molecular analysis using the PCR to identify T. lewisi infection in 31.1% of 95 wild rodents captured in a slum region in Venezuela. In contrast, the data presented by Molee et al. (2019) indicate a lower incidence of T. lewisi infection in Thailand (21% per 100 rodents captured), which is lower than the infection that occurred in Banyuwangi. Tanthanathipchai et al. (2023), who documented an 18% prevalence of T. lewisi in the same country, provide support for this report. In comparison to findings from other investigations conducted in various Indonesian cities, the prevalence of T. lewisi in Banyuwangi is comparatively higher. Winterhoff et al. (2020) identified T. lewisi infection in 7.7% of wild rodents in mountainous regions of Sulawesi. In contrast, Yesica et al. (2022) determined the infection rate to be approximately 17.5% among 74 wild rodents in Malang City. According to Mohammed et al. (2018), variations in T. lewisi prevalence levels in a region are influenced by several factors, including differences in geographic location, sample size, presence of vectors, and development status of T. lewisi in the host body. The experimentally infected rodents exhibited a consistent augmentation of parasitemia in the form of trypomastigotes until the ninth day after infection. By the tenth day, this parasitemia level had rapidly doubled and remained unchanged. After several weeks of stagnation and cessation of growth, the protozoa will subsequently vanish from the bloodstream. This natural state of development of T. lewisi could potentially account for discrepancies in prevalence findings across multiple studies. Kampung Mandar Village had the highest T. lewisi prevalence rate (31.18%; 29/93) in comparison to Lateng Village (15.71%; 11/70) and Kepatihan Village (16.6%; 1/6), as determined by the location of rodent captures. Geographic status and the quantity of samples collected from each location are hypothesised to account for this result. In comparison to Lateng Village (0.94 Km2) and Kepatihan Village (0.37 Km2), Kampung Mandar Village has the smallest area (0.12 Km2) among the three locations utilised as research subjects. However, due to Kampung Mandar Subdistrict having the most inhabitants among the Banyuwangi District, the proportion of houses with relatively tiny land ownership and their dense construction are observed (Central Statistics Agency, 2023). Pumhom et al. (2015) observed that rodents captured in close proximity to residential areas and densely populated regions exhibited a high prevalence of T. lewisi infection. The present observations are consistent with those findings. Given the substantial population size, Kampung Mandar Village becomes an optimal habitat for the proliferation and development of feral rodents due to the abundance of food sources in the vicinity. The statistical analysis revealed no significant difference in the capture of male and female rodents for R. norvegicus or R. tanezumi (p > 0.05). Gender dominance among captured rodents is correlated with their behaviour (Gumay et al., 2020). According to Yuliadi et al. (2016) and Dewi et al. (2020), female rodents exhibit heightened activity in their food search, particularly during the periods of lactation and childbirth, as they have a greater nutritional demand. Male rodent behaviour is more closely associated with their inclination to defend their nests and territorial regions, as well as their broader home range, as opposed to that of female rodents (Linardi and Botelho, 2002). In contrast, Linardi and Botelho (2002) stated that there is a tendency for the incidence of T. lewisi to be greater in male compared to female rodents. Male rodents have more expansive home ranges and exhibit behaviour to defend territorial areas. Consequently, X. cheopis exhibits a significantly higher abundance in male than in female rodents (Linardi et al., 1985). In addition, it has been established that the flea X. cheopis serves as a vector for trypanosomiasis, especially the transmission of T. lewisi from rodents to humans (Dahesh and Mikhail, 2016; Ortiz et al., 2018). This circumstance elevates the likelihood that male rodents will acquire T. lewisi at a greater rate than females. Statistical analysis revealed no significant differences between the number of R. tanezumi and R. norvegicus infected with T. lewisi (p > 0.05). According to these findings, Rattus tanezumi is more likely to contract T. lewisi (32.76%; 19/58) than R. norvegicus (19.82%; 22/111). According to Wardhana et al. (2024), the prevalence of T. lewisi infection among R. tanezumi is higher than R. norvegicus. Although R. tanezumi typically inhabits the exterior of a dwelling, they will migrate inside if food becomes scarce or unattainable outside. The diverse dynamic of R. tanezumi contributes to its capacity to transport zoonotic pathogenic agents, such as the pathogenic agent T. lewisi, within the residence (Loan et al., 2015; Widiastuti et al., 2021). Molecular analysisMolecular analysis can be utilised to identify and detect the accurate pathogenic agent. Compared to microscopic or conventional testing methods, this approach yields more precise results. The identical results obtained from molecular analysis using two distinct primers indicated that both primers were capable of detecting T. lewisi in rodents. The amplification of 623 base pairs of T. lewisi DNA was accomplished successfully using the TRYP1R-TRYP1S primer. One limitation of this primer is the presence of three DNA bands (unspecific bands) in the products, among which corresponds to the host DNA. Although this primer is capable of identifying numerous Trypanosoma spp. strains in livestock, it can distinguish T. lewisi from T. vivax (310 bp), T. brucei (520 bp), and T. congolense (680–750 bp) (Desquesnes et al., 2002; Desquesnes et al., 2011). According to Desquesnes et al. (2011), the LEWIR-LEWIR primer possesses the capacity to be a useful tool in subsequent inquiries concerning the following: (i) pathogen screening in laboratory rat colonies; (ii) sylvatic rodent investigation to identify the presence of T. lewisi; (iii) determination of infection prevalence in peri-domestic rodents to estimate the risk of human infection; and (iv) direct, single-step PCR confirmation of T. lewisi identity in animals and humans. Zoonotic aspectsMultiple pathogenic agents that induce rodent-borne diseases in humans reside in rodents. Various zoonotic pathogens, including parasites, viruses, bacteria, and fungi, have been identified and isolated from rodents. Historically, there was a prevailing belief that T. lewisi poses no zoonotic threat to humans due to its host-restricted nature. The organism, however, has been linked to several fatal human infections and has been described as an opportunistic pathogen in several instances (Sarataphan et al., 2005; Verma et al., 2011). As a zoonotic agent, T. lewisi possesses significant medical implications with regard to human health. While there have been no reported cases of human trypanosomiasis in Banyuwangi, the disease has been documented in a number of Asian and African nations, including Thailand, Malaysia, India, and the Gambia (Kumar et al., 2022). Rodents infected with T. lewisi do not exhibit significant clinical symptoms (Truc et al., 2013; Parashar et al., 2016). However, infection with T. lewisi in humans primarily affects infants, who manifest clinical symptoms such as fever, malaise, anaemia, vomiting, anorexia, and lethargy. The most recent documented instance of T. lewisi infection in humans occurred in Uttar Pradesh, India, where a 22-day-old infant presented with clinical manifestations including fever, appetite loss, and lethargy lasting for a duration of three days (Jain et al., 2023). The transmission of T. lewisi to humans correlates with rodents’ cohabitation and interaction, particularly R. norvegicus and R. tanezumi, within the same settlement (Tanthanathipchai et al., 2023). Considered a vector of trypanosomiasis, X. cheopis flea is accountable for transmitting T. lewisi from rodents to humans (Ortiz et al., 2018; Desquesnes et al., 2022). When fleas bite the human epidermis to withdraw blood, an initial infection ensues. Fleas will inevitably excrete faeces when they suck the blood of their host. This bite action will result in the development of open wounds on the epidermis. The expulsion of faeces containing the infective stage (trypomastigotes) is indicative of a positive infection of the flea with T. lewisi. Due to the proximity of the faeces to the bite wound, trypomastigotes are able to penetrate the body and circulate in the bloodstream of the host. Protozoa will undergo division to grow and develop while in the blood (Archer et al., 2018; Molee et al., 2019). The current investigation demonstrated that rats, specifically R. norvegicus and R. tanezumi, that are abundant in residential zones, may serve as vectors for T. lewisi. In order to mitigate the proliferation of pathogenic agents, which are becoming progressively more prevalent, it is imperative to implement control measures and strategies against rodents. It is advisable to restrict human contact with wild and peri-domestic rodents, improve building designs, wear protective gear during cleaning and hygiene practices, and store food properly in order to prevent rodent infestation (Issae et al., 2023). Furthermore, it is imperative that national and international research agencies, in conjunction with the Food and Agriculture Organisation (FAO), the World Organisation for Animal Health (WOAH), and the World Health Organisation (WHO), coordinate the monitoring of human disease cases and the implementation of routine surveillance programmes. This includes the development of diagnostic tools, detection mechanisms, and pharmaceuticals (Truc et al., 2013; Desquesnes et al., 2016). In addition, Parashar et al. (2006) proposed that medical personnel, including general practitioners and veterinarians, must receive comprehensive training to enhance their proficiency in conducting examinations and recognizing pathogenic agents and their vectors. Consequently, it is possible to proactively, efficiently, and effectively prevent T. lewisi infection, particularly in densely populated residential areas in the coastal region such as Kampung Mandar, Lateng, and Kepatihan Villages. The limitation of this study is we did not measure parasitemia levels in our study, as our objective was to ascertain the presence or absence of T. lewisi in captured wild rats. Additionally, we did not investigate the association between parasitemia levels and disease symptoms in rats. Therefore, our study solely focused on detecting the presence or absence of T. lewisi. In conclusion, rodents that are widely distributed along the coastal areas of Banyuwangi District, specifically Kampung Mandar, Lateng, and Kepatihan Villages, possess the potential to function as reservoirs of T. lewisi. This condition may transform into a latent menace that swiftly intensifies into an epidemic affecting the local human populace if immediate measures are not implemented to regulate the rodent population. AcknowledgmentAll authors would like to express our sincere gratitude to the National Research and Innovation Agency for the financial support given to the project and the Faculty of Health, Medicine, and Life Sciences, University of Airlangga, Banyuwangi, for providing all facilities to analyse all samples. The authors thank Mr. Ghofur, Mr. Eko, Mrs. Rosida, and Parasitina team for their support to rodent trapping during the study. FundingFinancial support was provided by the National Research and Innovation Agency. Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionsConceptualization: AHW, AY, DHS, and EW; sampling: AHW, FLP, AY, M, and SP; sample analyses: AHW, FLP, AY, DHS, EW, MS, and PHD; Data analyses: AHW, FLP, AY, DHS, MS, PJ, P, and MM; Writing—original draft preparation: AHW, FLP, AY, DHS, YRN and PHD; Writing—review and editing: AHW, FLP, AY, DHS, APD, MM, YRN, and AA; Supervision: AHW, APD, and MM. Data availabilityAll data are provided in the manuscript. ReferencesArcher, C.E., Schoeman, M.C., Appleton, C.C., Mukaratirwa, S., Hope, K.J. and Matthews, G.B., 2018. Predictors of Trypanosoma lewisi in Rattus norvegicus from Durban, South Africa. J. Parasitol. 104(3), 187–195. Azimi, T., Azimi, L., Fallah, F., Pormand, M.R., Dogehah, H.P. and Tabatabael S.R. 2021. Detection and distribution of zoonotic pathogens in wild Norway rats (Rattus norvegicus) from Tehran, Iran. New Microbe New infect. 42, 100908. Babyesiza, W.S., Katakweba, A., Fornuskova, A., Ssuunaf J., Akoth, S., Mpagi, J., Bellocq, J G., Bryja, J. and Votypka, J. 2024. Trypanosoma diversity in small mammals in Uganda and the spread of Trypanosoma lewisi to native species. Parasitol. Res. 124(54), 1–13. Central Statistics Agency. 2023. Kecamatan Banyuwangi dalam angka/Banyuwangi Subdistrict in figuree 2023. BPS Kapubaten Banyuwangi. Banyuwangi. Hal., pp: 3–9. Dahesh, S.M,A. and Mikhail, M.W. 2016. Surveillance of Trypanosoma spp. of rodents and studies in their transmission probability by fleas in some rural Egyptian areas. J. Egypt. Soc. Parasitol. 6(1), 157–166. Desquesnes, M., McLaughlin, G., Zoungrana, A. and Dávila, A.M.R. 2001. Detection and identification of Trypanosoma of African livestock through a single PCR based on internal transcribed spacer 1 of rDNA. Int. J. Parasitol. 31, 610–614 Desquesnes, M., Ravel, S. and Cuny, G. 2002. PCR identification of Trypanosoma lewisi, a common parasite of laboratory rats. Kin. Biol. Dis. BioMed. Central. 1:1–6. Desquesnes, M., Ketsarin, K., Sarawut,Y., Cristina, M., Sophie, R., Wang, MH, W., Lun, ZR., Serge, M. and Sathaporn, J. 2011. Spesific primers for PCR amplification of the ITS1 (ribosomal DNA) of Trypanosoma lewisi. Infec. Gen. Evol. 1, 1361–1367 Desquesnes, M., Yangtara, S., Kunphukhieo, P., Jittapalapong, S. and Herder, S. 2016. Zoonotic trypanosomes in South East Asia: attempts to control Trypanosoma lewisi using human and animal trypanocidal drugs. Infect. Genet. Evol. 44, 514–521. Dewi, W. M., Partaya, P. and dan Susanti, S. 2020. Prevalensi Ektoparasit pada Tikus sebagai Upaya Pemetaan Risiko Zoonosis di Kawasan Rob Kota Semarang. J. Ekol. Kesehat. 18(3), 171–182. Feng, A.Y. and Himsworth, C.G. 2014. The secret life of the city rat: a review of the ecology of urban Norway and black rats (Rattus norvegicus and Rattus Rattus). Urban Ecosyst. 17, 149–162. Garcia, H.A., Rangel, C.J., Ortíz, P.A., Calzadilla, C.O., Coronado, R.A., Silva, A.J. and Teixeira, M.M. 2019. Zoonotic trypanosomes in rats and fleas of venezuelan slums. Ecohealth. 16, 523–533. Griffiths, J., Yeo, H.L., Yap, G., Mailepessov, D., Johansson, P., Low, H.T., Siew, C.C., Lam, P. and Ng, L.C. 2022. Survey of rodent-borne pathogens in Singapore reveals the circulation of Leptospira spp., Seoul hantavirus, and Rikettsia typhi. Sci. Rep. 12(2692), 1–14. Gumay, D.P., Kanedi, M., Setyaningrum, E. and dan Busman, H. 2020. Keberhasilan Pemerangkapan Tikus (Rattus exulans) dengan Jenis Umpan Berbeda di Kebun Raya Liwa Lampung Barat. J. Med. Malahayati. 4(1), 25–32. Hardgrove, E., Zimmerman, D.M., Icken, A. and Deem, S. 2021. A scoping review of rodent-borne pathogen presence, exposure, and transmission at zoological institutions. Prev. Vet. Med. 193, 105345 Hoare, C.A. 1972. The trypanosomes of mammals. A zoological monograph. Oxford, UK: Black-well Scientific Publications, pp: 749. Hong, X.K., Zhang, X., Fusco, O.A., Lan, Y.G., Lun, Z.R. and Lai, D.H. 2017. PCR-based identification of Trypanosoma lewisi and Trypanosoma musculi using maxicircle kinetoplast DNA. Acta Trop. 171, 207-212. Herbreteau, V., Jittapalapong, S., Rerkamnuaychoke, W., Chaval, Y., Cosson, J.F. and Morand, S. 2011. Protocols for field and laboratory rodent studies. Bangkok, Thailand: Kasetsart University, pp: 5. Issae, A., Chengula, A., Kicheleri, R., Kasanga, C. and Katakweba, A. 2023. Knowledge, attitude and preventive practices toward rodent-borne diseases in Ngorongoro District, Tanzania. J. Public Health Africa. 14, 1–10. Jain, P., Goyal, V. and Agrawal, R. 2023. An atypical Trypanosoma lewisi infection in a 22-day-old neonate from india: an emergent zonosis. Indian J. Pathol. Microbiol. 66(1), 199. Kharisma, Y.I. 2020. Faktor Yang Mempengaruhi Kualitas Hidup Penduduk Permukiman Kumuh Perkotaan Di Kelurahan Kampung Mandar, Banyuwangi. J. Pendidikan. Ilmu. Sosial. 29(2), 118–130. Kelly, T.R., Machalaba, C., Karesh, W.B., Crook, P.Z., Gilardi, K., Nziza, J., Uhart, M.M., Robles, E.A., Saylors, K., Joly, D.O., Monagin, C., Mangombo, P.M., Kingebeni, P.M., Kazwala, R., Wolking, D., Smith, W., Mazet, J.A.K. and Consortium, P. 2020. Implementing one health approaches to confront emerging and re-emerging zoonotic disease threats: lessons from PREDICT. One Health Outlook 2(1), 1–7 Kumar R., Gupta S., Bhutia WD., Vaid RJ. and Kumar S. 2022. Atypical human trypanosomosis : pottentially emerging disease with lack of understanding. Zoonoses Public Health 69(4), 259–276. Linardi, P.M. and Botelho, J.R. 2002. Prevalence of Trypanosoma lewisi in Rattus norvegicus from Belo Horizonte, State of Minas Gerais, Brazil. Mem. Inst. Oswaldo. Cruz. 97(3), 411–413. Linardi, P.M., Botelho, J.R. and Cunha, C.H. 1985. Ectoparasitos de reodores da regiao urbana de Belo Horizonte, MG. II. Oscilacoes dos indices infestacao em Rattus norvegicus. Mem. Inst. Oswaldo. Cruz. 80, 277–284. Loan, H.K., Van Cuong, N., Takhampunya, R., Kiet, B.T., Campbell, J., Them, L.N. and Carrique-Mas, J.J. 2015. How important are rats as vectors of leptospirosis in the Mekong Delta of Vietnam? Vector-Borne Zoonotic Dis. 15(1), 56–64. Mohammed, E.S., El Kady, A.M., Youseef, A.G. and Hassan, A.A. 2018. Distribution pattern of Trypanosoma lewisi in (Rattus norvegicus) in Egypt. Biomed. J. 1(4), 36–39. Molee, P.W., Sakulsak, N. and Saengamnatdej, S. 2019. Detection of Trypanosoma spp. in Bandicota indica from the Thai-Myanmar border area, Mae Sot District Tak Province, Thailand. Asian Pac. J. Trop. Med. 12(10), 457–462. Monica, F., Jamika, F.I., Razak, A., Handayuni, L., Yuniarti, E. and Fauzi, M. 2023. Literatur review: Strategi Penanganan Pemukiman Kumuh di Kelurahan Batang Arau Kota Padang terkait Sanitasi dan Kesehatan Lingkungan. J. Serambi. Eng. 8(1), 65–72. Nguyen, L.K., Koizumi, N., Ung, T.H., Le, T.T., Hirayama, K., Hasebe, F. and Miura, K. 2022. Detection of Trypanosoma lewisi DNA from Rattus norvegicus and Rattus Rattus in Hanoi, Vietnam. Vector Borne Zoonotic Dis., 22(2), 159–161. Ortiz, P.A., Garcia, H.A., Lima, L., Silva, F.M., Campaner, M., Pereira, C.L., and Teixeira, M.M., 2018. Diagnosis and genetic analysis of the worldwide distributed Rattus-borne Trypanosoma (Herpetosoma) lewisi and its allied species in blood and fleas of rodents. Infect. Genet. Evol. 63, 380–390. Parashar, R., Singla, L. and Kaur, P. 2016. Is atypical human Trypanosomosis an emering threat to human society? A Debatable one health issue to public health experts and parasitologists. Int. J. Vet. Sci. 2(1), 36–41. Pumhom, P., Morand, S., Tran, A., Jittapalapong, S. and Desquesnes, M. 2015. Trypanosoma from rodents as potential source of infection in human-shaped landscapes of South-East Asia. Vet. Parasitol. 208(3–4), 174–180. Pumhom, P., Pognon, D., Yangtara, S., Thaprathorn, N., Milocco, C., Douangboupha, B. and Desquesnes, M. 2014. Molecular prevalence of Trypanosoma spp. in wild rodents of Southeast Asia: influence of human settlement habitat. Epidemiol. Infect. 142(6), 1221–1230. Ramdani, Y.R. and dan Prasetyo, B. 2011. Perumusan Pedoman Pengendalian Tikus Dan Mencit Di Balai Besar Karantina Pertanian Soekarno-Hatta. [e-book]. Available via https://scholar.google.com/ (Accessed 15 January 2024). Rodríguez, N., Tejedor-Junco, M., Hernández-Trujillo, Y., González, M., and Gutiérrez, C. 2010. The role of wild rodents in the transmission of Trypanosoma evansi infection in an endemic area of the Canary Islands (Spain). Vet. Parasitol. 174(3–4), 323–327. Sarataphan, N., Vongpakorn, M., Nuansrichay, B., Autarkool, N., Keowkarnkah, T., Rodtian, P. and Jittapalapong, S. 2007. Diagnosis of a Trypanosoma lewisi-like (Herpetosoma) infection in a sick infant from Thailand. J. Med. Microbiol. 56(Pt 8), 1118–1121. Setiati, N. and Fatmawati, L. 2023. Distribution of rats and endoparasites zoonoses risk in Tandang Village, Tembalang District, Semarang City. Inter. J. Sci. Res. Updates. 5(1), 152159. Setiati, N., Auliya, R., Partaya, P., Bodijantoro, F.P.M.H., Indriyanti, D.R. and Widiyaningrum, P. 2021. Types of rats and their parasites that potential to transmit disease in Tugu District, Semarang City. J. Biol. Educ. 13(3), 363–368. Shafiyyah, S.C., Jamaiah, I., Rohela, M., Lau, Y.L. and Aminah, F.S. 2012. Prevalence of intestinal and blood parasites among wild rats in Kuala Lumpur, Malaysia. Trop. Biomed. 29(4), 544–550. Silva, F.M., Marcili, A., Ortiz, P.A., Epiphanio, S., Campaner, M., Cata˜o-Dias, J.L. and Teixeira, M.M. 2010. Phylogenetic, morphological and behavioural analyses support host switching of Trypanosoma (Herpetosoma) lewisi from domestic rats to primates. Infect. Genet. Evol. 10(4), 522–529. Tanthanathipchai, N., Mitsuwan, W., Chaisiri, K., Thaikoed, S., de Lourdes Pereira, M., Paul, A. K. and Saengsawang, P. 2023. Trypanosoma lewisi in blood of Rattus Rattus complex residing in human settlements, Nakhon Si Thammarat, Thailand: Microscopic and molecular investigations. Comp. Immunol. Microbiol. Infect. Dis. 98, 102010. Truc, P., Bu¨scher, P., Cuny, G., Gonzatti, M. I., Jannin, J., Joshi, P. and Desquesnes, M. 2013. Atypical human infections by animal trypanosomes. PLoS Negl. Trop. Dis. 7(9), 1–7. Verma, A., Manchanda, S., Kumar, N., Sharma, A., Goel, M., and Banerjee PS. 2011. Case report: Trypanosoma lewisi or T. lewisi-like infection in a 37-day-old Indian infant. Am. J. Trop. Med. Hyg. 85(2), 221–224. Wardhana, A.H., Sawitri, D.H., Wiedosari, E., Mulyadi, A., Kurniawan, A., Sinaga, L.A., and Hamid, P.H., 2024. Molecular detection of Trypanosoma lewisi in rodents distributed in dairy cattle pens and residential areas. In IOP Conference Series: Earth and Environmental Science, 1292(1), 012038. Widiastuti, D., Pramestuti, N., Sholichah, Z., Setiani, E. and Rizki, R.L.P. 2021. Detection of pathogenic leptospires in rat and shallow populations and its spatial distribution in Bakaran Kulon Village, Pati District. Insights Public Health J. 2(1), 1–9. Winterhoff, M.L., Achmadi, A.S., Roycroft, E.J., Handika, H., Putra, R.T., Rowe, K.M. and Rowe, K.C. 2020. Native Introduced trypanosome parasites in endemic and introduced murine rodents of Sulawesi. J. Parasitol. 106(5), 523–536. Yesica, R., Holizah, Y.N., Pratiwi, H., Hardian, A.B., Kusumarini, S. and dan Wisesa, I.B.G.R. 2022. Data Prevalensi, Pemetaan Spasial, Analisis Morfologi, dan Morfometrik Trypanosoma lewisi Pada Tikus Liar Di Malang. Acta Vet. Indones. 1, 71–79. Yuliadi, B. and Muhidin, I.S. 2016. Tikus Jawa: Teknik Survei di Bidang Kesehatan, pp: 1–101. Zhang, K., Fu, Y., Li, J. and Zhang L. 2022. Public health and ecological significance of rodents in Cryptosporidium infection. One Health 14, 100364. | ||
| How to Cite this Article |
| Pubmed Style Wardhana AH, Putra FL, Yudhana A, Sawitri DH, Wiedosari E, Mujiyanto M, Priyambodo S, Mufasirin M, Hamid PH, Nugraheni YR, Awaludin A, Priyono P, Dargantes AP, Matsubayashi M. Detection of Trypanosoma lewisi from rodents residing in the densely populated residential regions along the coastal areas of Banyuwangi Sub District, Indonesia. Open Vet. J.. 2024; 14(8): 1808-1818. doi:10.5455/OVJ.2024.v14.i8.9 Web Style Wardhana AH, Putra FL, Yudhana A, Sawitri DH, Wiedosari E, Mujiyanto M, Priyambodo S, Mufasirin M, Hamid PH, Nugraheni YR, Awaludin A, Priyono P, Dargantes AP, Matsubayashi M. Detection of Trypanosoma lewisi from rodents residing in the densely populated residential regions along the coastal areas of Banyuwangi Sub District, Indonesia. https://www.openveterinaryjournal.com/?mno=196280 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i8.9 AMA (American Medical Association) Style Wardhana AH, Putra FL, Yudhana A, Sawitri DH, Wiedosari E, Mujiyanto M, Priyambodo S, Mufasirin M, Hamid PH, Nugraheni YR, Awaludin A, Priyono P, Dargantes AP, Matsubayashi M. Detection of Trypanosoma lewisi from rodents residing in the densely populated residential regions along the coastal areas of Banyuwangi Sub District, Indonesia. Open Vet. J.. 2024; 14(8): 1808-1818. doi:10.5455/OVJ.2024.v14.i8.9 Vancouver/ICMJE Style Wardhana AH, Putra FL, Yudhana A, Sawitri DH, Wiedosari E, Mujiyanto M, Priyambodo S, Mufasirin M, Hamid PH, Nugraheni YR, Awaludin A, Priyono P, Dargantes AP, Matsubayashi M. Detection of Trypanosoma lewisi from rodents residing in the densely populated residential regions along the coastal areas of Banyuwangi Sub District, Indonesia. Open Vet. J.. (2024), [cited January 25, 2026]; 14(8): 1808-1818. doi:10.5455/OVJ.2024.v14.i8.9 Harvard Style Wardhana, A. H., Putra, . F. L., Yudhana, . A., Sawitri, . D. H., Wiedosari, . E., Mujiyanto, . M., Priyambodo, . S., Mufasirin, . M., Hamid, . P. H., Nugraheni, . Y. R., Awaludin, . A., Priyono, . P., Dargantes, . A. P. & Matsubayashi, . M. (2024) Detection of Trypanosoma lewisi from rodents residing in the densely populated residential regions along the coastal areas of Banyuwangi Sub District, Indonesia. Open Vet. J., 14 (8), 1808-1818. doi:10.5455/OVJ.2024.v14.i8.9 Turabian Style Wardhana, April Hari, Frenky Laksana Putra, Aditya Yudhana, Dyah Haryuningtyas Sawitri, Ening Wiedosari, Mujiyanto Mujiyanto, Swastiko Priyambodo, Mufasirin Mufasirin, Penny Humaidah Hamid, Yudhi Ratna Nugraheni, Aan Awaludin, Priyono Priyono, Alan Payot Dargantes, and Makoto Matsubayashi. 2024. Detection of Trypanosoma lewisi from rodents residing in the densely populated residential regions along the coastal areas of Banyuwangi Sub District, Indonesia. Open Veterinary Journal, 14 (8), 1808-1818. doi:10.5455/OVJ.2024.v14.i8.9 Chicago Style Wardhana, April Hari, Frenky Laksana Putra, Aditya Yudhana, Dyah Haryuningtyas Sawitri, Ening Wiedosari, Mujiyanto Mujiyanto, Swastiko Priyambodo, Mufasirin Mufasirin, Penny Humaidah Hamid, Yudhi Ratna Nugraheni, Aan Awaludin, Priyono Priyono, Alan Payot Dargantes, and Makoto Matsubayashi. "Detection of Trypanosoma lewisi from rodents residing in the densely populated residential regions along the coastal areas of Banyuwangi Sub District, Indonesia." Open Veterinary Journal 14 (2024), 1808-1818. doi:10.5455/OVJ.2024.v14.i8.9 MLA (The Modern Language Association) Style Wardhana, April Hari, Frenky Laksana Putra, Aditya Yudhana, Dyah Haryuningtyas Sawitri, Ening Wiedosari, Mujiyanto Mujiyanto, Swastiko Priyambodo, Mufasirin Mufasirin, Penny Humaidah Hamid, Yudhi Ratna Nugraheni, Aan Awaludin, Priyono Priyono, Alan Payot Dargantes, and Makoto Matsubayashi. "Detection of Trypanosoma lewisi from rodents residing in the densely populated residential regions along the coastal areas of Banyuwangi Sub District, Indonesia." Open Veterinary Journal 14.8 (2024), 1808-1818. Print. doi:10.5455/OVJ.2024.v14.i8.9 APA (American Psychological Association) Style Wardhana, A. H., Putra, . F. L., Yudhana, . A., Sawitri, . D. H., Wiedosari, . E., Mujiyanto, . M., Priyambodo, . S., Mufasirin, . M., Hamid, . P. H., Nugraheni, . Y. R., Awaludin, . A., Priyono, . P., Dargantes, . A. P. & Matsubayashi, . M. (2024) Detection of Trypanosoma lewisi from rodents residing in the densely populated residential regions along the coastal areas of Banyuwangi Sub District, Indonesia. Open Veterinary Journal, 14 (8), 1808-1818. doi:10.5455/OVJ.2024.v14.i8.9 |