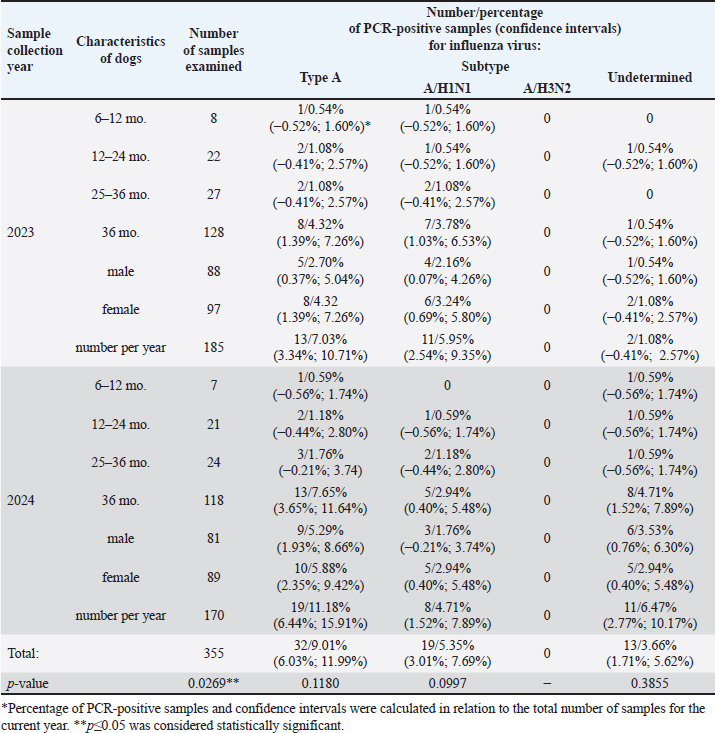
| Research Article | ||
Open Vet. J.. 2024; 14(8): 1896-1904 Open Veterinary Journal, (2024), Vol. 14(8): 1896–1904 Research Article Circulation of influenza viruses in the dog population in Kazakhstan (2023–2024)Tatyana I. Glebova1, Nailya G. Klivleyeva1*, Nurbol T. Saktaganov1, Mira G. Shamenova1, Galina V. Lukmanova1, Assem M. Baimukhametova1, Sagadat B. Baiseiit1, Nuray S. Ongarbayeva1, Kanat A. Orynkhanov2, Anna V. Ametova3 and Aitolkyn K. Ilicheva31The Research and Production Center for Microbiology and Virology, Almaty, Republic of Kazakhstan 2Kazakh National Agrarian University, Almaty, Republic of Kazakhstan 3LLP «CVM Clinic», Almaty, Republic of Kazakhstan *Corresponding Author: Nailya G. Klivleyeva. The Research and Production Center for Microbiology and Virology, Almaty, Republic of Kazakhstan. Email: i_nailya [at] list.ru Submitted: 26/04/2024 Accepted: 12/07/2024 Published: 31/08/2024 © 2024 Open Veterinary Journal
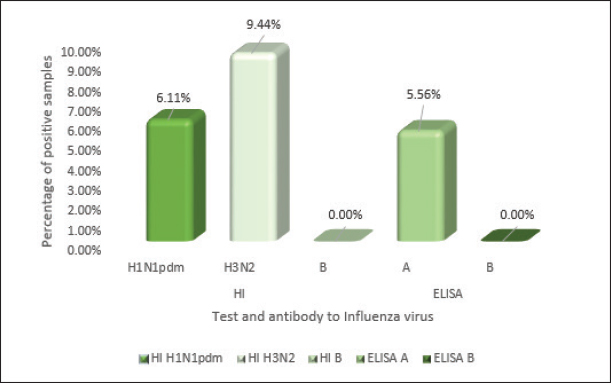
ABSTRACTBackground: Dogs in close contact with humans can serve as a source of potentially dangerous reassortant influenza viruses (IVs) with zoonotic potential. The dog’s body can serve as a vessel for the emergence of new IVs. These new viruses can become a source of infection for other animals and humans. The potential for zoonotic transmission of IVs from dogs to humans poses a public health risk. Aim: Study of the circulation of IVs in the dog population in Almaty, Kazakhstan. Methods: Biosamples (oropharyngeal swabs and blood serum) from dogs were collected from veterinary clinics in Almaty in 2023–2024. Samples were screened using RT-PCR, HI assay, and ELISA. Results: RT-PCR analysis of 355 nasopharyngeal swabs showed the presence of influenza A virus (IAV) in 32 samples (9.01% of the total number of samples analyzed). When subtyping IAV H1N1 RNA was detected in 19 swabs (5.35%). IAV subtype could not be determined in 13 PCR-positive samples (3.66%). The genetic material of IAV H3N2, H5, H7, and H9, as well as coronavirus, bocavirus, and adenovirus has not been identified. In a serological analysis of 180 blood sera using ELISA, antibodies to IAV were detected in 5.56% (n=10). The results of the HI assay showed the presence of antihemagglutinins to A/H1N1pdm in 6.11% (11 samples), to A/H3N2 in 9.44% (17 samples), and no antibodies to IAV H5, H7, and type B were detected. Conclusion: There is no information about human infection with any canine influenza virus. However, many cases of infection in dogs with human IAVs H1N1, H1N1pdm09, and H3N2 have been described. When dogs are co-infected with different IAVs, new recombinant IAVs may emerge that can infect humans and other animals. Therefore, ongoing global surveillance of animal populations is necessary to monitor the evolution and circulation of viruses dangerous to public health. This is also important for timely preparation for the emergence of a new zoonotic influenza virus that has pandemic potential for humans. Keywords: Canine influenza, Monitoring, Reassortment, Epizootology, Zoonotic infection. IntroductionInfluenza viruses (IVs) are widespread pathogens worldwide that infect both humans and a wide range of animals (Lvov, 1987; Webster, et al., 1992; Saktaganov, et al., 2020; Klivleyeva et al., 2021a,b, 2022). Due to their host diversity and zoonotic potential, IVs pose a serious threat to public health. Historically, the formation of zoonotic IVs occurred in pigs due to the expression of human and avian receptors. However, there are several other animals that carry both types of receptors and may act as potential mixing vessel hosts. Among other species of animals (humans, pigs, minks, ferrets, seals, cats, and birds), dogs can also be such mixer hosts with a high degree of probability (Abdelwhab and Mettenleiter, 2023). Unlike most pets, where influenza transmission is ineffective due to keeping in small numbers in residential homes and low frequency of contact with other members of their species, domestic dogs interact with a wide range of their pets because they require daily walking, attending training schools, or kept in dense populations (kennels and shelters). Due to the fact that in outdoor conditions contacts of domestic dogs with feral relatives, as well as with synanthropic animals, including birds, are inevitable, and in rural areas with domestic and wild animals, including generally recognized carriers of influenza A viruses (IAV) and sources of reassortant strains, the likelihood of these infectious agents entering the dog population increases. Despite existing evidence of IAV circulating in humans and other mammals, dogs have long been thought to be immune to this infection (Songserm et al., 2006). However, subsequently variants of the equine IAV H3N8 (Coppinger R. and Coppinger L., 2001; Said et al., 2011; Wasik et al., 2021), avian IVs (AIV) H3N2 (Payungporn et al., 2008; Song et al., 2008; Li et al., 2010), AIV H5N1 and H7N2, the 2009 pandemic virus H1N1pdm09 (Wang et al., 2019), as well as various reassortants of the canine influenza virus (CIV) carrying its gene) A/H3N2 has been identified in dogs (Song et al., 2012; Moon et al., 2015). Detection of other subtypes among dogs with signs of respiratory infection, in particular, H5N6, H5N2, H3N1, H9N2, and H10N8 (Sun et al., 2013; Song et al., 2013; Su et al., 2014; Zhang et al., 2013; Lee et al., 2016) was reported. It has been established that CIV H3N2 can spread among dogs and be transmitted to other animals (Song et al., 2011; Jeoung et al., 2013). In 2022, human infections with IAV H3N8 of avian origin occurred in China. In addition to the infected patient, viral RNA was detected in a nasopharyngeal swab of a practically healthy dog that had contact with him (Bao et al., 2022). Molecular and serological monitoring of the spread of IAVs among dogs is ongoing in the USA, East Asia, and some European countries (Kwasnik et al., 2020). However, there is no data on studying the IV circulation among these animals in Kazakhstan. Almaty is a city of republican significance, with a population of more than 2 million people. In addition, Almaty is first in the number of pets, including dogs. Of the 163,945 dogs registered in Kazakhstan, almost half are in Almaty and the Almaty region (73,780/45%). Due to the large number of pets in Almaty and the Almaty region, this region is the most indicative for studying IAV circulation among dogs. Materials and MethodsSample collectionBiological specimens from domestic dogs (oropharyngeal swabs and blood serum) were obtained according to the recommendations set by the International Association for the Study of Pain in large veterinary clinics in Almaty in 2023–2024. During the sampling efforts were made to reduce any pain or discomfort by the experienced animals. Veterinary clinics that see more than 500 dogs per year were selected for the study. The samples were collected randomly from animals that had undergone a microchip program, veterinary examinations, and surgical procedures and from animals with various respiratory symptoms. Dogs older than six months who had never received a canine influenza vaccination were chosen for the experiment. Each dog’s characteristics were added to an electronic database. These included the following: sex, age (6–12, 12–24, 25–36, and >36 months), location, activity (ornamental, hunting, or guard dog), and size (height at withers: short (<40 cm), medium (41–60 cm), or large (>60 cm). Owners of dogs provided permission for sampling. Oropharyngeal swabs from dogs were collected using a sterile cotton swab on a sterile plastic tube containing 1 ml of transport medium. The obtained clinical samples were stored at 4°C and transported to the laboratory for virological analysis within 72 hours. The remaining samples were stored at –80°C in the ultra-low temperature freezer. Blood was collected from small Saphenous veins of the leg, Cephalic Vein, or external jugular vein using a vacuum collection system. The serum was separated by centrifugation at 400 g for 15 min. All serum samples were then transferred to new tubes and stored at −20°C until assayed. Real-time polymerase chain reactionThe biomaterials were analyzed for the presence of sentinel viruses using real-time polymerase chain reaction (rtRT-PCR) on a Rotor-Gene Q6 plex device (QIAGEN, Germany) using RIBO-prep and AmpliSense kits® Influenzavirus A/B-FL. The kits “AmpliSens® Influenza virus A/H1-swine-FL,” “AmpliSens® Influenza virus A-type-FL,” and “AmpliSens® Influenza virus A-type-H5, H7, H9-FL” (Federal Budget Institute of Science Central Research Institute of Epidemiology of Rospotrebnadzor, Russia) were used to identify IAV subtypes, in accordance with the manufacturer’s instructions. Serological analysisSerum samples were treated with Receptor Destroying Enzyme II (DENKA SEIKEN, Japan) at a working dilution of 1:50 for removing of nonspecific inhibitors. The serum was combined with three volumes of a receptor-destroying enzyme and kept at 37°C for eighteen to twenty hours. The serum samples were then diluted to a 1:10 ratio by adding six parts of phosphate saline buffer, and heated for 30 minutes at 56°C. The level of specific antibodies to IAV hemagglutinins in blood sera was determined in HI assay using kits produced by LLC ‘Diagnostic Preparations Production Enterprise’ (St. Petersburg, Russia, in accordance with the manufacturer’s recommendations). The ELISA was conducted using test systems that contained nucleoprotein-specific monoclonal antibodies for IAV (IDEXX Influenza A, USA) as per the manufacturer’s instructions. When influenza A antibodies are not present in the test sample, the conjugate can freely associate with influenza A antigen. Conversely, the presence of antibodies to IAV in the sample blocked the binding of the conjugate to the antigen. In subsequent steps, the unbound conjugate was washed away, and then an enzymatic substrate was added to drive color development, the inverse ratio of the influenza A antibody. In the experiment, the average negative control (NCx) and average positive control (PCx) values were calculated, where the readings must meet the following criteria: NCx ≥ 0.600 and PCx < 0.50. The sample-to-negative (S/N) ratio for each sample was used to determine whether influenza A antibodies were present or not. An S/N value <0.60 indicates the presence of anti-IAV NP antibodies in the serum, whereas an S/N ≥0.60 was considered “negative,” respectively. The samples that were tested in ELISA were compared to the results of the HI assay. Isolation of IAVsIAVs were isolated by inoculating PCR-positive samples into embryonated chicken eggs and Madin-Darby canine kidney (MDCK) cells, as described previously (Klimov et al., 2012). Statistical analysisGraph Pad Prism software version 9.1 (Glants, 1998) and Microsoft Office Excel were used to analyze the data. Chi-square tests were used to determine the significance of group differences between IAV subtypes. Statistical significance for group differences in influenza virus types was defined as p > 0.05 and was considered statistically significant. Ethical approvalThe collection of biological samples was carried out under the authorization of the local ethical committee and in cooperation with licensed veterinarians (protocol no. 02-09-130, October 20, 2022). ResultsReal-time polymerase chain reactionBiological samples from dogs (oropharyngeal swabs and blood serum) were collected in the city of Almaty and the Almaty region from November 2023 to February 2024. As a result, a survey was conducted on biological specimens from 355 dogs (52.39% males and 47.61% females). The animals of 4.23% were under 12 months old, 12.11% were in 12–24 months old, 14.37%—in 25–36 months, and 69.30% presented animals older than 36 months. Most samples were collected from domestic animals of different breeds. The results of molecular genetic screening of collected biomaterials using real-time RT-PCR are presented in Table 1. As a result of a real-time RT-PCR study of 355 nasopharyngeal swabs, genetic material for IAV was detected in 32 samples (9.01% of the total number of samples studied). Subtyping allowed the detection of IAV H1N1 RNA in 19 swabs (5.35%). In 13 positive samples for IAV (3.66%), the virus subtype could not be determined. The genetic material of IAV H3N2, H5, H7, and H9, as well as coronavirus, bocavirus, and adenovirus, was not detected in the studied samples. Thus, the results of the initial screening of oropharyngeal swabs using RT-PCR indicate the predominance of strains with the antigenic formula A/H1N1 among IAV circulating on the territory of Kazakhstan in the dog population during the studied period. Serological analysisThe results of the serological analysis of 180 blood samples in the HI assay and ELISA are presented in Figure 1. As can be seen from the data in Figure 1, 5.56% (n=10) of the blood samples tested in ELISA (n=180) had antibodies to IAV. The results of the HI assay showed the presence of antihemagglutinins against influenza A/H1N1pdm virus in 6.11% of cases (11 samples) of the total number of samples; for A/H3N2 - in 9.44% (17 samples), no antibodies to IVA H5, H7, and type B were detected. Antibody titers ranged from 1:40 to 1:160. Thus, the ELISA was able to establish the presence of antibodies against influenza A; the HI assay identified antihemagglutinins to H1, in particular to the 2009 pandemic IAV H1pdm, and antihemagglutinins to H3. Unfortunately, it was not possible to determine the neuraminidase subtype using the methods used, and thus, it cannot be stated with complete confidence that there were no reassortment events in the dogs. Isolation of IAVsIt was not possible to isolate IAV by inoculating PCR-positive samples into 9–10-day-old chicken eggs and MDCK cell culture. DiscussionSince samples collected from domestic dogs were studied, it can be concluded that pets were likely to be infected by their owners by IAVs H1N1pdm and H3N2 circulating in humans. However, according to WHO, in the current epidemic season, IAV H3N2 was mainly detected in the population of Kazakhstan, the influenza B virus was detected to a lesser extent, and IAV H1N1pdm was detected very rarely (four cases out of 635 positive samples) (Fig. 2). Our RT-PCR results, which show a presence of IAV H1N1pdm09 among dogs throughout the 2023–2024 period, differ slightly from the WHO data, which are displayed in Figure 2 and showed the prevalence of IAV H3N2 among humans during this period. This difference may be due to the small sample of animals used in the study (n=355) relative to the general dog population in Kazakhstan (n=163,945). There is also a possibility that dogs could acquire this infectious agent through contact with the environment (Ali et al., 2011). Since the emergence of pandemic IAV A(H1N1)pdm09 in Kazakhstan in 2009–2010, this subtype almost every year caused a large number of human infection cases, being the dominant agent in the structure of the etiology of ARVI (Glebova et al., 2021; Klivleyeva et al., 2023; Shamenova et al., 2023). Kazakhstan is one of the Central Asian states where pets, including dogs, are registered. As available statistics show, the dog population in the republic is small compared to other countries in the world. However, in Almaty city there is a fairly large number of dogs of various breeds. The serological prevalence of influenza in dogs has been studied for quite some time in the USA, China, Korea, Japan, and some European countries (Italy, Germany, Poland) (Dundon et al., 2010; Song et al., 2012; Damiani et al., 2012; Anderson et al., 2013; Su et al., 2013; Jang et al., 2017; Kwasnik et al., 2020). Dogs are known to be more susceptible to infection with H3 CIV viruses. In the United States, serological studies have established the prevalence of human influenza H1N1, H3N2, and canine H3N8, as well as the presence of antibodies to two subtypes (H1N1 and H3N2; H1N1 and CIV H3N8) (Jang et al., 2017; Glebova et al., 2021). In Korea, serological evidence of infection with human H3N2 and pandemic IAV H1N1pdm09 has been obtained, both alone and in combination with canine H3N2 (Song et al., 2015). The whole genome analysis of the reassortant IAV H3N1 isolated in Korea from dogs established its 99.1%–99.9% relationship with IAV H1N1pdm09, apart from the HA gene, which showed 99.6% similarity to CIV H3N2 isolated from dogs in Korea and China (Song et al., 2012; Lyu et al., 2019). In European countries, dogs have been found to be seropositive for IAVs such as H3N8, H3N2, and H1N1pdm09. Antibodies in HI assay to IAV H1N1pdm09 ranged from 0.13% (Spain) (Jurado-Tarifa et al., 2020) to 1.6% (Germany, Poland) (Damiani et al., 2012; Kwasnik et al., 2020). A high percentage of positive responses was found in the HI assay for IAV H1N1pdm09 was found in the Netherlands (12.3%) (Zhao et al., 2020) and Hong Kong (9.5%) (Su et al., 2019). Table 1. Results of primary screening in real-time RT-PCR of nasopharyngeal swabs collected from dogs.
Fig. 1. Serological examination of serum samples in the HI assay and ELISA.
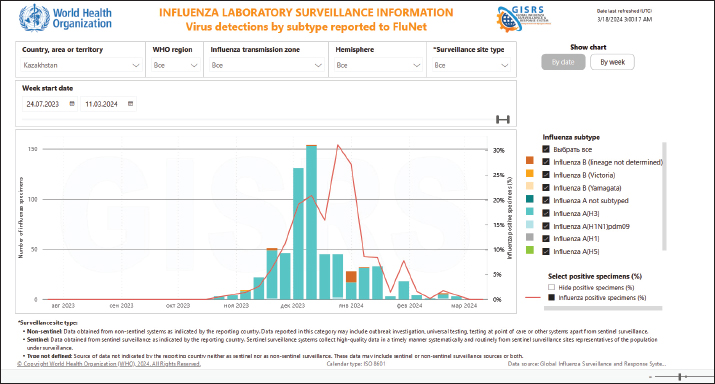
Fig. 2. Identification of influenza virus subtypes in Kazakhstan during the 2023–2024 epidemic season, FluNet data. To date, there is no data on the study of IV circulation among dogs in Kazakhstan, molecular or serological. This is the first study to identify IAV in dogs in Kazakhstan. The results of our studies indicate that out of 180 examined dog blood sera, seropositivity was revealed for both the H3 and A/H1N1pdm viruses with titers of 40–80. Only one serum (0.56%; 95% CI: 0.0–0.39) was positive for anti-A/H3N2 antibodies with a titer of 1:160. Our data correlate with the results of Jang et al. (2017), indicating higher seropositivity in a dog infected with the pandemic strain of IAV H1N1pdm09 with symptoms of respiratory infection than in healthy animals (Jang et al., 2017). From this, we can conclude that at the time of the study, the animal was probably infected with IAV H3. In our study, the percentage of dogs seropositive in the HI assay is higher than in the ELISA, so the likelihood of IAV-infected dogs may be significantly higher. This fact is confirmed by other studies (Damiani et al., 2012; Kwasnik et al., 2020; Jurado-Tarifa et al., 2020). Circulation in dogs of IAV H5, H7, H9, and type B was not detected either in RT-PCR or in serological studies. Finally, it should be noted that dogs, as companion animals, are in close contact with humans. In addition to the established CIV lineages H3N2 and H3N8, a large number of different IAVs have been identified in them: seasonal human H3N2 and pandemic H1N1, avian IAVs H5N6, H5N2, H3N1, H9N2, and H10N8 (Sun et al., 2013; Zhang et al., 2013; Lee et al., 2016; Wang et al., 2019; Wasik et al., 2021). There is information on the isolation of reassortant CIV A/H3N2 from dogs, in Korea (Song et al., 2012; Moon et al., 2015). Dogs inoculated with human IAV H3N2 are known to show no clinical signs of infection, but infection is confirmed by viruses shedding from the throat and seroconversion (Paniker and Nair, 1972). Serological studies in different countries have shown the presence of antibodies against human and avian IAVs such as H3N2, H1N1, H5N1, and H9N2 in dogs (Su et al., 2014; Wasik et al., 2021; Kovalenko et al., 2021; Jimenez-Bluhm et al., 2021). It is worth noting that receptors such as 2,3-SA, and 2,6-SA are observed in the respiratory tracts (goblet and subepithelial cell regions) and in the large intestine of dogs, which contribute to their susceptibility to animals and humans IAV (Ning et al., 2012). Considering that dogs are susceptible to various IAVs and are in close contact with humans, their potential as vessels for the formation of reassorted strains can be considered high (Abdelwhab and Mettenleiter, 2023). Undoubtedly, our data are not sufficient to obtain a complete picture of IAV circulation in the dog population in Kazakhstan. The relatively small sample size of individuals on the scale of Kazakhstan (only Almaty and the Almaty region were considered), and the limitations of the methods used do not allow us to draw definite conclusions about the genetic and phenotypic characteristics of the virus that contribute to interspecific transmission. Additional experiments are needed to understand the molecular biology of the different IAV subtypes circulating in the canine population. In addition, further experiments are required to identify mutations that can affect the transmission of new virus subtypes during human-animal interactions. However, the results obtained (real time RT-PCR and serological analysis) confirm that the dogs are infected with IAV. This is the first monitoring study in Kazakhstan, which needs to be continued in relation to obtaining a whole genome characterization of IAV in dogs. Such a research design will help provide the information necessary to understand the role of dogs in the natural chain of spread of IAV, predict the epidemiological and epizootological situation, and select the correct strategy and tactics for preventive and anti-epidemic measures. ConclusionDogs, which are susceptible to IAVs and in close contact with humans, can serve as a source of potentially dangerous reassortant strains of IV or subtypes with zoonotic potential. To date, there are no documented cases of human infection with any of the CIV subtypes. However, there are many described examples of infection in dogs with human IAV, including seasonal H1N1 and H1N1pdm09 IAVs, as well as seasonal H3N2 viruses (Murcia et al., 2010; Kim et al., 2013; Zhao et al., 2014; Klivleyeva, et al., 2022). Although these infections have not been observed to further spread among dogs, however, human IAV may reappear with CIV during natural coinfections in dogs (Jang et al., 2017; Chen et al., 2018, 2023). Under these conditions, due to ongoing genetic changes, new strains of the IVs may appear in dogs, which can become a source of infection for other animals and humans. This close contact between humans and dogs may pose a public health threat due to the potential for zoonotic transmission of IVs to humans (Abdelwhab and Mettenleiter, 2023). Unfortunately, very little is known about the genetic and phenotypic characteristics of the virus and host that contribute to interspecies transmission. There is also a lack of knowledge about the mechanisms by which these zoonotic viruses may subsequently adapt to efficiently replicate and spread among humans. Besides this, very few countries are dealing with this problem. Research in this area has shown that many countries in Asia and Africa have published few sequences and do not regularly sequence viruses as part of their monitoring programs (Escorcia et al, 2012). Genetic sequencing is known to identify changes that influence the phenotype of IV circulating, which in turn improves preparedness for epidemics and pandemics throughout the world (Escorcia et al, 2012). Preparations for the possible emergence of animal IV in humans include routine global surveillance of animal populations and monitoring the evolution and circulation of viruses with unknown public health risks. AcknowledgmentThe authors are grateful to the Ministry of Science and Higher Education of the Republic of Kazakhstan, for providing the funds for this study. Conflict of interestThe authors declare that there is no conflict of interest. FundingThe research was funded by the Ministry of Science and Higher Education of the Republic of Kazakhstan, the project ‘Virome of dogs (Canis familiaris) as a potential source of zoonotic infections’ (Grant No. AP19677712). Authors’ contributionThis project was conceived and designed by NS and NK, with data acquisition by KO, AA, AI, TG, GL, NO, AB, and SB. Analysis and interpretation of the data were performed by NS, TG, MS, and NK. The article was drafted by NS and NK, with the revision for intellectual content performed by TG, GL, MS, and NK. All authors gave final approval for article submission. Data availabilityThe data presented in this study are available in the manuscript and any further clarifications can be obtained on reasonable request from the corresponding author. ReferencesAbdelwhab, E. and Mettenleiter, T. 2023. Zoonotic animal influenza virus and potential mixing vessel hosts. Viruses. 15(4), 980. Ali, A., Daniels, J., Zhang, Y., Rodriguez-Palacios, A., Hayes-Ozello, K., Mathes, L. and Lee, C. 2011. Pandemic and seasonal human influenza virus infections in domestic cats: prevalence, association with respiratory disease, and seasonality patterns. J. Clin. Microbiol. 49(12):4101–4105. Anderson, T., Crawford, P., Dubovi, E., Gibbs, E. and Hernandez, J. 2013. Prevalence of and exposure factors for seropositivity to H3N8 canine influenza virus in dogs with influenza-like illness in the United States. J. Am. Vet. Med. Assoc. 242(2), 209–216. Bao, P., Liu, Y., Zhang, X., Fan, H., Zhao, J., Mu, M., Li, H., Wang, Y., Ge, H., and Li, S. 2022. Human infection with a reassortment avian influenza A H3N8 virus: An epidemiological investigation study. Nat. Commun. 13, 6817. Chen, M., Lyu, Y., Wu, F., Zhang, Y., Li, H., Wang, R., Liu, Y., Yang, X., Zhou, L., Zhang, M., Tong, Q., Sun, H., Pu, J., Liu, J. and Sun, Y. 2023. Increased public health threat of avian-origin H3N2 influenza virus caused by its evolution in dogs. Elife. 12, e83470. Chen, Y., Trovão, N., Wang, G., Zhao, W., He, P., Zhou, H., Mo, Y., Wei, Z., Ouyang, K., Huang, W., García-Sastre, A. and Nelson, M. 2018. Emergence and evolution of novel reassortants influenza A virus in canines in Southern China. MBio. 9(3): e00909–e00918. Coppinger, R. and Coppinger, L. 2001. Dogs: a startling new understanding of Canine origin, behavior and evolution. New York, NY: Scribner. pp: 352. Damiani, A., Kalthoff, D., Beer, M., Müller, E. and Osterrieder, N. 2012. Serological survey in dogs and cats for influenza A(H1N1)pdm09 in Germany. Zoonoses Public Health. 59(8), 549–552. Dundon, W., De Benedictis, P., Viale, E. and Capua, I. 2010. Serologic evidence of pandemic (H1N1) 2009 infection in dogs, Italy. Emerg. Infect. Dis. 16(12), 2019–2021. Escorcia, M., Attene-Ramos, M., Estrada, M. and Nava, G. 2012. Improving global influenza surveillance: trends of A(H5N1) virus in Africa and Asia. BMC Res. Notes. 5, 62. Glants, S. 1994. Mediko-biologicheskaja statistika. McGraw-Hill, Moscow: Praktika, 1998, pp: 459. Glebova, T., Klivleyeva, N., Baimukhametova, A., Saktaganov, N., Lukmanova, G., Ongarbayeva, N., Shamenova, M. and Baymakhanova, B. 2021. Circulation of influenza viruses in the epidemic season of 2018–2019 among people residing in Northern and Western Kazakhstan. Infect. Dis. 19(2), 70–75. Jang, H., Jackson, Y., Daniels, J., Ali, A., Kang, K., Elaish, M. and Lee, C. 2017. Seroprevalence of three influenza A viruses (H1N1, H3N2, and H3N8) in pet dogs presented to a veterinary hospital in Ohio. J. Vet. Sci. 18(S1), 291–298. Jeoung, H., Lim, S., Shin, B., Lim, J., Song, J., Song, D., Kang, B., Moon, H. and An, D. 2013. A novel canine influenza H3N2 virus isolated from cats in an animal shelter. Vet. Microbiol. 165(3–4), 281–286. Jimenez-Bluhm, P., Sepulveda, A., Baumberger, C., Di Pillo, F., Ruiz, S., Salazar, C., Marambio, V., Berrios, F., Galdames, P., Amaro, A., Tapia, D., Sharp, B., Freiden, P., Meliopoulos, V., Schultz-Cherry, S. and Hamilton-West, C. 2021. Evidence of influenza infection in dogs and cats in central Chile. Prev. Vet. Med. 191, 105349. Jurado-Tarifa, E., Cano-Terriza, D., Daly, J., Arenas, A. and García-Bocanegra, I. 2020. Serosurvey of pandemic H1N1 influenza A virus in dogs in Andalusia (southern Spain). Zoonoses Public Health. 67(8), 869–875. Kim, H., Song, D., Moon, H., Yeom, M., Park, S., Hong, M., Na, W., Webby, R., Webster, R., Park, B., Kim, J. and Kang, B. 2013. Inter- and intraspecies transmission of canine influenza virus (H3N2) in dogs, cats, and ferrets. Influenza Other Resp. Viruses. 7, 265–270. Klimov, A., Balish, A., Veguilla, V., Sun, H., Schiffer, J., Lu, X., Katz, J. M. and Hancock, K. 2012. Influenza virus titration, antigenic characterization, and serological methods for antibody detection. Methods in molecular biology (Clifton, N.J.), 865, 25–51. Available via https://link.springer.com/protocol/10.1007/978-1-61779-621-0_3 (Accessed 09 February 2023). Klivleyeva, N., Glebova, T., Shamenova, M. and Saktaganov, N. 2022. Influenza A viruses circulating in dogs: a review of the scientific literature. Open Vet. J. 12(5), 676–687. Klivleyeva, N., Lukmanova, G., Glebova, T., Shamenova, M., Ongarbayeva, N., Saktaganov, N., Baimukhametova, A., Baiseiit, S., Ismagulova, D., Kassymova, G., Rachimbayeva, A., Murzagaliyeva, A., Xetayeva, G., Isabayeva, R. and Sagatova, M. 2023. Spread of pathogens causing respiratory viral diseases before and during CoVID-19 pandemic in Kazakhstan. Indian J. Microbiol. 63(1), 129–138. Klivleyeva, N., Ongarbayeva, N., Baimukhametova, A., Saktaganov, N., Lukmanova, G., Glebova, T., Sayatov, M., Berezin, V., Nusupbaeva, G. and Aikimbayev, A. 2021a. Detection of influenza virus and pathogens of acute respiratory viral infections in population of Kazakhstan during 2018–2019 epidemic season. Russian J. Infect. Immun. 11(1), 137–147. Klivleyeva, N., Ongarbayeva, N., Korotetskiy, I., Glebova, T., Saktaganov, N., Shamenova, M., Baimakhanova, B., Shevtsov, A., Amirgazin, A., Berezin, V. and Webby, R. 2021b. Whole genome sequence of swine influenza virus isolate A/swine/Karaganda/04/2020 (H1N1) from Kazakhstan. Microbiol. Resour. Announc. 10(39), e0078621. Kovalenko, G., Galat, M., Ishchenko, L. and Halka, I. 2021. Serological evidence for influenza a viruses among domestic dogs and cats in Kyiv, Ukraine. Vector Borne Zoonotic Dis. 21(7), 483–489. Kwasnik, M., Smreczak, M., Rola, J., Urbaniak, K. and Rozek, W. 2020. Serologic investigation of influenza A virus infection in dogs in Poland. J. Vet. Diagn. Invest. 32(3), 420–422. Lee, I., Kim, Y., Lim, G., Kwon, H., Si, Y., Park, S., Kim, E., Kim, S., Nguyen, H., Song, M. and Choi, Y. 2018. Comparison of the virulence and transmissibility of canine H3N2 influenza viruses and characterization of their canine adaptation factors. Emerg. Microbes Infect. 7(1), 17. Lee, I, Le, T., Kim, H. and Seo, S. 2016. Isolation of a novel H3N2 influenza virus containing a gene of H9N2 avian influenza in a dog in South Korea in 2015. Virus Genes 52(1), 142–145. Li, S., Shi, Z., Jiao, P., Zhang, G., Zhong, Z., Tian, W., Long, L., Cai, Z., Zhu, X., Liao, M. and Wan, X. 2010. Avian-origin H3N2 canine influenza A viruses in Southern China. Infect. Genet. Evol. 10(8), 1286–1288. Lvov, D. 1987. Influenza A viruses a sum of populations with a common protected gene pool. Sov. Med. Rev. Virol. 2, 15–37. Lyu, Y., Song, S., Zhou, L., Bing, G., Wang, Q., Sun, H., Chen, M., Hu, J., Wang, M., Sun, H., Pu, J., Xia, Z., Liu, J. and Sun, Y. 2019. Canine influenza virus A(H3N2) clade with antigenic variation, China, 2016–2017. Emerg. Infect. Dis. 25(1), 161–165. Moon, H., Hong, M., Kim, J., Seon, B., Na, W., Park, S., An, D., Jeoung, H., Kim, D., Kim, J., Kim, S., Webby, R., Webster, R., Kang, B. and Song, D. 2015. H3N2 canine influenza virus with the matrix gene from the pandemic A/H1N1 virus: infection dynamics in dogs and ferrets. Epidemiol. Infect. 143(4), 772–780. Murcia, P., Baillie, G., Daly, J., Elton, D., Jervis, C., Mumford, J., Newton, R., Parrish, C., Hoelzer, K., Dougan, G., Parkhill, J., Lennard, N., Ormond, D., Moule, S., Whitwham, A., McCauley, J., McKinley, T., Holmes, E., Grenfell, B. and Wood, J. 2010. Intra- and interhost evolutionary dynamics of equine influenza virus. J. Virol. 84(14), 6943–6954. Ning, Z., Wu, X., Cheng, Y., Qi, W., An, Y., Wang, H., Zhang, G., and Li, S. 2012. Tissue distribution of sialic acid-linked influenza virus receptors in beagle dogs. J. Vet. Sci. 13(3), 219–222. Paniker, C. and Nair, C. 1972. Experimental infection of animals with influenzavirus types A and B. Bull. World Health Organ. 47(4), 461–463. Payungporn, S., Crawford, P., Kouo, T., Chen, L., Pompey, J., Castleman, W., Dubovi, E., Katz, J. and Donis, R. 2008. Influenza A virus (H3N8) in dogs with respiratory disease, Florida. Emerg. Infect. Dis. 14(6), 902–908. Said, A., Usui, T., Shinya, K., Ono, E., Ito, T., Hikasa, Y., Matsuu, A., Takeuchi, T., Sugiyama, A., Nishii, N. and Yamaguchi, T. 2011. A sero-survey of subtype H3 influenza A virus infection in dogs and cats in Japan. J. Vet. Med. Sci. 73(4), 541–544. Saktaganov, N., Klivleyeva, N., Ongarbayeva, N., Glebova, T., Lukmanova, G. and Baimukhametova, A. 2020. Study on antigenic relationships and biological properties of swine influenza А/H1N1 virus strains isolated in Northern Kazakhstan in 2018. Sel’skokhozyaistvennaya Biologiya [Agric. Biol.] 55(2), 355–363. Shamenova, M., Glebova T., Klivleyeva N., Baiseiit S., Baimukhametova A., Saktaganov N., Ongarbayeva N. and Ismagulova D. 2023. Serological studies of influenza infection among population in southern region of Kazakhstan during the 2018–2021 epidemic season. J. Pak. Med. Assoc. 73(4), 804–807. Song, D., An, D., Moon, H., Yeom, M., Jeong, H., Jeong, W., Park, S., Kim, H., Han, S., Oh, J., Park, B., Kim, J., Poo, H., Webster, R., Jung, K. and Kang, B. 2011. Interspecies transmission of the canine influenza H3N2 virus to domestic cats in South Korea, 2010. Gen. Virol. 92(Pt 10), 2350–2355. Song, D., Kang, B., Lee, C., Jung, K., Ha, G., Kang, D., Park, S., Park, B. and Oh, J. 2008. Transmission of avian influenza virus (H3N2) to dogs. Emerg. Infect. Dis. 14(5), 741–746. Song, D., Kim, H., Na, W., Hong, M., Park, S., Moon, H., Kang, B., Lyoo, K., Yeom, M., Jeong, D., An, D. and Kim, J. 2015. Canine susceptibility to human influenza viruses (A/pdm 09H1N1, A/H3N2 and B). J. Gen. Virol. 96(Pt 2), 254–258. Song, D., Moon, H., An, D., Jeoung, H., Kim, H., Yeom, M., Hong, M., Nam, J., Park, S., Park, B., Oh, J., Song, M., Webster, R., Kim, J. and Kang, B. 2012. A novel reassortant canine H3N1 influenza virus between pandemic H1N1 and canine H3N2 influenza viruses in Korea. Gen. Virol. 93, 551–554. Song, Q., Zhang, F., Liu, J., Ling, Z., Zhu, Y., Jiang, Sh. and Xie, Zh. 2013. Dog to dog transmission of a novel influenza virus (H5N2) isolated from a canine. Vet. Microbiol. 161(3–4), 331–333. Songserm, T., Amonsin, A., Jam-on, R., Sae-Heng, N., Pariyothorn, N., Payungporn, S., Theamboonlers, A., Chutinimitkul, S., Thanawongnuwech, R. and Poovorawan, Y. 2006. Fatal avian influenza A H5N1 in a dog. Emerg. Infect. Dis. 12(11), 1744–1747. Su, S., Chen, Y., Zhao, F., Chen, J., Xie, J., Chen, Z., Huang, Z., Hu, Y., Zhang, M., Tan, L., Zhang, G. and Li, S. 2013. Avian-origin H3N2 canine influenza virus circulating in farmed dogs in Guangdong, China. Infect. Genet. Evol. 19, 251–256. Su, W., Kinoshita, R., Gray, J., Ji, Y., Yu, D., Peiris, J. and Yen, H. 2019. Seroprevalence of dogs in Hong Kong to human and canine influenza viruses. Vet. Rec. Open. 6(1). е000327. Su, S., Qi, W., Zhou, P., Xiao, C., Yan, Z., Cui, J., Jia, K., Zhang, G., Gray, G., Liao, M. and Li, S. 2014. First evidence of H10N8 avian influenza virus infections among feral dogs in live poultry markets in Guangdong province, China. Clin. Infect. Dis. 59(5), 748–750. Su, S., Zhou, P., Fu, X., Wang, L., Hong, M., Lu, G., Sun, L., Qi, W., Ning, Z., Jia, K., Yuan, Z., Wang, H., Ke, C., Wu, J., Zhang, G., Gray, G. and Li, S. 2014. Virological and epidemiological evidence of avian influenza virus infections among feral dogs in live poultry markets, china: a threat to human health? Clin. Infect. Dis. 58(11), 1644–1646. Sun, X., Xu, X., Liu, Q., Liang, D., Li, C., He, Q., Jiang, J., Cui, Y., Li, J., Zheng, L., Guo, J., Xiong, Y. and Yan, J. 2013. Evidence of avian like H9N2 influenza A virus among dogs in Guangxi, China. Infect. Genet. Evol. 20, 471–475. Voorhees, I., Dalziel, B., Glaser, A., Dubovi, E., Murcia, P., Newbury, S., Toohey-Kurth, K., Su, S., Kriti, D., Van Bakel, H., Goodman, L., Leutenegger, C., Holmes, E. and Parrish, C. 2018. Multiply incursions and recurrent epidemic fade-out of H3N2 canine influenza A virus in the United States. J. Virol. 92(16), e00323–18. Wang, G., Borges, L.G., Stadlbauer, D., Ramos, I., González, M., He, J., Ding, Y., Wei, Z., Ouyang, K., Huang, W., Simon, V., Fernandez-Sesma, A., Krammer, F., Nelson, M., Chen Y. and GarcíaSastre, A. 2019. Characterization of swine-origin H1N1 canine influenza viruses. Emerg. Microbes Infect. 8(1), 1017–1026. Wasik, B., Voorhees, I. and Parrish, C. 2021. Canine and feline influenza. Cold Spring Harb. Perspect. Med. 11(1), a038562. Webster RG, Bean WJ, Gorman OT, Chambers TM, and Kawaoka Y. 1992. Evolution and ecology of influenza A viruses. Microbiol. Rev. 56(1), 152–179. Zhang, K., Zhang, Z., Yu, Z., Li, L., Cheng, K., Wang, T., Huang, G., Yang, S., Zhao, Y., Feng, N., Fu, J., Qin, C., Gao, Y. and Xia, X. 2013. Domestic cats and dogs are susceptible to H9N2 avian influenza virus. Virus Res. 175(1), 52–57. Zhao, F., Liu, C., Yin, X., Zhou, D., Wei, P. and Chang, H. 2014. Serological report of pandemic (H1N1) 2009 infection among cats in northeastern China in 2012-02 and 2013-03. Virol. J. 11, 49. Zhao, S., Schuurman, N., Tieke, M., Quist, B., Zwinkels, S., van Kuppeveld, F., de Haan CAM, and Egberink, H. 2020. Serological screening of influenza A virus antibodies in cats and dogs indicates frequent infection with different subtypes. J. Clin. Microbiol. 58(11), e01689–20. | ||
| How to Cite this Article |
| Pubmed Style Glebova T, Klivleyeva N, Saktaganov N, Shamenova M, Lukmanova G, Baimukhametova A, Baiseiit S, Ongarbayeva N, Orynkhanov K, Ametova A, Ilicheva A. Circulation of influenza viruses in the dog population in Kazakhstan (2023-2024). Open Vet. J.. 2024; 14(8): 1896-1904. doi:10.5455/OVJ.2024.v14.i8.17 Web Style Glebova T, Klivleyeva N, Saktaganov N, Shamenova M, Lukmanova G, Baimukhametova A, Baiseiit S, Ongarbayeva N, Orynkhanov K, Ametova A, Ilicheva A. Circulation of influenza viruses in the dog population in Kazakhstan (2023-2024). https://www.openveterinaryjournal.com/?mno=196578 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i8.17 AMA (American Medical Association) Style Glebova T, Klivleyeva N, Saktaganov N, Shamenova M, Lukmanova G, Baimukhametova A, Baiseiit S, Ongarbayeva N, Orynkhanov K, Ametova A, Ilicheva A. Circulation of influenza viruses in the dog population in Kazakhstan (2023-2024). Open Vet. J.. 2024; 14(8): 1896-1904. doi:10.5455/OVJ.2024.v14.i8.17 Vancouver/ICMJE Style Glebova T, Klivleyeva N, Saktaganov N, Shamenova M, Lukmanova G, Baimukhametova A, Baiseiit S, Ongarbayeva N, Orynkhanov K, Ametova A, Ilicheva A. Circulation of influenza viruses in the dog population in Kazakhstan (2023-2024). Open Vet. J.. (2024), [cited January 25, 2026]; 14(8): 1896-1904. doi:10.5455/OVJ.2024.v14.i8.17 Harvard Style Glebova, T., Klivleyeva, . N., Saktaganov, . N., Shamenova, . M., Lukmanova, . G., Baimukhametova, . A., Baiseiit, . S., Ongarbayeva, . N., Orynkhanov, . K., Ametova, . A. & Ilicheva, . A. (2024) Circulation of influenza viruses in the dog population in Kazakhstan (2023-2024). Open Vet. J., 14 (8), 1896-1904. doi:10.5455/OVJ.2024.v14.i8.17 Turabian Style Glebova, Tatyana, Nailya Klivleyeva, Nurbol Saktaganov, Mira Shamenova, Galina Lukmanova, Assem Baimukhametova, Sagadat Baiseiit, Nuray Ongarbayeva, Kanat Orynkhanov, Anna Ametova, and Aitolkyn Ilicheva. 2024. Circulation of influenza viruses in the dog population in Kazakhstan (2023-2024). Open Veterinary Journal, 14 (8), 1896-1904. doi:10.5455/OVJ.2024.v14.i8.17 Chicago Style Glebova, Tatyana, Nailya Klivleyeva, Nurbol Saktaganov, Mira Shamenova, Galina Lukmanova, Assem Baimukhametova, Sagadat Baiseiit, Nuray Ongarbayeva, Kanat Orynkhanov, Anna Ametova, and Aitolkyn Ilicheva. "Circulation of influenza viruses in the dog population in Kazakhstan (2023-2024)." Open Veterinary Journal 14 (2024), 1896-1904. doi:10.5455/OVJ.2024.v14.i8.17 MLA (The Modern Language Association) Style Glebova, Tatyana, Nailya Klivleyeva, Nurbol Saktaganov, Mira Shamenova, Galina Lukmanova, Assem Baimukhametova, Sagadat Baiseiit, Nuray Ongarbayeva, Kanat Orynkhanov, Anna Ametova, and Aitolkyn Ilicheva. "Circulation of influenza viruses in the dog population in Kazakhstan (2023-2024)." Open Veterinary Journal 14.8 (2024), 1896-1904. Print. doi:10.5455/OVJ.2024.v14.i8.17 APA (American Psychological Association) Style Glebova, T., Klivleyeva, . N., Saktaganov, . N., Shamenova, . M., Lukmanova, . G., Baimukhametova, . A., Baiseiit, . S., Ongarbayeva, . N., Orynkhanov, . K., Ametova, . A. & Ilicheva, . A. (2024) Circulation of influenza viruses in the dog population in Kazakhstan (2023-2024). Open Veterinary Journal, 14 (8), 1896-1904. doi:10.5455/OVJ.2024.v14.i8.17 |