| Case Report | ||
Open Vet. J.. 2024; 14(8): 2085-2091 Open Veterinary Journal, (2024), Vol. 14(8): 2085–2091 Case Report Renal abscess in a Lionhead rabbit due to Encephalitozoon cuniculi and Escherichia coli: A case reportWarisraporn Tangchang1, Sang-Hun Kim2, Su-young Park1, Eun-Hye Jung1, Hyo-Jung Kwon1 and Hwa-Young Son1*1College of Veterinary Medicine, Chungnam National University, Daejeon, South Korea 2Hanaro Animal Hospital, Daejeon, South Korea *Corresponding Author: Hwa-Young Son. College of Veterinary Medicine, Chungnam National University, Daejeon, South Korea. Email: hyson [at] cnu.ac.kr Submitted: 03/04/2024 Accepted: 16/07/2024 Published: 31/08/2024 © 2024 Open Veterinary Journal
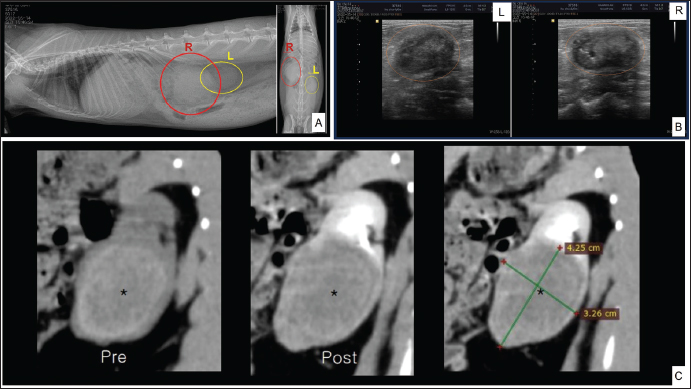
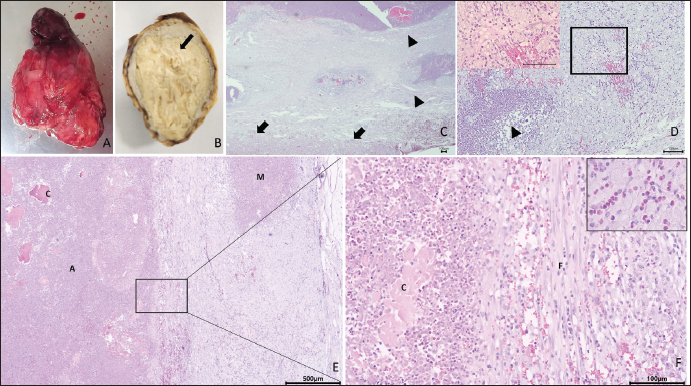
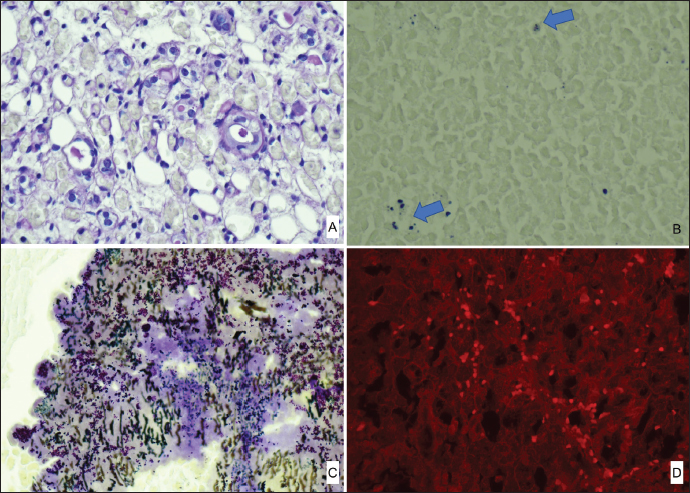
ABSTRACTBackground: In rabbits, renal abscesses (pus-filled sores) are rare and diagnosis remains challenging. Therefore, in this study, we aimed to determine the clinical manifestation and diagnostic tests associated with renal abscess identification in rabbits. Case Description: A four-and-a-half-year-old castrated male Lionhead rabbit with a history of poor appetite and abdominal distension was admitted to the animal hospital. Blood analysis, radiography, ultrasonography, and computed tomography scans revealed a kidney abscess found within the renal parenchyma, with severe loss of the cortex and medulla, extending toward the capsule. Consequently, the rabbit underwent nephrectomy. The enlarged right kidney was surgically removed. Histopathological examination of the affected kidney showed severe necrosis and ischemic zones, atrophy of the renal tubules, and prominent heterophils with mixed inflammatory cell infiltrates. Immunohistochemistry and polymerase chain reaction confirmed Encephalitozoon cuniculi and Escherichia coli infections, respectively. Conclusion: This report provides novel insights into the diagnosis of renal abscesses in Lionhead rabbits. Keywords: Encephalitozoon cuniculi, Escherichia coli, Lionhead rabbit, Renal abscess. IntroductionAbscesses, accumulations of pus formed by tissue degeneration surrounded by a thick scar tissue capsule that can be formed in any tissue in the body (Harcourt, 2009), are common. If untreated, abscesses can lead to serious, potentially life-threatening complications. The effects of an abscess are dependent on the abscess location and the affected organs. There are several types of abscesses, including skin or internal abscesses (Walter, 2013). In animals, several abscess cases have been reported: brain abscesses in the Japanese monkey (Macaca fuscata), domestic animals (horses, cattle, goats, and alpacas), companion animals (dogs and cats), and laboratory nonhuman primates (Kimura, 2023); superficial skin abscesses in cattle (Fesseha, 2020), cutaneous abscess in a companion dog (Antunes, et al., 2015), liver abscess in cattle (Amachawadi and Nagaraja, 2022; Pinnell et al., 2022), and dogs (Köhler et al., 2012); and kidney abscesses in dogs (Lewis et al., 1988; Hess and Ilan, 2003; Agut et al., 2004; Guedes et al., 2018; Hwang et al., 2020) and cats (Zatelli and D’Ippolito, 2004; Lee et al, 2010; Faucher et al., 2017). However, in rabbits, reports have been limited to mandibular and maxillary (Tyrrell et al, 2002), facial (Pagliarani et al., 2014), intra-abdominal (Etsuko et al., 2004), prostatic (Mariann, 2019), and dental (Zahid, 2021) abscesses, whereas renal parenchymal abscesses are rare (Bradley et al., 2021). In humans, several conditions predispose patients to renal parenchymal abscesses. Abscesses of the skin, oral cavity, lungs, or bones may result in bacteremia and metastasis of the infection to the renal parenchyma (Saiki et al., 1982). Infections of the kidneys and perirenal space can occur in a variety of clinical entities and can be divided into intrarenal and perirenal pathologies. Urinary system infections occur in the intrarenal and perirenal space, resulting in various treatment and diagnosis implications. (Dembry and Andriole, 1997). Furthermore, intrarenal abscesses are classified as acute focal and multifocal bacterial nephritis, renal cortical or corticomedullary abscesses, and xanthogranulomatous pyelonephritis (Dembry and Andriole, 1997). Escherichia coli (E. coli), the Proteus species, and Staphylococcus aureus (S. aureus) are the most common causative pathogens of renal and perirenal abscesses (Dembry and Andriole, 1997). According to Hinton, (1981) the renal histopathological results associated with infectious processes in rabbits up to 5 months of age are renal abscesses; inflammatory cells infiltrate in intrarenal tissues and pyelonephritis. The abscesses in many rabbit organs consist of various bacteria, including Pasteurella multocida, Staphylococcus species, Pseudomonas aeroginosa, E. coli, the Bacteroides species, Proteus species, and Fusiformis species (Harcourt, 2009). Encephalitozoon cuniculi leads to high morbidity rates in rabbit farms; therefore, serves as a potential candidate for rabbit health (Morsy et al., 2020). In addition, it is an infectious pathogen to rabbits (Latney et al., 2014; Reavill and Lennox, 2020). In this study, we aimed to identify the pathogens present in a renal abscess affecting a Lionhead rabbit and determine the clinical manifestation and diagnostic tests associated with renal abscess identification. To the best of our knowledge, this is one of only a few case reports of a renal abscess in a rabbit in Korea. Case DetailsA 4.5-year-old castrated male Lionhead rabbit with a history of poor appetite and abdominal distension was admitted to an animal hospital. The complete blood count and blood chemistry test results suggested mild dehydration. The complete blood count also revealed leukocytopenia with lymphocytopenia and high hematocrit levels. Abnormal serum biochemistry findings included hyperglycemia, hyperproteinemia, hyperglobulinemia, and hypocholesterolemia. In contrast, blood urea nitrogen (BUN), creatinine, BUN/creatinine ratio, and other profiles were normal. We performed radiography (X-rays), ultrasound, and computed tomography, which revealed a significantly enlarged right kidney (Fig. 1A) with a mass-like area (Fig. 1B). A contrast media agent was intravenously injected into the mass; the mass measured 4.25 × 3.26 cm with calcification originating from a suspected area in the right kidney. There was no contrast enhancement, suggestive of a benign lesion such as an abscess or granuloma (Fig. 1C). The right kidney was removed and organ enlargement was confirmed (Fig. 2A). The obtained kidney was fixed in 10% neutral-buffered formalin. When sectioned, a firm yellowish mass within the parenchyma with loss of the cortex and medulla extending towards the capsule was observed (Fig. 2B). The mass was trimmed and stained with hematoxylin and eosin (H&E) for routine histopathological examination. Microscopically, the areas of abscess formation were characterized by parenchymal necrosis and dense mixed inflammatory cell infiltrates (Fig. 2C), mainly heterophilic infiltrates (Fig. 2D), surrounded by thick fibrous tissue (Fig. 2E). Scattered glomeruli, tubules, and degenerating areas were observed around the abscesses (Fig. 2C). These areas showed coagulative inflammation, abscesses, and microabscesses (Fig. 2E). Co-coagulative inflammatory cell infiltrates were observed with frequent heterophils (Fig. 2F). Periodic acid-Schiff staining (PAS) was positive and observed in some tubule lumens that appeared pink or purple (Fig. 3A). However, there was no conclusive evidence of fungal hyphae formation. Modified Brown Brenn staining indicated blue particles among the inflammatory cells and necrotic debris, which were identified as Gram-positive bacteria (Fig. 3B). Silver staining revealed short pink rod-shaped Gram-negative bacteria during suppuration (Fig. 3C). Immunohistochemistry showed E. cuniculi spores presenting as round to ovoid organisms that reacted with the specific anti-E. cuniculi antibodies (Fig. 3D).
Fig. 1. Diagnostic imaging tests. Lateral (left) and ventrodorsal (right) abdominal radiographic findings in the rabbit (A). Ultrasonographic results of the left (L) and right (R) kidneys (B). computerized tomography (CT) scan comparing the pre- and post-stain injection and measuring the suspected abscess area of the right kidney (C).
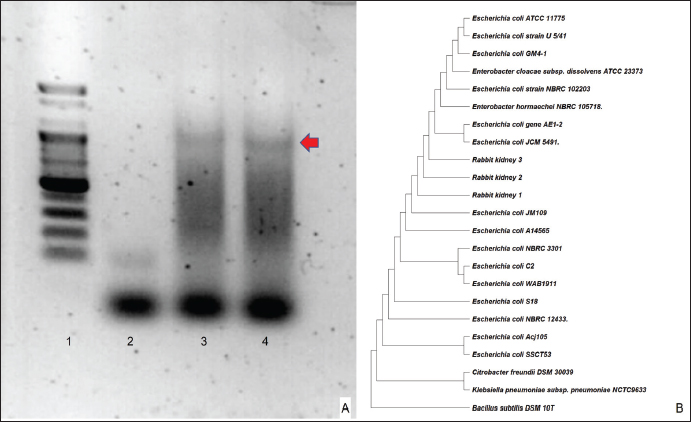
Fig. 2. Pathohistological findings of the kidney. Gross findings: enlarged right kidney (A). Cross-sectional view: creamy mass (arrow) within the thickened capsule (B). Histopathological section of the affected kidney showing fibrosis (arrowhead) and the normal areas (arrow) using H&E staining. Bar=100 μm (C). The image shows an affected heterophil infiltrate zone (arrowhead) next to the area of degenerated tubules with diffuse heterophils infiltrate (rectangular), and the insert shows foamy cytoplasm among the heterophils infiltrate H&E. Bar=100 μm. (D). Image depicting the following, using H&E staining: A: abscess, C: coagulative necrosis, M: micro-abscess, and F: Fibrosis. Bar=500 μm (E). High magnification of Fig. 2E showing the following, using H&E staining: C: coagulative necrosis, and F: Fibrosis. The inserted figure contains heterophil infiltrates. Bar=100 μm (F). The bacterial 16S rRNA gene was amplified from the DNA extracted from rabbit tissue using polymerase chain reaction (PCR), and the sequences were analyzed (Fig. 4A). All three clones contained identical sequences, and a phylogenetic tree based on the 16S rRNA sequences showed that all the selected clones were within the radius of the cluster comprising E. coli. (Fig. 4B). DiscussionIn domestic rabbits, urinary tract diseases and abscesses in non-renal organs in rabbits are common, whereas renal parenchymal abscesses are rare (Walter, 2013). Generally, there are many predisposing factors such as calculi, neoplasm, ureterovesical reflux, trauma, polycystic kidney disease, diabetes mellitus, glucocorticoid therapy, and intravenous drug abuse that lead to the development of renal abscesses (Saiki, 1982). In this study, based on the rabbit’s history, clinical tests, and histological findings, the observed renal parenchymal mass was an abscess. H&E and Gram staining results were in agreement, and we concluded that there was bacterial contrast (Becerra et al., 2016). To identify the causative agent, we performed modified Brown–Brenn staining, wherein E. cuniculi spores appeared purple in color with prominent and obvious particles (Joseph et al., 2006). However, this characteristic is non-specific; therefore, accurate identification remains challenging (Rodríguez et al., 2017). In this study, we confirmed the identity of the candidate spores via immunohistochemical examination. Positive antibody expression confirmed E. cuniculi infection in the kidney, whereas, in a previous study on three rabbit farms in Egypt, E. cuniculi was detected in the kidney using transmission electron microscopy which was confirmed by conventional PCR (Morsy et al., 2020). To the best of our knowledge, E. cuniculi is a common pathogen in rabbits (Oryctolagus cuniculus) that causes central nervous and urinary (kidney) system diseases and manifests as granulomatous and fibrosis (Latney et al., 2014). In pet rabbits, the primary cause of renal disease is E. cuniculi infection with chronic renal failure and urolithiasis (Reavill and Lennox, 2020). In this study, the rod-shaped Gram-negative bacteria identified using the silver staining method indicated that the pathogenic strain was E. coli or Pasteurella spp. According to a previous study, pathogenic E. coli can be divided into diarrheagenic and extraintestinal pathogenic (ExPEC) E. coli. A uropathogenic E. coli (UPEC) is defined as an ExPEC that causes urinary tract infections (UTIs) and is the most common cause of UTIs worldwide (Tanabe et al., 2022). Rabbit-origin enteropathogenic E. coli causes substantial diarrhea-associated morbidity and has zoonotic potential (Swennes et al., 2013) which is not a spontaneous infection in rabbit kidneys, and E. coli is generally absent from rabbit gut flora and attaches to cecal epithelial cells (Varga, 2014). In addition, in a previous experimental study, E. coli infection caused acute kidney failure; however, no significant lesions were observed in the kidney sections of the infected rabbits (Panda et al., 2010). Although UPEC is the most common cause of infection in both outpatients and inpatients worldwide (Foxman, 2010), a rare case of UPEC infection was reported in rabbits. Additionally, a previous study that induced UPEC into the urinary bladder of female rabbits led to the development of urinary infections, in the absence of abscesses (Othman et al., 2021). Moreover, acute uropathogenic E. coli (UPEC) cystitis in C57BL/6 and C3H/HeN male mice causes severe pyelonephritis and 100% penetrant renal abscesses (Olson et al., 2016). Pasteurella spp commonly presents in the nasal and respiratory regions, whereas in the urinary tract, it is uncommon (Varga, 2014). However, Pasteurella spp infection-induced severe perirenal abscessation occurs in one out of 60 wild European rabbits (Lamalle, 2023).
Fig. 3. Special stains. PAS (A). Modified Brown Brenn staining expressing Gram-positive bacteria (arrow) (B). Silver staining (C) and immunohistochemistry of E. Cuniculii antibody (D) were performed. In addition, silver staining followed by Sodium Dodecyl Sulfate polyacrylamide gel electrophoresis is extensively used to accurately detect bacterial lipopolysaccharides. PCR was used to classify the candidate pathogens. Finally, 16S rDNA sequences were amplified and confirmed the positions of E. coli rRNA in this rabbit kidney, along with other E. coli strains. Therefore, pasteurellosis was ruled out. This study indicates that the abnormality found in the Lionhead rabbit kidney was an abscess, identified using clinical history and gross and histopathological findings. In addition, we detected E. coli and E. Cuniculii spores in the abscess, which are emerging pathogens. Therefore, this study provides informative data to allow for a definitive diagnosis that is essential for abscess treatment in rabbits. This case report describes a rare renal abscess in a Lionhead rabbit in Korea, along with a review of the literature on abscesses in both humans and animals. The results of this case study may enhance our understanding of diagnostic approaches for renal abscesses and other urinary tract disorders in rabbits.
Fig. 4. Molecular tests.16S rRNA gene sequencing was carried out using two primers: 27F (5’-AGAGTTTGATCCTGGCTCAG-3’) and 1392R (5’-GGTTACCTTGTTACGACTT-3’). The sequences (from left to right): Lane 1: 100bp size marker, Lane 2 negative control, Lane 3-4 rabbit renal sample (A) and phylogenetic tree based on 16S rDNA sequences showing the positions of Escherichia coli rRNA from rabbit kidney with other E. coli strains and the representatives of some other taxa. at the branch points shown the Bootstrap values were greater than 50 % (B). AcknowledgmentsNone. FundingThis study was supported by a research fund from Chungnam National University. Conflict of interestAll authors declare no potential conflicts of interest. Author contributionsConceptualization: Kim SH, Kwon HY, Son HY; Formal analysis: Park SY, Jung EH; Funding acquisition: Kwon HY, Son HY; Methodology: Kwon HY, Son HY; Supervision: Son HY; Writing–original draft: Tangchang W, Kim SH; Writing–review and editing: Tangchang W, Kwon HY, Son HY. Data availabilityAll data are provided in the manuscript. ReferencesAgut, A., Laredo, F., Belda, E., Soler, M. and Seva, J. 2004. Left perinephric abscess associated with nephrolithiasis and bladder calculi. Vet. Rec. 154, 562–565. Amachawadi, R.G. and Nagaraja, T.G. 2022. Pathogenesis of liver abscesses in cattle. Vet. Clin. North Am. Food Anim. Pract. 38(3), 335–346. Antunes, J.M., Ribeiro, M.G., Demoner, L.C., Ramos, J.N., Baio, P.V.P., Simpson-Louredo, L., Santos, C.S., Hirata Jr., R., Ferioli, R.B., Romera, A.R.C., Vieira, V.V. and Mattos-Guaraldi, A.L. 2015. Cutaneous abscess caused by Corynebacterium lactis in a companion dog. Vet. Microbiol. 178(1-2), 163–166. Becerra, S.C., Roy, D.C., Sanchez, C.J., Christy, R.J. and Burmeister, D.M. 2016. An optimized staining technique for the detection of gram-positive and gram-negative bacteria within tissues. BMC Res. Notes. 9, 216. Bradley, A.E., Wancket, L.M., Rinke, M., Gruebbel, M.M., Saladino, B.H., Schafer, K., Katsuta, O., Garcia, B., Chanut, F., Hughes, K., Nelson, K., Himmel, L., McInnes, E., Schucker, A. and Uchida, K. 2021. International harmonization of nomenclature and diagnostic criteria (INHAND): nonproliferative and proliferative lesions of the rabbit. J. Toxicol. Pathol. 34(3 Suppl), 183S–292S. Dembry, L.M. and Andriole, V.T. 1997. Renal and perirenal abscesses. Infect. Dis. Clin. North Am. 11(3), 663–680. Etsuko, T., Kazuaki, T., Tomonori, K. and Yoshihisa, Y. 2004. Case report: intraabdominal abscess in a rabbit. J. Anim. Clin. Med. 12(4), 197–199. Faucher, M.R., Theron, M.-L. and Reynolds, B.S. 2017. Renal abscesses in cats: six cases. J. Feline Med. Surg. 19(4), 484–492. Fesseha, H. 2020. Management of superficial skin abscess in cattle: a case report. Open Access J. Biol. Sci. Res. 2(2), 1–4. Foxman, B. 2010. The epidemiology of urinary tract infection. Nat. Rev. Urol. 7, 653–660. Guedes, R.L., Dornbusch, P.T., Costa, B.N., Froes, T.R., Sousa, M.G. and Oliveira, S.T. 2018. Renal capsulotomy associated with omentopexy for treatment of bilateral perinephric abscesses in a bitch: a case report. Semin. Cienc. Agrar. 39, 2301–2306. Harcourt-Brown, F. 2009. Dental disease in pet rabbits 2. Diagnosis and treatment. Pract. 31, 432–445. Hess, R. and Ilan, I. 2003. Renal abscess in a dog with transient diabetes mellitus. J. Small Anim. Pract. 44, 13–16. Hinton, M. 1981. Kidney disease in the rabbit: a histological survey. Lab. Anim. 15(3), 263–265. Hwang, T.S., An, S., Choi, M., Song, J.H., Jung, D. and Lee, H.C. 2020. Renal subcapsular abscess associated with pyometra in a dog. J. Vet. Clin. 37(6), 360–362. Joseph, J., Sridhar, M.S., Murthy, S. and Sharma, S. 2006. Clinical and microbiological profile of microsporidial keratoconjunctivitis in southern India. Ophthalmology. 113, 531–537. Kimura, T. 2023. Case report on successful treatment for brain abscess in a Japanese monkey (Macaca fuscata). Lab. Anim. Res. 39(1), 13. Köhler, C., Jopp, I., Bosch, B. and Pfeifer, S. 2012. Leberabszess bei einem Hund. Ein Fallbericht [Liver abscess in a dog. A case report]. Tierarztl. Prax. Ausg. K Kleintiere Heimtiere. 40(3), 211–218. Lamalle, A., Haverson, V.A. and Hughes, K. 2023. Renal pathology in wild European rabbits. Vet. Rec. 193(2), e2948. Latney, L.V., Wyre, N.N.R. and Bradley, C.W., 2014. Encephalitozoon cuniculi in pet rabbits: diagnosis and optimal management. Vet. Med. Res. Rep. 5, 169–180. Lee, H., Chang, J., Jung, J., Oh, S., Kim, J., Kim, W., Yoon, J., and Choi, M. 2010. Unilateral renal subcapsular abscess associated with pyelonephritis in a cat. J. Vet. Clin. 27, 79–82. Lewis, D.C., Adamson, D.R., Jacobs, K.A. and Lamb, W.A. 1988. Pyelonephritis, nephrolithiasis and perinephric abscessation in a dog. Aust. Vet. J. 65, 195–196. Mariann, L. 2019. Urinary obstruction due to a prostatic abscess in a young neutered rabbit. J. Exotic Pet Med. 29, 15–21. Morsy, E.A., Salem, H.M., Khattab, M.S., Hamza, D.A. and Abuowarda, M.M. 2020. Encephalitozoon cuniculi infection in farmed rabbits in Egypt. Acta Vet. Scand. 62(1), 11. Olson, P.D., Hruska, K.A., and Hunstad, D.A. 2016. Androgens enhance male urinary tract infection severity in a new model. J. Am. Soc. Nephrol. 27(6), 1625–1634. Othman, M.A., Ezzat, H.M., Rizk, D.E.E., Kamal, A.H., Al-Mahameed, A.E., Marwani, A.M., Bindyna, K.M. and Salvatore, S. 2021. Induction of bacterial cystitis in female rabbits by uropathogenic Escherichia coli and the differences between the bladder dome and trigone. Ultrastruct. Pathol. 45(3), 159–166. Pagliarani, S., Del Duca, V., Selleri, P. and Di Girolamo, N. 2014. Facial abscess in a rabbit secondary to sewing-pin ingestion and cheek perforation. J. Small Anim. Pract. 55, 597–597. Panda, A., Tatarov, I., Melton-Celsa, A.R., Kolappaswamy, K., Kriel, E.H., Petkov, D., Coksaygan, T., Livio, S., McLeod, C.G., Nataro, J.P., O’Brien, A.D. and DeTolla, L.J. 2010. Escherichia coli O157:H7 infection in Dutch belted and New Zealand white rabbits. Comp. Med. 60(1), 31–37. Pinnell, L.J., Whitlow, C.W., Huebner, K.L., Bryant, T.C., Martin, J., Belk, K.E. and Morley, P.S. 2022. Not all liver abscesses are created equal: the impact of tylosin and antibiotic alternatives on bovine liver abscess microbial communities and a first look at Bacteroidetes-dominated communities. Front. Microbiol. 13, 882419. Reavill, D.R. and Lennox, A.M. 2020. Disease overview of the urinary tract in exotic companion mammals and tips for clinical management. Vet. Clin. North Am. Exotic Anim. Pract. 23, 169–193. Rodríguez-Tovar, L.E., Villarreal-Marroquín, A., Nevárez-Garza, A.M., Castillo-Velázquez, U., Rodríguez-Ramírez, H.G., Navarro-Soto, M.C., Zárate-Ramos, J.J., Hernández-Vidal, G. and Trejo-Chávez, A. 2017. Histochemical study of Encephalitozoon cuniculi spores in the kidneys of naturally infected New Zealand rabbits. J. Vet. Diagn. Invest. 29(3), 269–277. Saiki, J., Vaziri, N.D. and Barton, C. 1982. Perinephric and intranephric abscesses: a review of the literature. West. J. Med. 136(2), 95–102. Swennes, A.G., Buckley, E.M., Madden, C.M., Byrd, C.P., Donocoff, R.S., Rodriguez, L., Parry, N.M. and Fox, J.G. 2013. Enteropathogenic Escherichia coli prevalence in laboratory rabbits. Vet. Microbiol. 163(3-4), 395–398. Tanabe, R.H.S., Dias, R.C.B., Orsi, H., de Lira, D.R.P., Vieira, M.A., Dos Santos, L.F., Ferreira, A.M., Rall, V.L.M., Mondelli, A.L., Gomes, T.A.T., Camargo, C.H. and Hernandes, R.T. 2022. Characterization of uropathogenic Escherichia coli reveals hybrid isolates of uropathogenic and diarrheagenic (UPEC/DEC) E. coli. Microorganisms. 10(3), 645. Tyrrell, K.L., Citron, D.M., Jenkins, J.R. and Goldstein, E.J. 2002. Periodontal bacteria in rabbit mandibular and maxillary abscesses. J. Clin. Microbiol. 40(3), 1044–1047. Varga, M. 2014. Infectious diseases of domestic rabbits. Textbook. Rabbit. Med.. 435–471. Walter, N. 2013. Abscesses. In Clinical veterinary advisor. Eds., Vella, D., Mayer, J. and Donnelly, T.M, Saint Louis, MO: W.B. Saunders, pp: 331–332. Zahid, B.K. 2021. Management of abscess in right para lumbar fossa in a cow: a case report. Acta Sci. Vet. Sci. 3(10), 72–74. Zatelli, A. and D’Ippolito, P. 2004. Bilateral perirenal abscesses in a domestic neutered shorthair cat. J. Vet. Intern. Med. 18, 902–903. | ||
| How to Cite this Article |
| Pubmed Style Tangchang W, Kim S, Park S, Jung E, Kwon H, Son H. Renal abscess in a Lionhead rabbit due to Encephalitozoon cuniculi and Escherichia coli: A case report. Open Vet. J.. 2024; 14(8): 2085-2091. doi:10.5455/OVJ.2024.v14.i8.38 Web Style Tangchang W, Kim S, Park S, Jung E, Kwon H, Son H. Renal abscess in a Lionhead rabbit due to Encephalitozoon cuniculi and Escherichia coli: A case report. https://www.openveterinaryjournal.com/?mno=196317 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i8.38 AMA (American Medical Association) Style Tangchang W, Kim S, Park S, Jung E, Kwon H, Son H. Renal abscess in a Lionhead rabbit due to Encephalitozoon cuniculi and Escherichia coli: A case report. Open Vet. J.. 2024; 14(8): 2085-2091. doi:10.5455/OVJ.2024.v14.i8.38 Vancouver/ICMJE Style Tangchang W, Kim S, Park S, Jung E, Kwon H, Son H. Renal abscess in a Lionhead rabbit due to Encephalitozoon cuniculi and Escherichia coli: A case report. Open Vet. J.. (2024), [cited January 25, 2026]; 14(8): 2085-2091. doi:10.5455/OVJ.2024.v14.i8.38 Harvard Style Tangchang, W., Kim, . S., Park, . S., Jung, . E., Kwon, . H. & Son, . H. (2024) Renal abscess in a Lionhead rabbit due to Encephalitozoon cuniculi and Escherichia coli: A case report. Open Vet. J., 14 (8), 2085-2091. doi:10.5455/OVJ.2024.v14.i8.38 Turabian Style Tangchang, Warisraporn, Sang-hun Kim, Su-young Park, Eun-hye Jung, Hyo-jung Kwon, and Hwa-young Son. 2024. Renal abscess in a Lionhead rabbit due to Encephalitozoon cuniculi and Escherichia coli: A case report. Open Veterinary Journal, 14 (8), 2085-2091. doi:10.5455/OVJ.2024.v14.i8.38 Chicago Style Tangchang, Warisraporn, Sang-hun Kim, Su-young Park, Eun-hye Jung, Hyo-jung Kwon, and Hwa-young Son. "Renal abscess in a Lionhead rabbit due to Encephalitozoon cuniculi and Escherichia coli: A case report." Open Veterinary Journal 14 (2024), 2085-2091. doi:10.5455/OVJ.2024.v14.i8.38 MLA (The Modern Language Association) Style Tangchang, Warisraporn, Sang-hun Kim, Su-young Park, Eun-hye Jung, Hyo-jung Kwon, and Hwa-young Son. "Renal abscess in a Lionhead rabbit due to Encephalitozoon cuniculi and Escherichia coli: A case report." Open Veterinary Journal 14.8 (2024), 2085-2091. Print. doi:10.5455/OVJ.2024.v14.i8.38 APA (American Psychological Association) Style Tangchang, W., Kim, . S., Park, . S., Jung, . E., Kwon, . H. & Son, . H. (2024) Renal abscess in a Lionhead rabbit due to Encephalitozoon cuniculi and Escherichia coli: A case report. Open Veterinary Journal, 14 (8), 2085-2091. doi:10.5455/OVJ.2024.v14.i8.38 |