| Research Article | ||
Open Vet. J.. 2024; 14(8): 1836-1842 Open Veterinary Journal, (2024), Vol. 14(8): 1836–1842 Research Article Monitoring of bone matrix acidification by TRAP and ERK biomarkers in the chronic hypercholesterolemia male ratsDuaa Raad Abd-Al-Ameer1*, Wefak Albazi1 and Hayder Ali muhammed21Department of Physiology, Veterinary Medicine College, University of Kerbala, Karbala, Iraq 2Department of Microbiology, Veterinary Medicine College, University of Kerbala, Karbala, Iraq *Corresponding Author: Duaa Raad Abd-Al-Ameer. Department of Physiology, Veterinary Medicine College, University of Kerbala, Karbala, Iraq. Email: duaa.r [at] s.uokerbala.edu.iq Submitted: 15/04/2024 Accepted: 29/07/2024 Published: 31/08/2024 © 2024 Open Veterinary Journal
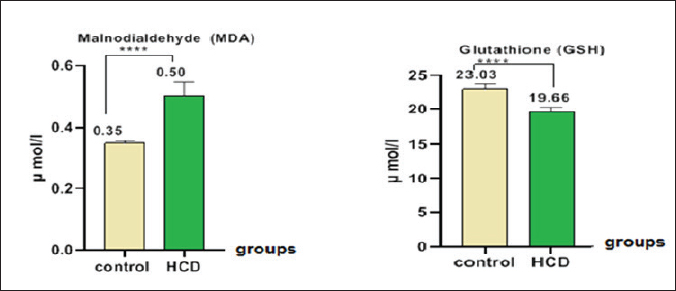
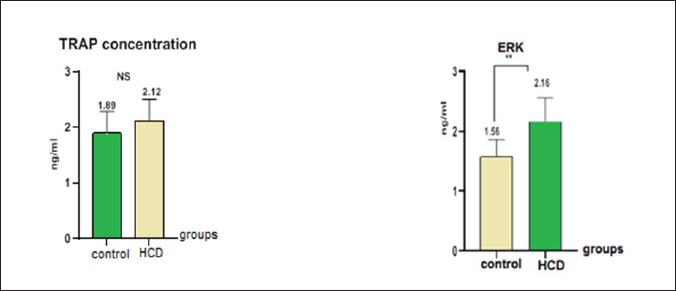
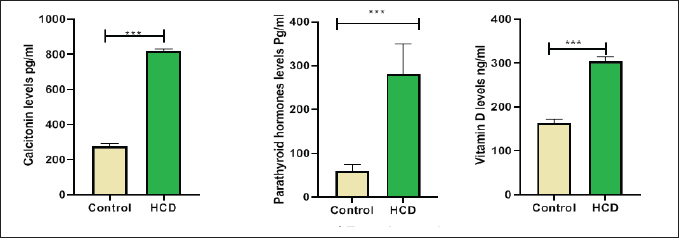
ABSTRACTBackground: Hypercholesterolemia is frequently linked to an elevated risk of cardiovascular diseases, including heart attacks and strokes. Additionally, it could be connected to a higher susceptibility to osteoporosis. Hypercholesterolemia can stimulate the differentiation and activity of osteoclasts, leading to enhanced bone reabsorption and a subsequent net loss of bone tissue. Aim: The purpose of this study was to examine the influence of a high-cholesterol diet on osteoporosis in male rats with differences in biological and oxidative indicators in the hypercholesterolemia diet in male rats. Methods: The samples in this study were twenty male rats, ranging between 1.5 and 2 months, were separated into two groups. In one group, 10 rats were fed a regular diet, while in another group, 10 rats were fed a high-cholesterol diet (2%) over the course of 8 weeks. Samples of blood were obtained at the last stage of the experiment. To calculate physiological and biological markers including extracellular signal-regulated kinase (ERK), tartrate-resistant acid phosphatase (TRAP), hormones, malondialdehyde (MDA), and glutathione (GSH). Results: The results of this study demonstrated a decrease in GSH levels, an increase in ERKs, no significant change in serum TRAP levels, an increase in MDA levels in the blood, and elevated levels of parathyroid hormone, calcitonin, and vitamin D in the cholesterol group. Conclusion: Increased oxidative stress, altered signaling, and disruptions in calcium/bone metabolism associated with cholesterol-related conditions and monitoring biomarker ERK can provide valuable information about disease progression. Keywords: Hypercholesterolemia, Osteoporosis, ERK, TRAP. IntroductionOsteoporosis is a metabolic bone condition that affects many people around the globe. It is represented by a major reduction in the mineral and protein content of the bones. The prevalence of the disease increases with age, creating a substantial strain on healthcare systems and economies worldwide (Sobh et al., 2022). Osteoporosis is a prevalent, long-term metabolic bone disorder characterized by a decrease in bone mass, degradation of bone structure, and heightened vulnerability to fracture (Wawrzyniak and Balawender, 2022). Elevated levels of cholesterol can contribute to a range of Physiological disorders like fat accumulation, Cardiovascular disorders, and Alzheimer’s disease. leading to adverse impacts on the body (Jeong et al., 2018; Raheem et al., 2023). Additionally, high cholesterol levels can negatively affect the microstructure of bones, leading to conditions like osteopenia and osteoporosis, as well as reduced bone strength. These, in turn, increase the risk of fracture, particularly in animals that have low calcium and vitamin D levels or engage in excessive dieting with cholesterol (Perticone et al., 2019). Hypercholesterolemia is a complex disorder influenced by a combination of lifestyle choices and genetic predisposition. Furthermore, it serves as a contributing factor to cardiovascular diseases (CVDs), which result in approximately e172 million fatalities each year) (Fang et al., 2021). A correlation exists between a decrease in bone mineral density (BMD) and the consumption of a high-cholesterol diet (Qiao et al., 2021). Emerging studies have revealed that the consumption of a high-fat diet (HFD), which is rich in fat content. Contributes to both obesity and interferes with metabolic processes. and impacts bone health. Specifically, the effect of this is a decrease in bone density and reduced bone integrity. thereby elevating the potential for both spontaneous and traumatic bone fractures (Duran et al., 2020). During this process, the generation of reactive oxygen species (ROS) can exacerbate inflammation and contribute to the progression of atherosclerosis (Nowak et al., 2017). Increased lipid levels, particularly cholesterol, in the bloodstream, along with the production of the ROS, play a vital role in the advancement of coronary artery disease and atherosclerosis, in order to counteract the detrimental effects of various oxidizing agents, the organism bodies have developed an intricate defense mechanism involving antioxidant enzymes. This defensive system consists of three enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) works in tandem to protect the organism from oxidative damage (Demirci et al., 2022). In rats, hypercholesterolemia was found to be linked to a decrease in bone density, an increase in bone resorption, and a decline in bone formation. Moreover, in vitro investigations demonstrated that free cholesterol hindered the proliferation and differentiation of osteoclast, while simultaneously elevating levels of malondialdehyde (MDA) and reducing the activity of glutathione (GSH). Additionally, hypercholesterolemia resulted in increased osteoblast activity (Arai et al., 2007; Jähn and Bonewald, 2012). Three primary types of bone cells play crucial roles in maintaining bone homeostasis. The types of cells are osteoblasts, osteocytes, and osteoclasts. They are actively involved in bone formation and remodeling processes (Sims and Vrahnas, 2014). The cell types mentioned are derived from two separate stem cell lineages (Triffitt et al., 2022). Osteoblasts and osteocytes are derived from the mesenchymal lineage, while the hematopoietic lineage is accountable for the generation of osteoclasts (Kim et al., 2020). Osteoclasts are large, multinucleated cells derived from hematopoietic origins, and their primary function is the resorption of bone tissue (Wojda and Donahue, 2018). Maintaining a delicate equilibrium between osteoclastic bone resorption and osteoplastic bone formation is crucial for maintaining overall bone homeostasis. Direct connections exist between parathyroid hormone (PTH) and cholesterol metabolism, where vitamin D (regulated by PTH) affects cholesterol production. Nevertheless, they lack a direct relationship or influence over each other. PTH primarily regulates calcium and phosphate levels, while cholesterol has its own metabolic pathways and regulatory mechanisms (Song, 2017). PTH is a hormone secreted by the parathyroid glands; very small glands situated in the neck. PTH is essential for controlling calcium and phosphate levels in the body. It affects the structure of the skeleton. maintain appropriate levels of calcium and phosphate in the bloodstream. PTH helps increase calcium levels by promoting its release from bones (Wein and Kronenberg, 2018). Calcitonin is a hormone secreted by the thyroid gland. The primary role is to control the amounts of calcium and phosphate in the body. When blood calcium levels are too high, calcitonin works to decrease them by inhibiting calcium release from the bones and increasing calcium excretion by the kidneys. Calcitonin also plays a role in inhibiting the activity of osteoclasts, which are cells responsible for breaking down bone tissue (Sheweita and Khoshhal, 2007). Vitamin D plays a vital function in maintaining skeletal health by influencing the process of bone mineralization and the balance of calcium and phosphate levels (Xia, 2022). It also helps regulate the PTH (Warren et al., 2021). Vitamin D and cholesterol metabolism are closely linked through the same biosynthetic route. Cholesterol is a multifunctional lipid. It is an essential element of cell membranes. (Zidovetzki and Levitan., 2007). The study aimed to determine some physiological parameters and hormones in the hypercholesterolemic diet in male rats. Materials and MethodsExperimental designThis study exploited an experiment of twenty Wister male rats. The rats were acquired from the Faculty of Pharmaceutical at the University of Kerbala in Iraq. Each rat had an average and standard deviation weight of 100 ± 7.5 g, with a standard deviation of 75 g. The rats, aged between 4 and 6 weeks, were housed in sterile, specialized plastic enclosures. To initiate the experimental design, the rodents were placed in a clean compartment within a box. The study employed a 12-hour photoperiod and maintained a moisture content of the air at 50% ± 5%. The participants were kept for two weeks to adapt to the Typical testing conditions. The experiment commenced on September 25th. and ended on November 23rd. The experiment involved regulating the room temperature to a range of 23°C–26°C. Utilizing a room thermostat. The room’s air was always replenished through continuous ventilation suction, and the animal was provided with nourishment through pre-prepared fresh feed pellets. Twenty whitish male rats were allocated at random and administered the following treatments for eight weeks. The control group consisted of half of the rats in this group who were given a normal diet that was consumed orally. The remaining half of the rats were administered a high-cholesterol diet containing 2% cholesterol by weight for eight weeks (Cunha et al., 2021). Collecting blood samplesAfter 8 weeks, the blood samples were taken from animals following the administration of ketamine and xylazine to ensure they were calm and still. The collected serum was subjected to centrifugation for 5 minutes at a speed of 4,000 revolutions per minute using a specialized gel tube, following the heart puncture method. Upon separation, the serum was directly transferred into 1.5 microcentrifuge tubes for preservation and stored in a freezer at a temperature of −30°C. The concentrations of the extracellular signal-regulated kinase (ERK, tartrate-resistant acid phosphatase (TRAP)), GSH, and MDA were measured using ELISA kits (Fine Test, Wuhan, China) and a special blood analysis company (SQLab, Seoul, Korea) assessed the levels of PTH, calcitonin and vitamin D in the blood. Statistical analysisGraphPad Prism 8.0 was used to conduct the statistical analysis. The standard of significance for the analysis was p–0.05, and the data points were reported as mean–standard error. Ethical approvalThe study was conducted at the anatomical facility of the College of Veterinary Medicine at Kerbala University in Iraq, under the reference number (UOK.VET.PH.2023.076). ResultsThe study showed the concentration of antioxidants in rats dosed with cholesterol, it was found a significant increase (p < 0.05) in the rate of MDA (0.5014 ± 0.083) concurrent with a significant decrease (p < 0.05) in the rate of GSH (19.67 ± 1.46) compare with control groups (0.3500 ± 0.046) and (23.03 ± 2.74), respectively (Fig. 1). On the other hand, the study found a significant increase in membrane proteins responsible for building bones in mice, as we found a significant increase (p < 0.05) in (ERK) in the hypercholesteremic diet group (2.161 ± 0.057 ) ng/ml compared with control group (1.566 ± 0.034 ) ng/ml and this study show a no-significant (p ≤ 0.05) in (tartrate-resistant acid phosphatase (TRAP)) in the hypercholesteremic diet group (2.120 ± 0.036) ng/ml compared with control group (1.899 ± 0.045) ng/ml, respectively (Fig. 2). The results from this study of the present examination revealed significant variations in the concentration of hormones, including PTH and Calcitonin and vitamin D levels in the rats’ blood. DiscussionHypercholesterolemia can cause the buildup of lipids, including cholesterol, in bone tissue, this lipid accumulation within the bone can disrupt the normal bone structure, leading to compromised bone strength. Consequently, the bones become more susceptible to fractures (AL-tememy, 2023). Both hypercholesterolemia and a diet high in cholesterol have detrimental impacts on bone health tissues (Anagnostis et al., 2022). High-cholesterol diets also decrease bone marrow stromal cell proliferation and differentiation, which results in decreased osteoblastogenesis. Under these circumstances, the functionality and equilibrium of osteoblasts are disrupted, leading to an increase in osteoclast activity and quantity, ultimately resulting in a decrease in bone mass (Akhmetshina et al., 2023). Hypercholesterolemia can impair the function of endothelial cells lining the blood vessels. Healthy endothelial cells produce antioxidants and regulate vascular tone, but dysfunction of these cells can disrupt antioxidant production and promote oxidative stress (Queiroz et al., 2024). The current study shows a significant reduction in serum GSH in the treated group as compared with the control group as shown in Figure 1, also there is a significant increase in serum MDA in the HCD group as compared with the control group. ROS generation may rise because of elevated blood cholesterol levels, oxidation can occur with elevated cholesterol levels, especially with low-density lipoprotein cholesterol (LDL-C) (Murphy and Johnson, 2008). ROS can be generated as a result of oxidative stress caused by oxidises low-density lipoproteins, known as ox-LDL (Safo and AlDulaimi, 2022).
Fig. 1. Malnodialdehyde and GPx concentrations in male rats after hypercholesteremic diet, rats in control group were fed normal diets daily for eight weeks, while rats in hypercholesteremic-diet (0.2 mg/kg BW cholesterol) were fed normal diets daily for eight weeks.
Fig. 2. TRAP and ERK concentrations in male rats after hypercholesteremic diet; TRAP concentration in ng/ml; ERK concentration in ng/ml; rats in control group were fed normal diets daily for eight weeks, while rats in hypercholesteremicdiet (0.2 mg/kg BW cholesterol) were fed normal diets daily for eight weeks.
Fig. 3. PTH and calcitonine and vitamin D in male rats after hypercholesteremic diet, rats in control group were fed normal diets daily for eight weeks, while rats in hypercholesteremic-diet (0.2 mg/kg BW cholesterol) were fed normal diets daily for eight week. The endothelial cells that line blood arteries may suffer harm from high cholesterol levels and this results in an increase of oxidative stress by inflammation (Fernández-Sánchez et al., 2011). On the other hand, high cholesterol can affect how well mitochondria operate, and when they malfunction, the total amount of the ROS produced might go up which leads to a reduction in GSH levels and elevation in MDA levels (Xiao et al., 2022). The enzyme NADPH oxidase is linked to elevated cholesterol due to its generation of ROS (Masoud et al., 2014). The current investigation revealed no significant disparity in serum TRAP levels between the control group and the treated group, as depicted in Figure 2. This outcome may be attributed to rats having individual variability in their TRAP response to osteoporosis induction. Some rats may see a notable rise in serum TRAP levels, while others may not. This variance may be attributed to hereditary causes, hormone changes, or other underlying variables that impact bone metabolism (Garnero, 2008). This study was in disagreement with some of the studies that have shown that TRAP levels and activity are elevated in individuals with osteoporosis compared to healthy controls. This indicates increased osteoclast activity and bone resorption, which are characteristic features of osteoporosis. TRAP levels have been correlated with BMD and can be used as an indicator of bone turnover and disease severity (Chung et al., 2005). Also, results demonstrated that the hypercholesterolemic diet groups exhibited elevated ERK activity compared to the control groups (Fig. 3). The ERK signaling pathway has been extensively investigated in the context of osteoporosis, and its activation or inhibition can potentially impact bone health, activation of the ERK signaling pathway has been linked to enhanced osteoblast (cells responsible for bone formation) activity and increased bone formation and conversely, inhibiting the ERK signaling pathway may lead to reduced osteoblast activity and a consequent loss of bone mass (Matsushita et al., 2009). Hypercholesterolemia can lead to alterations in lipid raft composition affecting the localization and activation of signaling molecules including those involved in the ERK pathway. The arrangement of lipid rafts may enhance the clustering and activation of RTKs and downstream effectors, leading to sustained ERK activation (Degirmenci et al., 2020). Also, High cholesterol levels can induce oxidative stress which can activate ERK signaling pathways. Oxidative stress is known to activate various intracellular signaling cascades including ERK in a variety of cell types. Therefore, oxidative stress induced by a hypercholesterolemic diet may contribute to increased ERK activation in bone cells (Rezatabar et al., 2019). In the current study, there was a significant increase in the (PTH, vitamin D, and calcitonin) in the cholesterol group as compared with the control group as shown in Figure 3. Calcium-sensing receptors (CaSRs) play a crucial role in regulating PTH secretion by sensing changes in extracellular calcium levels and the intracellular. Oxidative stress has been associated with changes in the function of the CaSR (Mroczko et al., 2023). The CaSR is a G protein-coupled receptor that plays a crucial role in maintaining calcium homeostasis in the body by sensing changes in extracellular calcium levels. It is primarily expressed in parathyroid glands (Chavez-Abiega et al., 2020). Oxidative stress can impact the expression levels of the CaSR. Complex intracellular pathways regulate CaSR signaling, and oxidative stress syndrome may impair the receptor’s signal-transmitting capacity in response to changes in calcium levels (Kosiba et al., 2020). Prolonged exposure to oxidative stress may contribute to parathyroid hyperplasia, a condition characterized by the hyperactivity of the parathyroid glands, this actiate could be a compensatory response to oxidative damage, aiming to maintain adequate PTH production. However, the hypertrophic changes may lead to dysregulation of PTH secretion, leading to the connection between oxidative stress and the endocrine function of the parathyroid glands. This can interrupt these signaling cascades, which can lead to an imbalance in the level of the (PTH, vitamin D, and calcitonin) in the cholesterol grouping as compared with the control group (Hong et al., 2021). ConclusionIn conclusion, these results provide insights into the biochemical and hormonal alterations associated with cholesterol-related conditions; and concluded that increased oxidative stress, altered signaling, and disruptions in calcium/bone metabolism are associated with cholesterol-related conditions. AcknowledgmentThe authors would like to thank the University of Kerbala, College of Veterinary Medicine for facilitating this research. Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionsDuaa Raad Abd Al Ameer was data collection and curation of study; Wefak Albazi was formal analysis and interpretation of results and Hayder ALi muhammed writing the original draft and paragraph and designing the statistical figures. FundingThis research received no external funding. Data availabilityAll data supporting the findings of this study are available within the manuscript and no additional data sources are required. ReferencesAl-Safo, A.A. and AlDulaimi, L.H. 2022. Effect of orlistat and aquatic extract of Rosmarinus officinalis leaves in histopathological changes in kidney of albino rat. Iraqi. J. Vet. Sci. 36(2), 393–400. AL-tememy, H.A.H. 2023. Effect of folic acid on avascular bone necrosis in mice associated with hypercholesterolemia. J. Popul. Therap. Clin. Pharma. 30(6), 407–413. Anagnostis, P., Florentin, M., Livadas, S., Lambrinoudaki, I. and Goulis, D.G. 2022. Bone health in patients with dyslipidemias: an underestimated aspect. Inter. J. Mol. Sci. 23(3), 1639. Akhmetshina, E.S. and Khursan, S.L. 2023. Theoretical determination of the standard enthalpies of formation of alkyl radicals using the concept of a complete set of homodesmotic reactions. J. Mol. Graph. Model. 125, 108615. Arai, M., Shibata, Y., Pugdee, K., Abiko, Y. and Ogata, Y. 2007. Effects of reactive oxygen species (ROS) on antioxidant system and osteoblastic differentiation in MC3T3-E1 cells. IUBMB Life 59(1), 27–33. Chavez-Abiega, S., Mos, I., Centeno, P.P., Elajnaf, T., Schlattl, W., Ward, D.T. and Kallay, E. 2020. Sensing extracellular calcium–an insight into the structure and function of the calcium-sensing receptor (CaSR). Ca Signal. 2020, 1031–1063. Chung, S.L., Chu, M.F., Zhang, Y., Xie, Y., Lo, C.H., Lee, T.Y. and Wang, Y. 2005. Tibetan tectonic evolution inferred from spatial and temporal variations in post-collisional magmatism. Earth Sci. Rev. 68(3–4), 173–196. Cunha, L.F., Ongaratto, M.A., Endres, M. and Barschak, A.G. 2021. Modelling hypercholesterolaemia in rats using high cholesterol diet. Intern. J. Exper. Patho. 102(2), 74–79. Degirmenci, U., Wang, M. and Hu, J. 2020. Targeting aberrant RAS/RAF/MEK/ERK signaling for cancer therapy. Cells 9(1), 198. Demirci-Cekic, S., Özkan, G., Avan, A.N., Uzunboy, S., Çapanoğlu, E. and Apak, R. 2022. Biomarkers of oxidative stress and antioxidant defense. J. Pharm. Boil. Anal. 209, 114477. Duran, E.K., Aday, A.W., Cook, N.R., Buring, J.E., Ridker, P.M. and Pradhan, A.D. 2020. Triglyceride-rich lipoprotein cholesterol, small dense LDL cholesterol, and incident cardiovascular disease. J. Am. College Cardiol. 75(17), 2122–2135. Fang, Y., Zhu, J., Fan, J., Sun, L., Cai, S., Fan, C. and Li, Y. 2021. Dietary inflammatory index in relation to bone mineral density, osteoporosis risk and fracture risk: a systematic review and meta-analysis. Osteoporosis Inter. 32, 633–643. Fernández-Sánchez, A., Madrigal-Santillán, E., Bautista, M., Esquivel-Soto, J., Morales-González, Á., Esquivel-Chirino, C. and Morales-González, J.A. 2011. Inflammation, oxidative stress, and obesity. Intern. J. Mol. Sci. 12(5), 3117–3132. Garnero, P. 2008. Biomarkers for osteoporosis management: utility in diagnosis, fracture risk prediction and therapy monitoring. Mol. Diagn. Therap. 12, 157–170. Hong, G., Chen, Z., Han, X., Zhou, L., Pang, F., Wu, R. and Wei, Q. 2021. A novel RANKL-targeted flavonoid glycoside prevents osteoporosis through inhibiting NFATc1 and reactive oxygen species. Clin. Trans. Med. 11(5), e392. Jähn, K. and Bonewald, L.F. 2012. Bone cell biology: osteoclasts, osteoblasts, osteocytes. In Pediatric bone. Cambridge, MA: Academic Press, pp: 1–8. Jeong, S.M., Choi, S., Kim, K., Kim, S.M., Lee, G., Park, S.Y. and Park, S.M. 2018. Effect of change in total cholesterol levels on cardiovascular disease among young adults. J. Am. Heart Assoc. 7(12), e008819. Kim, J.M., Lin, C., Stavre, Z., Greenblatt, M.B. and Shim, J.H. 2020. Osteoblast-osteoclast communication and bone homeostasis. Cells 9(9), 2073. Kosiba, A.A., Wang, Y., Chen, D., Wong, C.K.C., Gu, J. and Shi, H. 2020. The roles of calcium-sensing receptor (CaSR) in heavy metals-induced nephrotoxicity. Life Sci. 242, 117183. Masoud, R., Bizouarn, T. and Houée-Levin, C. 2014. Cholesterol: a modulator of the phagocyte NADPH oxidase activity-A cell-free study. Redox Biol. 3, 16–22. Matsushita, T., Chan, Y.Y., Kawanami, A., Balmes, G., Landreth, G.E. and Murakami, S. 2009. Extracellular signal-regulated kinase 1 (ERK1) and ERK2 play essential roles in osteoblast differentiation and in supporting osteoclastogenesis. Mol. Cell. Biol. 29(21), 5843–5857. Mroczko, B., Kulczynska-Przybik, A., Borawska, R., Dulewicz, M., Doroszkiewicz, J., Karpiuk, M. and Slowik, A. 2023. The significance of the calbindin-D in Alzheimer’s disease. Alzheimer’s Dementia 19, e062132. Murphy, R.C. and Johnson, K.M. 2008. Cholesterol, reactive oxygen species, and the formation of biologically active mediators. J. Bio. Chem. 283(23), 15521–15525. Nowak, W.N., Deng, J., Ruan, X.Z. and Xu, Q. 2017. ROS generation and atherosclerosis. Arteriosclerosis Thrombosis vascul. Biol. 37(5), e41–e52. Perticone, M., Maio, R., Sciacqua, A., Suraci, E., Pinto, A., Pujia, R. and Perticone, F. 2019. Ketogenic diet-induced weight loss is associated with an increase in vitamin D levels in obese adults. Molecules 24(13), 2499. Qiao, J., Wu, Y. and Ren, Y. 2021. The impact of a high fat diet on bones: potential mechanisms. Food Funct. 12(3), 963–975. Queiroz, M.I., Lazaro, C.M., Dos Santos, L.M., Rentz, T., Virgilio-da-Silva, J.V., Moraes-Vieira, P.M. and Oliveira, H.C. 2024. In vivo chronic exposure to inorganic mercury worsens hypercholesterolemia, oxidative stress and atherosclerosis in the LDL receptor knockout mice. Ecotoxicol. Environ. Safe. 275, 116254. Raheem, H.A., Albazi, W., Altaee, R., Al-Thuwaini, T.M. and Jhoni, G.H. 2023. Effect of hypercholestermic diet on the β-amyloid deposition and microglial cells with some biomarkers alterations in male rats. Iraqi. J. Vet. Sci. 37(Suppl. I–IV), 251–257. Rezatabar, S., Karimian, A., Rameshknia, V., Parsian, H., Majidinia, M., Kopi, T. A. and Yousefi, B. 2019. RAS/MAPK signaling functions in oxidative stress, DNA damage response and cancer progression. J. Cell. Physiol. 234(9), 14951–14965. Sheweita, S.A. and Khoshhal, K.I. 2007. Calcium metabolism and oxidative stress in bone fractures: role of antioxidants. Curr. Drug Metab. 8(5), 519–525. Sims, N.A. and Vrahnas, C. 2014. Regulation of cortical and trabecular bone mass by communication between osteoblasts, osteocytes and osteoclasts. Arch. Biochem. Biophys. 561, 22–28. Sobh, M.M., Abdalbary, M., Elnagar, S., Nagy, E., Elshabrawy, N., Abdelsalam, M. and El-Husseini, A. 2022. Secondary osteoporosis and metabolic bone diseases. J. Clin. Med. 11(9), 2382. Song, L. 2017. Calcium and bone metabolism indices. Adv. Clin. Chem. 82, 1–46. Triffitt, J. T. and Wang, Q. 2022. Stem cell fate and microenvironment. Biomater. Transl. 3(1), 1. Warren, T., McAllister, R., Morgan, A., Rai, T.S., McGilligan, V., Ennis, M. and Watterson, S. 2021. The interdependency and co-regulation of the vitamin D and cholesterol metabolism. Cells 10(8), 2007. Wawrzyniak, A. and Balawender, K. 2022. Structural and metabolic changes in bone. Animals 12(15), 1946. Wein, M.N. and Kronenberg, H.M. 2018. Regulation of bone remodeling by parathyroid hormone. Cold Spring Harbor Perspectives Med. 8(8), a031237. Wojda, S.J. and Donahue, S.W. 2018. Parathyroid hormone for bone regeneration. J. Orthopaedic Res. 36(10), 2586–2594. Xiao, Z., Yu, X., Zhang, S. and Liang, A. 2022. The expression levels and significance of GSH, MDA, SOD, and 8-OHdG in osteochondral defects of rabbit knee joints. Bio. Med. Res. Intern. 2022:6916179. Xia, Z. 2022. Skeletal interoception: an emerging area for musculoskeletal research. Biomater. Translation. 3(4), 237. Zidovetzki, R. and Levitan, I. 2007. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim. Biophys. Acta Biomembran. 1768(6), 1311–1324. | ||
| How to Cite this Article |
| Pubmed Style Abd-al-ameer DR, Albazi W, Muhammed HA. Monitoring of bone matrix acidification by TRAP and ERK biomarkers in the chronic hypercholesterolemia male rats. Open Vet. J.. 2024; 14(8): 1836-1842. doi:10.5455/OVJ.2024.v14.i8.11 Web Style Abd-al-ameer DR, Albazi W, Muhammed HA. Monitoring of bone matrix acidification by TRAP and ERK biomarkers in the chronic hypercholesterolemia male rats. https://www.openveterinaryjournal.com/?mno=196924 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i8.11 AMA (American Medical Association) Style Abd-al-ameer DR, Albazi W, Muhammed HA. Monitoring of bone matrix acidification by TRAP and ERK biomarkers in the chronic hypercholesterolemia male rats. Open Vet. J.. 2024; 14(8): 1836-1842. doi:10.5455/OVJ.2024.v14.i8.11 Vancouver/ICMJE Style Abd-al-ameer DR, Albazi W, Muhammed HA. Monitoring of bone matrix acidification by TRAP and ERK biomarkers in the chronic hypercholesterolemia male rats. Open Vet. J.. (2024), [cited January 25, 2026]; 14(8): 1836-1842. doi:10.5455/OVJ.2024.v14.i8.11 Harvard Style Abd-al-ameer, D. R., Albazi, . W. & Muhammed, . H. A. (2024) Monitoring of bone matrix acidification by TRAP and ERK biomarkers in the chronic hypercholesterolemia male rats. Open Vet. J., 14 (8), 1836-1842. doi:10.5455/OVJ.2024.v14.i8.11 Turabian Style Abd-al-ameer, Duaa Raad, Wefak Albazi, and Hayder Ali Muhammed. 2024. Monitoring of bone matrix acidification by TRAP and ERK biomarkers in the chronic hypercholesterolemia male rats. Open Veterinary Journal, 14 (8), 1836-1842. doi:10.5455/OVJ.2024.v14.i8.11 Chicago Style Abd-al-ameer, Duaa Raad, Wefak Albazi, and Hayder Ali Muhammed. "Monitoring of bone matrix acidification by TRAP and ERK biomarkers in the chronic hypercholesterolemia male rats." Open Veterinary Journal 14 (2024), 1836-1842. doi:10.5455/OVJ.2024.v14.i8.11 MLA (The Modern Language Association) Style Abd-al-ameer, Duaa Raad, Wefak Albazi, and Hayder Ali Muhammed. "Monitoring of bone matrix acidification by TRAP and ERK biomarkers in the chronic hypercholesterolemia male rats." Open Veterinary Journal 14.8 (2024), 1836-1842. Print. doi:10.5455/OVJ.2024.v14.i8.11 APA (American Psychological Association) Style Abd-al-ameer, D. R., Albazi, . W. & Muhammed, . H. A. (2024) Monitoring of bone matrix acidification by TRAP and ERK biomarkers in the chronic hypercholesterolemia male rats. Open Veterinary Journal, 14 (8), 1836-1842. doi:10.5455/OVJ.2024.v14.i8.11 |